Green Extraction of Volatile Terpenes from Artemisia annua L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Content and Composition of Essential Oil of Artemisia annua Obtained by Hydrodistillation and Steam Distillation
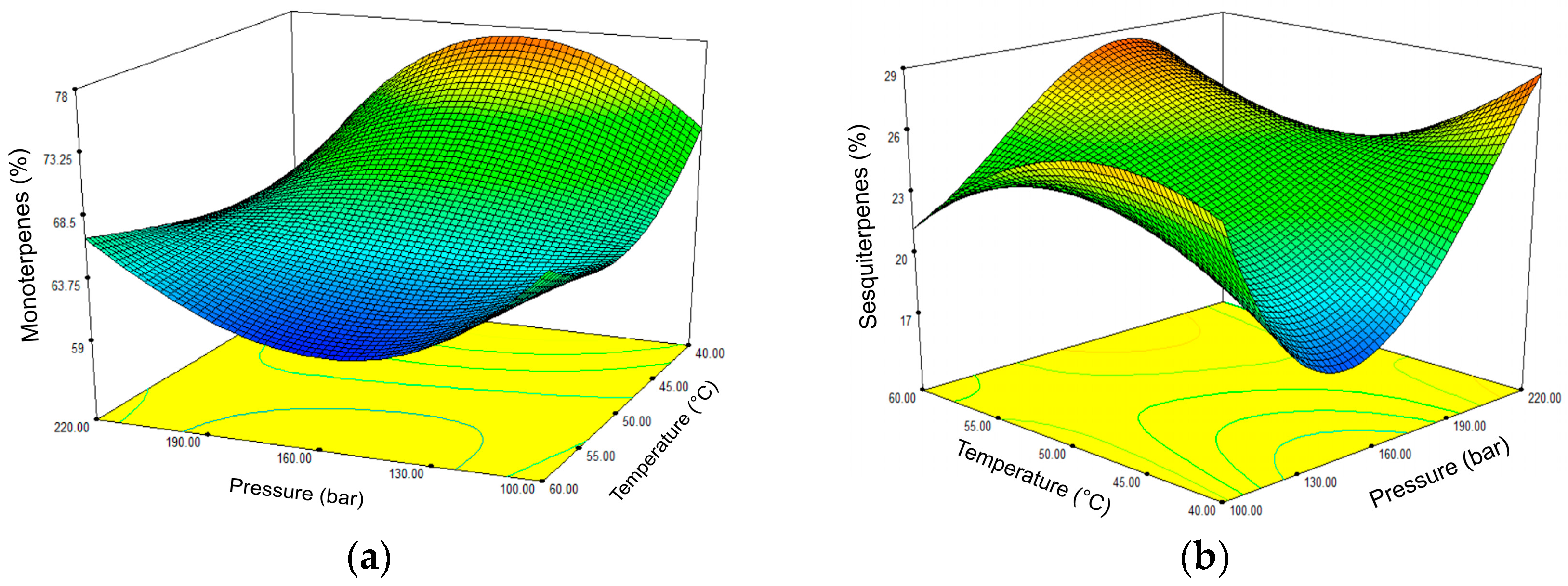
2.2. Content and Composition of sc-CO2 Extracts of Artemisia annua Depending on the Applied Pressure and Temperature—Full Factorial Design Approach
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Essential Oil by Hydrodistillation and Steam Distillation
3.3. Supercritical Carbon Dioxide (sc-CO2) Extraction
3.4. GC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, M.; Vilatersana, R.; Hidalgo, O.; Garcia-Jacas, N.; Susanna, A.; Schneeweiss, G.M.; Vallès, J. Molecular phylogeny and evolution of floral characters of Artemisia and allies (Anthemideae, Asteraceae): Evidence from NrDNA ETS and ITS sequences. Taxon 2008, 57, 66–78. [Google Scholar]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, isolation and characterization of bioactive compounds from Artemisia and their biological significance: A review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Kumar Chellappan, D. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Prasad Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Hussain, M.; Thakurn, R.K.; Khazir, J.; Ahmed, S.; Khan, M.I.; Rahi, P.; Peer, L.A.; Shanmugam, P.V.; Kaur, S.; Raina, S.N.; et al. Traditional uses, phytochemistry, pharmacology, and toxicology of the genus Artemisia L. (Asteraceae): A high-value medicinal plant. Curr. Top. Med. Chem. 2024, 24, 301–342. [Google Scholar]
- Su, X.-Z.; Miller, L.H. The discovery of artemisinin and Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qui, F.; Zang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a traditional plant brought to light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Baggieri, M.; Gioacchini, S.; Borgonovo, G.; Catinella, G.; Marchi, A.; Picone, P.; Vasto, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; et al. Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2. Biomed. Pharmacother. 2023, 168, 115682. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Salimov, A.; Numonov, S.; Safomuddin, A.; Bakri, M.; Salimov, T.; Setzer, W.N.; Habasi, M. Chemical composition, antioxidant, and antimicrobial activities of the essential oils from Artemisia annua L. growing wild in Tajikistan. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential oil of Artemisia annua L.: An extraordinary component with numerous antimicrobial properties. Evid. Based Complement. Alternat. Med. 2014, 2014, 159819. [Google Scholar]
- Hong, M.; Kim, M.; Jang, H.; Bo, S.; Deepa, P.; Sowndhararajan, K.; Kim, S. Multivariate analysis of essential oil composition of Artemisia annua L. collected from different locations in Korea. Molecules 2023, 28, 1131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, L.; Vazquez, J.F.T.; Song, S. Optimization of supercritical CO2 extraction of essential oil from Artemisia annua L. by means of response surface methodology. J. Essent. Oil Bear. Plants 2017, 20, 314–327. [Google Scholar] [CrossRef]
- Rao, A.B.; Sardeshpande, V.R. A hydrodistillation-based essential oils extraction: A quest for the most effective and cleaner technology. Sustain. Chem. Pharm. 2023, 36, 101270.TrAC. [Google Scholar]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Cheriyan, B.V.; Karunakar, K.K.; Anandakumar, R.; Murugathirumal, A.; Kumar, A.S. Eco-friendly extraction technologies: A comprehensive review of modern green analytical methods. Sustain. Chem. Clim. Action. 2025, 6, 100054. [Google Scholar]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Tecnol. 2014, 133, 443–451. [Google Scholar]
- Šulniūtė, V.; Baranauskienė, R.; Ragažinskien, O.; Rimantas Venskutonis, P. Comparison of composition of volatile compounds in ten Salvia species isolated by different methods. Flavor. Fragr. J. 2017, 32, 254–264. [Google Scholar]
- Bendif, H.; Adouni, K.; Miara, M.D.; Baranauskienė, R.; Kraujalis, P.; Venskutonis, P.R.; Nabavi, S.M.; Maggi, F. Essential oils (EOs),pressurized liquid extracts (PLE) and carbon dioxide supercritical fluid extracts (SFE-CO2) from Algerian Thymus munbyanus as valuable sources of antioxidants to be used on an industrial level. Food Chem. 2018, 260, 289–292. [Google Scholar] [CrossRef]
- Donato, R.; Santomauro, F.; Bilia, A.R.; Flamini, G.; Sacco, C. Antibacterial activity of Tuscan Artemisia annua essential oil and its major components against some foodborne pathogens. LWT-Food Sci. Technol. 2015, 64, 1251–1254. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar]
- Grifoni, L.; Sacco, C.; Donato, R.; Tziakas, S.; Tomou, E.-M.; Skaltsa, H.; Vanti, G.; Bergonzi, M.C.; Bilia, A.R. Environmentally friendly microemulsions of essential oils of Artemisia annua and Salvia fruticosa to protect crops against Fusarium verticillioides. Nanomaterials 2024, 14, 1715. [Google Scholar] [CrossRef]
- Chebbac, K.; Benziane Ouaritini, Z.; El Moussaoui, A.; Chalkha, M.; Lafraxo, S.; Bin Jardan, Y.A.; Nafidi, H.-A.; Bourhia, M.; Guemmouh, R. Antimicrobial and antioxidant properties of chemically analyzed essential oil of Artemisia annua L. (Asteraceae) native to Mediterranean area. Life 2023, 13, 807. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Chifiriuc, M.C.; Badea, I.A.; Buleandra, M.; Lazar, V. Chemical composition and antipathogenic activity of Artemisia annua essential oil from Romania. Chem. Biodivers. 2015, 12, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Risaliti, L.; Pini, G.; Ascrizzi, R.; Donato, R.; Sacco, C.; Bergonzi, M.C.; Salvatici, M.C.; Bilia, A.R. Artemisia annua essential oil extraction, characterization, and incorporation in nanoliposomes, smart drug delivery systems against Candida species. J. Drug Deliv. Sci. Technol. 2020, 59, 101849. [Google Scholar]
- Vidic, D.; Čopra-Janićijević, A.; Miloš, M.; Maksimović, M. Effects of different methods of isolation on volatile composition of Artemisia annua L. Int. J. Anal. Chem. 2018, 2018, 9604183. [Google Scholar]
- Bedinim, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhigzhitzhapova, S.V.; Dylenova, E.P.; Gulyaev, S.M.; Randalova, T.E.; Taraskin, V.V.; Tykheev, Z.A.; Radnaeva, L.D. Composition and antioxidant activity of the essential oil of Artemisia annua L. Nat. Prod. Res. 2020, 34, 2668–2671. [Google Scholar] [CrossRef]
- Santomauro, F.; Donato, R.; Sacco, C.; Pini, G.; Flamini, G.; Bilia, A.F. Vapour and liquid-phase Artemisia annua essential oil activities against several clinical strains of Candida. Planta Med. 2016, 82, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Stanković Jeremić, J.; Todosijević, M.; Kiprovski, B.; Vidović, S.; Vladić, J.; Pezo, L. Comparative study of the essential oil and hydrosol composition of sweet wormwood (Artemisia annua L.) from Serbia. Chem. Biodivers. 2022, 19, e202100954. [Google Scholar] [CrossRef]
- Ćavar, S.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crop Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Liu, H.; Guo, S.-S.; Lu, L.; Li, D.; Liang, J.; Huang, Z.-H.; Zhou, Y.-M.; Zhang, W.-J.; Du, S. Essential oil from Artemisia annua aerial parts: Composition and repellent activity against two storage pests. Nat. Prod. Res. 2021, 35, 822–825. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, M. Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, F.F.; Carvalho, J.C.T.; Rehder, V.L.G. Central properties of the essential oil and the crude ethanol extract from aerial parts of Artemisia annua L. Pharmacol. Res. 2003, 48, 497–502. [Google Scholar] [CrossRef]
- Habibi, Z.; Ghanian, S.; Ghasemi, S.; Yousefi, M. Chemical composition and antibacterial activity of the volatile oil from seeds of Artemisia annua L. from Iran. Nat. Prod. Res. 2013, 27, 198–200. [Google Scholar] [CrossRef]
- Ma, L.; Wei, L.; Chen, X.; Wang, W.; Lu, J.; Li, Y.; Yao, L. Chemical composition, antioxidative and antimicrobial activities of essential oil of wild Artemisia annua from Ningxia, China. Nat. Prod. Res. 2024, 38, 4340–4346. [Google Scholar] [CrossRef]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Kőszegi, T.; Széchenyi, A. Antimicrobial activity of different Artemisia essential oil formulations. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef] [PubMed]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Haerdi, W.; Christen, P.; Veuthey, J.L. Extraction of artemisinin and artemisinic acid from Artemisia annua L. using supercritical carbon dioxide. J. Chromatogr. A 1997, 785, 353–360. [Google Scholar] [PubMed]
- Baldino, L.; Reverchon, E.; Della Porta, G. An optimized process for SC-CO2 extraction of antimalarial compounds from Artemisia annua L. J. Supercrit. Fluids 2017, 128, 89–93. [Google Scholar]
- Martinez-Correa, H.A.; Bitencourt, R.G.; Kayano, A.C.A.V.; Magalhães, P.M.; Costa, F.T.M.; Cabral, F.A. Integrated extraction process to obtain bioactive extracts of Artemisia annua L. leaves using supercritical CO2, ethanol and water. Ind. Crops Prod. 2017, 95, 535–542. [Google Scholar]
- Ciftci, O.N.; Cahyadi, J.; Guigard, S.E.; Saldaña, M.D.A. Optimization of artemisinin extraction from Artemisia annua L. with supercritical carbon dioxide + ethanol using response surface methodology. Electrophoresis 2018, 39, 1926–1933. [Google Scholar]
- Vidović, S.; Simić, S.; Gavarić, A.; Aćimović, M.; Vladic, J. Extraction of sweet wormwood (Artemisia annua L.) by supercritical carbon dioxide. Lek. Sirovine 2020, 40, 22–26. [Google Scholar] [CrossRef]
- Confortin, T.C.; Todero, I.; Canabarro, N.; Luft, L.; Ugalde, G.A.; Neto, J.R.C.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Supercritical CO2 extraction of compounds from different aerial parts of Senecio brasiliensis: Mathematical modeling and effects of parameters on extract quality. J. Supercrit. Fluids 2019, 153, 104589. [Google Scholar]
- Vladić, J.; Jerković, I.; Svilović, S.; Pavić, V.; Pastor, K.; Paiva, A.; Jokić, S.; Rebocho, S.; Duarte, A.R. Evaluation of the volatiles’ chemical profile and antibacterial activity of Lavandula stoechas L. extracts obtained by supercritical carbon dioxide. Sustain. Chem. Pharm. 2023, 33, 101126. [Google Scholar] [CrossRef]
- Banožić, M.; Wronska, A.W.; Jakovljević Kovač, M.; Aladić, K.; Jerković, I.; Jokić, S. Comparative evaluation of different extraction techniques for separation of artemisinin from sweet wormwood (Artemisia annua L.). Horticulturae 2023, 9, 629. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Wang, N.; Ouyang, D.; Xiao, L.; Zhang, S.; Ou, X.; He, T.; Yu, R.; Song, L. Development of arteannuin B sustained-release microspheres for anti-tumor therapy by integrated experimental and molecular modeling approaches. Pharmaceutics 2021, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; He, T.; Zhu, J.; Li, J.; Zhang, S.; Zhu, Y.; Xu, Y.; Xu, L.; Wang, H.; et al. Arteannuin B enhances the effectiveness of cisplatin in non-small cell lung cancer by regulating connexin 43 and MAPK pathway. Am. J. Chin. Med. 2022, 50, 1963–1992. [Google Scholar] [PubMed]
- Varela, K.; Arman, H.D.; Berger, M.S.; Sponsel, V.M.; Lin, C.A.; Yoshimoto, F.K. Inhibition of cysteine proteases via thiol-Michael addition explains the anti-SARS-CoV-2 and bioactive properties of arteannuin B. J. Nat. Prod. 2023, 86, 1654–1666. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Q.; Wen, T.; Luo, L.; Liu, L.; Wang, L.; Shen, X. Arteannuin B, a sesquiterpene lactone from Artemisia annua, attenuates inflammatory response by inhibiting the ubiquitin-conjugating enzyme UBE2D3-mediated NF-kappaB activation. Phytomedicine 2024, 124, 155263. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-rich essential oils from the parts of Callicarpa macrophylla and their antioxidant and pharmacological activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef]
- Maiolini, T.C.S.; Nicácio, K.J.; Rosa, W.; Miranda, D.O.; Santos, M.F.C.; Bueno, P.C.P.; Lago, J.H.G.; Sartorelli, P.; Dias, D.F.; de Chagas Paula, D.A.; et al. Potential anti-inflammatory biomarkers from Myrtaceae essential oils revealed by untargeted metabolomics. Nat. Prod. Res. 2025, 39, 985–999. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online (11.6). Available online: https://pheur.edqm.eu/home (accessed on 19 February 2025).
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]


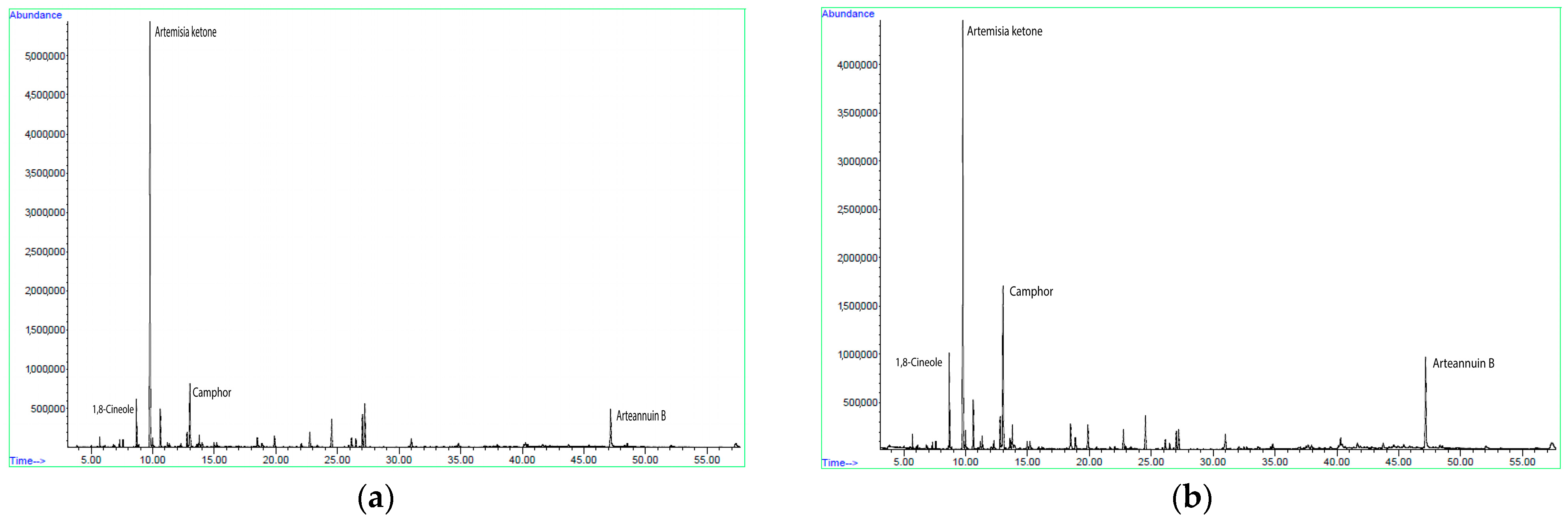

| Yield (%) | |||
|---|---|---|---|
| Compound | RI | Hydrodistillation | Steam Distillation |
| Tricyclene | 922 | 0.17 | 0.11 |
| α-Thujene | 926 | - | 0.20 |
| α-Pinene | 933 | 7.64 | 17.78 |
| 2-Methylpropyl butanoate | 943 | 0.17 | 0.27 |
| Camphene | 947 | 3.86 | 2.55 |
| Sabinene | 973 | 1.51 | 1.25 |
| β-Pinene | 976 | 1.29 | 1.84 |
| Myrcene | 991 | 7.73 | 1.11 |
| Yomogi alcohol | 1001 | 0.93 | 0.39 |
| α-Terpinene | 1016 | 0.17 | 0.31 |
| p-Cymene | 1024 | - | 0.72 |
| Limonene | 1028 | 0.26 | 0.23 |
| 1,8-Cineole | 1030 | 9.88 | 7.47 |
| γ-Terpinene | 1057 | 0.22 | 0.42 |
| Artemisia ketone | 1061 | 29.20 | 42.04 |
| cis-Sabinene hydrate | 1067 | 0.19 | - |
| Artemisia alcohol | 1083 | 3.22 | 0.81 |
| trans-Sabinene hydrate | 1099 | 0.19 | - |
| 1,3,8-p-Mentatriene | 1103 | 1.59 | - |
| α-Campholenal | 1126 | 0.24 | 0.47 |
| trans-Pinocarveol | 1138 | 0.92 | 1.79 |
| 2-Methyl-6-methylene-1,7-octadien-3-one | 1140 | 0.72 | - |
| Camphor | 1143 | 17.67 | 4.89 |
| Ocimenol | 1157 | 1.17 | - |
| Pinocarvone | 1162 | 1.20 | 2.46 |
| Terpinen-4-ol | 1177 | 0.54 | 0.29 |
| α-Terpineol | 1190 | 0.56 | - |
| Myrtenol | 1196 | 0.55 | 0.73 |
| Eugenol | 1357 | 0.22 | - |
| α-Copaene | 1374 | 0.37 | 0.33 |
| trans-β-Caryophyllene | 1417 | 0.92 | - |
| trans-β-Farnesene | 1456 | 0.31 | - |
| Artemisia triene | 1465 | 0.20 | 0.35 |
| Germacrene D | 1478 | 1.10 | - |
| β-Selinene | 1483 | 1.74 | 3.70 |
| Caryophyllene oxide | 1580 | 0.90 | 2.26 |
| Monoterpenes | 91.62 | 88.13 | |
| Hydrocarbons | 24.82 | 26.79 | |
| Oxidized forms | 66.80 | 61.34 | |
| Sesquiterpenes | 5.54 | 6.64 | |
| Hydrocarbons | 4.64 | 4.38 | |
| Oxidized forms | 0.90 | 2.26 | |
| Others | 0.39 | 0.27 | |
| TOTAL | 97.55 | 95.04 | |
| Sequence | Pressure (bar) | Temperature (°C) | Yield (%) |
|---|---|---|---|
| 1 | 220 | 60 | 1.43 |
| 2 | 130 | 50 | 0.90 |
| 3 | 100 | 60 | 1.92 |
| 4 | 160 | 60 | 1.61 |
| 5 | 220 | 40 | 1.05 |
| 6 | 190 | 50 | 1.46 |
| 7 | 190 | 60 | 1.88 |
| 8 | 130 | 40 | 0.62 |
| 9 | 220 | 50 | 1.27 |
| 10 | 160 | 40 | 0.89 |
| 11 | 100 | 50 | 1.59 |
| 12 | 160 | 50 | 1.10 |
| 13 | 130 | 60 | 1.55 |
| 14 | 190 | 40 | 0.66 |
| 15 | 100 | 40 | 1.02 |
| Compound | RI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Santolina triene | 908 | - | - | - | - | - | - | - | - | 0.22 | - | - | - | - | - | - |
| α-Pinene | 933 | 0.77 | 2.75 | 2.34 | 2.60 | 1.31 | 2.11 | 3.30 | 2.48 | 2.99 | 1.08 | 3.99 | 2.44 | 1.05 | 0.95 | 0.76 |
| Camphene | 947 | - | 0.39 | 0.52 | 0.40 | 0.26 | - | - | - | 0.51 | 0.39 | - | - | - | - | - |
| Sabinene | 973 | - | 0.39 | 0.33 | 0.39 | 0.27 | - | 0.57 | 0.53 | 0.50 | 0.35 | 0.70 | 0.53 | 0.34 | - | - |
| β-Pinene | 976 | - | 0.43 | 0.37 | 0.39 | 0.24 | - | 0.44 | - | 0.43 | - | 0.54 | 0.35 | - | - | - |
| Myrcene | 991 | 0.45 | 1.35 | 1.32 | 0.87 | 0.71 | 0.81 | 0.60 | 0.93 | 0.80 | 0.80 | 0.82 | 1.46 | 0.89 | 0.83 | 0.68 |
| Yomogi alcohol | 1001 | 0.50 | 0.27 | - | - | 0.42 | - | - | - | 0.34 | 0.56 | - | 0.43 | 0.49 | 0.52 | 0.68 |
| 1,8-Cineole | 1031 | 6.64 | 8.05 | 8.00 | 8.24 | 6.79 | 10.35 | 11.29 | 11.24 | 7.06 | 7.44 | 11.89 | 7.62 | 8.59 | 6.94 | 4.77 |
| Artemisia ketone | 1061 | 31.55 | 19.28 | 19.57 | 16.93 | 27.06 | 38.04 | 19.90 | 30.13 | 27.43 | 48.49 | 25.31 | 31.75 | 29.81 | 37.56 | 46.76 |
| cis-Sabinene hydrate | 1066 | 1.24 | 1.84 | 1.80 | 1.91 | 1.31 | 1.41 | 2.30 | 2.04 | 1.01 | 0.68 | 2.35 | 1.49 | 1.43 | 0.90 | 0.94 |
| Artemisia alcohol | 1083 | 3.64 | 2.70 | 2.43 | 2.25 | 3.08 | 4.03 | 2.81 | 3.60 | 2.50 | 3.44 | 3.24 | 2.72 | 2.97 | 2.94 | 4.10 |
| trans-Sabinene hydrate | 1099 | 0.65 | 0.76 | 0.55 | 0.83 | 0.67 | - | 1.01 | 0.91 | 0.58 | 0.38 | 1.03 | 0.69 | 0.81 | 0.56 | 0.44 |
| 1,3,8-p-Mentatriene | 1103 | 1.11 | 2.18 | 2.41 | 2.17 | 1.66 | 0.93 | 0.93 | 1.25 | 0.89 | - | 0.93 | 1.00 | 1.10 | 0.75 | - |
| α-Campholenal | 1126 | 0.78 | 1.22 | 1.31 | 1.37 | 0.82 | 0.78 | 1.24 | 0.90 | 0.78 | 0.37 | 1.15 | 0.75 | 0.71 | - | - |
| trans-Pinocarveol | 1138 | 2.66 | 2.96 | 2.80 | 3.52 | 3.01 | 2.59 | 5.08 | 3.68 | 2.74 | 1.39 | 4.81 | 2.90 | 2.54 | 1.42 | 1.74 |
| Camphor | 1143 | 13.94 | 14.17 | 18.44 | 13.15 | 11.88 | 16.05 | 3.29 | 12.88 | 9.39 | 12.22 | 4.17 | 7.17 | 6.77 | 14.36 | 7.83 |
| Ocimenol | 1157 | 1.09 | 1.99 | 2.32 | 2.01 | 1.37 | 1.02 | 1.13 | 1.20 | 0.97 | - | 1.08 | 1.02 | 1.28 | 0.81 | - |
| Pinocarvone | 1162 | 1.86 | 1.86 | 1.79 | 2.23 | 1.93 | 2.03 | 3.31 | 2.45 | 1.85 | 1.28 | 3.10 | 1.97 | 1.95 | 1.15 | 1.53 |
| Borneole | 1165 | - | 0.13 | - | - | - | - | 0.23 | - | 0.12 | - | - | - | 0.75 | - | - |
| α-Terpineol | 1190 | 0.60 | 0.77 | 0.55 | 0.68 | 0.51 | - | 0.88 | 0.98 | 0.59 | 0.36 | 0.98 | 0.83 | 0.85 | - | 0.54 |
| Myrtenol | 1196 | 0.70 | 0.77 | 0.66 | 0.88 | 0.70 | - | 1.09 | 0.86 | 0.71 | 0.41 | 1.06 | 0.80 | 0.72 | - | - |
| Eugenol | 1357 | - | - | - | - | - | - | - | - | 0.48 | - | - | - | - | - | - |
| α-Copaene | 1374 | 1.76 | 1.62 | 1.23 | 1.50 | 2.32 | 1.76 | 1.82 | 1.78 | 1.24 | 1.33 | 1.82 | 1.98 | 1.64 | 1.70 | 1.90 |
| trans-β-Caryophyllene | 1417 | 3.15 | 2.87 | 2.68 | 3.23 | 5.41 | 3.95 | 4.04 | 3.37 | 2.59 | 2.53 | 3.86 | 3.75 | 3.33 | 4.19 | 3.78 |
| trans-β-Farnesene | 1457 | 0.96 | 0.98 | 0.77 | 0.89 | 1.53 | 1.23 | 0.88 | 0.86 | 0.91 | 0.52 | 0.75 | 1.32 | 1.03 | 1.29 | 1.31 |
| Artemisia triene | 1465 | 0.50 | 0.68 | 0.48 | 0.78 | 0.56 | - | 0.68 | 0.60 | 0.75 | 0.53 | 0.58 | 0.98 | - | 0.78 | 1.06 |
| Germacrene D | 1478 | 1.71 | 1.71 | 1.66 | 1.85 | 3.79 | 3.48 | 2.54 | 2.75 | 1.74 | 1.98 | 2.56 | 3.42 | 3.38 | 5.33 | 4.34 |
| β-Selinene | 1483 | 1.87 | 5.26 | 5.16 | 6.41 | 4.98 | 1.05 | 7.42 | 3.80 | 4.91 | 3.60 | 7.14 | 6.72 | 6.23 | 4.26 | 5.88 |
| Caryophyllene oxide | 1580 | 1.82 | 2.00 | 1.36 | 1.83 | 2.10 | 1.55 | 2.88 | - | 2.22 | 1.14 | 2.43 | 2.08 | 1.53 | 1.65 | 1.40 |
| Eudesma-4(15),7-dien-1-β-ol | 1683 | - | 0.58 | - | 0.57 | 0.55 | - | - | - | - | - | - | 0.92 | 1.02 | - | - |
| Arteannuic acid | 1840 | 0.80 | 0.55 | 1.55 | 0.62 | 0.58 | - | 0.61 | 1.72 | 0.55 | - | 0.74 | 0.20 | 0.52 | - | - |
| Arteannuin B | 2054 | 10.03 | 6.10 | 5.82 | 7.55 | 6.23 | 4.23 | 8.86 | 3.98 | 9.52 | 4.38 | 6.70 | 7.05 | 7.54 | 8.32 | 5.86 |
| Monoterpenes | 68.18 | 62.08 | 67.51 | 60.80 | 63.98 | 80.16 | 59.43 | 76.06 | 62.41 | 79.66 | 67.13 | 65.90 | 63.06 | 69.68 | 70.75 | |
| Hydrocarbons | 4.23 | 7.91 | 9.64 | 9.56 | 6.44 | 5.26 | 9.17 | 8.13 | 7.92 | 3.68 | 10.34 | 7.95 | 5.63 | 3.99 | 2.82 | |
| Oxidized forms | 63,95 | 54,17 | 57,87 | 51,24 | 57,54 | 74,90 | 50,26 | 67,93 | 54,49 | 75,98 | 56,79 | 57,95 | 57,43 | 65,69 | 67,93 | |
| Sesquiterpenes | 22.59 | 22.34 | 20.69 | 25.24 | 28.05 | 17.24 | 29.73 | 18.85 | 24.43 | 15.99 | 26.57 | 28.41 | 26.22 | 27.51 | 25.52 | |
| Hydrocarbons | 9.95 | 13.11 | 11.96 | 14.66 | 18.59 | 11.46 | 17.38 | 13.16 | 12.14 | 10.47 | 16.70 | 18.16 | 15.61 | 17.54 | 18.26 | |
| Oxidized forms | 1.64 | 9.23 | 8.73 | 10.58 | 9.46 | 5.78 | 12,35 | 5.71 | 12.29 | 5.52 | 9.87 | 10.25 | 10.61 | 9.97 | 7.26 | |
| Phenylpropanes | - | - | - | - | - | - | - | - | 0.48 | - | - | - | - | - | - | |
| TOTAL | 90.77 | 84.43 | 88.20 | 86.04 | 92.03 | 97.39 | 89.16 | 94.91 | 87.32 | 95.64 | 93.70 | 94.30 | 89.27 | 97.19 | 96.27 |
| Target | Pressure (bar) | Temperature (°C) | Desirability | |
|---|---|---|---|---|
| 1. | High extraction yield and high monoterpenes | 100 | 60 | 0.660 |
| 2. | High extraction yield and high artemisia ketone | 100 | 45 | 0.473 |
| 3. | High extraction yield and high artemisia ketone, camphor and 1,8-cineole | 160 | 50 | 0.509 |
| 4. | High extraction yield and high sesquiterpenes | 181 | 60 | 0.809 |
| 5. | High extraction yield and high arteannuin B | 220 | 60 | 0.899 |
| 6. | High extraction yield and high arteannuin B and β-selinene | 220 | 53 | 0.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, M.; Ivančić, I.; Cvetnić, M.; Ferrante, C.; Orlando, G.; Vladimir-Knežević, S. Green Extraction of Volatile Terpenes from Artemisia annua L. Molecules 2025, 30, 1638. https://doi.org/10.3390/molecules30071638
Mandić M, Ivančić I, Cvetnić M, Ferrante C, Orlando G, Vladimir-Knežević S. Green Extraction of Volatile Terpenes from Artemisia annua L. Molecules. 2025; 30(7):1638. https://doi.org/10.3390/molecules30071638
Chicago/Turabian StyleMandić, Marta, Ivona Ivančić, Matija Cvetnić, Claudio Ferrante, Giustino Orlando, and Sanda Vladimir-Knežević. 2025. "Green Extraction of Volatile Terpenes from Artemisia annua L." Molecules 30, no. 7: 1638. https://doi.org/10.3390/molecules30071638
APA StyleMandić, M., Ivančić, I., Cvetnić, M., Ferrante, C., Orlando, G., & Vladimir-Knežević, S. (2025). Green Extraction of Volatile Terpenes from Artemisia annua L. Molecules, 30(7), 1638. https://doi.org/10.3390/molecules30071638









