Impact of Co-Inoculation Patterns of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Cider Quality and Aromatic Profiles
Abstract
1. Introduction
2. Results and Discussion
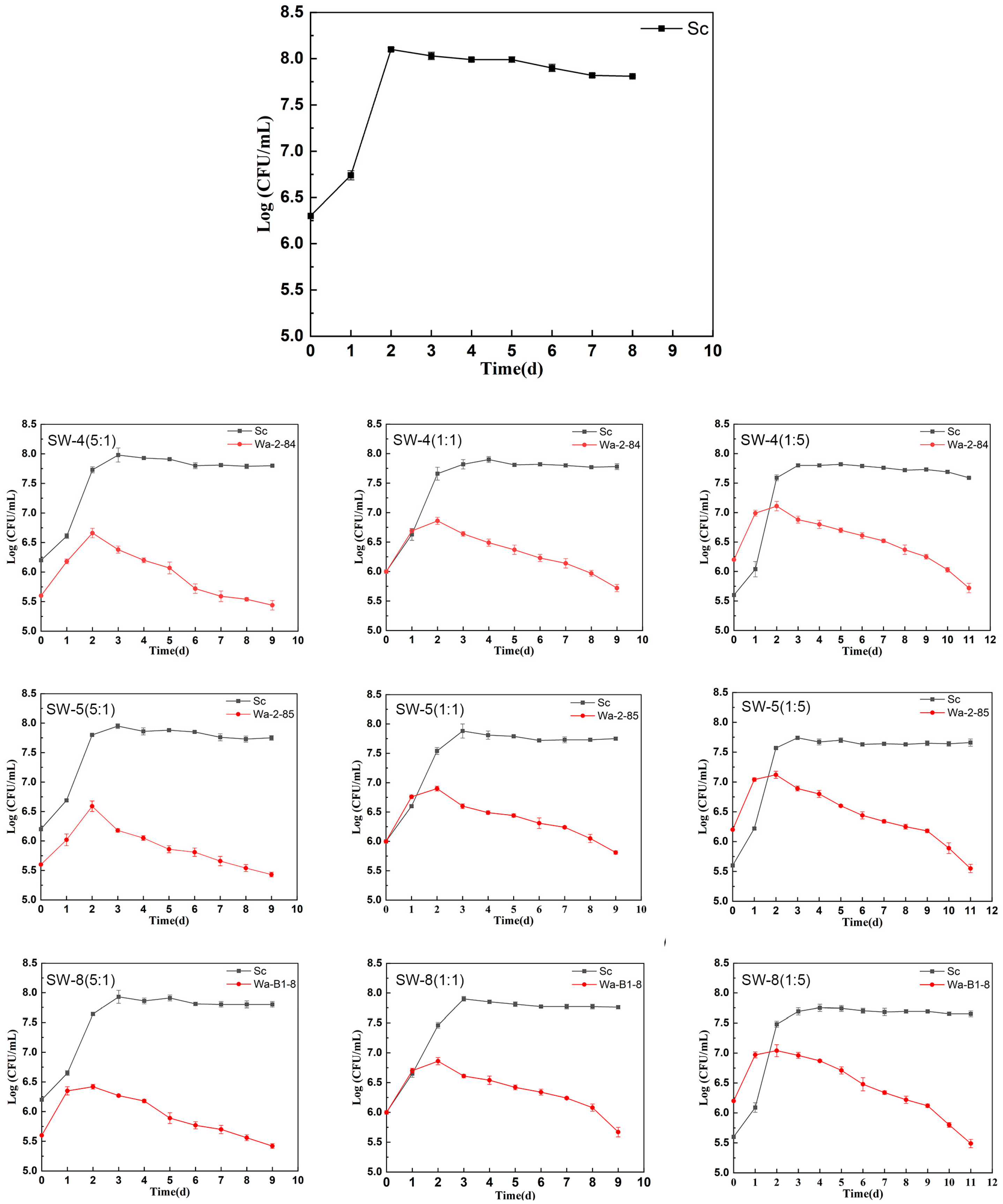
2.1. Fermentation Kinetics
2.2. Physicochemical Parameters
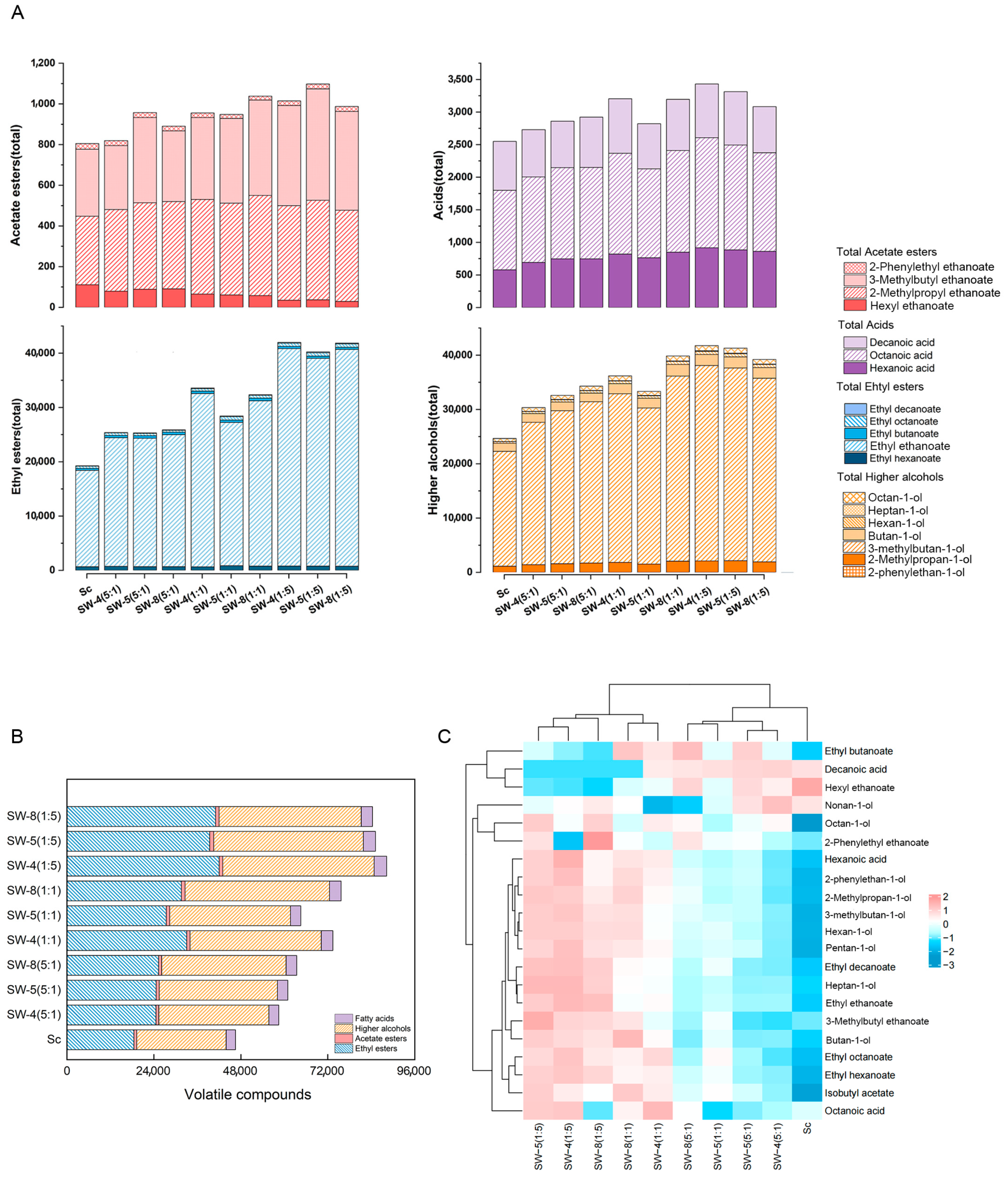
2.3. Volatile Profiles
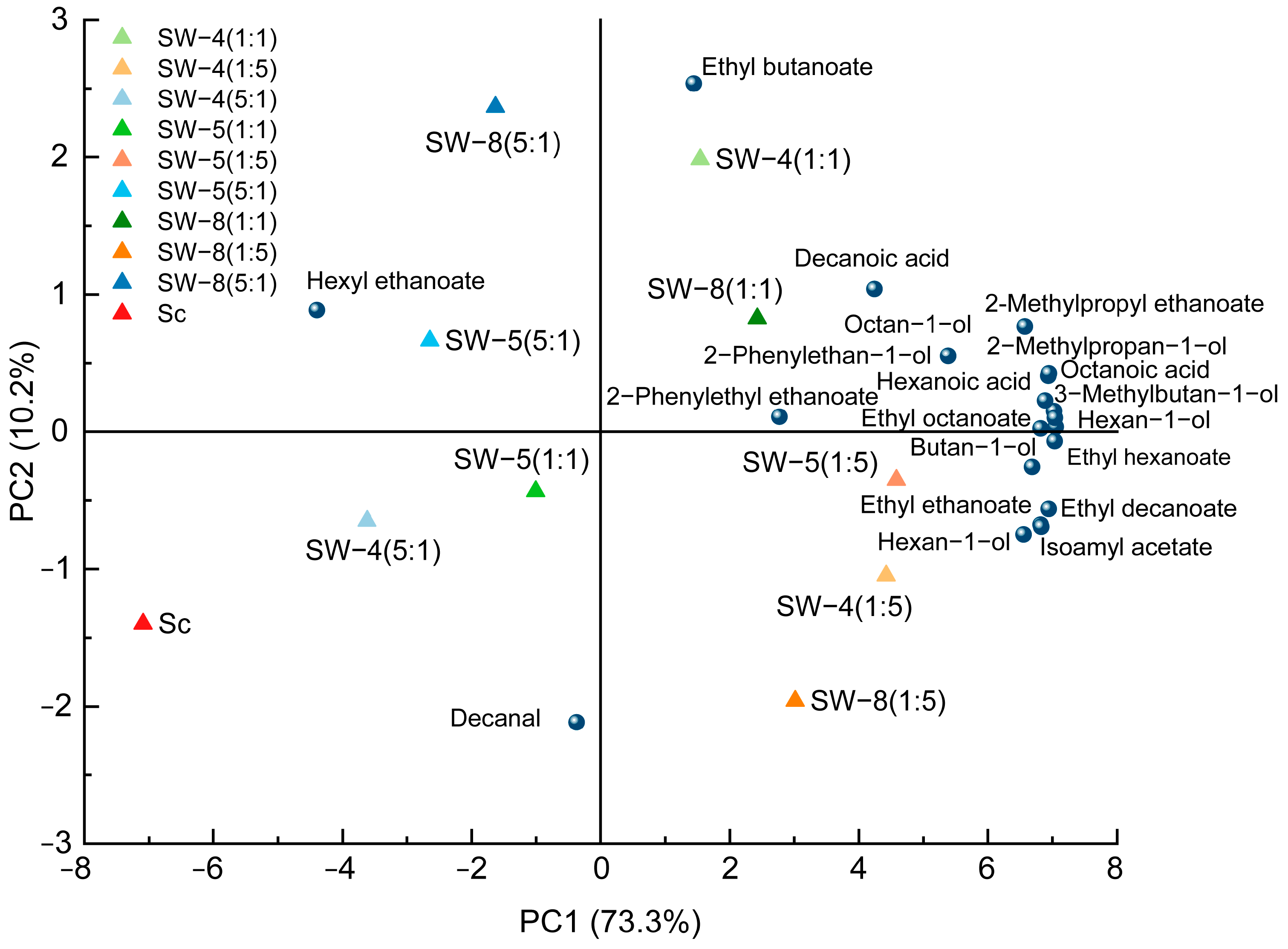
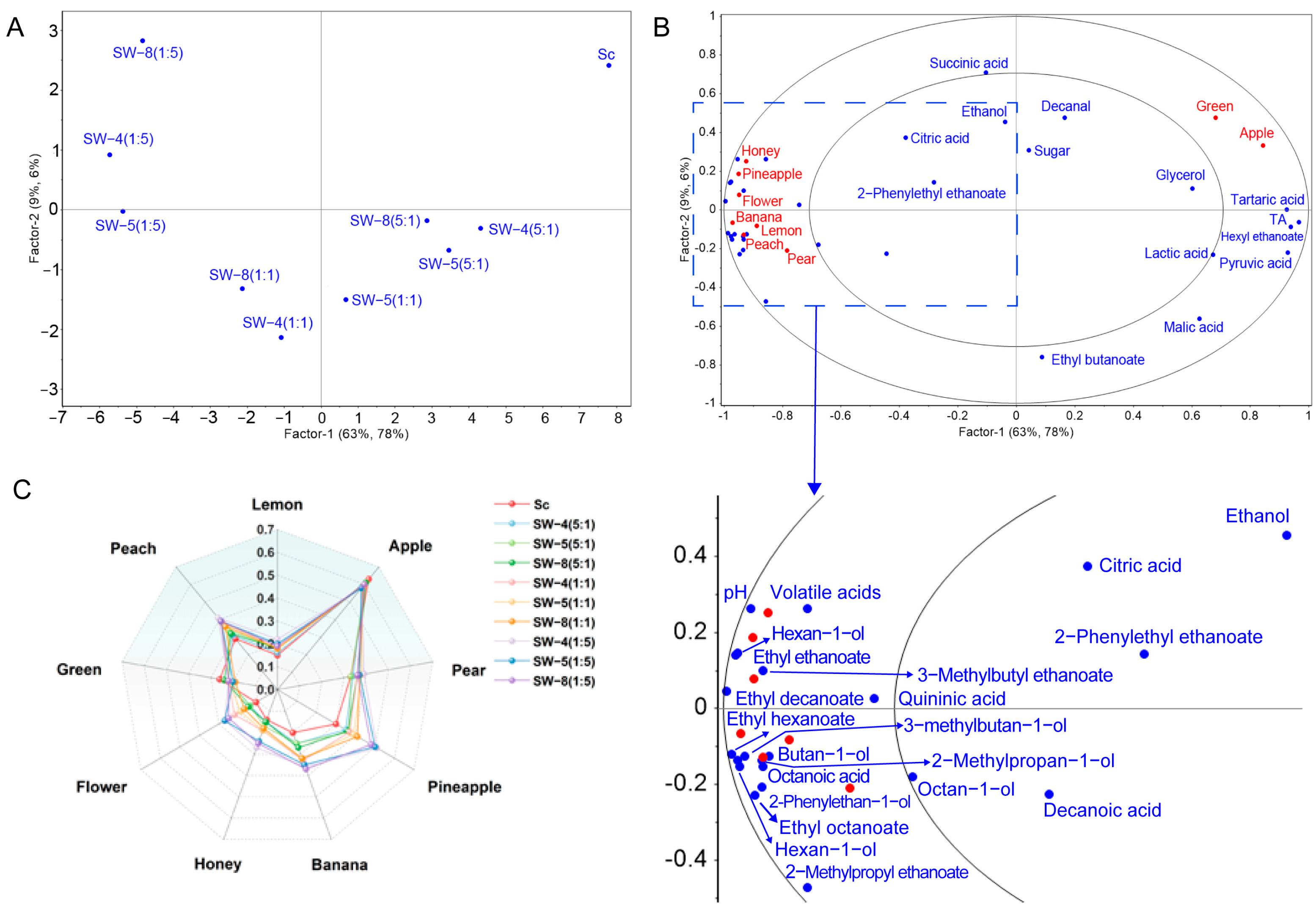
2.4. Multivariate Analysis of the Chemical Parameters
2.5. Sensory Profiles of Ciders
3. Materials and Methods
3.1. Apple and Yeast Strains
3.2. Fermentation Setup
3.3. Determination of Physicochemical Parameters and Volatile Compounds
3.4. Determination of Volatile Compounds
3.5. Sensory Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARTP | atmospheric room temperature plasma |
| OIV | international organization of vine and wine |
| PCA | principal component analysis |
| PLSR | partial least squares regression |
| ARTP | atmospheric room temperature plasma |
References
- Guiné, R.P.F.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple Fermented Products: An Overview of Technology, Properties and Health Effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of Apple Cultivar, Ripening Stage, Fermentation Type and Yeast Strain on Phenolic Composition of Apple Ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic Fruit Wine Production via Reciprocal Selection of Juice and Non-Saccharomyces Species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. Pichia anomala: Cell Physiology and Biotechnology Relative to Other Yeasts. Antonie Van Leeuwenhoek 2011, 99, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Effect of Sequential Fermentation with Four Non-Saccharomyces and Saccharomyces cerevisiae on Nutritional Characteristics and Flavor Profiles of Kiwi Wines. J. Food Compos. Anal. 2022, 109, 104480. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-B.; Jeon, J.-Y.; Park, H.-D. Development of Air-Blast Dried Non-Saccharomyces Yeast Starter for Improving Quality of Korean Persimmon Wine and Apple Cider. Int. J. Food Microbiol. 2019, 290, 193–204. [Google Scholar] [CrossRef]
- Cai, W.; Li, B.; Chen, Y.; Fu, G.; Fan, H.; Deng, M.; Wan, Y.; Liu, N.; Li, M. Increase the Content of Ester Compounds in Blueberry Wine Fermentation with the Ester-Producing Yeast: Candida glabrata, Pichia anomala, and Wickerhamomyces anomalus. Foods 2022, 11, 3655. [Google Scholar] [CrossRef]
- Hu, L.; Chen, X.; Lin, R.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Mu, Y.; Yuan, J.; Zhang, H.; Song, J.; Kang, S. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider. Molecules 2024, 29, 1750. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of Yeasts for Apple Juice Fermentation and Production of Cider Volatile Compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Zhang, S.; Ji, C.; Liang, H. Chemical Composition and Flavor Characteristics of Cider Fermented with Saccharomyces cerevisiae and Non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational Selection of Non-Saccharomyces Wine Yeasts for Mixed Starters Based on Ester Formation and Enological Traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Bauer, F.F.; Setati, M.E. The Impact of Saccharomyces cerevisiae on a Wine Yeast Consortium in Natural and Inoculated Fermentations. Front. Microbiol. 2017, 8, 1988. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of Sequential Mixed Cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Apple Cider Fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of Non-Saccharomyces Yeasts Affects Nutrient Availability for Saccharomyces cerevisiae during Wine Fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Satora, P.; Tarko, T.; Sroka, P.; Blaszczyk, U. The Influence of Wickerhamomyces anomalus Killer Yeast on the Fermentation and Chemical Composition of Apple Wines. FEMS Yeast Res. 2014, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe Strains on Chemical Composition and Sensory Quality of Ciders Made from Finnish Apple Cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of Fermentation Temperature on Key Aroma Compounds and Sensory Properties of Apple Wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef] [PubMed]
- da Mota, R.V.; Peregrino, I.; Rivera, S.P.T.; Hassimotto, N.M.A.; de Souza, A.L.; de Souza, C.R. Characterization of Brazilian Syrah Winter Wines at Bottling and after Ageing. Sci. Agric. 2020, 78, e20190233. [Google Scholar] [CrossRef]
- Braga, C.M.; Zielinski, A.A.F.; da Silva, K.M.; de Souza, F.K.F.; Pietrowski, G.d.A.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of Juices and Fermented Beverages Made from Unripe, Ripe and Senescent Apples Based on the Aromatic Profile Using Chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, Y.; Zhu, R.; Bai, J.; Qiu, J.; Wu, Y.; Zhong, K.; Gao, H. Insight into the Characteristics of Cider Fermented by Single and Co-Culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe Based on Metabolomic and Transcriptomic Approaches. LWT 2022, 163, 113538. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile Components of Negroamaro Red Wines Produced in Apulian Salento Area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on Chemical Composition and Sensory Profiles of Merlot Wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.A.; Bredie, W.L.P. Flavor Profiling of Apple Ciders from the UK and Scandinavian Region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Liu, Y.; Wang, X.; Gao, C.; Mu, J.; Wang, W.; Wang, J. Correlations between Microbes with Volatile Compounds and Physicochemical Indicators of Cabernet Sauvignon Wines Fermented with Different Starters. LWT 2024, 198, 115918. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Mao, Y.; Zhang, X.; Xu, B.; Yang, X. Effect of Different Inoculation Strategies of Mixed Culture Saccharomyces cerevisiae/Oenococcus oeni on the Aroma Quality of Chardonnay Wine. Food Res. Int. 2024, 190, 114636. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.M.E.; Alberti, A.; Pietrowski, G.d.A.M.; Zielinski, A.A.F.; Wosiacki, G.; Nogueira, A.; Jorge, R.M.M. Supplementation of Amino Acids in Apple Must for the Standardization of Volatile Compounds in Ciders. J. Inst. Brew. 2016, 122, 334–341. [Google Scholar] [CrossRef]
- Stribny, J.; Querol, A.; Pérez-Torrado, R. Differences in Enzymatic Properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum Alcohol Acetyltransferases and Their Impact on Aroma-Active Compounds Production. Front. Microbiol. 2016, 7, 897. [Google Scholar] [CrossRef]
- Antón, M.J.; Suárez Valles, B.; García Hevia, A.; Picinelli Lobo, A. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014, 79, S92–S99. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Weng, L.; Zou, L.; Jiang, H.; Qiu, J.; Fu, J. Analysis of Sucrose Addition on the Physicochemical Properties of Blueberry Wine in the Main Fermentation. Front. Nutr. 2023, 9, 1092696. [Google Scholar] [CrossRef] [PubMed]
- Master, O.I.V.; Patronage, O.I.V. Compendium of International Methods of Wine and Must Analysis. International Organisation of Vine and Wine. 2024. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis (accessed on 20 March 2025).
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of Medium-Chain Fatty Acid Ethyl Ester Content in Mixed H. uvarum/S. cerevisiae Fermentation Leads to Wine Fruity Aroma Enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, J.; Song, Y.; Zang, X.; Wang, G.; Pei, Y.; Song, Y.; Qin, Y.; Liu, Y. Yeast Diversity during Spontaneous Fermentations and Oenological Characterisation of Indigenous Saccharomyces cerevisiae for Potential as Wine Starter Cultures. Microorganisms 2022, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking Changes in Volatile Profiles at Sub-Zero Temperatures during over-Ripening of ‘Beibinghong’ Grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Dravnieks, A. Odor Quality: Semantically Generated Multidimensional Profiles Are Stable. Science 1982, 218, 799–801. [Google Scholar] [CrossRef] [PubMed]

| Yeast Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC | SW-4 (5:1) | SW-5 (5:1) | SW-8 (5:1) | SW-4 (1:1) | SW-5 (1:1) | SW-8 (1:1) | SW-4 (1:5) | SW-5 (1:5) | SW-8 (1:5) | |
| Residual sugar (g/L) | 2.53 ± 0.09 ab | 2.60 ± 0.21 a | 2.52 ± 0.24 ab | 2.71 ± 0.23 a | 2.45 ± 0.16 ab | 2.19 ± 0.11b | 2.61 ± 0.16 a | 2.70 ± 0.20 a | 2.38 ± 0.16 ab | 2.53 ± 0.09 ab |
| Glycerol (g/L) | 3.20 ± 0.12 ab | 3.10 ± 0.09 bc | 3.16 ± 0.10 ab | 3.33 ± 0.14 a | 2.78 ± 0.05 d | 2.71 ± 0.17 d | 3.24 ± 0.03 ab | 2.71 ± 0.09 d | 2.88 ± 0.08 d | 2.92 ± 0.10 cd |
| Ethanol (% v/v) | 6.35 ± 0.07 a | 6.30 ± 0.07 a | 6.22 ± 0.11 a | 6.35 ± 0.13 a | 6.29 ± 0.08 a | 6.24 ± 0.13 a | 6.36 ± 0.20 a | 6.33 ± 0.16 a | 6.27 ± 0.18 a | 6.34 ± 0.07 a |
| pH | 3.69 ± 0.01 e | 3.71 ± 0.01 de | 3.69 ± 0.02 e | 3.71 ± 0.02cd | 3.73 ± 0 bc | 3.72 ± 0.02 bcd | 3.74 ± 0.01b | 3.79 ± 0.01 a | 3.79 ± 0.01 a | 3.79 ± 0.01 a |
| TA(g/L) | 4.53 ± 0.03 a | 4.40 ± 0.15 abc | 4.46 ± 0.07 ab | 4.45 ± 0.19 ab | 4.31 ± 0.03 bcd | 4.39 ± 0.10 abc | 4.30 ± 0.03 bcd | 4.17 ± 0.05 d | 4.30 ± 0.14 bcd | 4.21 ± 0.03 cd |
| Volatile acids (g/L) | 0.27 ± 0.01 d | 0.27 ± 0.02 cd | 0.29 ± 0.01 bcd | 0.28 ± 0.01 bcd | 0.30 ± 0 bc | 0.28 ± 0.01 bcd | 0.31 ± 0.02b | 0.30 ± 0.01 b | 0.33 ± 0 a | 0.34 ± 0.02 a |
| Malic acid (g/L) | 2.34 ± 0.06 a | 2.38 ± 0.18 a | 2.32 ± 0.12 a | 2.38 ± 0.09 a | 2.39 ± 0.14 a | 2.32 ± 0.07 a | 2.28 ± 0.10 a | 2.20 ± 0.08 a | 2.33 ± 0.17 a | 2.17 ± 0.15 a |
| Pyruvic acid (g/L) | 0.34 ± 0.04 a | 0.34 ± 0.01 ab | 0.34 ± 0.02 a | 0.34 ± 0.02 a | 0.28 ± 0 c | 0.27 ± 0.02 c | 0.29 ± 0.03 bc | 0.21 ± 0.01d | 0.22 ± 0.01d | 0.22 ± 0d |
| Acetic acid (g/L) | 0.35 ± 0.04 b | 0.36 ± 0.02 b | 0.37 ± 0.03 b | 0.37 ± 0.04 b | 0.36 ± 0.03 b | 0.35 ± 0.02 b | 0.37 ± 0.04 b | 0.46 ± 0.02 a | 0.43 ± 0.02 a | 0.46 ± 0.01 a |
| Lactic acid (g/L) | 0.14 ± 0.01 a | 0.15 ± 0.02 a | 0.14 ± 0.01 a | 0.15 ± 0.03 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a | 0.15 ± 0.02 a | 0.13 ± 0.02 a | 0.14 ± 0.02 a | 0.13 ± 0.01 a |
| Citric acid (g/L) | 0.90 ± 0.07 a | 0.87 ± 0.09 a | 0.93 ± 0.05 a | 0.95 ± 0.01 a | 0.89 ± 0.02 a | 0.87 ± 0.05 a | 0.93 ± 0.09 a | 0.92 ± 0.04 a | 0.94 ± 0.02 a | 0.95 ± 0.03 a |
| Succinic acid (g/L) | 0.55 ± 0.05 ab | 0.53 ± 0.02 ab | 0.55 ± 0.06 ab | 0.58 ± 0.03 a | 0.48 ± 0.01 b | 0.48 ± 0.03b | 0.53 ± 0.07 ab | 0.54 ± 0.06 ab | 0.56± 0.02 ab | 0.59 ± 0.03 a |
| Quininic acid (g/L) | 0.15 ± 0.01 d | 0.17 ± 0 bc | 0.18 ± 0.01 abc | 0.18 ± 0 ab | 0.16 ± 0 cd | 0.17 ± 0.01 bc | 0.19 ± 0.01 a | 0.18 ± 0 ab | 0.19 ± 0 ab | 0.19 ± 0.02 a |
| Compounds (µg/L) | Yeast Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC | SW-4 (5:1) | SW-5 (5:1) | SW-8 (5:1) | SW-4 (1:1) | SW-5 (1:1) | SW-8 (1:1) | SW-4 (1:5) | SW-5 (1:5) | SW-8 (1:5) | |
| Ethyl ethanoate | 17,756 ± 441 f | 23,686 ± 732 e | 23,663 ± 697 e | 24,292 ± 1551 de | 31,928 ± 1704 c | 26,388 ± 669 d | 30,456 ± 939 c | 40,860 ± 1176 a | 38,272 ± 1305 b | 39,940 ± 969 ab |
| Ethyl butanoate | 381 ± 21 e | 433 ± 24 bcd | 481 ± 8 ab | 493 ± 17 a | 467 ± 17 abc | 434 ± 7 bcd | 488 ± 18 a | 406 ± 20 de | 428 ± 5 cde | 396 ± 5 de |
| Ethyl hexanoate | 132 ± 3 f | 173 ± 8 e | 178 ± 7 de | 192 ± 8 d | 228 ± 10 b | 210 ± 6 c | 224 ± 12 bc | 255 ± 5 a | 249 ± 4 a | 245 ± 3 a |
| Ethyl octanoate | 170 ± 14 c | 207 ± 21 bc | 227 ± 21 b | 227 ± 7 b | 321 ± 20 a | 288 ± 8 a | 297 ± 8 a | 341 ± 17 a | 320 ± 20 a | 305 ± 25 a |
| Ethyl decanoate | 47 ± 4 d | 69 ± 3 c | 71 ± 5 c | 80 ± 4 c | 108 ± 12 b | 102 ± 6 b | 111 ± 9 b | 147 ± 8 a | 146 ± 8 a | 138 ± 9 a |
| Ʃ Ethyl esters | 18,487 ± 445 f | 24,567 ± 681 e | 24,620 ± 670 e | 25,285 ± 1516 de | 33,052 ± 1762 c | 27,423 ± 670 d | 31,576 ± 910 c | 42,009 ± 1140 a | 39,415 ± 1272 b | 41,025 ± 938 ab |
| 2-Methylpropyl ethanoate | 337 ± 24c | 402 ± 12b | 425 ± 6 ab | 429 ± 20 ab | 465 ± 8 ab | 451 ± 4 ab | 492 ± 15 a | 465 ± 23 ab | 489 ± 22 a | 448 ± 41 ab |
| 3-Methylbutyl ethanoate | 329 ± 11 e | 314 ± 23 e | 319 ± 7 e | 347 ± 13 e | 403 ± 17 d | 417 ± 8 cd | 469 ± 30 bc | 492 ± 21 b | 548 ± 25 a | 486 ± 14 b |
| Hexyl ethanoate | 111 ± 3 a | 79 ± 0 c | 89 ± 1 b | 91 ± 1 b | 65 ± 4 d | 61 ± 1 de | 58 ± 2 e | 35 ± 1 f | 37 ± 2 f | 29 ± 0 g |
| 2-Phenylethyl ethanoate | 4 ± 0 a | 5 ± 0 a | 5 ± 1 a | 5 ± 1 a | 5 ± 0 a | 5 ± 0 a | 5 ± 0 a | 4 ± 0 a | 5 ± 0 a | 6 ± 0 a |
| Ʃ Acetate esters | 782 ± 37 d | 800 ± 35 d | 839 ± 13 d | 872 ± 33 cd | 938 ± 14 bc | 934 ± 5 bc | 1024 ± 46 ab | 996 ± 5 ab | 1079 ± 2 a | 969 ± 54 b |
| 2-Methylpropan-1-ol | 1126 ± 51 f | 1394 ± 44 e | 1583 ± 112 de | 1720 ± 30 cd | 1835 ± 46 bc | 1528 ± 87 de | 2063 ± 115 ab | 2092 ± 44 a | 2122 ± 45 a | 1922 ± 119 abc |
| Butan-1-ol | 1497 ± 117 d | 1609 ± 56 cd | 1604 ± 148 cd | 1602 ± 78 cd | 1872 ± 36 abc | 1787 ± 28 bcd | 2126 ± 151 a | 2002 ± 46 ab | 2049 ± 57 ab | 1967 ± 77 ab |
| 3-methylbutan-1-ol | 21,163 ± 1143 f | 26,231 ± 138 e | 28,199 ±771 d | 29,702 ± 150 cd | 31,044 ± 403 c | 28,750 ± 358 d | 34,097 ± 390 ab | 36,013 ± 681 a | 35,507 ± 233 ab | 33,821 ± 1094 b |
| Hexan-1-ol | 260 ± 19 f | 376 ± 4 e | 412 ± 13 d | 441 ± 2 cd | 493 ± 7 b | 460 ± 6 c | 567 ± 9 a | 595 ± 11 a | 593 ± 3 a | 565 ± 17 a |
| Heptan-1-ol | 44 ± 2 d | 53 ± 3 cd | 52 ± 2 cd | 55 ± 1 c | 69 ± 6 b | 58 ± 2 c | 72 ± 2 b | 93 ± 1 a | 92 ± 4 a | 86 ± 3 a |
| Octan-1-ol | 5 ± 0 c | 5 ± 0 ab | 5 ± 0 b | 5 ± 0 b | 6 ± 0 ab | 6 ± 0 ab | 6 ± 0 ab | 6 ± 0 ab | 6 ± 0 a | 6 ± 0 ab |
| Nonan-1-ol | 2 ± 0 d | 2 ± 0 d | 2 ± 0 d | 2 ± 0 d | 2 ± 0 b | 2 ±0 c | 2 ± 0 b | 2 ± 0 a | 2 ± 0 a | 2 ± 0 a |
| 2-phenylethan-1-ol | 558 ± 38 e | 676 ± 16 b | 753 ± 21 c | 782 ± 17 c | 862 ± 19 b | 737 ± 13 c | 918 ± 21 ab | 976 ± 14 a | 940 ± 3 a | 854 ± 32 b |
| Pentan-1-ol | ND | 13 ± 1c | 12 ± 0c | 22 ± 2 a | 15 ± 1 bc | 16 ± 0 b | 16 ± 0 b | 14 ± 0 bc | 15 ± 0 bc | 16 ± 0 b |
| Ʃ Higher alcohols | 24,655 ± 1348 f | 30,358 ± 207 e | 32,622 ± 1064 d | 34,330 ± 272 cd | 36,198 ± 516 c | 33,344 ± 490 d | 39,866 ± 601 ab | 41,791 ± 786 a | 41,325 ± 281 ab | 39,237 ± 1306 b |
| Hexanoic acid | 579 ± 40 f | 691 ± 7 e | 748 ± 25 d | 747 ± 12 d | 821 ± 20 c | 763 ± 8 d | 849 ± 10 bc | 917 ± 9 a | 883 ± 8 ab | 861 ± 22 abc |
| Octanoic acid | 1220 ± 41 d | 1313 ± 16 c | 1398 ± 39 c | 1404 ± 29 c | 1547 ± 42 b | 1365 ± 22c | 1561 ± 11 b | 1690 ± 24 a | 1610 ± 16 ab | 1516 ± 44 b |
| Decanoic acid | 750 ± 8 ab | 726 ± 27 b | 714 ± 10 b | 771 ± 32 ab | 837 ± 30 a | 693 ± 30 b | 785 ± 41 ab | 824 ± 19 a | 820 ± 46 a | 707 ± 19 b |
| Ʃ Fatty acids | 2549 ± 87 f | 2730 ± 45 e | 2860 ± 72 e | 2922 ± 63 de | 3205 ± 91 bc | 2821 ± 59 e | 3195 ± 61 bc | 3431 ± 15 a | 3313 ± 54 ab | 3084 ± 58 cd |
| Inoculation Modalities | S. cerevisiae | W. anomalus | Inoculation Proportion |
|---|---|---|---|
| SW-4 (5:1) | CECA | Wa-2-84 | 5:1 |
| SW-5 (5:1) | CECA | Wa-2-85 | 5:1 |
| SW-8 (5:1) | CECA | Wa-B1-8 | 5:1 |
| SW-4 (1:1) | CECA | Wa-2-84 | 1:1 |
| SW-5 (1:1) | CECA | Wa-2-85 | 1:1 |
| SW-8 (1:1) | CECA | Wa-B1-8 | 1:1 |
| SW-4 (1:5) | CECA | Wa-2-84 | 1:5 |
| SW-5 (1:5) | CECA | Wa-2-85 | 1:5 |
| SW-8 (1:5) | CECA | Wa-B1-8 | 1:5 |
| Sc | CECA | — | 1:0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Mou, J.; Zhang, H.; Gao, A.; Qin, Y. Impact of Co-Inoculation Patterns of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Cider Quality and Aromatic Profiles. Molecules 2025, 30, 1620. https://doi.org/10.3390/molecules30071620
Wei Y, Mou J, Zhang H, Gao A, Qin Y. Impact of Co-Inoculation Patterns of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Cider Quality and Aromatic Profiles. Molecules. 2025; 30(7):1620. https://doi.org/10.3390/molecules30071620
Chicago/Turabian StyleWei, Yue, Jianguo Mou, Haoran Zhang, Aiying Gao, and Yi Qin. 2025. "Impact of Co-Inoculation Patterns of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Cider Quality and Aromatic Profiles" Molecules 30, no. 7: 1620. https://doi.org/10.3390/molecules30071620
APA StyleWei, Y., Mou, J., Zhang, H., Gao, A., & Qin, Y. (2025). Impact of Co-Inoculation Patterns of Wickerhamomyces anomalus and Saccharomyces cerevisiae on Cider Quality and Aromatic Profiles. Molecules, 30(7), 1620. https://doi.org/10.3390/molecules30071620






