The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone
Abstract
1. Introduction
2. Plant Families That Synthesize Plumbagin
3. Detection of Plumbagin in Diverse Taxa
4. Plumbagin in Human Healthcare
4.1. Anticancer Activity
4.2. Mode of Action of Plumbagin Against Cancer Cells
4.3. Plumbagin and Its Ability to Re-Sensitize Chemo and Radiation-Resistant Cancer Cells
4.4. Anticoagulation, and Cardiovascular Diseases
4.5. Antidiarrheal Activity
4.6. Antifertility
4.7. Anti-Inflammatory
4.8. Antimalarial
4.9. Antimicrobial and Antibiofilm Activities
4.10. Antimutagenic
4.11. Antinephrotic
4.12. Antiobesity and Antidiabetic
4.13. Antirheumatic
4.14. Aphrodisiac
4.15. Arteriosclerosis and Cough
4.16. Digestive Problems, Piles, and Liver Disorders
4.17. Neuroprotective
4.18. Limitations of Plumbagin for Use in the Clinics and the Ways to Improve Its Bioavailability
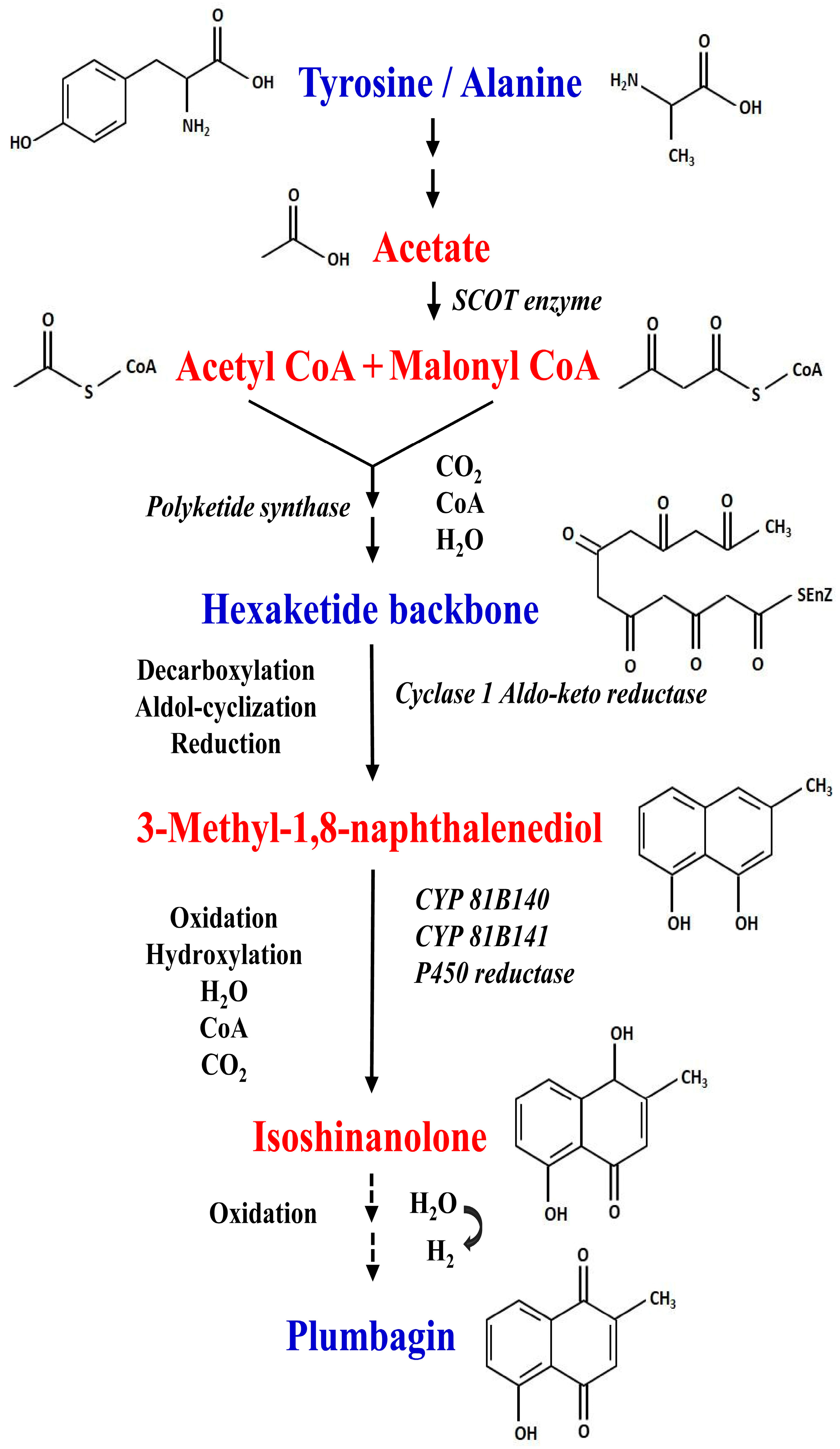
5. Elucidation of Biosynthetic Pathway of Plumbagin
6. Need to Explore Biotechnological Methods to Produce Plumbagin
7. Induction of Callus and Suspensions from Plumbago Species and Plumbagin Accumulation
8. Cell Aggregate Size Optimization as a Novel Method for Plumbagin Accumulation
9. Screening and Selection of a Large Number of Stable Cell Lines for Plumbagin Accumulation in Suspensions of P. rosea
10. Source of Light, Precursor Feeding and Accumulation of Plumbagin in In Vitro Cultures of Plumbago rosea, Drosera burmannii and D. indica
11. Feeding Conditioned Medium and Combination of Metabolic Modules
12. Elicitation of Callus and Suspension Cultures for Plumbagin Accumulation
13. Elicitation in Adventitious Root Cultures
14. Simultaneous and Sequential Dual Elicitations, an Innovative Approach
15. Immobilization, Combination of Elicitation, Immobilization and In Situ Adsorption of Plumbagin by Amberlite XAD-7 and Diaion® HP-20, a Critical Tool for Enhancing Secondary Metabolite
16. Induction and Influence of Ammonium, Potassium, Calcium on Hairy Root Cultures of P. rosea
17. Elicitation of Hairy Roots
18. Cell and Hairy Root Cultures of P. rosea, and Plumbagin Accumulation in Bioreactors
19. Semicontinuous Production of Plumbagin with Total Cell Recycle in a Bioreactor: An Alternative, Key Strategy
20. Addition of Micronutrients, and Cell Wall Inhibitors for the Accumulation of Bioactive Compounds
21. Tasks That Require Immediate Attention
22. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Mohan Jain, S.; Suprasanna, P. Plant cell cultures: Biofactories for the production of bioactive compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Jawahar, G.; Priyadarshani, K.A.; Suprasanna, P.; Suravajhala, P.; Rathnagiri, P.; Charumathi, P.; Kavi Kishor, P.B. Accumulation of Anticancer Compounds in Cultured Cells and Hairy Roots. In Biotechnological Production of Bioactive Phytochemicals of Medicinal Value: A Comprehensive Treatise; Kavi Kishor, P.B., Pullaiah, T., Suprasanna, P., Ranga Rao, A., Anabaela, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 471–505. ISBN 978-0-443-21818-7. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Pullaiah, T.; Ranga Rao, A.; Anabela, R.; Rathnagiri, P.; Suprasanna, P. Plants to Pharmacy: Recapitulation of Natural Compounds Transmuting Human Health; Kavi Kishor, P.B., Pullaiah, T., Suprasanna, P., Ranga Rao, A., Anabaela, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–16. ISBN 978-0-443-21818-7. [Google Scholar] [CrossRef]
- Government of India, Medicinal Plants Introduction, Indian System of Medicine and Homoeopathy (ISMH), Department of ISMH, Ministry of Health and Family Welfare, Govt. of India. 2000. Available online: http://indianmedicine.nic.in/html/plants/mimain.htm (accessed on 18 October 2024).
- WHO. Traditional Medicine: Growing Needs and Potential. In WHO Policy Perspectives on Medicines; WHO: Geneva, Switzerland, 2002; pp. 1–6. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Reisman, S.E.; Maimone, T.J. Total synthesis of complex natural products: More than a race for molecular summits. Acc. Chem. Res. 2021, 54, 1815–1816. [Google Scholar] [CrossRef]
- Mahajan, Y.A.; Shinde, B.A.; Shirke, H.A.; Jawahar, G.; Prashanth, S.; Kavi Kishor, P.B.; Kadoo, N.Y.; Nikam, T.D. Unlocking the genetic and biotechnological potential of Gloriosa superba to enhance its alkaloid production. Ind. Crops Prod. 2024, 211, 118144. [Google Scholar] [CrossRef]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Sandur, S.K. Chemopreventive and Anticancer Effects of Plumbagin: Novel Mechanism(S) Via Modulation of Cellular Redox. In Cancer Sensitizing Agents for Chemotherapy, Role of Nutraceuticals in Cancer Chemosensitization; Bharti, A.C., Aggarwal, B.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 325–341. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Sahu, G.; Muralidhar, J. Screening of Plumbago species for the bioactive marker plumbagin. Pharm. Biol. 2002, 40, 508–511. [Google Scholar] [CrossRef]
- Hsieh, Y.; Lin, L.; Tsai, T. Determination and identification of plumbagin from the roots of Plumbago zeylanica L. by liquid chromatography with tandem mass spectrometry. J. Chromatograph. 2005, 1083, 141–145. [Google Scholar] [CrossRef]
- Marczak, L.; Kawiak, A.; Lojkowska, E.; Stobiecki, M. Secondary metabolites in in vitro cultured plants of the genus Drosera. Phytochem. Anal. 2005, 16, 143–149. [Google Scholar] [CrossRef]
- Dorni, A.I.C.; Vidyalakshmi, K.; Vasanthi, R.; Rajamanickam, G.; Dubey, G. HPTLC method for the quantification of plumbagin in three Plumbago species. Res. J. Phytochem. 2007, 1, 46–51. [Google Scholar]
- Roy, A.; Bharadvaja, N. A review on pharmaceutically important medical plant: Plumbago zeylanica. J. Ayurvedic Herb. Med. 2017, 3, 225–228. [Google Scholar]
- Badwaik, H.R.; Kumari, L.; Nakhate, K.; Verma, V.S.; Sakure, K. Phytoconstituent plumbagin: Chemical, biotechnological and pharmaceutical aspects. Stud. Nat. Prod. Chem. 2019, 63, 415–460. [Google Scholar] [CrossRef]
- Rahman-Soad, A.; Dávila-Lara, A.; Paetz, C.; Mithöfer, A. Plumbagin, a potent naphthoquinone from Nepenthes plants with growth inhibiting and larvicidal activities. Molecules 2021, 26, 825. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.A.; Hu, J.; Lei, T.; Chen, Q.; Li, J.; Yang, L.; Hu, D.; Gao, S. MeJA induced hairy roots in Plumbago auriculata L. by RNA-seq profiling and key synthase provided new insights into the sustainable production of plumbagin and saponins. Front. Plant Sci. 2024, 15, 1411963. [Google Scholar] [CrossRef]
- Khatun, A. Plumbago indica L.: A review of its medicinal uses, phytochemistry, pharmacology, and toxicology. Internat. J. Herb. Med. 2023, 11, 31–37. [Google Scholar] [CrossRef]
- Pant, M.; Lal, A.; Rana, S.; Rani, A. Plumbago zeylanica L.: A mini review. Int. J. Pharm. Appl. 2012, 3, 399–405. [Google Scholar]
- Sharma, A.; Singh, N.A. Multifarious potent herb: Plumbago zeylanica: A mini review. Int. J. Recent Sci. Res. 2015, 6, 4825–4829. [Google Scholar]
- Jijhotiya, A.; Madhuri Goyal, S. Qualitative and quantitative phytochemical estimation of leaves extracts of plant Plumbago zeylanica. Int. J. Recent Sci. Res. 2018, 9, 23249–23252. [Google Scholar]
- Chitrak, (Plumbago zeylanica)—Properties, Benefits & Dosage. 2019. Available online: https://www.planetayurveda.com (accessed on 21 October 2024).
- Andhale, N.B.; Shahnawaz, M.; Ade, A.B. Fungal endophytes of Plumbago zeylanica L. enhances plumbagin content. Bot. Stud. 2019, 60, 21. [Google Scholar] [CrossRef]
- Shukla, B.; Saxena, S.; Usmani, S.; Kushwaha, P. Phytochemistry and pharmacological studies of Plumbago zeylanica L.: A medicinal plant review. Clin. Phytosci. 2021, 7, 34. [Google Scholar] [CrossRef]
- Mohtashami, L.; Shakeri, A.; Javadi, B. Neuroprotective natural products against experimental autoimmune encephalomyelitis: A review. Neurochem. Internat. 2019, 129, 104516. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Mishra, B.; Tiwari, V.; Tripathi, V. An account of phytochemials from Plumbago zeylanica (Family-Plumbaginaceae): A natural gift to human being. Chronical Young Sci. 2012, 3, 178–198. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Velmurugan, P.; Raja, R.B. Anti-inflammatory and cytotoxic effects of extract from Plumbago zeylanica. Afr. J. Microbiol. Res. 2010, 4, 1239–1245. [Google Scholar]
- Budavari, S.; O’Neal, M.; Smith, A.; Heckelman, P.; Kinneary, J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biological, 12th ed.; Merck & Co. Inc.: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- Komaraiah, P.; Amrutha, R.N.; Sita Lakshmi, G.J.; Rama Krishna, S.V.; Kavi Kishor, P.B. Production of Plumbagin-A multifarious Medicinal Compound from Cultured Cells of Plumbago rosea L. In Plant Biotechnology and Its Applications in Tissue Culture; Aswani, K., Shikha, R., Eds.; I.K. International Pvt. Ltd.: New Delhi, India, 2005; pp. 123–139. ISBN 8188237507. [Google Scholar]
- Komaraiah, P.; Kavi Kishor, P.B.; Maria, C.; Karl-Eric, M.; Carl-Fredrik, M. Enhancement of anthraquinone accumulation in Morinda citrifolia suspension cultures. Plant Sci. 2005, 168, 1337–1344. [Google Scholar] [CrossRef]
- Komaraiah, P.; Jogeswar, G.; Ramakrishna, S.V.; Kavi Kishor, P.B. Naphthoquinone Production from Cultured Cells of Plumbago indica L.; Janardhan Reddy, K., Bir Bahadur, B., Rao, M.L.N., Eds.; Advances in Medicinal Plants; Universities Press (India) Private Limited: Hyderabad, India, 2007; pp. 185–193. ISBN 8173715882. [Google Scholar]
- Roy, A.; Bharadvaja, N. Establishment of root suspension culture of Plumbago zeylanica and enhanced production of plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Satheeshkumar, K.; Jose, B.; Soniya, E.V.; Seeni, S. Isolation of morphovariants through plant regeneration in Agrobacterium rhizogenes induced hairy root cultures of Plumbago rosea L. Indian J. Biotech. 2009, 8, 435–441. [Google Scholar]
- Pillai, D.B.; Jose, B.; Satheeshkumar, K.; Krishnan, P.N. Optimization of inoculum density in hairy root cultures of Plumbago rosea L. for enhanced growth and plumbagin production towards scaling up in bioreactor. Ind. J. Biotech. 2015, 14, 264–269. [Google Scholar]
- Beigmohamadi, M.; Movafeghi, A.; Sharafi, A.; Jafari, S.; Danafar, H. Cell suspension culture of Plumbago europaea L. towards production of plumbagin. Iran J. Biotechnol. 2019, 17, e2169. [Google Scholar] [CrossRef]
- Pandey, D.K.; Katoch, K.; Das, T.; Majumder, M.; Dhama, K.; Mane, A.B.; Gopalakrishnan, A.V.; Dey, A. Approaches for in vitro propagation and production of plumbagin in Plumbago spp. Appl. Microb. Biotechnol. 2023, 107, 4119–4132. [Google Scholar] [CrossRef]
- Bringmann, G.; Wohlfarth, M.; Rischer, H.; Rückert, M.; Schlauer, J. The polyketide folding mode in the biogenesis of isoshinanolone and plumbagin from Ancistrocladus heyneanus (Ancistrocladaceae). Tetrahed. Lett. 1998, 39, 8445–8448. [Google Scholar] [CrossRef]
- Hook, I.; Mills, C.; Sheridan, H. Bioactive naphthoquinones from higher plants. Stud. Nat. Prod. Chem. 2014, 41, 119–160. [Google Scholar] [CrossRef]
- Aronsson, P.; Munissi, J.J.E.; Gruhonjic, A.; Fitzpatrick, P.A.; Landberg, G.; Nyandoro, S.S.; Erdelyi, M. Phytoconstituents with radical scavenging and cytotoxic activities from Diospyros shimbaensis. Diseases 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Lee, H.S. Acaricidal activity and function of mite indicator using plumbagin and its derivatives isolated from Diospyros kaki Thunb. roots (Ebenaceae). J. Microbiol. Biotechnol. 2008, 18, 314–321. [Google Scholar]
- Babula, P.; Mikelova, R.; Potesil, D.; Adam, V.; Kizek, R.; Havel, L.; Sladký, Z. Simultaneous determination of 1,4-naphtoquinone, lawsone, juglone and plumbagin by liquid chromatography with UV detection. Biomed. Pap. 2005, 149, 25–28. [Google Scholar]
- Krolicka, A.; Szpitter, A.; Gilgenast, E.; Romanik, G.; Kaminski, M.; Lojkowska, E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzym. Microb. Technol. 2008, 42, 216–221. [Google Scholar] [CrossRef]
- Ziaratnia, S.; Kunert, K.; Lall, N. Elicitation of 7-methyljuglone in Drosera capensis. S. Afr. J. Bot. 2009, 75, 97–103. [Google Scholar] [CrossRef][Green Version]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmannii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef]
- Thaweesak, J.; Sakamoto, S.; Tanaka, H.; Waraporn, P. Elicitation effect on production of plumbagin in in vitro culture of Drosera indica L. J. Med. Plant Res. 2011, 5, 4949–4953. [Google Scholar]
- Boonsnongcheep, P.; Sae-foo, W.; Banpakoat, K.; Channarong, S.; Chitsaithan, S.; Uafua, P.; Putha, W.; Kerdsiri, K.; Putalun, W. Artificial color light sources and precursor feeding enhance plumbagin production of the carnivorous plants Drosera burmannii and Drosera indica. J. Photochem. Photobiol. B Biol. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Finnie, J.F.; van Staden, J. Drosera spp. (Sundew): Micropropagation and the in vitro production of plumbagin. In Medicinal and Aromatic Plants, V. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 24, pp. 164–177. [Google Scholar] [CrossRef]
- Grevenstuk, T.; Gonçalves, S.; Domingos, T.; Quintas, C.; van der Hooft, J.J.; Vervoort, J.; Romano, A. Inhibitory activity of plumbagin produced by Drosera intermedia on food spoilage fungi. J. Sci. Food Agric. 2012, 92, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Krychowiak-Masnicka, M.; Grinholc, M.; Banasiuk, R.; Krauze-Baranowska, M.; Glód, D.; Kawiak, A.; Krolicka, A. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS ONE 2014, 9, e115727. [Google Scholar] [CrossRef]
- Tian, J.; Chen, Y.; Ma, B.; He, J.; Tong, J.; Wang, Y. Drosera peltata Smith var. lunata (Buch.-Ham.) C.B. Clarke as a feasible source of plumbagin: Phytochemical analysis and antifungal activity assay. World J. Microbiol. Biotechnol. 2014, 30, 737–745. [Google Scholar] [CrossRef]
- Sanhueza, V.; Fajardo, V.; Cuadra, P. Occurrence of plumbagin and 7-methyljuglone in the Patagonian sundew Drosera uniflora. Chem. Nat. Compd. 2019, 55, 322–323. [Google Scholar] [CrossRef]
- Norman, E.O.; Tuohey, H.; Pizzi, D.; Saidah, M.; Bell, R.; Brkljaca, R.; White, J.M.; Gasser, R.B.; Taki, A.C.; Urban, S. Phytochemical profiling and biological activity of the Australian carnivorous plant, Drosera magna. J. Nat. Prod. 2021, 84, 964–971. [Google Scholar] [CrossRef]
- Krychowiak-Masnicka, M.; Krauze-Baranowska, M.; Godlewska, S.; Kaczynski, Z.; Bielicka-Giełdon, A.; Grzegorczyk, N.; Narajczyk, M.; Frackowiak, J.E.; Krolicka, A. Potential of silver nanoparticles in overcoming the intrinsic resistance of Pseudomonas aeruginosa to secondary metabolites from carnivorous plants. Int. J. Mol. Sci. 2021, 22, 4849. [Google Scholar] [CrossRef]
- Crouch, I.J.; Finnie, J.F.; Staden, J. Studies on the isolation of plumbagin from in vitro and in vivo grown Drosera species. Plant Cell Tissue Organ Cult. 1990, 21, 79–82. [Google Scholar] [CrossRef]
- Grevenstuk, T.; Goncalves, S.; Nogueira, J.M.F.; Romano, A. Plumbagin recovery from field specimens of Drosophyllum lusitanicum (L.) link. Phytochem. Anal. 2008, 19, 229–235. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Campbell, B.C. Regulation of aflatoxin production by naphthoquinones of walnut (Juglans regia). J. Agric. Food Chem. 2000, 48, 4418–4421. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 2006, 281, 17023–17033. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Kaewamatawong, R.; Ruangrungsi, N.; Krungkrai, J. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Medica 1998, 64, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lee, S.; Cha, B. Antifungal activity of plumbagin purified from leaves of Nepenthes ventricosa x maxima against phytopathogenic fungi. Plant Pathol. J. 2007, 23, 113. [Google Scholar] [CrossRef][Green Version]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Buch, F.; Rott, M.; Rottloff, S.; Paetz, C.; Hilke, I.; Raessler, M.; Mithöfer, A. Secreted pitfall-trap fluid of carnivorous Nepenthes plants is unsuitable for microbial growth. Ann. Bot. 2013, 111, 375–383. [Google Scholar] [CrossRef]
- Ou-Yang, F.; Tsai, I.H.; Tang, J.Y.; Yen, C.Y.; Cheng, Y.B.; Farooqi, A.A.; Chen, S.R.; Yu, S.Y.; Kao, J.K.; Chang, H.W. Antiproliferation for breast cancer cells by ethyl acetate extract of Nepenthes thorellii x (ventricosa x maxima). Int. J. Mol. Sci. 2019, 20, 3238. [Google Scholar] [CrossRef]
- Davila-Lara, A.; Rahman-Soad, A.; Reichelt, M.; Mithofer, A. Carnivorous Nepenthes x ventrata plants use a naphthoquinone as phytoanticipin against herbivory. PLoS ONE 2021, 16, e0258235. [Google Scholar] [CrossRef]
- Jaksova, J.; Adamec, L.; Petrík, I.; Novák, O.; Šebela, M.; Pavlovic, A. Contrasting effect of prey capture on jasmonate accumulation in two genera of aquatic carnivorous plants (Aldrovanda, Utricularia). Plant Physiol. Biochem. 2021, 166, 459–465. [Google Scholar] [CrossRef]
- Raj, G.; Kurup, R.; Hussain, A.A.; Baby, S. Distribution of naphthoquinones, plumbagin, droserone, and 5-O-methyl droserone in chitin-induced and uninduced Nepenthes khasiana: Molecular events in prey capture. J. Exp. Bot. 2011, 62, 5429–5436. [Google Scholar] [CrossRef]
- Bringmann, G.; Schlauer, J.; Wolf, K.; Rischer, H.; Buschbom, U.; Kreiner, A.; Thiele, F.; Duschek, M.; Assi, L. Cultivation of Triphyophyllum peltatum (Dioncophyllaceae), the part-time carnivorous plant. Carniv. Plant Newsl. 1999, 28, 7–13. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Cartwright-Jones, C.; Viljoen, A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014, 155, 80–103. [Google Scholar] [CrossRef]
- Kubo, I.; Uchida, M.; Klocke, J.A. An insect ecdysis inhibitor from African medicinal plant Plumbago capensis (Plumbaginaceae). Agric. Biol. Chem. 1983, 47, 911. [Google Scholar] [CrossRef]
- Sharma, I.; Gusain, D.; Dixit, V. Hypolipidaemic and antiatherosclerotic effects of plumbagin in rabbits. Indian J. Physiol. Pharmacol. 1991, 35, 10–14. [Google Scholar] [PubMed]
- Luo, P.; Wong, Y.F.; Ge, L.; Zhang, Z.F.; Liu, Y.; Liu, L.; Zhou, H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor κB activation. J. Pharmacol. Exp. Therapeut. 2010, 335, 735–742. [Google Scholar] [CrossRef]
- Sunil, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antidiabetic effect of plumbagin isolated from Plumbago zeylanica L. root and its effect on GLUT4 translocation in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2012, 50, 4356–4363. [Google Scholar] [CrossRef]
- Gwee, P.S.; Khoo, K.S.; Ong, H.C.; Sit, N.W. Bioactivity guided isolation and structural characterization of the antifungal compound, plumbagin, from Nepenthes gracilis. Pharmaceut. Biol. 2014, 52, 1526–1531. [Google Scholar] [CrossRef]
- Wang, T.; Qiao, H.; Zhai, Z.; Zhang, J.; Tu, J.; Zheng, X.; Qian, N.; Zhou, H.; Lu, E.; Tang, T. Plumbagin ameliorates collagen-induced arthritis by regulating Treg/Th17 cell imbalances and suppressing osteoclastogenesis. Front. Immunol. 2018, 9, 3102. [Google Scholar] [CrossRef]
- Shao, Y.; Dang, M.; Lin, Y.; Xue, F. Evaluation of wound healing activity of plumbagin in diabetic rats. Life Sci. 2019, 231, 116422. [Google Scholar] [CrossRef]
- Periasamy, H.; Iswarya, S.; Pavithra, N.; Senthilnathan, S.; Gnanamani, A. In vitro antibacterial activity of plumbagin isolated from Plumbago zeylanica L. against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef]
- Yap, J.K.Y.; Tan, S.Y.Y.; Tang, S.Q.; Thien, V.K.; Chan, E.W.L. Synergistic antibacterial activity between 1,4-naphthoquinone and β-lactam antibiotics against methicillin-resistant. Microb. Drug Resist. 2020, 27, 234–240. [Google Scholar] [CrossRef]
- Li, Z.; Chinnathambi, A.; Ali Alharbi, S.; Yin, F. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats. Environ. Toxicol. 2020, 35, 1374–1385. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer effects and mechanisms of action of plumbagin: Review of research advances. BioMed Res. Int. 2020, 6940953. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, T.Y.; Tang, T.T. Advances in tumor suppression by plumbagin and its mechanism. Tumor 2014, 34, 957–962. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Dandawate, P.; Yusuf, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Vyawahare, N.; Pawar, A.; Mahaparale, P.; Chellampillai, B. Current development in novel drug delivery systems of bioactive molecule plumbagin. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 209–218. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural plants compounds as modulators of epithelial-to-mesenchymal transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Roy, A. Plumbagin: A potential anti-cancer compound. Mini Rev. Med. Chem. 2021, 21, 731–737. [Google Scholar] [CrossRef]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Sarkar, F.H. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J. Cell Biochem. 2008, 105, 1461–1471. [Google Scholar] [CrossRef]
- Kawiak, A.; Zawacka-Pankau, J.; Lojkowska, E. Plumbagin induces apoptosis in Her2-overexpressing breast cancer cells through the mitochondrial-mediated pathway. J. Nat. Prod. 2012, 75, 747–751. [Google Scholar] [CrossRef]
- Sankara, A.J.V.S.P.K.; Naresh, K.L.; Animisha, M. In vitro anticancer activities of few plant extracts against MCF-7 and HT-29 cell lines. Internat. J. Pharma Sci. 2013, 3, 185–188. [Google Scholar]
- Eldhose, B.; Gunawan, M.; Rahman, M.; Latha, M.S.; Notario, V. Plumbagin reduces human colon cancer cell survival by inducing cell cycle arrest and mitochondria-mediated apoptosis. Int. J. Oncol. 2014, 45, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Wojciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Biological potential of carnivorous plants from Nepenthales. Molecules 2023, 28, 3639. [Google Scholar] [CrossRef] [PubMed]
- Binoy, A.; Nedungadi, D.; Katiyar, N.; Bose, C.; Shankarappa, S.A.; Nair, B.G.; Mishra, N. Plumbagin induces paraptosis in cancer cells by disrupting the sulfhydryl homeostasis and proteasomal function. Chem. Biologic. Interact. 2019, 310, 108733. [Google Scholar] [CrossRef]
- Mani, H.; Jayachitra, A. Anti-cancer activity of ethanolic extract of Plumbago zeylanica against Dalton’s ascitic lymphoma in mice. Int. J. Appl. Eng. Res. 2019, 14, 1715–1721. [Google Scholar]
- Kawiak, A.; Domachowska, A. Plumbagin suppresses the invasion of HER2-overexpressing breast cancer cells through inhibition of IKKα-mediated NF-κB activation. PLoS ONE 2016, 11, e0164064. [Google Scholar] [CrossRef]
- Sakunrangsit, N.; Kalpongnukul, T.; Pisitkun, T.; Ketchart, W. Plumbagin enhances tamoxifen sensitivity and inhibits tumor invasion in endocrine resistant breast cancer through EMT regulation. Phytother. Res. 2016, 30, 1968–1977. [Google Scholar] [CrossRef]
- Sameni, S.; Hande, M.P. Plumbagin triggers DNA damage response, telomere dysfunction and genome instability of human breast cancer cells. Biomed. Pharmacother. 2016, 82, 256–268. [Google Scholar] [CrossRef]
- Sharma, B.; Dhiman, C.; Hasan, G.M.; Shamsi, A.; Hassan, M.I. Pharmacological features and therapeutic implications of plumbagin in cancer and metabolic disorders: A narrative review. Nutrients 2024, 16, 3033. [Google Scholar] [CrossRef]
- Pearlman, R.L.; Pal, H.C.; Elmets, C.A.; Afaq, F. Plumbagin induces apoptosis in melanoma cells by ROS-mediated disruption of mitochondrial membrane potential and inhibition of PI3K/AKT/mTOR signaling. J. Investig. Dermatol. 2016, 136, 15. [Google Scholar] [CrossRef]
- Majiene, D.; Kuseliauskyte, J.; Stimbirys, A.; Jekabsone, A. Comparison of the effect of native 1,4-naphthoquinones plumbagin, menadione, and lawsone on viability, redox status, and mitochondrial functions of c6 glioblastoma cells. Nutrients 2019, 11, 1294. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Rajendran, P.; Li, F.; Ramachandran, L.; Hay, H.S.; Kannaiyan, R.; Swamy, S.N.; Vali, S.; Kapoor, S.; et al. Plumbagin inhibits invasion and migration of breast and gastric cancer cells by downregulating the expression of chemokine receptor CXCR4. Mol. Cancer 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Liu, J.; Zhai, D.; Lin, Q.; He, L.; Dong, Y.; Zhang, J.; Lu, B.; Chen, Y.; Yi, Z.; et al. Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2. Br. J. Pharmacol. 2012, 165, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sharma, D.; Sandur, S.K.; Subrahmanyam, G.; Krishnan, S.; Poduval, T.B.; Sainis, K.B. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J. Cell Biochem. 2010, 110, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, F.; Jin, Z.; Zhai, Z.; Wang, Y.; Tu, B.; Yan, W.; Tang, T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 2014, 64, 177–183. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yu, J.; Liu, T.T.; Yang, K.X.; Yang, L.Y.; Chen, Q.; Shi, F.; Hao, J.J.; Cai, Y.; Wang, M.R.; et al. Plumbagin inhibits the proliferation and survival of esophageal cancer cells by blocking STAT3-PLK1-AKT signaling. Cell Death Dis. 2018, 9, 17. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; You, W.; Zhou, F.; Guo, Z.; Qian, K.; Xiao, Y.; Wang, X. Suppressive effects of plumbagin on the growth of human bladder cancer cells via PI3K/AKT/mTOR signaling pathways and EMT. Cancer Cell Int. 2020, 20, 520. [Google Scholar] [CrossRef]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Plumbagin, a natural product with potent anticancer activities, binds to and inhibits dihydroorotase, a key enzyme in pyrimidine biosynthesis. Int. J. Mol. Sci. 2021, 22, 6861. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sawant, A.V.; Prassanawar, S.S.; Panda, D. Copper-plumbagin complex produces potent anticancer effects by depolymerizing microtubules and inducing reactive oxygen species and DNA damage. ACS Omega 2023, 8, 3221–3235. [Google Scholar] [CrossRef]
- El Oirdi, M. Plumbagin’s antiproliferative mechanism in human cancer cells: A copper-dependent cytotoxic approach. Chem. Biol. Drug Des. 2024, 104, e14606. [Google Scholar] [CrossRef]
- Li, X.X.; Liu, C.; Dong, S.L.; Ou, C.S.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, M.; Li, Z.; Jiang, H.; Shi, J.; Shi, X.; Liu, S.; Zhao, J.; Kong, L.; Zhang, W.; et al. Intake of soy, soy isoflavones and soy protein and risk of cancer incidence and mortality. Front. Nutr. 2022, 9, 847421. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Olivas-Aguirre, F.J.; Dufoo-Hurtado, E.; Castaneda-Moreno, R.; Villegas-Quintero, H.; Medina-Franco, J.L.; Mendoza, S.; Wall-Medrano, A. Potential anticancer activity of pomegranate (Punica granatum L.) fruits of different color: In vitro and in silico evidence. Biomolecules 2022, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, R.; Kahingalage, I.N.; Archibald, D.; Casari, I.; Falasca, M. Synergistic anticancer activity of plumbagin and xanthohumol combination on pancreatic cancer models. Int. J. Mol. Sci. 2024, 25, 2340. [Google Scholar] [CrossRef]
- Andres, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as antioxidant/prooxidant compounds and donors of reducing species: Relationship with human antioxidant metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Eltahir, S.; Ahmad, A. Flavonoids on the frontline against cancer metastasis. Cancers 2023, 15, 4139. [Google Scholar] [CrossRef]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef]
- Ahmad, I.; Tabrez, S. Exploring natural resources: Plumbagin as a potent anticancer agent. S. Afr. J. Bot. 2024, 174, 167–179. [Google Scholar] [CrossRef]
- Kawiak, A.; Domachowska, A.; Lojkowska, E. Plumbagin increases paclitaxel-induced cell death and overcomes paclitaxel resistance in breast cancer cells through ERK-mediated apoptosis induction. J. Nat. Prod. 2019, 82, 878–885. [Google Scholar] [CrossRef]
- Giacomini, I.; Cocetta, V.; Carrara, M.; Ragazzi, E.; Montopoli, M. Plumbagin induces cell cycle arrest and apoptosis in A431 cisplatin-resistant cancer cells. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Senthilvelan, M.; Ravindran, R.; Devi, S.R. Plumbago zeylanica action on blood coagulation profile with and without blood volume reduction. Vascul. Pharmacol. 2006, 45, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhu, X.; Yang, X.; Jin, L.; Xu, J.; Ma, T.; Yang, H. Plumbagin prevents secretory diarrhoea by inhibiting CaCC and CFTR channel activities. Front. Pharma. 2019, 10, 1181. [Google Scholar] [CrossRef]
- Edwin, S.; Siddheswar, J.B.; Dharam, C.J. Antifertility activity of leaves of Plumbago zeylanica Linn. In female albino rats. Eur. J. Contracept Reprod. Health Care 2009, 14, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Vishnukanta, S.; Rana, A.C. Evaluation of the antifertility activity of the hydroalcoholic extract of the leaves of Plumbago zeylanica L. (Plumbaginaceae) in female wistar rats. Indian J. Pharm. Educ. Res. 2015, 44, 49–55. [Google Scholar]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Sandur, S.K.; Sainis, K.B.; Poduval, T.B. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-κB suppression. Inflammation 2014, 37, 542–554. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Paramasivam, V. Potential anti-inflammatory activity of Plumbago zeylanica. Asian J. Pharm. Clin. Res. 2017, 10, 372–375. [Google Scholar] [CrossRef]
- Poosarla, A. Effect of Plumbago zeylanica ethyl acetate extract in prevention or treatment of arthritis using adjuvant induced arthritic rat model. Ind. J. Appl. Res. 2017, 7, 44–46. [Google Scholar] [CrossRef]
- Zaki, A.M.; El-Tanbouly, D.M.; Abdelsalam, R.M.; Zaki, H.F. Plumbagin ameliorates hepatic ischemia-reperfusion injury in rats: Role of high mobility group box 1 in inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 106, 785–793. [Google Scholar] [CrossRef]
- Pradeepa, V.; Senthil-Nathan, S.; Sathish-Narayanan, S.; Selin-Rani, S.; Vasantha-Srinivasan, P.; Thanigaivel, A.; Ponsankar, A.; Edwin, E.S.; Sakthi-Bagavathy, M.; Kalaivani, K.; et al. Potential mode of action of a novel plumbagin as a mosquito repellent against the malarial vector Anopheles stephensi (Culicidae: Diptera). Pestic. Biochem. Physiol. 2016, 134, 84–93. [Google Scholar] [CrossRef]
- Sumsakul, W.; Plengsuriyakarn, T.; Chaijaroenkul, W.; Viyanant, V.; Karbwang, J.; Na-Bangchang, K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement Altern. Med. 2014, 14, 15. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Owais, H. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) and synergy of some bioactive plant extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Aqil, F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol. Res. 2007, 162, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.; Sarkar, P.; Sultana, F.; Saikia, M.; Dey, A. In vitro antimicrobial screening with phytochemical study of Plumbago zeylanica L. collected from two regions of Eastern Himalayas—A comparative study. Int. J. Phytopharm. 2014, 4, 120–123. [Google Scholar]
- Shweta, S.; Dubey, S. Antimicrobial activity of leaves extract of Plumbago zeylanica plant against known drugs. Int. J. Res. Stud. Biosci. 2015, 3, 1–6. [Google Scholar]
- Qais, F.A.; Khan, M.S.; Ahmad, I.; Husain, F.M.; Al-Kheraif, A.A.; Arshad, M.; Alam, P. Plumbagin inhibits quorum sensing-regulated virulence and biofilms of Gram-negative bacteria: In vitro and in silico investigations. Biofouling 2021, 37, 724–739. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, J.; Zhang, X.; Liu, Y.; Huang, Z.; Yuan, L.; Zhang, Y.; Cao, J.; Chen, L.; Liu, Y.; et al. Plumbagin resurrect colistin susceptible against colistin-resistant Pseudomonas aeruginosa in vitro and in vivo. Front. Microbiol. 2022, 13, 1020652. [Google Scholar] [CrossRef]
- Qian, W.; Wang, W.; Zhang, J.; Fu, Y.; Liu, Q.; Li, X.; Wang, T.; Zhang, Q. Exploitation of the antifungal and antibiofilm activities of plumbagin against Cryptococcus neoformans. Biofouling 2022, 38, 558–574. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Ma, Z.; Zhang, Y.; Zhang, S.; Zhao, D.; Yao, Z.; Zhou, T.; Wang, Z. Plumbagin enhances antimicrobial and anti-biofilm capacities of chlorhexidine against clinical Klebsiella pneumoniae while reducing resistance mutations. Microbiol Spectr. 2024, 12, e0089624. [Google Scholar] [CrossRef]
- Edenharder, R.; Tang, X. Inhibition of the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-nitropyrene by flavonoids, coumarins, quinones and other phenolic compounds. Food Chem. Toxicol. 1997, 35, 357–372. [Google Scholar] [CrossRef]
- Santhakumari, G.; Saralamma, P.G.; Radhakrishnan, N. Effect of plumbagin on cell growth and mitosis. Indian J. Exp. Biol. 1980, 18, 215–218. [Google Scholar]
- Rajakrishnan, R.; Lekshmi, R.; Benil, P.B.; Thomas, J.; Farhan, A.H.; Rakesh, V.; Khalaf, S. Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephroprotective agent. Saudi J. Biol. Sci. 2017, 24, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, M.; Rao, K.S. Clinical evaluation of haridra and chitrak in the management of medoroga (obesity). J. Ayurveda 2007, 1, 226–228. [Google Scholar]
- Zarmouh, M.M.; Subramaniyam, K.; Viswanathan, S.; Kumar, P.G. Cause and effect of Plumbago zeylanica root extract on blood glucose and hepatic enzymes in experimental diabetic rats. Afr. J. Microbiol. Res. 2010, 4, 2674–2677. [Google Scholar]
- Chauhan, M. A review on morphology, phytochemistry and pharmacological activities of medicinal herb Plumbago zeylanica Linn. Int. J. Pharmacogn. Phytochem. 2014, 3, 95–118. [Google Scholar]
- Vishnukanta, S.; Rana, A.C. Plumbago zeylanica: A phytopharmacological review. Int. J. Pharm. Sci. Res. 2011, 2, 247–255. [Google Scholar] [CrossRef]
- Ganesan, K.; Gani, S.B. Ethnomedical and pharmacological potentials of Plumbago zeylanica L.—A review. Am. J. Phytomed. Clin. Ther. 2013, 1, 313–337. [Google Scholar]
- Jangra, A.; Chadha, V.; Kumar, D.; Kumar, V.; Arora, M.K. Neuroprotective and acetylcholinesterase inhibitory activity of plumbagin in ICV-LPS induced behavioral deficits in rats. Curr. Res. Behavior. Sci. 2021, 2, 100060. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Lin, L.C.; Tsai, T.H. Measurement and pharmacokinetic study of plumbagin in a conscious freely moving rat using liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 844, 1–5. [Google Scholar] [CrossRef]
- Chrastina, A.; Baron, V.; Abedinpour, P.; Rondeau, G.; Welsh, J.; Borgstrom, P. Plumbagin-loaded nanoemulsion drug delivery formulation and evaluation of antiproliferative effect on prostate cancer cells. BioMed Res. Internat. 2018, 2018, 9035452. [Google Scholar] [CrossRef]
- Kamble, P.R.; Shaikh, K.S. Optimization and evaluation of self-nanoemulsifying drug delivery system for enhanced bioavailability of plumbagin. Planta Med. 2022, 88, 79–90. [Google Scholar] [CrossRef]
- Durand, R.; Zenk, M.H. Biosynthesis of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) via the acetate pathway in higher plants. Tetrahedron Lett. 1971, 12, 3009–3012. [Google Scholar] [CrossRef]
- Rischer, H.; Hamm, A.; Bringmann, G. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 2002, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.; Zenk, M.H. The homogentisate ring cleavage pathway in the biosynthesis of acetate derived naphthoquinones of Droseraceae. Phytochemistry 1974, 13, 1483–1492. [Google Scholar] [CrossRef]
- Jadhav, S.; Phapale, P.; Thulasiram, H.V.; Bhargava, S. Polyketide synthesis in tobacco plants transformed with a Plumbago zeylanica type III hexaketide synthase. Phytochemistry 2014, 98, 92–100. [Google Scholar] [CrossRef]
- Vasav, A.P.; Pable, A.A.; Barvkar, V.T. Differential transcriptome and metabolome analysis of Plumbago zeylanica L. reveal putative genes involved in plumbagin biosynthesis. Fitoterapia 2020, 147, 104761. [Google Scholar] [CrossRef]
- Muralidharan, K.S.; Lalitha, R.; Girija, S.; Kumar, P.R.; Akashi, P.S.; Swamy, M.N.; Nayana, M.; Jayanthi, M. Identification of a reaction intermediate and mechanism of action of intermediary enzymes in plumbagin biosynthetic pathway using molecular dynamics simulation. Catalysts 2020, 10, 280. [Google Scholar] [CrossRef]
- Ved, D.K.; Goraya, G.S. Demand and Supply of Medicinal Plants in India; The National Medicinal Plant Board (NMPB): New Delhi, India, 2007; p. 18. [Google Scholar]
- Gangopadhyay, M.; Dewanjee, S.; Bhattacharya, S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J. Biosci. Bioeng. 2011, 111, 706–710. [Google Scholar] [CrossRef]
- Kaewbumrung, S.; Panichayupakaranant, P. Isolation of three antibacterial naphthoquinones from Plumbago indica roots and development of a validated quantitative HPLC analytical method. Nat. Prod. Res. 2012, 26, 2020–2023. [Google Scholar]
- Komaraiah, P.; Jogeswar, G.; Amrutha, R.N.; Sri Laxmi, P.; Lavanya, B.; Rama Krishna, S.V.; Kavi Kishor, P.B. Influence of hormones and selection of stable cell lines of Plumbago rosea for accumulation of plumbagin. J. Plant Biotechnol. 2003, 5, 171–175. [Google Scholar]
- Komaraiah, P.; Ramakrishna, S.V.; Reddanna, P.; Kavi Kishor, P.B. Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and in situ adsorption. J. Biotechnol. 2003, 101, 181–187. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, M.; Gokulanathan, A.; Haribalan, P.; Ashakiran, K.; Kumar, C.D.; Kamal, D.; Girija, S.; Renukadevi, B.S. Plumbagin from two Plumbago species inhibits the growth of stomach and breast cancer cell lines. Indust. Crops Prod. 2020, 146, 112147. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.M.; Ritala, A.; Cardon, F. Hairy root cultures: A versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy root culture: A potent method for improved secondary metabolite production of Solanaceous plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef]
- Fieser, L.F.; Dunn, J.T. Synthesis of plumbagin. J. Am. Chem. Soc. 1936, 58, 572–575. [Google Scholar] [CrossRef]
- Xu, X.; Meier, F.; Blount, B.A.; Pretorius, I.; Ellis, T.; Paulsen, I.T.; Williams, T.C. Trimming the genomic fat: Minimising and re-functionalising genomes using synthetic biology. Nat. Commun. 2023, 14, 1984. [Google Scholar] [CrossRef]
- Gabrielli, P.; Rosa, L.; Gazzani, M.; Meys, R.; Bardow, A.; Mazzotti, M.; Sansavini, G. Net-zero emissions chemical industry in a world of limited resources. One Earth 2023, 6, 682–704. [Google Scholar] [CrossRef]
- IPCC. Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Tian, D.S.; Zhang, X.; Cox, R.J. Comparing total chemical synthesis and total biosynthesis routes to fungal specialized metabolites. Nat. Prod. Rep. 2025. [Google Scholar] [CrossRef]
- Fujita, Y. Industrial production of shikonin and berberine. In Book Series: Novartis Foundation Symposia; Bock, G., Marsh, J., Eds.; Ciba Foundation Symposium 137; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Ahuja, P.S. Biotechnological approaches to the production of shikonins: A critical review with recent updates. Crit. Rev. Biotechnol. 2016, 36, 327–340. [Google Scholar] [CrossRef]
- Motolinia-Alcantara, E.A.; Castillo-Araiza, C.O.; Rodríguez Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Ogita, S. Plant cell, tissue and organ culture: The most flexible foundations for plant metabolic engineering applications. Nat. Prod. Commun. 2015, 10, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Karioti, A.; Rohr, D.; Bilia, A.R.; Efferth, T. Production of rosmarinic acid and salvianolic acid B from callus culture of Salvia miltiorrhiza with cytotoxicity towards acute lymphoblastic leukemia cells. Food Chem. 2016, 201, 292–297. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Fischer, R.; Emans, N.; Schuster, F.; Hellwig, S.; Drossard, J. Towards molecular farming in the future: Using plant-cell-suspension cultures as bioreactors. Biotechnol. Appl. Biochem. 1999, 30, 109–112. [Google Scholar] [CrossRef]
- Xu, J.; Ge, X.; Dolan, M.C. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol. Adv. 2011, 29, 278–299. [Google Scholar] [CrossRef]
- Verdu-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plant Res. 2009, 3, 1222–1239. [Google Scholar]
- Murthy, H.N.; Lee, E.J.; Paek, Y.K. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Szopa, A.; Kubica, P.; Ekiert, H. Agitated shoot cultures of Aronia arbutifolia and Aronia× prunifolia: Biotechnological studies on the accumulation of phenolic compounds and biotransformation capability. Plant Cell Tissue Organ Cult. 2018, 134, 467–479. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, Y.; Vento, M.; Pistelli, L.; Lombardi, T.; Pistelli, L. Halophyte Artemisia caerulescens L.: Metabolites from in vitro shoots and wild plants. Plants 2022, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Thakore, D.; Srivastava, A.K.; Sinha, A.K. Mass production of ajmalicine by bioreactor cultivation of hairy roots of Catharanthus roseus. Biochem. Eng. J. 2017, 119, 84–91. [Google Scholar] [CrossRef]
- Li, C.; Wang, M. Application of hairy root culture for bioactive compounds production in medicinal plants. Curr. Pharm. Biotechnol. 2021, 22, 592–608. [Google Scholar] [CrossRef]
- Nahalka, J.; Blanarik, P.; Gemeiner, P.; Matusova, E.; Partlova, I. Production of plumbagin by cell suspension cultures of Drosophyllum lusitanicum Link. J. Biotechnol. 1996, 49, 153–163. [Google Scholar]
- Komaraiah, P.; Kavi Kishor, P.B.; Ramakrishna, S.V. Production of plumbagin from cell cultures of Plumbago rosea L. Biotechnol. Lett. 2001, 23, 1269–1272. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, U.; Agrawal, V. Isolation and optimization of plumbagin production in root callus of Plumbago zeylanica L. augmented with chitosan and yeast extract. Ind. Crops Prod. 2020, 151, 112446. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Komaraiah, P.; Jogeswar, J.; Rama Krishna, S.V.; Kavi Kishor, P.B. Acetylsalicylic acid and ammonium induced somatic embryogenesis and enhanced plumbagin production in suspension cultures of Plumbago rosea L. Vitr. Cell. Develop. Biol. 2004, 40, 230–234. [Google Scholar] [CrossRef]
- Komaraiah, P. In Vitro Production of Plumbagin, a Pharmaceutically Important Compound from Cell and Hairy Root Cultures of Plumbago rosea L. Ph.D. Thesis, Osmania University, Hyderabad, India, 2001. [Google Scholar]
- Silja, P.K.; Satheeshkumar, K. Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind. Crops Prod. 2015, 76, 479–486. [Google Scholar] [CrossRef]
- Jose, B.; Silja, P.K.; Pillai, D.B.; Satheeshkumar, K. In vitro cultivation of hairy roots of Plumbago rosea L. in a customized reaction kettle for the production of plumbagin-an anticancer compound. Ind. Crops Prod. 2016, 87, 89–95. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Demiwal, P.; Roy, P.; Sircar, D. Enhanced production of bioactive plumbagin in hairy root cultures and adventitious root cultures of Plumbago zeylanica L. by a novel apocarotenoid elicitor, α-ionone. Ind. Crops Prod. 2023, 203, 117140. [Google Scholar] [CrossRef]
- Komaraiah, P.; Amrutha, R.N.; Kavi Kishor, P.B.; Ramakrishna, S.V. Elicitor enhanced production of plumbagin in suspension cultures of Plumbago rosea L. Enzym. Microbiol. Technol. 2002, 31, 634–639. [Google Scholar] [CrossRef]
- Heble, M.R.; Narayanaswamy, S.; Chadba, M.S. Tissue differentiation and plumbagin synthesis in variant cell strains of Plumbago zeylanica L. in vitro. Plant Sci. Lett. 1974, 2, 405–409. [Google Scholar] [CrossRef]
- Budzianowski, J. Naphthohydroquinone glucosides of Drosera rotundifolia and D. intermedia from in vitro cultures. Phytochemistry 1996, 42, 1145–1147. [Google Scholar] [CrossRef]
- Nahalka, J.; Nahálková, J.; Gemeiner, P.; Blanarik, P. Elicitation of plumbagin by chitin and its release into the medium in Drosophyllum lusitanicum Link. suspension cultures. Biotechnol. Lett. 1998, 20, 841–845. [Google Scholar] [CrossRef]
- Samaj, J.; Blehová, A.; Repcak, M.; Ovecka, M.; Bobak, M. Drosera species (Sundew): In vitro culture and the production of plumbagin and other secondary metabolites. In Medicinal and Aromatic Plants XI. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 43. [Google Scholar] [CrossRef]
- Hook, I.L. Naphthoquinone contents of in vitro cultured plants and cell suspensions of Dionaea muscipula and Drosera species. Plant Cell Tissue Organ Cult. 2001, 67, 281–285. [Google Scholar] [CrossRef]
- Komaraiah, P.; Amrutha, R.N.; Jogeswar, G.; Rama Krishna, S.V.; Kavi Kishor, P.B. Production of plumbagin from hairy root cultures of Plumbago rosea L. Plant Cell Biotechnol. Mol. Biol. 2002, 3, 65–68. [Google Scholar]
- Satheeshkumar, K.; Seeni, S. Production of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) in callus and cell suspension cultures of Plumbago indica Linn. Ind. J. Biotechnol. 2002, 1, 305–308. [Google Scholar]
- Panichayupakaranant, P.; Tewtrakul, S. Plumbagin production by root cultures of Plumbago rosea. Electron. J. Biotechnol. 2002, 5, 228–232. [Google Scholar]
- Verma, P.C.; Singh, D.; Rahman, L.; Gupta, M.M.; Banerjee, S. In vitro studies in Plumbago zeylanica: Rapid micropropagation and establishment of higher plumbagin yielding hairy root cultures. J. Plant Physiol. 2002, 159, 547–552. [Google Scholar] [CrossRef]
- Jaisi, A.; Sakunphueak, A.; Panichayupakaranant, P. Increased production of plumbagin in Plumbago indica root cultures by gamma ray irradiation. Pharm. Biol. 2013, 51, 1047–1051. [Google Scholar] [CrossRef]
- Deshpande, J.; Labade, D.; Shankar, K.; Kata, N.; Chaudhari, M.; Wani, M.; Khetmalas, M. In vitro callus induction and estimation of plumbagin content from Plumbago auriculata Lam. Ind. J. Exp. Biol. 2014, 52, 1122–1127. [Google Scholar]
- Wongsa, T.; Nakkuntod, M.; Premjet, D.; Inthima, P.; Kongbangkerd, A. Enhancement of plumbagin production from Drosera peltata Thumb. using different elicitors. NU Int. J. Sci. 2018, 15, 135–147. [Google Scholar]
- Sharma, U.; Agrawal, V. In vitro shoot regeneration and enhanced synthesis of plumbagin in root callus of Plumbago zeylanica L.-an important medicinal herb. In Vitro Cell Dev. Biol. Plant 2018, 54, 423–435. [Google Scholar] [CrossRef]
- Wongwanich, S.; Inthima, P. Effect of LED Light Quality on Shoot Organogenesis of Leaf-Disc Culture and Elicitation of Plumbagin Production in Drosera spp. and Dionaea muscipula J. Ellis. Doctoral Dissertation, Naresuan University, Phitsanulok, Thailand, 2020. [Google Scholar]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of plumbagin production through elicitation in in vitro-regenerated shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; McGrew, J.J.; Vivanco, J.M. Factors affecting growth of cell suspension cultures of Hypericum perforatum L. (St. John’s Wort) and production of hypericin. In Vitro. Cell. Develop. Biol. Plant 2002, 38, 58–65. [Google Scholar]
- Rao, B.R.; Kumar, D.V.; Amrutha, R.N.; Jalaja, N.; Vaidyanath, K.; Rao, A.M.; Rao, S.K.; Rathnagiri, P.; Kavi Kishor, P.B. Effect of growth regulators, carbon source and cell aggregate size on berberine production from cell cultures of Tinospora cordifolia Miers. Curr. Trends Biotechnol. Pharm. 2008, 2, 269–276. [Google Scholar]
- Kolewe, M.E.; Henson, M.A.; Roberts, S.C. Analysis of aggregate size as a process variable affecting paclitaxel accumulation in Taxus suspension cultures. Biotechnol. Prog. 2011, 27, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhu, C.S.; Feng, J.T.; Han, J.; Song, X.W.; Zhang, X. Aggregate cell suspension cultures of Tripterygium wilfordii Hook. f. for triptolide, wilforgine and wilforine production. Plant Cell Tissue Organ Cult. 2013, 112, 109–116. [Google Scholar] [CrossRef]
- Capataz-Tafur, J.; Trejo-Tapia, G.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Arabinogalactan proteins are involved in cell aggregation of cell suspension cultures of Beta vulgaris L. Plant Cell Tissue Organ Cult. 2011, 106, 169–177. [Google Scholar] [CrossRef]
- Patil, R.A.; Kolewe, M.E.; Roberts, S.C. Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures. Plant Cell Tissue Organ Cult. 2013, 112, 303–310. [Google Scholar] [CrossRef]
- Haida, Z.; Syahida, A.; Ariff, S.M.; Maziah, M.; Hakiman, M. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in cell suspension culture system. Sci. Rep. 2019, 9, 9533. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Scheid, O.M.; Jakovleva, L.; Afsar, K.; Maluszynska, J.; Paszkowski, J. A change of ploidy can modify epigenetic silencing. Proc. Natl. Acad. Sci. USA 1996, 93, 7114–7119. [Google Scholar] [CrossRef]
- Baebler, S.; Hren, M.; Camloh, M.; Ravnikar, M.; Bohaneck, B.; Plaper, I.; Ucman, R.; Zel, J. Establishment of cell suspension cultures of yew (Taxus × media Rehd.) and assessment of their genomic stability. Vitr. Cell. Dev. Biol. Plant 2005, 41, 338–343. [Google Scholar] [CrossRef]
- Zhang, K.R.; John, P.C.L. Raised level of cyclin dependent kinase A after prolonged suspension culture of Nicotiana plumbaginifolia is associated with more rapid growth and division, diminished cytoskeleton and lost capacity for regeneration: Implications for instability of cultured plant cells. Plant Cell Tissue Organ Cult. 2005, 82, 295–308. [Google Scholar] [CrossRef]
- Landey, R.B.; Cenci, A.; Guyot, R.; Bertrand, B.; Georget, F.; Dechamp, E.; Herrera, J.C.; Aribi, J.; Lashermes, P.; Etienne, H. Assessment of genetic and epigenetic changes during cell culture ageing and relations with somaclonal variation in Coffea arabica. Plant Cell Tissue Organ Cult. 2015, 122, 517–531. [Google Scholar] [CrossRef]
- Fu, C.; Li, L.; Wu, W.; Li, M.; Yu, X.; Yu, L. Assessment of genetic and epigenetic variation during long-term Taxus cell culture. Plant Cell Rep. 2012, 31, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, G.; Yang, F.; Liang, Y.; Gao, Q.; Xiang, C.; Li, X.; Yang, R.; Zhang, G.; Jiang, H.; et al. Multilayered regulation of secondary metabolism in medicinal plants. Mol. Hortic. 2023, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Shanks, J.V. Phytochemical engineering: Combining chemical reaction engineering with plant science. Aiche J. 2005, 51, 2–7. [Google Scholar] [CrossRef]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klockner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Buchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef]
- Dhawan, S.; Shasany, A.K.; Naqvi, A.A.; Kumar, S.; Suman, P.S. Menthol tolerant clones of Mentha arvensis: Approach for in vitro selection of menthol rich genotypes. Plant Cell Tissue Organ Cult. 2003, 75, 87–94. [Google Scholar] [CrossRef]
- Georgiev, M.; Pavlov, A.; Ilieva, M. Selection of high rosmarinic acid producing Lavandula vera MM cell lines. Process Biochem. 2006, 41, 2068–2071. [Google Scholar] [CrossRef]
- Qu, J.G.; Zhang, W.; Yu, X.J.; Jin, M.F. Instability of anthocyanin accumulation in Vitis vinifera L. var. Gamay Freaux suspension cultures. Biotechnol. Bioprocess Eng. 2005, 10, 155–161. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Tokarz, K.; Makowski, W.; Banasiuk, R. Response of Dionaea muscipula J. Ellis to light stress in in vitro: Physiological study. Plant Cell Tissue Organ Cult. 2018, 134, 65–77. [Google Scholar] [CrossRef]
- John, R.; Shajitha, P.P.; Devassy, A.; Mathew, L. Effect of elicitation and precursor feeding on accumulation of 20-hydroxyecdysone in Achyranthes aspera Linn. cell suspension cultures. Physiol. Mol. Biol. Plants 2018, 24, 275–284. [Google Scholar] [CrossRef]
- Szewczyk, A.; Paździora, W.; Ekiert, H. The influence of exogenous phenylalanine on the accumulation of secondary metabolites in agitated shoot cultures of Ruta graveolens L. Molecules 2023, 28, 727. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, A.; Panichayupakaranant, P. Enhanced plumbagin production in Plumbago indica root cultures by ʟ-alanine feeding and in situ adsorption. Plant Cell Tissue Organ Cult. 2017, 129, 53–60. [Google Scholar] [CrossRef]
- Rajabi, F.; Heene, E.; Maisch, J.; Nick, P. Combination of plant metabolic modules yields synthetic synergies. PLoS ONE 2017, 12, e0169778. [Google Scholar] [CrossRef]
- Dewey, R.E.; Xie, J. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 2013, 94, 10–27. [Google Scholar] [CrossRef]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Praveen, N. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Kamalipourazad, M.; Sharifi, M.; Maivan, H.Z.; Behmanesh, M.; Chashmi, N.A. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiol. Biochem. 2016, 107, 374–384. [Google Scholar] [CrossRef]
- Silja, P.K.; Gisha, G.P.; Satheeshkumar, K. Enhanced plumbagin accumulation in embryogenic cell suspension cultures of Plumbago rosea L. following elicitation. Plant Cell Tissue Organ Cult. 2014, 119, 469–477. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Bibi, A.; Idrees, M.Z.; Zaman, G.; Farooq, U.; Bhatti, T.T. Elicitation, a mechanistic approach to change the metabolic pathway of plants to produce pharmacological important compounds in in-vitro cell cultures. Glob. J. Eng. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Increased production of plumbagin in Plumbago indica root cultures by biotic and abiotic elicitors. Biotechnol. Lett. 2016, 38, 351–355. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Simultaneous heat shock and in situ adsorption enhance plumbagin production in Plumbago indica root cultures. Eng. Life Sci. 2016, 16, 417–423. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Chitosan elicitation and sequential Diaion® HP-20 addition a powerful approach for enhanced plumbagin production in Plumbago indica root cultures. Process Biochem. 2017, 53, 210–215. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Krishnamurthy, R.; Azeez, M.A. Efect of biofertilizer on growth, yield and bioactive component of Plumbago zeylanica (Lead Wort). J. Agric. Sci. 2016, 8, 141–155. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, R. Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, J.Y.; Liu, R. Modeling of tanshinone synthesis and phase distribution under the combined effect of elicitation and in situ adsorption in Salvia miltiorrhiza hairy root cultures. Biotechnol. Lett. 2011, 33, 813–819. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In Biotechnology of Hairy Root Systems; Doran, P.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 134, pp. 55–89. [Google Scholar] [CrossRef]
- Jaisi, A.; Panichayupakaranant, P. Enhanced plumbagin production in Plumbago indica root culture by simultaneous and sequential dual elicitations using chitosan with ʟ-alanine and methyl-β-cyclodextrin. Bioresour. Bioprocess 2020, 7, 10. [Google Scholar] [CrossRef]
- Doran, P.M. (Ed.) Biotechnology of Hairy Root Systems; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Santoyo-Garcia, J.H.; Walls, L.E.; Valdivia-Cabrera, M.; Malcı, K.; Jonguitud-Borrego, N.; Halliday, K.J.; Rios-Solis, L. The synergetic effect from the combination of different adsorption resins in batch and semi-continuous cultivations of S. cerevisiae cell factories to produce acetylated taxanes precursors of the anticancer drug taxol. Biotechnol Bioeng. 2023, 120, 2160–2174. [Google Scholar] [CrossRef]
- Wang, C.G.; Wu, J.Y.; Mei, X.G. Enhanced taxol production and release in Taxus chinensis cell suspension cultures with selected organic solvents and sucrose feeding. Biotechnol. Prog. 2001, 17, 89–94. [Google Scholar] [CrossRef]
- Yan, Q.; Hu, Z.D.; Tan, R.X.; Wu, J.Y. Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi-continuous operation. J. Biotechnol. 2005, 119, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, W.; Deng, M. Hyper-production of 13C-labeled trans-resveratrol in Vitis vinifera suspension cell culture by elicitation and in situ adsorption. Biochem. Eng. J. 2011, 53, 292–296. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Madhavi, D.; Abhayankar, G.; Reddy, V.D.; Kavi Kishor, P.B. Carbohydrate and elicitor enhanced withanolide (withaferin A and withanolide A) accumulation in hairy root cultures of Withania somnifera (L.). Ind. J. Exp. Biol. 2012, 50, 484–490. [Google Scholar]
- Khazaei, A.; Bahramnejad, B.; Mozafari, A.A.; Dastan, D.; Mohammadi, S. Hairy root induction and farnesiferol B production of endemic medicinal plant Ferula pseudalliacea. 3 Biotech 2019, 9, 407. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Jawahar, G.; Punita, D.L.; Rajasheker, G.; Manoharachary, C.; Venkatachalam, P.; Kavi Kishor, P.B. Feeding elicitors and precursors enhance colchicine accumulation in morphogenic cultures of Gloriosa superba L. Plant Cell Tissue Organ Cult. 2018, 135, 235–245. [Google Scholar] [CrossRef]
- Sivanesan, I.; Jeong, B.R. Induction and establishment of adventitious and hairy root cultures of Plumbago zeylanica L. Afr. J. Biotechnol. 2009, 8, 5294–5300. [Google Scholar]
- Beigmohamadi, M.; Movafeghi, A.; Jafari, S.; Sharafi, A. Potential of the genetically transformed root cultures of Plumbago europaea for biomass and plumbagin production. Biotechnol. Prog. 2020, 36, e2905. [Google Scholar] [CrossRef]
- Martin, K.P.; Sabovljevic, A.; Madassery, J. High-frequency transgenic plant regeneration and plumbagin production through methyl jasmonate elicitation from hairy roots of Plumbago indica L. J. Crop Sci. Biotechnol. 2011, 14, 205–212. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Sircar, D.; Mitra, A.; Bhattacharya, S. Hairy root culture of Plumbago indica as a potential source for plumbagin. Biol. Plant. 2008, 52, 533–537. [Google Scholar] [CrossRef]
- Yogananth, N.; Basu, M.J. TLC method for the determination of plumbagin in hairy root cultures of Plumbago rosea L. Glob. J. Biotechnol. Biochem. 2009, 4, 66–69. [Google Scholar]
- Gangopadhyay, M.; Chakraborty, D.; Bhattacharyya, S.; Bhattacharya, S. Regeneration of transformed plants from hairy roots of Plumbago indica. Plant Cell Tissue Organ Cult. 2010, 102, 109–114. [Google Scholar] [CrossRef]
- Satheeshkumar, K.; Jose, B.; Dhanya, B.P.; Krishnan, P.N. Prospects of Plumbago rosea L. hairy root culture in traditional preparations: A phytochemical comparison with tuberous roots. Plant Root 2014, 8, 13–23. [Google Scholar] [CrossRef]
- Nayak, P.; Sharma, M.; Behera, S.N.; Thirunavoukkarasu, M.; Chand, P.K. High-performance liquid chromatographic quantification of plumbagin from transformed rhizoclones of Plumbago zeylanica L.: Inter-clonal variation in biomass growth and plumbagin production. Appl. Biochem. Biotechnol. 2015, 175, 1745–1770. [Google Scholar] [CrossRef]
- Phisalaphong, M.; Linden, J.C. Kinetic studies of paclitaxel production by Taxus canadensis cultures in batch and semicontinuous with total cell recycle. Biotechnol. Prog. 1999, 15, 1072–1077. [Google Scholar] [CrossRef]
- Sheng, H.; Lei, Y.; Wei, J.; Yang, Z.; Peng, L.; Li, W.; Liu, Y. Analogy of silicon and boron in plant nutrition. Front. Plant Sci. 2024, 15, 1353706. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Chandrakanth, N.N.; Zhang, C.; Freeman, J.; de Souza, W.R.; Bartley, L.E.; Mitchell, R.A.C. Modification of plant cell walls with hydroxycinnamic acids by BAHD acyltransferases. Front. Plant Sci. 2023, 13, 1088879. [Google Scholar] [CrossRef] [PubMed]
- Chormova, D.; Messenger, D.J.; Fry, S.C. Rhamnogalacturonan-II cross-linking of plant pectins via boron bridges occurs during polysaccharide synthesis and/or secretion. Plant Signal. Behav. 2014, 9, e28169. [Google Scholar] [CrossRef]
- Camacho-Cristobal, J.; Lunar, L.; Lafont, F.; Baumert, A.; González-Fontes, A. Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J. Plant Physiol. 2004, 161, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, G.; Karabourniotis, G. Boron deficiency and concentrations and composition of phenolic compounds in Olea europaea leaves: A combined growth chamber and field study. Tree Physiol. 2005, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Rostkowska, C.; Mota, C.M.; Oliveira, T.C.; Santiago, F.M.; Oliveira, L.A.; Korndörfer, G.H.; Lana, R.M.Q.; Rossi, M.L.; Nogueira, N.L.; Simonnet, X.; et al. Si-accumulation in Artemisia annua glandular trichomes increases artemisinin concentration, but does not interfere in the impairment of Toxoplasma gondii growth. Front. Plant Sci. 2016, 7, 1430. [Google Scholar] [CrossRef]
- Tateno, M.; Brabham, C.; DeBolt, S. Cellulose biosynthesis inhibitors—A multifunctional toolbox. J. Exp. Bot. 2016, 67, 533–542. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Melida, H.; Álvarez, J.; Acebes, J.L.; Encina, A.; Fry, S.C. Changes in cinnamic acid derivatives associated with the habituation of maize cells to dichlobenil. Mol. Plant 2011, 4, 869–878. [Google Scholar] [CrossRef]
- Gachon, C.M.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, G.; Zhou, H.; Gu, C.; Vimolmangkang, S.; Liao, L.; Han, Y. Unraveling the mechanism underlying the glycosylation and methylation of anthocyanins in peach. Plant Physiol. 2014, 166, 1044–1058. [Google Scholar] [CrossRef]
- He, L.; Tang, R.; Shi, X.; Wang, W.; Cao, Q.; Liu, X.; Wang, T.; Sun, Y.; Zhang, H.; Li, R.; et al. Uncovering anthocyanin biosynthesis related microRNAs and their target genes by small RNA and degradome sequencing in tuberous roots of sweetpotato. BMC Plant Biol. 2019, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Singh, N.; Khan, S.A.; Mathur, A.K.; Sharma, A.; Jamal, F. TIAs pathway genes and associated miRNA identification in Vinca minor: Supporting aspidosperma and eburnamine alkaloids linkage via transcriptomic analysis. Physiol. Mol. Biol. Plants 2020, 26, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
| Species | Explant | Type of Culture | Media | Plumbagin Accumulation | Reference |
|---|---|---|---|---|---|
| Plumbago zeylanicum | Stem | Callus | - | Plumbagin accumulation depends on anthocyanin pigment | [200] |
| Drosera rotundifolia and D. intermedia | Whole plants | Whole plants grown in culture | - | Detection of plumbagin | [201] |
| Drosophyllum lusitanicum | - | Suspensions | MS medium | 3.5% fresh wt | [189] |
| Drosophyllum lusitanicum | - | Suspensions | MS + chitin | Plumbagin released into the medium | [202] |
| Drosera spathulata, D. rotundifolia | - | In vitro cultured plants | - | Detection of plumbagin | [203] |
| P. rosea | Leaf | Cell aggregate size of 500 µm | MS + 1.5 mg/L IAA + 0.5 mg/L NAA + 0.3 mg/L BAP | 1.83 mg/g dry wt | [190] |
| P. rosea | Leaf | Cell aggregate size of 1500 µm | MS + 1.5 mg/L IAA + 0.5 mg/L NAA + 0.3 mg/L BAP | 4.57 mg/g dry wt | [190] |
| Drosera binata | Whole plantlets | Plant culture | MS medium + no hormones | 1.4% dry wt | [204] |
| Dionaea muscipula | Whole plantlets | Plant culture | McCowns Woody Plant’s medium + no hormones | 5.3% dry wt | [204] |
| P. rosea | Leaf | Suspensions | MS + 1 mg/L IAA + 0.3 mg/L BAP + 30 g/L glucose | 4.06 mg/g dry wt | [194] |
| P. rosea | Leaf | Suspensions | MS + 1 mg/L IAA + 0.3 mg/L BAP + 30 g/L sucrose | 3.85 mg/g dry wt | [194] |
| P. rosea | Leaf | Suspension-derived cell line PR10 | MS + 1 mg/L IAA + 0.3 mg/L BAP | 5.496 mg/g dry wt | 190] |
| P. rosea | Leaf | Suspensions | MS + 1 mg/L IAA, 0.5 mg/L NAA, 0.3 mg/L BAP + 200 mg/L chitosan | 28.94 mg/g dry wt (645% increase over that of control) | [205] |
| P. rosea | Stem, leaf | Callus and suspension cultures | MS + 1.5–2.5 mg/L 2,4-D + 0.5–1.5 mg/L KN | 0.05 mg/g dry wt and 0.028 mg/g dry wt | [206] |
| P. rosea | - | Root cultures | Gamborg’s (B5) medium + 1 mg/L NAA + 0.1 ng/L kinetin | 0.016% dry wt | [207] |
| P. rosea | Young leaf | Root cultures | Gamborg’s + 1 mg/L NAA + 0.1 mg/L kinetin | 0.023% dry wt | [207] |
| P. rosea | Young leaf | Root cultures | Gamborg’s + 1 mg/L NAA + 0.1 mg/L kinetin + (NH4)2SO4 | 0.020% dry wt | [207] |
| P. zeylanica | Nodal explant | Axillary buds | MS + 8.87 mmol/L BAP + 0.49 mmol/L IBA | 0.017% fresh wt | [208] |
| P. rosea | Leaf | Suspensions | MS + 1 mg/L, 0.5 mg/L NAA + 0.3 mg/L BAP | 3.97 mg/g dry wt | [160] |
| P. rosea | Leaf | Suspensions | MS + 1 mg/L IAA + 0.3 mg/L BAP | 4.92 mg/g dry wt | [160] |
| P. roae | Leaf | Suspensions | MS + 1 mg L/L IAA, 0.5 mg/L NAA, 0.3 mg/L BAP + Immobilization + Elicitation + In situ product removal by Amberlite XAD-7 | 92.13 mg/g dry wt | [161] |
| Diospyros melanoxylon | Leaf or petioles | Callus | MS + 2 mg/L 2,4-D + 1 mg/L BAP | 2.2 mg/g dry wt | [63] |
| Diospyros melanoxylon | Leaf or petioles | Suspensions | MS + 2 mg/L NAA + 0.5 mg/L kinetin + 30 g/L sucrose + 100 µM jasmonic acid | 3.1 mg/g dry wt | [63] |
| Nepenthes khasiana | Leaf, shoot tips, or roots | Callus | MS + 2 mg/L 2,4-D + 1 mg/L kinetin | 1.8 mg/g dry wt | [68] |
| N. khasiana | Leaf, shoot tips, or roots | Suspensions | MS + 2 mg/L NAA + 1 mg/L BAP + 30 g/L sucrose + 100 µM jasmonic acid | 3.4 mg/g dry wt | [68] |
| Drosera indica | Stem segments | Whole plant culture | ¼ MS + 0.5 mg/L BA + 0.5 mg/mL yeast extract | 2.69 mg/g dry wt (5.4-fold over the control plant) | [48] |
| P. indica | Young leaf | Root cultures | B5 + 0.1 mg/L NAA elicited by 20 Gy γ-ray irradiation | 1.04 mg/g dry wt | [209] |
| P. auriculata | In vitro generated roots | Callus | MS + G2 media (0.2 mg/L BAP + 0.02 mg/L NAA) | 0.35% dry wt | [210] |
| P. auriculata | Stem | Callus | MS + 1 mg/L 2,4-D + 1.75 mg/L NAA + 0.5 mg/L BAP + 1.5 mg/L NAA + 1 mg/L IAA | 0.023% dry wt | [210] |
| P. auriculata | Leaf | Callus | MS + 1 mg/L 2,4-D + 1.5 mg/L NAA + 1 mg/L IBA | 0.0145% dry wt | [210] |
| P. rosea | Leaf | Adventitious root cultures | MS + 1.5 mg/L IAA + 1 mg/L IBA + 50 μM jasmonic acid | 1.23% dry wt | [195] |
| P. zeylanica | Leaf | Adventitious roots | MS solid medium + 1 mg/L IBA, 10 µM α-ionone | 3.1 fold increase | [198] |
| Drosera peltata D. burmannii | Shoot clumps | Shoot culture | MS medium + 5 mg/L 2,4-D + 30 g/L sucrose | 3.45 ± 0.90 mg/g dry wt | [211] |
| P. zeylanica | Nodal explants | Callus | MS medium + 5 μM IBA/TDZ/IAA + 100 mg/L yeast extract and 25 μM salicylic acid | 0.55 mg/g dry wt with yeast extract and 0.32 mg/g dry wt with salicylic acid | [212] |
| D. peltata | Shoot tips | Shoot tip cultures | Half strength MS + 0.1 mg/L BA | 12.04 mg/g dry wt from regenerated shoots | [211] |
| P. zeylanica | Nodal explants | Root cultures | Half strength liquid MS + 3% sucrose + 2 g/L inoculum density + 150 mg/L yeast extract | 3-fold enhancement | [38] |
| P. europaea | Seeds, roots | Callus and suspensions | MS + 2,4-D + Kin/BA | 0.9 mg/g dry wt | [38] |
| Drosera indica | Leaf, stem or root | Callus | MS + 5 mg/L 2,4-D + 1 mg/L BAP + 30 g/L sucrose | 2.0 mg/g dry wt | [49] |
| Drosera indica | Leaf, stem or root | Suspensions | MS + 1 mg/L NAA + 0.5 mg/L BAP + 100 µM methyl jasmonate | 4.0 mg/g dry wt | [49] |
| Dionaea muscipula | Leaf, petiole or root | Callus | MS + 2 mg/L 2,4-D + 1 mg/L BAP + 30 g/L sucrose | 1.4 mg/g dry wt | [213] |
| Dionaea muscipula | Leaf, petiole or root | Suspensions | MS + 1 mg/L NAA + 0.5 mg/L BAP + 30 g/L sucrose + 100 µM methyl jasmonate | 3.5 mg/g dry wt | [213] |
| P. indica P. indica | - | Regenerated shoots Regenerated shoots | MS + 1 mg/L BA + 50 mg/L yeast extract MS + 1 mg/L BA + 100 mg/L yeast extract | 3.88% dry wt 3.81% dry wt | [214] |
| Species | Medium | Elicitor Used If Any | Strain | Plumbagin Accumulation | Reference |
|---|---|---|---|---|---|
| P. rosea | MS + 3% sucrose | Grown in a stirred tank reactor for 28 days | Agrobacterium rhizogenes | ~12.5 mg/L | [194] |
| P. rosea | MS + 3% sucrose | Grown in a tapered airlift reactor for 28 days | Agrobacterium rhizogenes | ~30 mg/L | [194] |
| P. rosea | MS + 3% sucrose | CaCl2 (0.25X–3X) | Agrobacterium rhizogenes | 2.1–2.53 mg/g dry wt | [205] |
| P. zeylanica | Half-strength MS with 4% sucrose | - | Agrobacterium rhizogenes A4 strain | 0.042% fresh wt 2.5 times higher amounts of plumbagin | [208] |
| P. rosea | Hormone-free liquid MS + 3% sucrose | - | Agrobacterium rhizogenes ATCC 15834 | 7.8 mg/g dry wt | [271] |
| P. zeylanica | MS basal without hormones | - | Agrobacterium rhizogenes MTCC 532 | Not estimated | [268] |
| P. rosea | Hairy roots grown in half-strength MS medium | - | Agrobacterium rhizogenes strain ATCC 15834 | Identified by TLC, but not estimated | [272] |
| P. rosea | Hairy roots grown on 0.5 mg/L GA3 + 0.5 mg/L NAA | - | Hairy root clone H13 | 7.90 mg/L dry wt | [158] |
| P. rosea | MS basal liquid in a bioreactor | - | Hairy roots | Plumbagin obtained in bioreactor as against 5.39-fold in shake flasks with 1% w/v inoculum over 3-weeks | [273] |
| P. zeylanica | MS medium free from hormones | - | Agrobacterium rhizogenes A4 and LBA9402 strains | A4 transformed HRA2B5 2.26 mg/g dry wt | [274] |
| P. rosea | MS basal in a bioreactor | - | Agrobacterium rhizogenes A4 (ATCC43057) | 1.425% in a bioreactor | [37] |
| P. rosea | MS basal liquid in a 2L reaction kettle | Customized reaction kettle | Agrobacterium rhizogenes A4 strain | 1.5% dry weight | [196] |
| P. zeylanica | MS + 1 mg/L IBA. Hairy roots grown in a bioreactor | 10 µM α-ionone | Agrobacterium rhizogenes LBA1334, and R1000 | 3.6 fold increase in plumbagin in a bioreactor | [198] |
| P. auriculata | 1/2 MS liquid medium | 100 μmol/L methyl jasmonate | Agrobacterium rhizogenes (PAHR) 15834 | 8.24 mg/g dry wt at 25 days | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavi Kishor, P.B.; Thaddi, B.N.; Guddimalli, R.; Nikam, T.D.; Sambasiva Rao, K.R.S.; Mukhopadhyay, R.; Singam, P. The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone. Molecules 2025, 30, 1618. https://doi.org/10.3390/molecules30071618
Kavi Kishor PB, Thaddi BN, Guddimalli R, Nikam TD, Sambasiva Rao KRS, Mukhopadhyay R, Singam P. The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone. Molecules. 2025; 30(7):1618. https://doi.org/10.3390/molecules30071618
Chicago/Turabian StyleKavi Kishor, Polavarapu B., Bangaru Naidu Thaddi, Rajasheker Guddimalli, Tukaram Dayaram Nikam, Krothapalli Raja Surya Sambasiva Rao, Rupasree Mukhopadhyay, and Prashant Singam. 2025. "The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone" Molecules 30, no. 7: 1618. https://doi.org/10.3390/molecules30071618
APA StyleKavi Kishor, P. B., Thaddi, B. N., Guddimalli, R., Nikam, T. D., Sambasiva Rao, K. R. S., Mukhopadhyay, R., & Singam, P. (2025). The Occurrence, Uses, Biosynthetic Pathway, and Biotechnological Production of Plumbagin, a Potent Antitumor Naphthoquinone. Molecules, 30(7), 1618. https://doi.org/10.3390/molecules30071618








