Epigenetic and Gene Expression Responses of Daphnia magna to Polyethylene and Polystyrene Microplastics
Abstract
1. Introduction
2. Results and Discussion
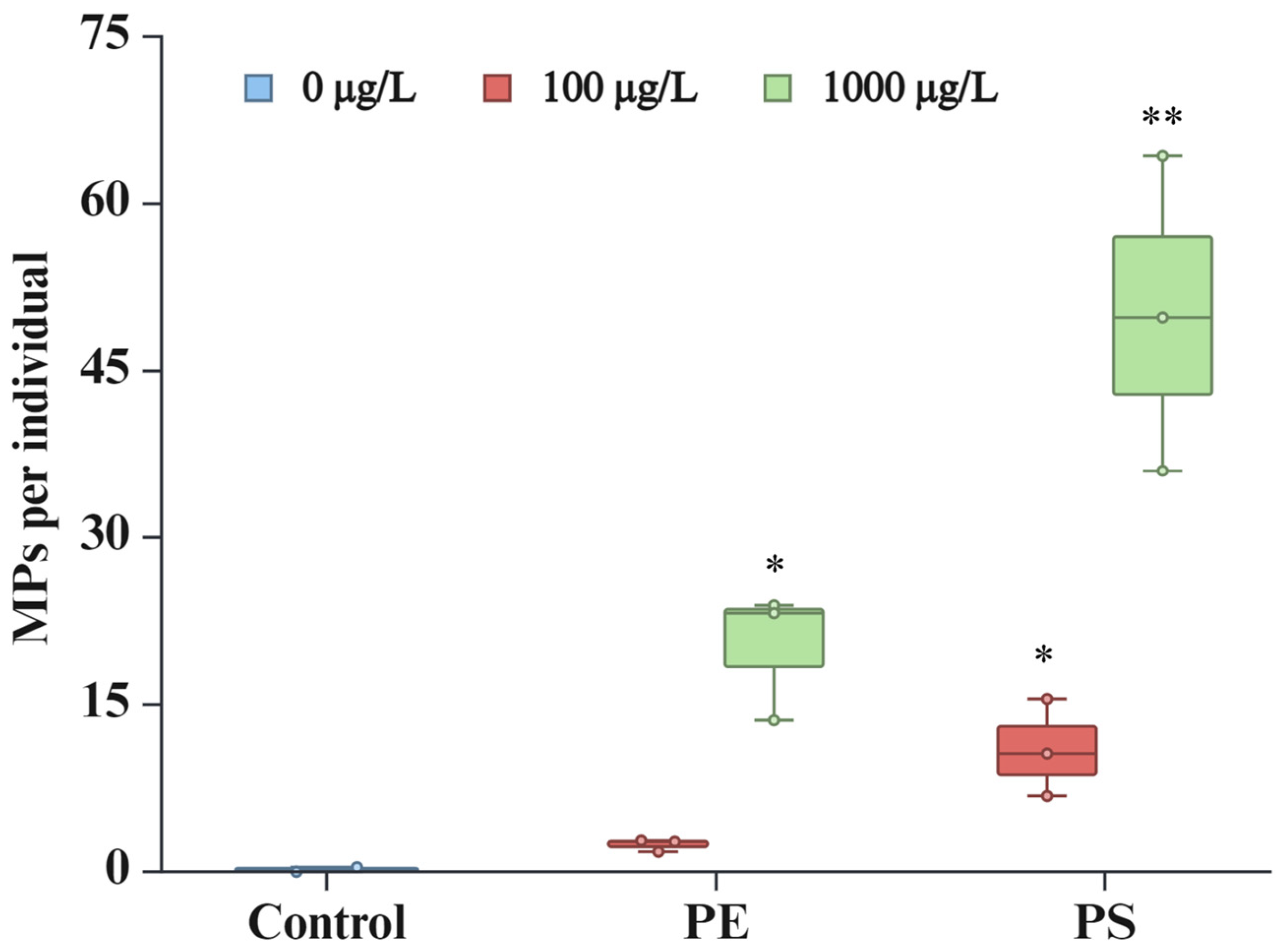
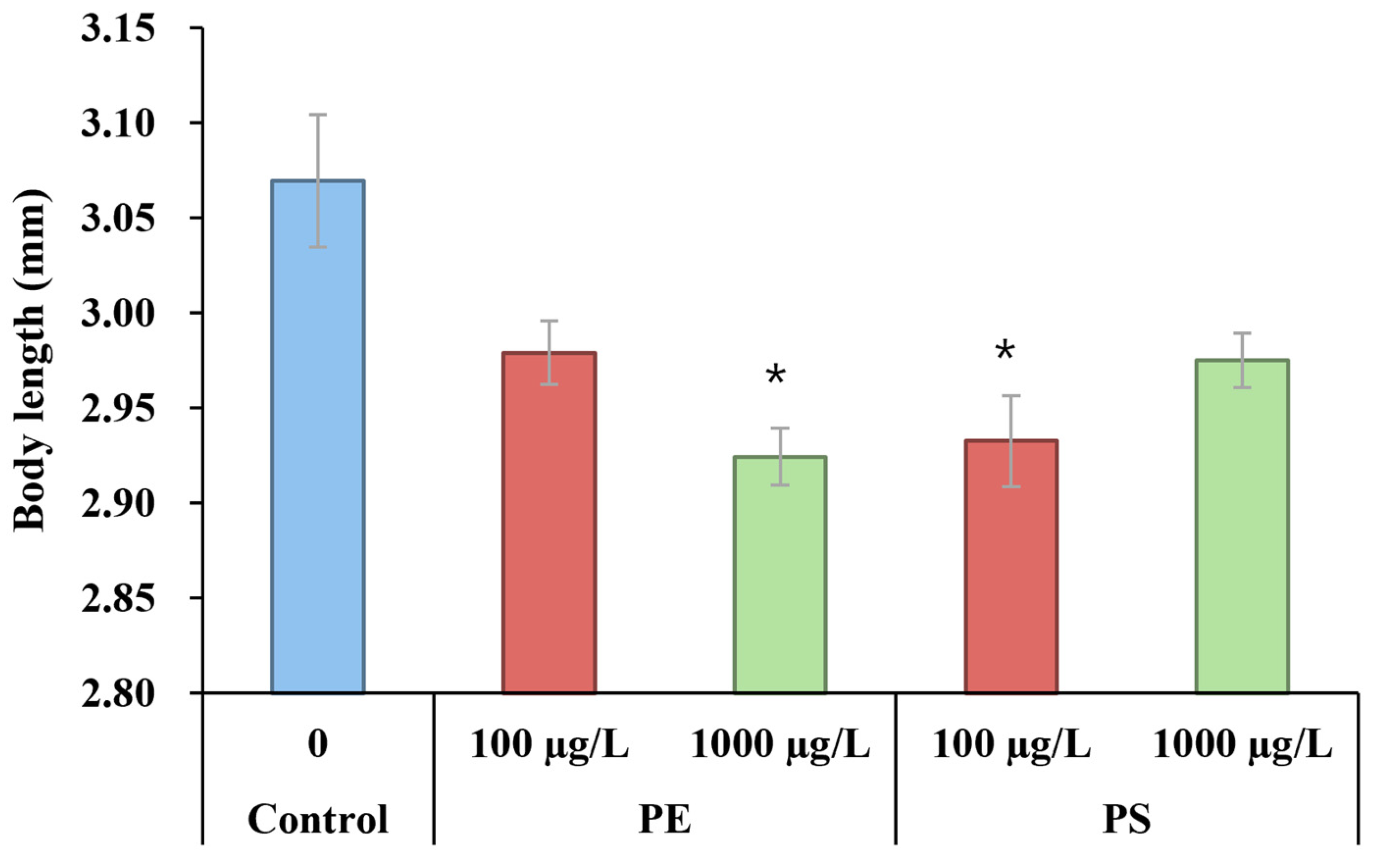
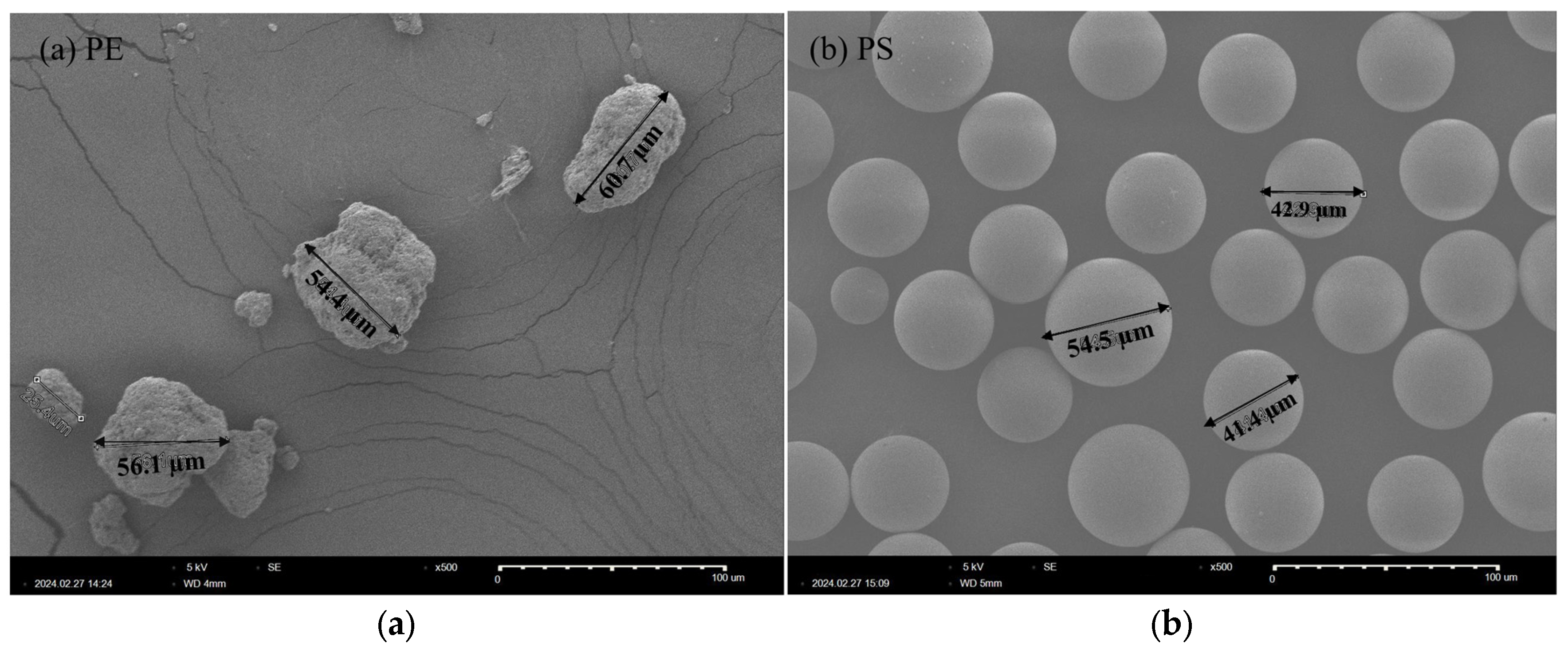
2.1. Microplastic Ingestion by D. magna
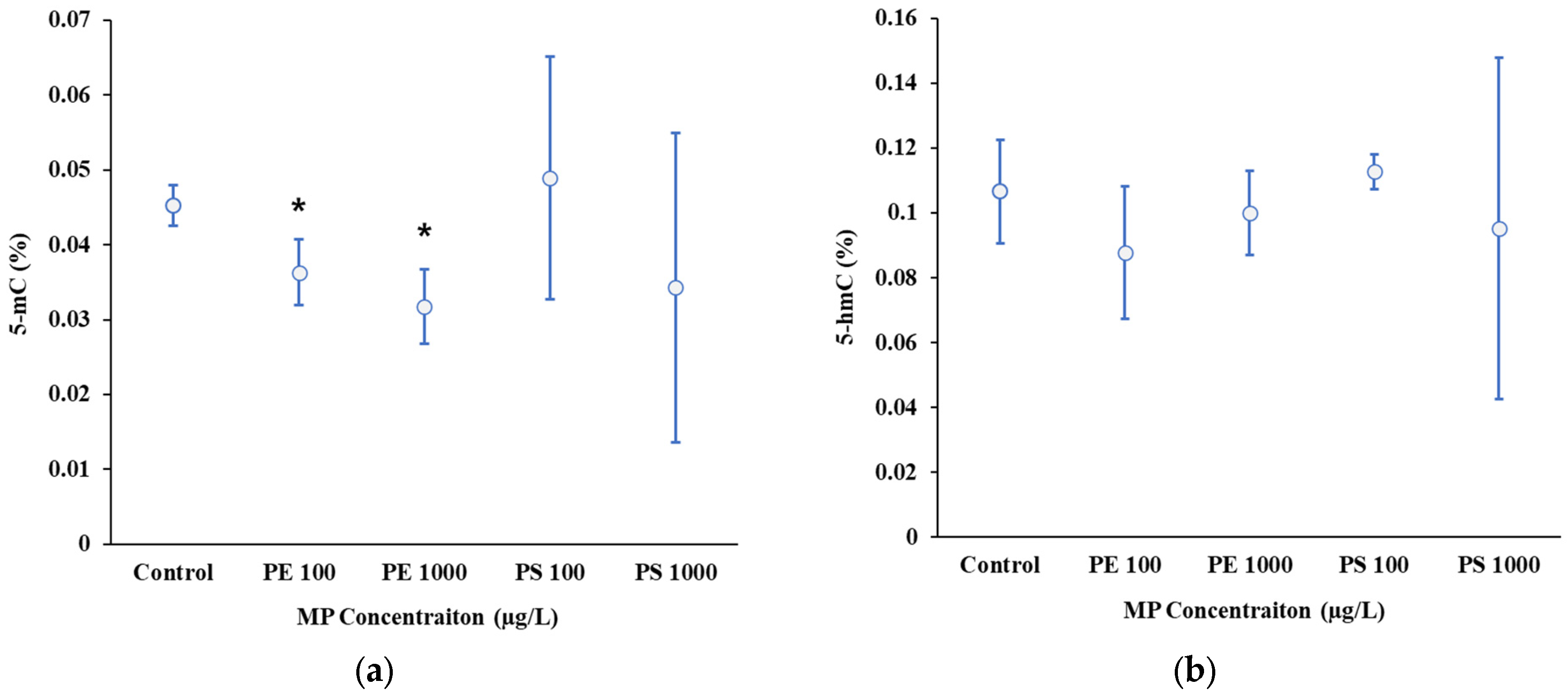
2.2. Epigenetic Responses to Microplastic Exposure in D. magna
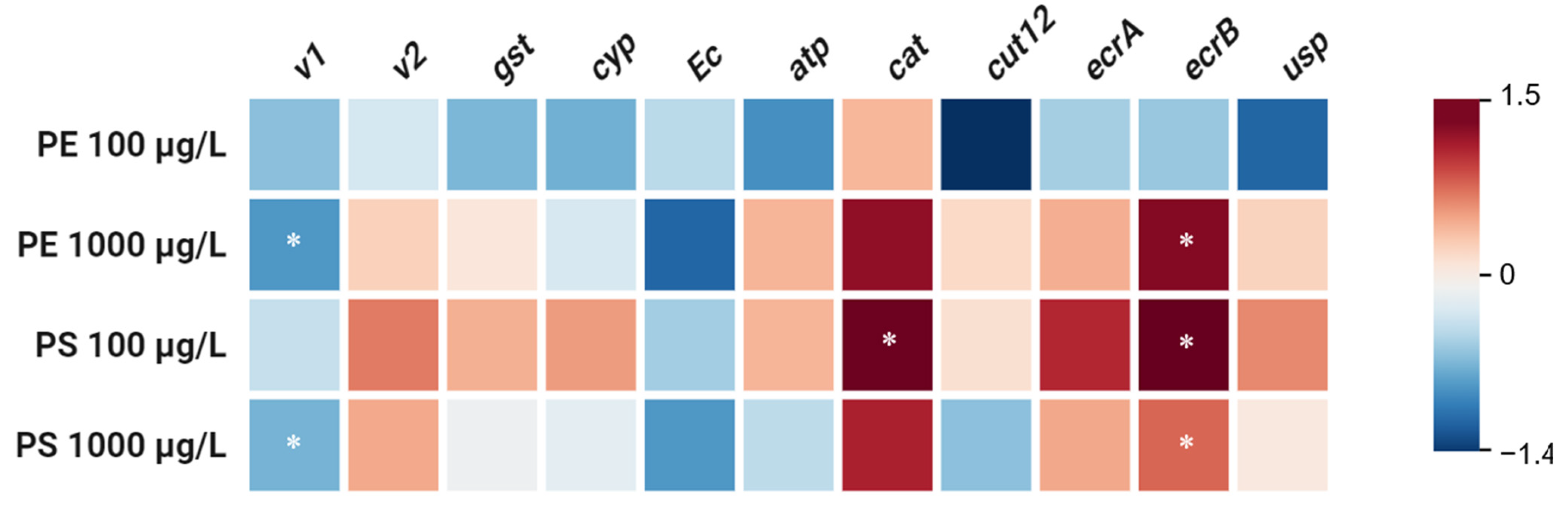
2.3. Transcriptional Response to MP Exposure in D. magna
3. Materials and Methods
3.1. Test Organisms
3.2. Microplastic Exposure to D. magna
3.3. Gene Expression Analysis (RT-qPCR)
3.4. Global DNA Methylation (5-mc) and DNA Hydroxymethylation (5-hmc) Analysis
3.5. Statistical Analysis
3.6. Microplastic Analysis
3.6.1. Sample Preparation
3.6.2. Micro-FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MP | Microplastic particle |
| PE | polyethylene |
| PS | polystyrene |
| TRxR | Thioredoxin reductase |
| SEM | Scanning electron microscope |
| RT-PCR | Real-time PCR |
| DLS | Dynamic Light Scattering |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and Spatial Distribution of Microplastics in Sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.R.; Chadwick, D.D.A.; Eagle, L.J.B.; Gould, A.N.; Harwood, M.; Sayer, C.D.; Rose, N.L. Microplastic Burden in Invasive Signal Crayfish (Pacifastacus leniusculus) Increases along a Stream Urbanization Gradient. Ecol. Evol. 2023, 13, e10041. [Google Scholar] [CrossRef]
- Khan, A.A.; Bose, K. Microplastics: An Overview on Ecosystem and Human Health. Int. J. Res. Publ. Rev. 2024, 5, 3691–3702. [Google Scholar] [CrossRef]
- Liu, W.; Liao, H.; Wei, M.; Junaid, M.; Chen, G.; Wang, J. Biological Uptake, Distribution and Toxicity of Micro(Nano)Plastics in the Aquatic Biota: A Special Emphasis on Size-Dependent Impacts. TrAC Trends Anal. Chem. 2024, 170, 117477. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater Microplastic Concentrations Vary through Both Space and Time. Environ. Pollut. 2020, 263, 114481. [Google Scholar] [CrossRef]
- Fabricant, L.; Edelstein, O.; Dispigno, J.; Weseley, A. The Effect of Microplastics on the Speed, Mortality Rate, and Swimming Patterns of Daphnia magna. J. Emerg. Investig. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Wen, M.; Ji, G. Toxic Effects of Methylene Blue on the Growth, Reproduction and Physiology of Daphnia magna. Toxics 2023, 11, 594. [Google Scholar] [CrossRef]
- Aljaibachi, R.; Callaghan, A. Impact of Polystyrene Microplastics on Daphnia magna Mortality and Reproduction in Relation to Food Availability. PeerJ 2018, 6, e4601. [Google Scholar] [CrossRef]
- Canniff, P.M.; Hoang, T.C. Microplastic Ingestion by Daphnia magna and Its Enhancement on Algal Growth. Sci. Total Environ. 2018, 633, 500–507. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and Effects of Microplastic Textile Fibers on Freshwater Crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Martins, A.; Guilhermino, L. Transgenerational Effects and Recovery of Microplastics Exposure in Model Populations of the Freshwater Cladoceran Daphnia magna Straus. Sci. Total Environ. 2018, 631–632, 421–428. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of Micro- and Nanoplastics in Daphnia magna—Quantification of Body Burdens and Assessment of Feeding Rates and Reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Long, Y.; Xiao, W.; Liu, D.; Tian, Q.; Li, Y.; Liu, C.; Chen, L.; Pan, Y. Ecotoxicology of Microplastics in Daphnia: A Review Focusing on Microplastic Properties and Multiscale Attributes of Daphnia. Ecotoxicol. Environ. Saf. 2023, 249, 114433. [Google Scholar] [CrossRef] [PubMed]
- Berlino, M.; Sarà, G.; Mangano, M.C. Functional Trait-Based Evidence of Microplastic Effects on Aquatic Species. Biology 2023, 12, 811. [Google Scholar] [CrossRef]
- An, D.; Na, J.; Song, J.; Jung, J. Size-Dependent Chronic Toxicity of Fragmented Polyethylene Microplastics to Daphnia magna. Chemosphere 2021, 271, 129591. [Google Scholar] [CrossRef] [PubMed]
- Isinibilir, M.; Eryalçın, K.M.; Kideys, A.E. Effect of Polystyrene Microplastics in Different Diet Combinations on Survival, Growth and Reproduction Rates of the Water Flea (Daphnia magna). Microplastics 2023, 2, 27–38. [Google Scholar] [CrossRef]
- Song, J.; Kim, C.; Na, J.; Sivri, N.; Samanta, P.; Jung, J. Transgenerational Effects of Polyethylene Microplastic Fragments Containing Benzophenone-3 Additive in Daphnia magna. J. Hazard. Mater. 2022, 436, 129225. [Google Scholar] [CrossRef]
- Mao, X.; Xu, Y.; Cheng, Z.; Yang, Y.; Guan, Z.; Jiang, L.; Tang, K. The Impact of Microplastic Pollution on Ecological Environment: A Review. Front. Biosci. (Landmark Ed.) 2022, 27, 46. [Google Scholar] [CrossRef]
- Strepetkaitė, D.; Alzbutas, G.; Astromskas, E.; Lagunavičius, A.; Sabaliauskaitė, R.; Arbačiauskas, K.; Lazutka, J. Analysis of DNA Methylation and Hydroxymethylation in the Genome of Crustacean Daphnia Pulex. Genes 2016, 7, 1. [Google Scholar] [CrossRef]
- He, B.; Zhang, C.; Zhang, X.; Fan, Y.; Zeng, H.; Liu, J.; Meng, H.; Bai, D.; Peng, J.; Zhang, Q.; et al. Tissue-Specific 5-Hydroxymethylcytosine Landscape of the Human Genome. Nat. Commun. 2021, 12, 4249. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, H. Hydroxymethylation and Tumors: Can 5-Hydroxymethylation Be Used as a Marker for Tumor Diagnosis and Treatment? Hum. Genom. 2020, 14, 15. [Google Scholar] [CrossRef]
- Okamoto, Y.; Iwai-Shimada, M.; Nakai, K.; Tatsuta, N.; Mori, Y.; Aoki, A.; Kojima, N.; Takada, T.; Satoh, H.; Jinno, H. Global DNA Methylation in Cord Blood as a Biomarker for Prenatal Lead and Antimony Exposures. Toxics 2022, 10, 157. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Tang, W.; Shang, Y.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Ledesma, M.; Leon, M.; Laclaustra, M.; Pollak, J.; et al. Association of Global DNA Methylation and Global DNA Hydroxymethylation with Metals and Other Exposures in Human Blood DNA Samples. Environ. Health Perspect. 2014, 122, 946–954. [Google Scholar] [CrossRef]
- Faraji, M.; Pourpak, Z.; Naddafi, K.; Nodehi, R.N.; Nicknam, M.H.; Shamsipour, M.; Rezaei, S.; Ghozikali, M.G.; Ghanbarian, M.; Mesdaghinia, A. Effects of Airborne Particulate Matter (PM10) from Dust Storm and Thermal Inversion on Global DNA Methylation in Human Peripheral Blood Mononuclear Cells (PBMCs) in Vitro. Atmos. Environ. 2018, 195, 170–178. [Google Scholar] [CrossRef]
- Chatterjee, N.; Jeong, J.; Park, M.-S.; Ha, M.; Cheong, H.-K.; Choi, J. Cross-Sectional and Longitudinal Associations between Global DNA (Hydroxy) Methylation and Exposure Biomarkers of the Hebei Spirit Oil Spill Cohort in Taean, Korea. Environ. Pollut. 2020, 263, 114607. [Google Scholar] [CrossRef]
- Im, H.; Kang, J.; Jacob, M.F.; Bae, H.; Oh, J.-E. Transgenerational Effects of Benzotriazole on the Gene Expression, Growth, and Reproduction of Daphnia magna. Environ. Pollut. 2023, 323, 121211. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, G.; Barbosa, J.; Marques, S.M.; De Schamphelaere, K.A.C.; Van Nieuwerburgh, F.; Deforce, D.; Gonçalves, F.J.M.; Pereira, J.L.; Asselman, J. Transgenerational Inheritance of DNA Hypomethylation in Daphnia magna in Response to Salinity Stress. Environ. Sci. Technol. 2018, 52, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Litoff, E.J.; Garriott, T.E.; Ginjupalli, G.K.; Butler, L.; Gay, C.; Scott, K.; Baldwin, W.S. Annotation of the Daphnia magna Nuclear Receptors: Comparison to Daphnia Pulex. Gene 2014, 552, 116–125. [Google Scholar] [CrossRef]
- Asselman, J.; De Coninck, D.I.M.; Vandegehuchte, M.B.; Jansen, M.; Decaestecker, E.; De Meester, L.; Vanden Bussche, J.; Vanhaecke, L.; Janssen, C.R.; De Schamphelaere, K.A.C. Global Cytosine Methylation in Daphnia magna Depends on Genotype, Environment, and Their Interaction. Environ. Toxicol. Chem. 2015, 34, 1056–1061. [Google Scholar] [CrossRef]
- Kvist, J.; Gonçalves Athanàsio, C.; Shams Solari, O.; Brown, J.B.; Colbourne, J.K.; Pfrender, M.E.; Mirbahai, L. Pattern of DNA Methylation in Daphnia: Evolutionary Perspective. Genome Biol. Evol. 2018, 10, 1988–2007. [Google Scholar] [CrossRef]
- Nelson, H.H.; Marsit, C.J.; Kelsey, K.T. Global Methylation in Exposure Biology and Translational Medical Science. Environ. Health Perspect. 2011, 119, 1528–1533. [Google Scholar] [CrossRef]
- Valente, A.; Vieira, L.; Silva, M.J.; Ventura, C. The Effect of Nanomaterials on DNA Methylation: A Review. Nanomaterials 2023, 13, 1880. [Google Scholar] [CrossRef]
- Feiner, N.; Radersma, R.; Vasquez, L.; Ringnér, M.; Nystedt, B.; Raine, A.; Tobi, E.W.; Heijmans, B.T.; Uller, T. Environmentally Induced DNA Methylation Is Inherited across Generations in an Aquatic Keystone Species. iScience 2022, 25, 104303. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Van Damme, K.; Gosálvez, J.; Morán, E.S.; Colbourne, J.K. Male Meiosis in Crustacea: Synapsis, Recombination, Epigenetics and Fertility in Daphnia magna. Chromosoma 2016, 125, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Barrick, A.; Boardwine, A.J.; Hoang, T.C. Accumulation, Depuration, and Potential Effects of Environmentally Representative Microplastics towards Daphnia magna. Sci. Total Environ. 2024, 950, 175384. [Google Scholar] [CrossRef]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene Microplastics Ingestion Induced Behavioral Effects to the Cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef]
- Luangrath, A.; Na, J.; Kalimuthu, P.; Song, J.; Kim, C.; Jung, J. Ecotoxicity of Polylactic Acid Microplastic Fragments to Daphnia magna and the Effect of Ultraviolet Weathering. Ecotoxicol. Environ. Saf. 2024, 271, 115974. [Google Scholar] [CrossRef]
- Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Feeding Type and Development Drive the Ingestion of Microplastics by Freshwater Invertebrates. Sci. Rep. 2017, 7, 17006. [Google Scholar] [CrossRef]
- Doskocz, N.; Skwarska, D.; Affek, K.; Kucharska, M.; Hua, J.; Załęska-Radziwiłł, M. Polystyrene Microbeads in Freshwater Ecosystems—Ecotoxicological Effects on Daphnia magna. Ecohydrol. Hydrobiol. 2025, 100647. [Google Scholar] [CrossRef]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, Size, and Polymer Dependent Effects of Microplastics on Daphnia magna. J. Hazard. Mater. 2022, 426, 128136. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, J.; Elagami, H.; Gilfedder, B.S.; Fleckenstein, J.H.; Papastavrou, G.; Mansfeld, U.; Peiffer, S. Heteroaggregation of PS Microplastic with Ferrihydrite Leads to Rapid Removal of Microplastic Particles from the Water Column. Environ. Sci. Process. Impacts 2022, 24, 1782–1789. [Google Scholar] [CrossRef]
- Okutan, H.M.; Sağir, Ç.; Fontaine, C.; Nauleau, B.; Kurtulus, B.; Le Coustumer, P.; Razack, M. One-Dimensional Experimental Investigation of Polyethylene Microplastic Transport in a Homogeneous Saturated Medium. Front. Environ. Sci. 2022, 10, 885875. [Google Scholar] [CrossRef]
- Long, M.; Paul-Pont, I.; Hégaret, H.; Moriceau, B.; Lambert, C.; Huvet, A.; Soudant, P. Interactions between Polystyrene Microplastics and Marine Phytoplankton Lead to Species-Specific Hetero-Aggregation. Environ. Pollut. 2017, 228, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Sakhon, E.G.; Mukhanov, V.S.; Khanaychenko, A.N. Phytoplankton Exopolymers Enhance Adhesion of Microplastic Particles to Submersed Surfaces. Ecol. Montenegrina 2019, 23, 60–69. [Google Scholar] [CrossRef]
- Summers, S.; Henry, T.; Gutierrez, T. Agglomeration of Nano- and Microplastic Particles in Seawater by Autochthonous and de Novo-Produced Sources of Exopolymeric Substances. Mar. Pollut. Bull. 2018, 130, 258–267. [Google Scholar] [CrossRef]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid Aggregation of Biofilm-Covered Microplastics with Marine Biogenic Particles. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef]
- Hassan, M.D.A.; Shammi, M.; Tareq, S.M. The Deciphering of Microplastics-Derived Fluorescent Dissolved Organic Matter in Urban Lakes, Canals, and Rivers Using Parallel Factor Analysis Modeling and Mimic Experiment. Water Environ. Res. 2024, 96, e11041. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Frère, L.; Maignien, L.; Chalopin, M.; Huvet, A.; Rinnert, E.; Morrison, H.; Kerninon, S.; Cassone, A.-L.; Lambert, C.; Reveillaud, J.; et al. Microplastic Bacterial Communities in the Bay of Brest: Influence of Polymer Type and Size. Environ. Pollut. 2018, 242, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Athanasio, C.G.; Sommer, U.; Viant, M.R.; Chipman, J.K.; Mirbahai, L. Use of 5-Azacytidine in a Proof-of-Concept Study to Evaluate the Impact of Pre-Natal and Post-Natal Exposures, as Well as within Generation Persistent DNA Methylation Changes in Daphnia. Ecotoxicology 2018, 27, 556–568. [Google Scholar] [CrossRef]
- Macha, F.J.; Im, H.; Kim, K.; Park, K.; Oh, J.-E. Effect of Cresol Isomers on Reproduction and Gene Expression in Daphnia magna. Sci. Total Environ. 2023, 893, 164910. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; De Coninck, D.; Vandenbrouck, T.; De Coen, W.M.; Janssen, C.R. Gene Transcription Profiles, Global DNA Methylation and Potential Transgenerational Epigenetic Effects Related to Zn Exposure History in Daphnia magna. Environ. Pollut. 2010, 158, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, X.; Yin, J.; Han, Y.; Yang, J.; Lu, X.; Xie, T.; Akbar, S.; Lyu, K.; Yang, Z. Molecular Characterization of Thioredoxin Reductase in Waterflea Daphnia magna and Its Expression Regulation by Polystyrene Microplastics. Aquat. Toxicol. 2019, 208, 90–97. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Matsuura, T.; Kato, Y.; Watanabe, H. Caloric Restriction Upregulates the Expression of DNMT3.1, Lacking the Conserved Catalytic Domain. Genesis 2020, 58, e23396. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Olthof, G.; Vijver, M.G.; Baas, J.; Barmentlo, S.H. Significant Decline of Daphnia magna Population Biomass Due to Microplastic Exposure. Environ. Pollut. 2019, 250, 669–675. [Google Scholar] [CrossRef]
- Mueller, M.-T.; Fueser, H.; Trac, L.N.; Mayer, P.; Traunspurger, W.; Höss, S. Surface-Related Toxicity of Polystyrene Beads to Nematodes and the Role of Food Availability. Environ. Sci. Technol. 2020, 54, 1790–1798. [Google Scholar] [CrossRef]
- Kim, C.; Song, J.; Jung, J. Maternal Effect of Polyethylene Microplastic Fragments Containing Benzophenone-3 in Different Ages and Broods of Daphnia magna. Bull. Environ. Contam. Toxicol. 2023, 110, 66. [Google Scholar] [CrossRef]
- Eltemsah, Y.S.; Bøhn, T. Acute and Chronic Effects of Polystyrene Microplastics on Juvenile and Adult Daphnia magna. Environ. Pollut. 2019, 254, 112919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Zhao, H.; Cai, J.; Sultan, Y.; Fang, H.; Zhang, B.; Ma, J. Effects of Polyvinyl Chloride Microplastics on Reproduction, Oxidative Stress and Reproduction and Detoxification-Related Genes in Daphnia magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109269. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Zhou, Q.; Gu, L.; Sun, Y.; Zhang, L.; Yang, Z. Artificial Light Pollution with Different Wavelengths at Night Interferes with Development, Reproduction, and Antipredator Defenses of Daphnia magna. Environ. Sci. Technol. 2022, 56, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Lee, S.; Kwak, K.; Chung, W.-J.; Choi, K. Determination of mRNA Expression of DMRT93B, Vitellogenin, and Cuticle 12 in Daphnia magna and Their Biomarker Potential for Endocrine Disruption. Ecotoxicology 2011, 20, 1741–1748. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). OECD Guidelines for the Testing of Chemicals: Test Guideline No. 211 Daphnia magna Reproduction Test; OECD: Paris, France, 2012. [Google Scholar]
- Lasee, S.; Mauricio, J.; Thompson, W.A.; Karnjanapiboonwong, A.; Kasumba, J.; Subbiah, S.; Morse, A.N.; Anderson, T.A. Microplastics in a Freshwater Environment Receiving Treated Wastewater Effluent. Integr. Environ. Assess. Manag. 2017, 13, 528–532. [Google Scholar] [CrossRef]
- Guilhermino, L.; Martins, A.; Cunha, S.; Fernandes, J.O. Long-Term Adverse Effects of Microplastics on Daphnia magna Reproduction and Population Growth Rate at Increased Water Temperature and Light Intensity: Combined Effects of Stressors and Interactions. Sci. Total Environ. 2021, 784, 147082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tian, W. Study on the Microplastics’ Effect on the Life History of Daphnia magna. E3S Web Conf. 2024, 598, 01011. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to Polystyrene Microplastics Impairs Gonads of Zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.; Lee, J.; Oh, J.-E.; Song, J.; Jeong, S. Epigenetic and Gene Expression Responses of Daphnia magna to Polyethylene and Polystyrene Microplastics. Molecules 2025, 30, 1608. https://doi.org/10.3390/molecules30071608
Im H, Lee J, Oh J-E, Song J, Jeong S. Epigenetic and Gene Expression Responses of Daphnia magna to Polyethylene and Polystyrene Microplastics. Molecules. 2025; 30(7):1608. https://doi.org/10.3390/molecules30071608
Chicago/Turabian StyleIm, Hyungjoon, Jieun Lee, Jeong-Eun Oh, Jinyoung Song, and Sanghyun Jeong. 2025. "Epigenetic and Gene Expression Responses of Daphnia magna to Polyethylene and Polystyrene Microplastics" Molecules 30, no. 7: 1608. https://doi.org/10.3390/molecules30071608
APA StyleIm, H., Lee, J., Oh, J.-E., Song, J., & Jeong, S. (2025). Epigenetic and Gene Expression Responses of Daphnia magna to Polyethylene and Polystyrene Microplastics. Molecules, 30(7), 1608. https://doi.org/10.3390/molecules30071608







