Magnesium Molybdate: An Efficient Nanosorbent for Methylene Blue Cationic Dye Removal from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. MB Removal
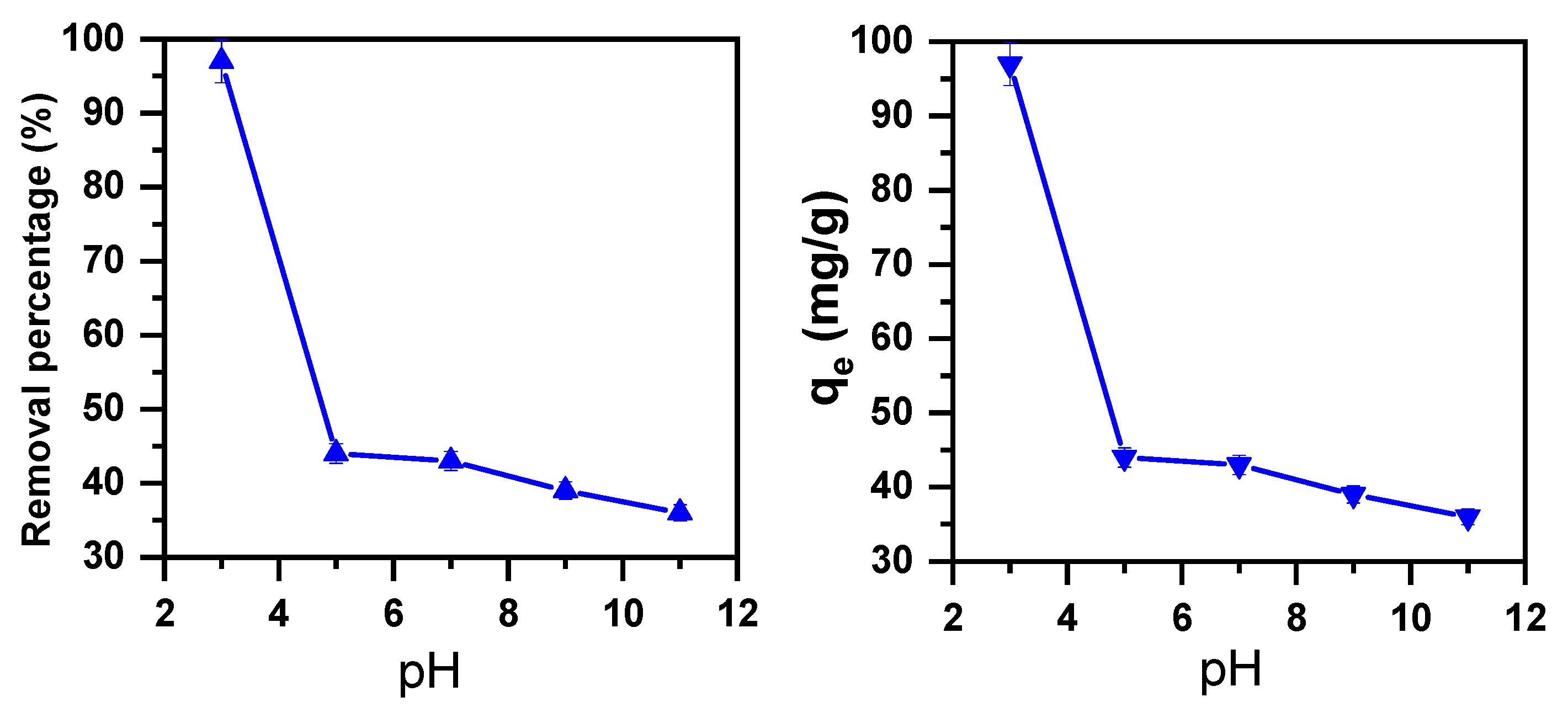
2.1.1. pH Effect
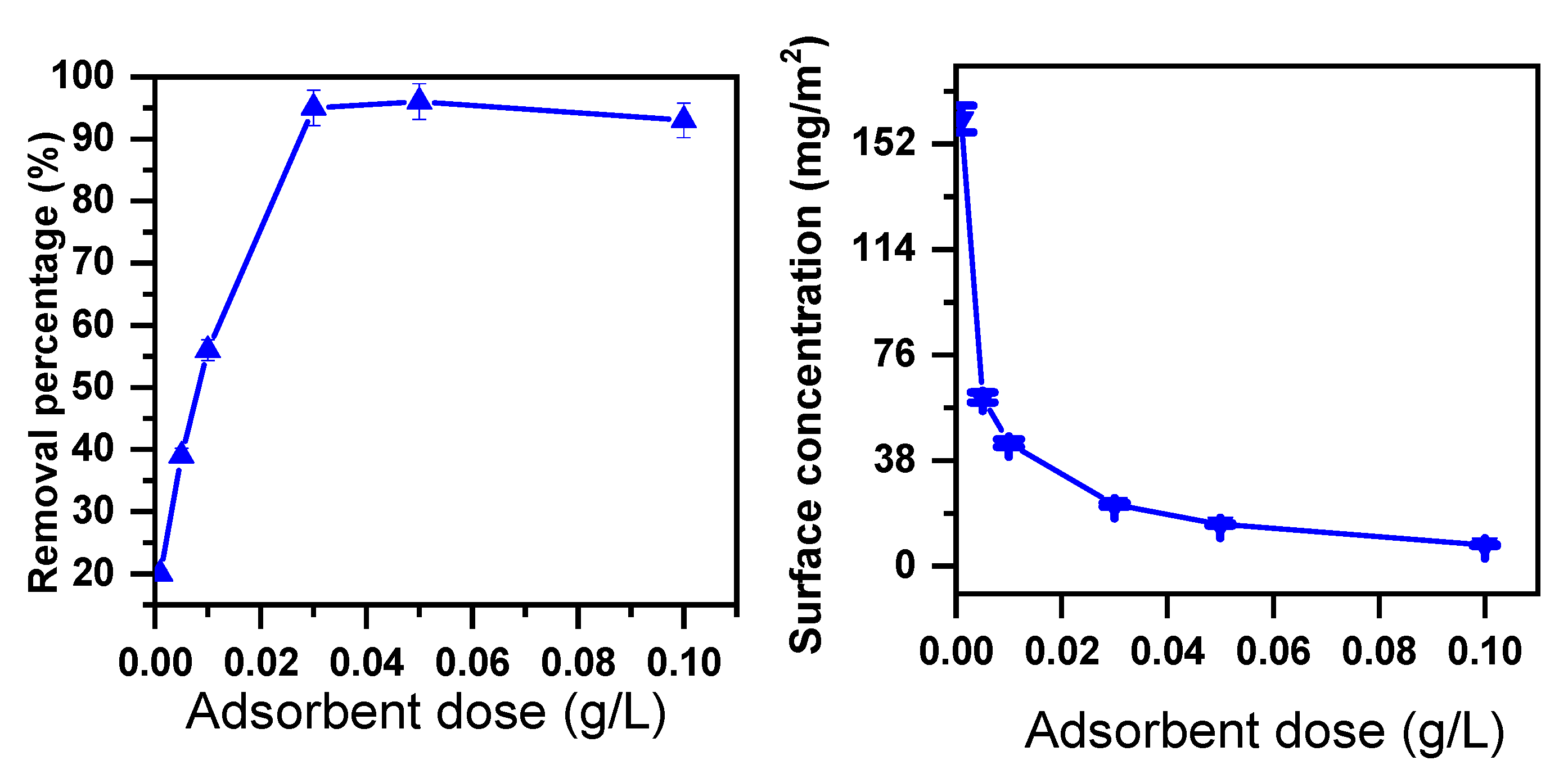
2.1.2. Effect of Adsorbent Dose
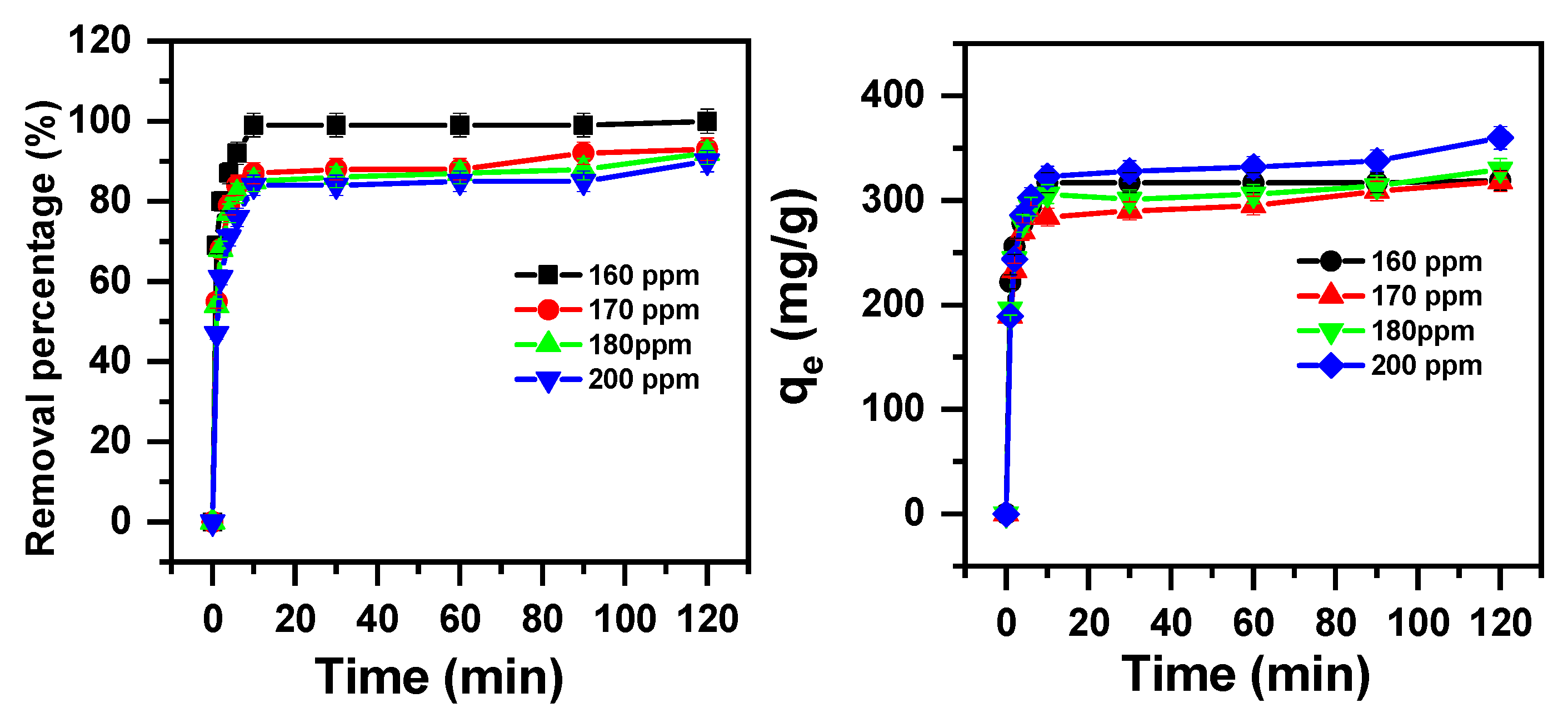
2.1.3. Initial Cationic Dye Concentration and Contact Time Effect
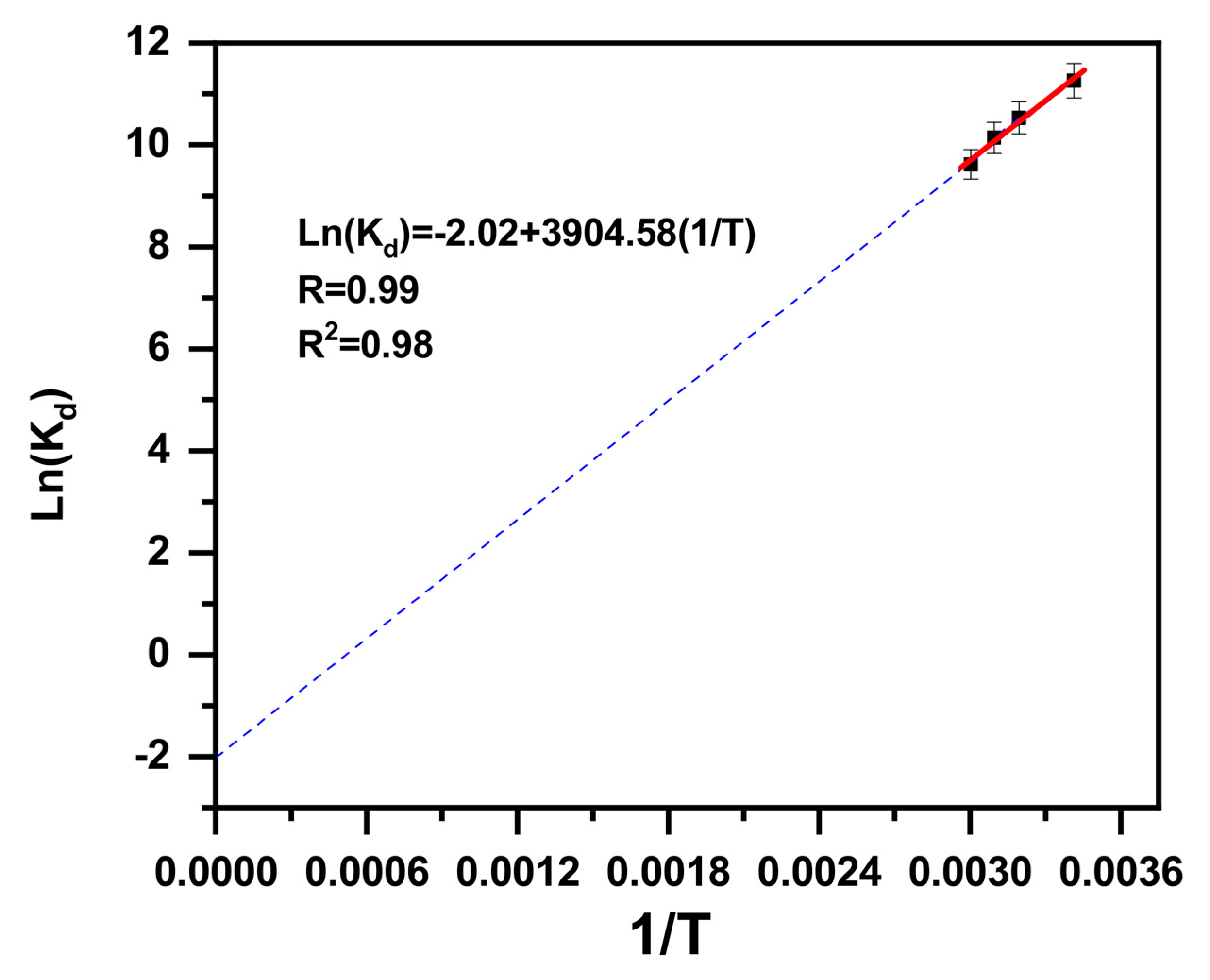
2.1.4. Temperature Effect
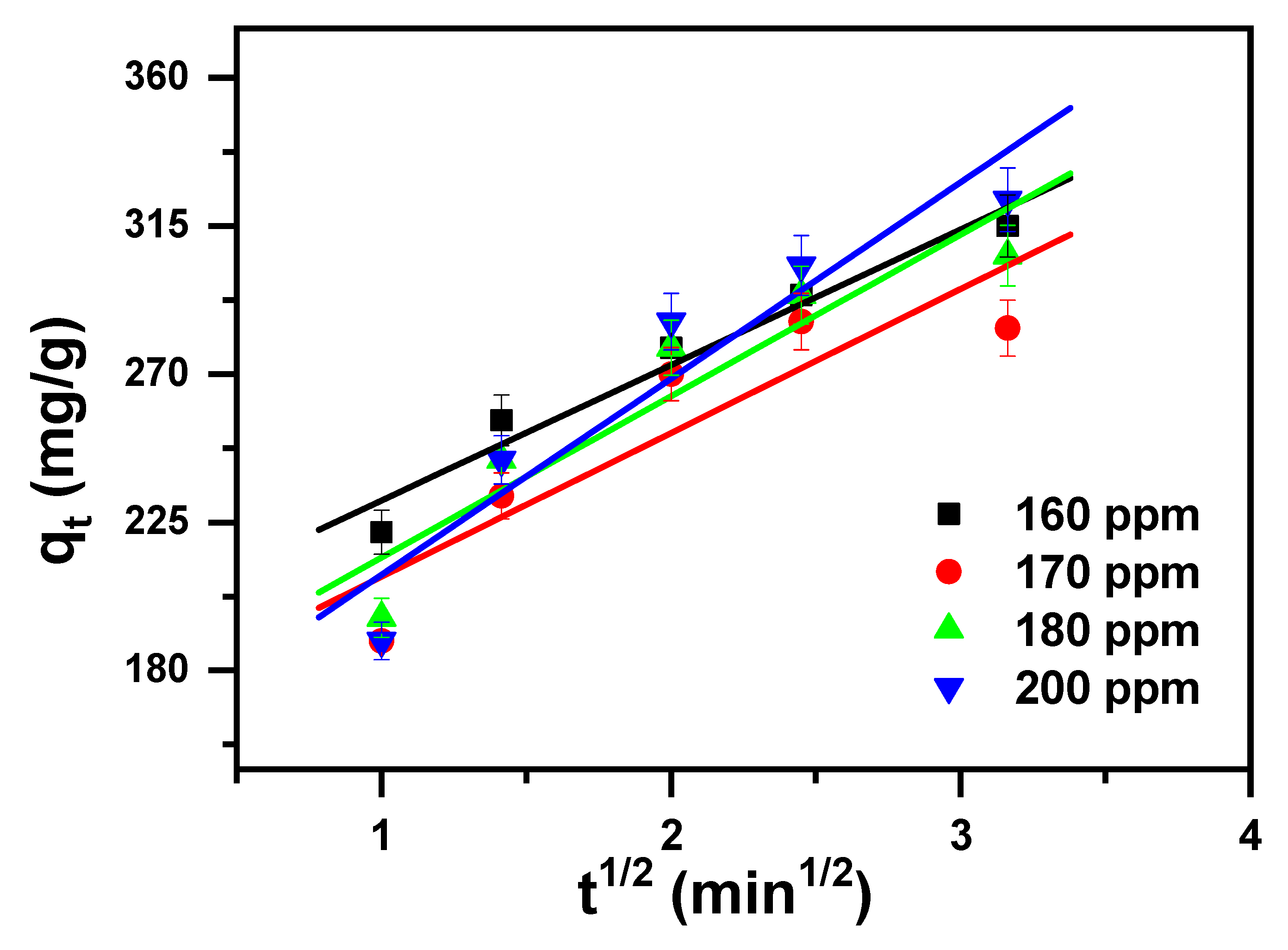
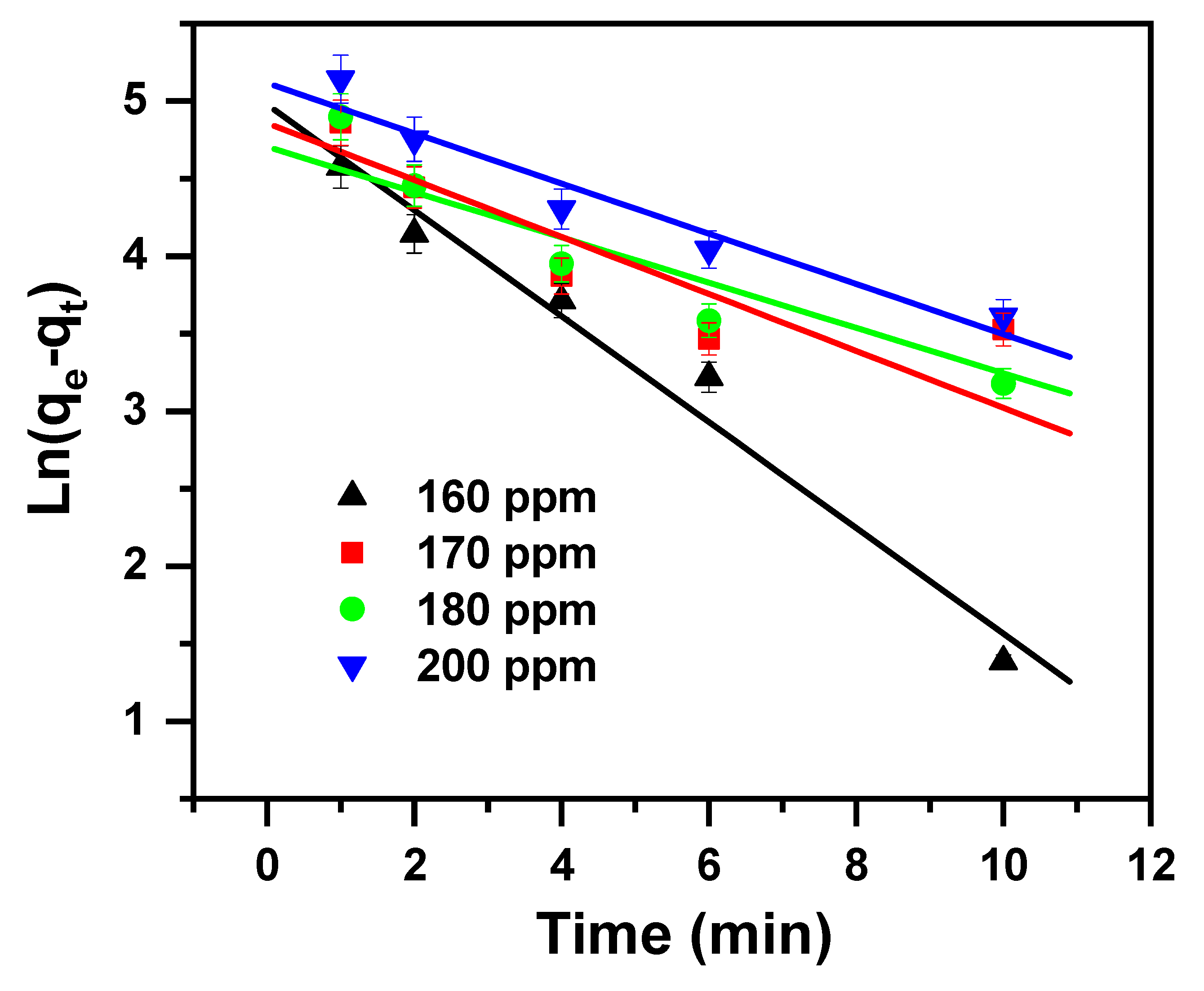
2.2. Study of Kinetics
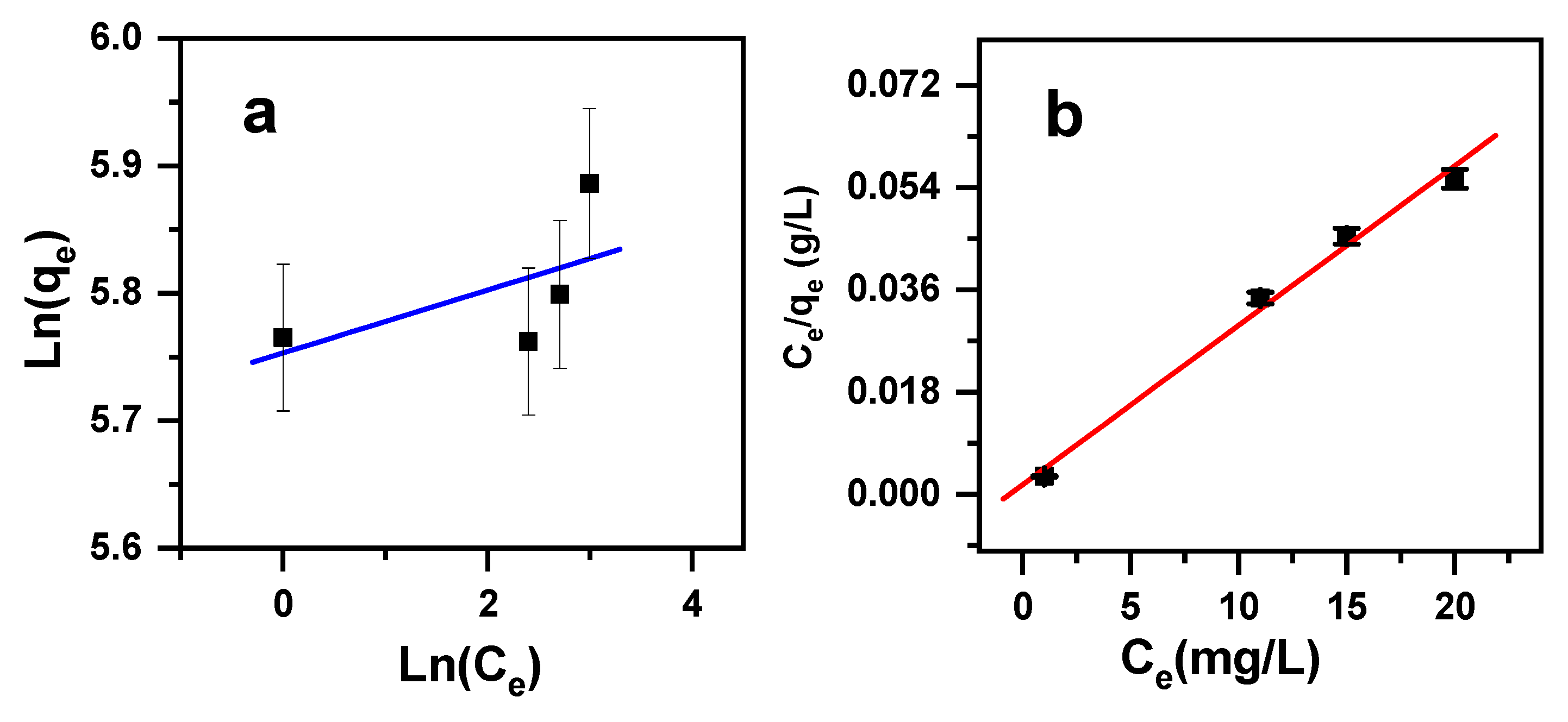
2.3. Adsorption Isotherms
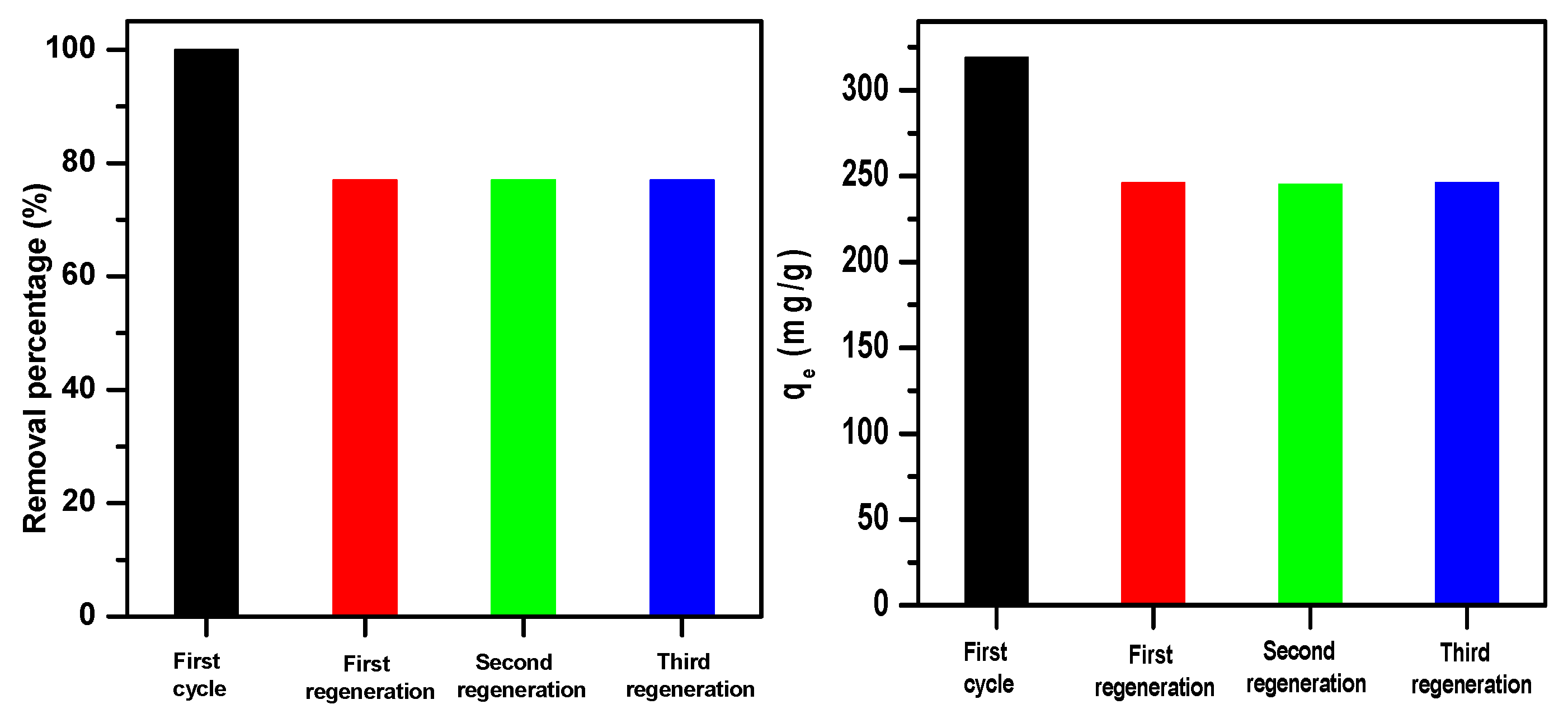
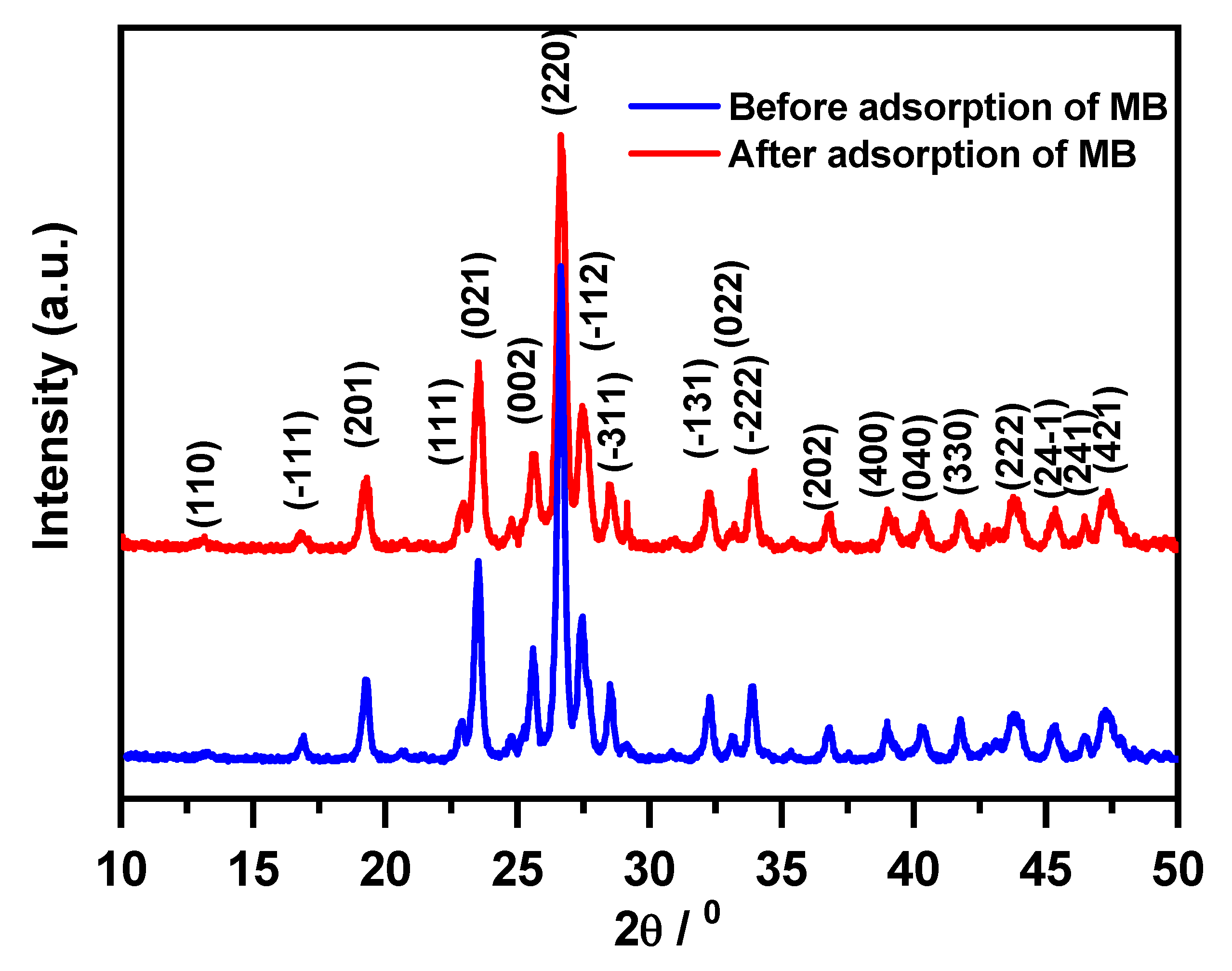
2.4. Magnesium Molybdate (β-MgMoO4) Regeneration and Characterization
2.4.1. Regeneration Efficacy
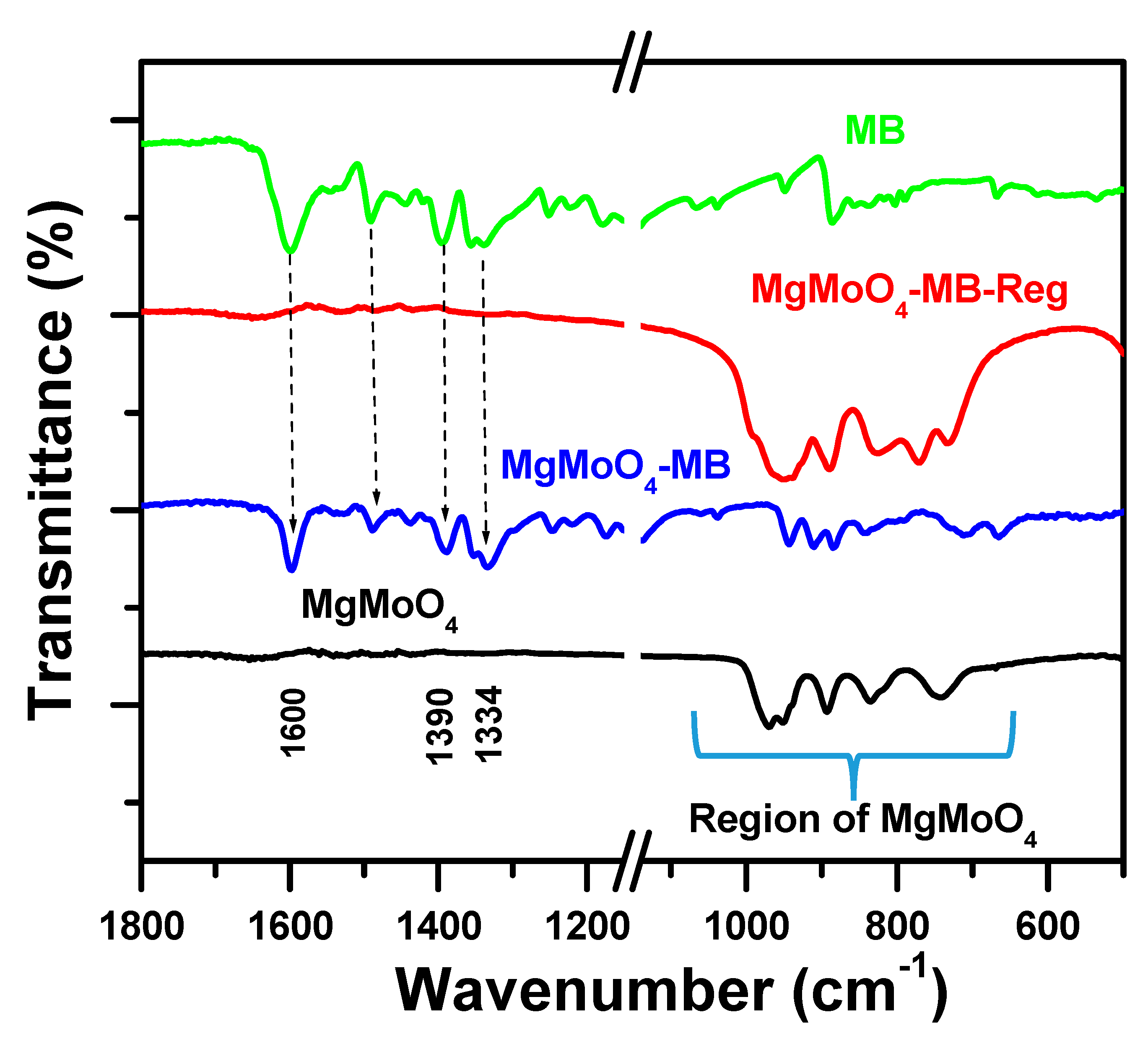
2.4.2. FT-IR Spectroscopy
2.5. MB Removal Mechanism
3. Experimental Materials and Methods
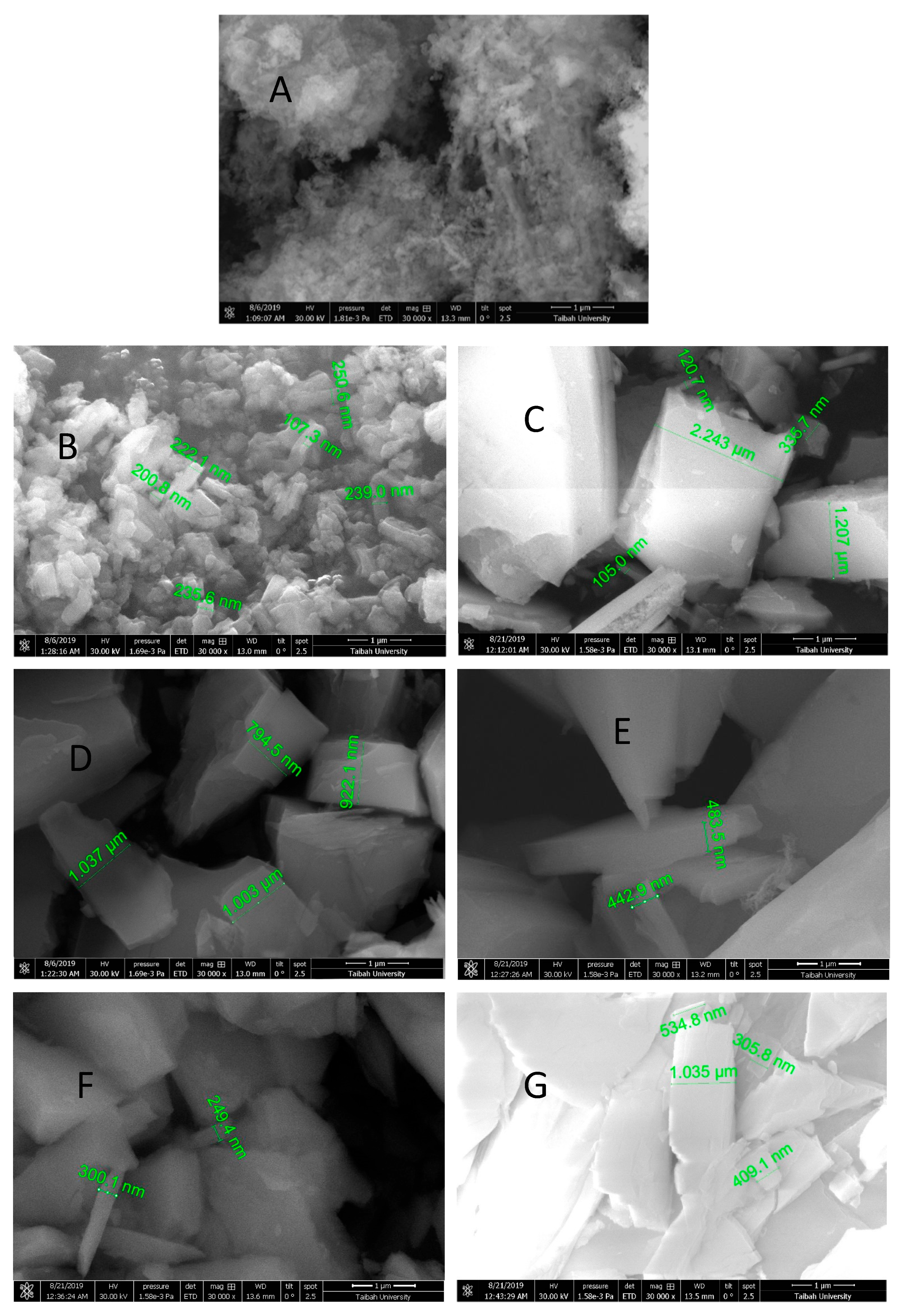
3.1. Magnesium Molybdate Preparation
3.2. Experimental Adsorptions
3.3. Adsorbent Regeneration Procedure
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and Characterization of Organic Dyes Containing Various Donors and Acceptors. Int. J. Mol. Sci. 2010, 11, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Prokein, M.; Dyes, T.; Renner, M.; Weidner, E. Waterless Leather Dyeing with Dense Carbon Dioxide as Solvent for Dyes. J. Supercrit. Fluids 2021, 178, 105377. [Google Scholar] [CrossRef]
- Fatimah, I.; Citradewi, P.W.; Iqbal, R.M.; Ghazali, S.A.I.S.M.; Yahya, A.; Purwiandono, G. Geopolymer from Tin Mining Tailings Waste Using Salacca Leaves Ash as Activator for Dyes and Peat Water Adsorption. S. Afr. J. Chem. Eng. 2023, 43, 257–265. [Google Scholar] [CrossRef]
- Sivasangari, S.; Suseendhar, S.; Suresh Kumar, K.; Vijayaprasath, N.; Thirumurugan, M. Characteristic Study of Electroplating and Dye Industrial Effluents. Int. J. Innov. Res. Sci. Eng. Technol. ISO 2007, 3297, 20810–20816. [Google Scholar]
- Magdy, Y.H.; Altaher, H. Kinetic Analysis of the Adsorption of Dyes from High Strength Wastewater on Cement Kiln Dust. J. Environ. Chem. Eng. 2018, 6, 834–841. [Google Scholar] [CrossRef]
- Rusmini, R.; Aquastin, D.; Manullang, R.R.; Daryono, D. Tingkat Kesukaan Konsumen Terhadap Serat Kenaf Organik Dengan Pewarna Alami. J. Penelit. Pertan. Terap. 2019, 19, 58–65. [Google Scholar] [CrossRef]
- Karimov, K.S.; Ahmad, Z.; Bhadra, J.; Nabi, J.U.; Hong, M.; Bashir, M.M.; Ahkmedov, K.M.; Murtaza, I.; Khan, M.U. Design of a Semiconductor Photoelectrochemical Cells Using Orange Dye and NaCl Aqueous Solutions. Int. J. Electrochem. Sci. 2020, 15, 3189–3195. [Google Scholar] [CrossRef]
- Hong, I.H.; Shen, Z.; Chen, S.C.; Chen, A.; Tsai, K.C.; Lin, Y.T. Manufacturing Parameters Optimization in Functional Textile Dyeing Processes. Procedia Manuf. 2017, 11, 619–624. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef]
- Pinzon, I.A.P.; Razal, R.A.; Mendoza, R.C.; Carpio, R.B.; Eusebio, R.C.P. Parametric Study on Microwave-Assisted Extraction of Runo (Miscanthus Sinensis Andersson) Dye and Its Application to Paper and Cotton Fabric. Biotechnol. Rep. 2020, 28, e00556. [Google Scholar] [CrossRef]
- Olas, B.; Białecki, J.; Urbańska, K.; Bryś, M. The Effects of Natural and Synthetic Blue Dyes on Human Health: A Review of Current Knowledge and Therapeutic Perspectives. Adv. Nutr. 2021, 12, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jiang, H.; Chen, W.; Cui, Z. Design, Synthesis, Characterization of Water-Soluble Indophenine Dyes and Their Application for Dyeing of Wool, Silk and Nylon Fabrics. Dye. Pigment. 2020, 179, 108385. [Google Scholar] [CrossRef]
- De, I.; Pahuja, M.; Wani, H.M.U.D.; Dey, A.; Dube, T.; Ghosh, R.; Kankan, N.; Mishra, J.; Panda, J.J.; Maruyama, T.; et al. In-Vitro Toxicity Assessment of a Textile Dye Eriochrome Black T and Its Nano-Photocatalytic Degradation through an Innovative Approach Using Mf-NGr-CNTs-SnO2 Heterostructures. Ecotoxicol. Environ. Saf. 2022, 243, 113985. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.; Abel, A. Cationic (Basic) Dye Complex Pigments. Phys. Sci. Rev. 2021, 6, 557–567. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Long, J.J. Development of a Novel Method for Investigation the Uptake Behaviors of Acid Dye on Silk Fabric at Various Flow Statuses in a Coloration Circular Pipe. Arab. J. Chem. 2022, 15, 104175. [Google Scholar] [CrossRef]
- Takijiri, K.; Morita, K.; Nakazono, T.; Sakai, K.; Ozawa, H. Highly Stable Chemisorption of Dyes with Pyridyl Anchors over TiO2: Application in Dye-Sensitized Photoelectrochemical Water Reduction in Aqueous Media. Chem. Commun. 2017, 53, 3042–3045. [Google Scholar] [CrossRef]
- Gorjanc, M.; Gerl, A.; Kert, M. Screen Printing of PH-Responsive Dye to Textile. Polymers 2022, 14, 447. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, V.; Kesari, K.K. Microbial and Lignocellulosic Biomass Based Dye Decolourization. Biomass Convers. Biorefinery 2022, 13, 16643–16666. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and Impact of Synthetic Textile Dyes on Aquatic Flora: A Review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Pierrat, É.; Laurent, A.; Dorber, M.; Rygaard, M.; Verones, F.; Hauschild, M. Advancing Water Footprint Assessments: Combining the Impacts of Water Pollution and Scarcity. Sci. Total Environ. 2023, 870, 161910. [Google Scholar] [CrossRef]
- Rakass, S.; Mohmoud, A.; Hassani, H.O.; Abboudi, M.; Kooli, F.; Al Wadaani, F. Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules 2018, 23, 1950. [Google Scholar] [CrossRef] [PubMed]
- Greluk, M.; Hubicki, Z. Effect of Basicity of Anion Exchangers and Number and Positions of Sulfonic Groups of Acid Dyes on Dyes Adsorption on Macroporous Anion Exchangers with Styrenic Polymer Matrix. Chem. Eng. J. 2013, 215–216, 731–739. [Google Scholar] [CrossRef]
- Türgay, O.; Ersöz, G.; Atalay, S.; Forss, J.; Welander, U. The Treatment of Azo Dyes Found in Textile Industry Wastewater by Anaerobic Biological Method and Chemical Oxidation. Sep. Purif. Technol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A Review on Chemical Coagulation/Flocculation Technologies for Removal of Colour from Textile Wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C. Degradation of Azo Dyes by Laccase: Biological Method to Reduce Pollution Load in Dye Wastewater. Clean. Technol. Environ. Policy 2015, 17, 1443–1456. [Google Scholar] [CrossRef]
- Pǎcurariu, C.; Paşka, O.; Ianoş, R.; Muntean, S.G. Effective Removal of Methylene Blue from Aqueous Solution Using a New Magnetic Iron Oxide Nanosorbent Prepared by Combustion Synthesis. Clean. Technol. Environ. Policy 2016, 18, 705–715. [Google Scholar] [CrossRef]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; Van Der Lelie, D.; Van Aken, B.; Foret, M.; Onderwater, R.C.A.; Wesenberg, D.; et al. Decolorization, Cytotoxicity, and Genotoxicity Reduction during a Combined Ozonation/Fungal Treatment of Dye-Contaminated Wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scalese, S. Graphene Oxide and Titania Hybrid Nation Membranes for Efficient Removal of Methyl Orange Dye from Water. Carbon 2015, 82, 489–499. [Google Scholar]
- Kandisa, R.V.; Saibaba KV, N. Dye Removal by Adsorption: A Review. J. Bioremediat Biodegrad. 2016, 07, 371. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Mohmoud, A.; Rakass, S.; Hassani, H.O.; Kooli, F.; Abboudi, M.; Aoun, S. Ben Iron Molybdate Fe2(MoO4)3 Nanoparticles: Efficient Sorbent for Methylene Blue Dye Removal from Aqueous Solutions. Molecules 2020, 25, 3390. [Google Scholar] [CrossRef] [PubMed]
- Madeley, R.A.; Wanke, S.E. Variation of the Dispersion of Active Phases in Commercial Nickel-Molybdenum/γ-Alumina Hydrotreating Catalysts during Oxidative Regeneration. Appl. Catal. 1988, 39, 295–314. [Google Scholar] [CrossRef]
- Gates, B.C.; Katzer, J.R.; Schuit, G.C.A. Chemistry of Catalytic Processes; McGraw-Hill: New York, NY, USA, 1979; Volume 60. [Google Scholar]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2 and H2S with CoMoO4 and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. B 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Sundaram, R.; Nagaraja, K.S. Solid State Electrical Conductivity and Humidity Sensing Studies on Metal Molybdate-Molybdenum Trioxide Composites (M = Ni2+, Cu2+ and Pb2+). Sens. Actuators B Chem. 2004, 101, 353–360. [Google Scholar] [CrossRef]
- Kaddouri, A.; Anouchinsky, R.; Mazzocchia, C.; Madeira, L.M.; Portela, M.F. Oxidative Dehydrogenation of Ethane on the α and β Phases of NiMoO4. Catal. Today 1998, 40, 201–206. [Google Scholar] [CrossRef]
- Pillay, B.; Mathebula, M.R.; Friedrich, H.B. The Oxidative Dehydrogenation of N-Hexane over Ni-Mo-O Catalysts. Appl. Catal. A Gen. 2009, 361, 57–64. [Google Scholar] [CrossRef]
- Mi, Y.; Huang, Z.; Hu, F.; Jiang, J.; Li, Y. Controlled Synthesis and Growth Mechanism of Alpha Nickel Molybate Microhombohedron. Mater. Lett. 2010, 64, 695–697. [Google Scholar] [CrossRef]
- Brito, J.L.; Barbosa, A.L.; Albornoz, A.; Severino, F.; Laine, J. Nickel Molybdate as Precursor of HDS Catalysts: Effect of Phase Composition. Catal. Lett. 1994, 26, 329–337. [Google Scholar] [CrossRef]
- Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M. Preparation of Cobalt Molybdate Nanoparticles; Taguchi Optimization and Photocatalytic Oxidation of Reactive Black 8 Dye. Powder Technol. 2013, 249, 378–385. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Seeta Rama Raju, G.; Pavitra, E.; Kwak, C.H.; Han, Y.K.; Huh, Y.S. Controlled Synthesis of Hierarchical α-Nickel Molybdate with Enhanced Solar-Light-Responsive Photocatalytic Activity: A Comprehensive Study on the Kinetics and Effect of Operational Factors. Ceram. Int. 2019, 45, 12041–12052. [Google Scholar] [CrossRef]
- Ferreira, E.A.C.; Andrade Neto, N.F.; Bomio, M.R.D.; Motta, F.V. Influence of Solution PH on Forming Silver Molybdates Obtained by Sonochemical Method and Its Application for Methylene Blue Degradation. Ceram. Int. 2019, 45, 11448–11456. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ibrahim, A.A.; Rayan, D.A.; Sanad, M.M.S.; Helmy, I.M. Photo-Fenton-like Degradation of Rhodamine B Dye from Waste Water Using Iron Molybdate Catalyst under Visible Light Irradiation. Environ. Nanotechnol. Monit. Manag. 2017, 8, 175–186. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, Characterization and Application of Chromium Molybdate for Oxidation of Methylene Blue Dye. J. Mater. Environ. Sci. 2018, 9, 1051–1057. [Google Scholar] [CrossRef]
- Esmaeili, H.; Foroutan, R.; Jafari, D.; Aghil Rezaei, M. Effect of Interfering Ions on Phosphate Removal from Aqueous Media Using Magnesium Oxide@ferric Molybdate Nanocomposite. Korean J. Chem. Eng. 2020, 37, 804–814. [Google Scholar] [CrossRef]
- Santiago, A.A.G.; Oliveira, M.C.; Ribeiro, R.A.P.; Tranquilin, R.L.; Longo, E.; De Lázaro, S.R.; Motta, F.V.; Bomio, M.R.D. Atomistic Perspective on the Intrinsic White-Light Photoluminescence of Rare-Earth Free MgMoO4 Nanoparticles. Cryst. Growth Des. 2020, 20, 6592–6603. [Google Scholar] [CrossRef]
- Cadus, L.E.; Abello, M.C.; Gomez, M.F.; Rivarola, J.B. Oxidative Dehydrogenation of Propane over Mg-Mo-O Catalysts. Ind. Eng. Chem. Res. 1996, 35, 14–18. [Google Scholar] [CrossRef]
- Miller, J.E.; Jackson, N.B.; Evans, L.; Sault, A.G.; Gonzales, M.M. The Formation of Active Species for Oxidative Dehydrogenation of Propane on Magnesium Molybdates. Catal. Lett. 1999, 58, 147–152. [Google Scholar] [CrossRef]
- Al Wadaani, F. Nano-Structured ß-MgMoO4: Synthesis, Characterization and Catalytic Efficiency in the 3-Nitrophenol Reduction. Moroc. J. Chem. 2016, 4, 332–338. [Google Scholar]
- Xavier, C.S.; de Moura, A.P.; Longo, E.; Varela, J.A.; Zaghete, M.A. Synthesis and Optical Property of MgMoO4 Crystals. In Proceedings of the Advanced Materials Research; 2014; Volume 975. [Google Scholar]
- Mikhailik, V.B.; Kraus, H.; Kapustyanyk, V.; Panasyuk, M.; Prots, Y.; Tsybulskyi, V.; Vasylechko, L. Structure, Luminescence and Scintillation Properties of the MgWO 4-MgMoO4 System. J. Phys. Condens. Matter 2008, 20, 365219. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Wei, J.S.; Yi, L.H.; Gong, F.Z.; Huang, J.L.; Wang, W. A Promising Red Phosphor MgMoO4:Eu3+ for White Light Emitting Diodes. Mater. Res. Bull. 2009, 44, 1411–1414. [Google Scholar] [CrossRef]
- Mo, F.; Chen, P.; Guan, A.; Zhang, W.; Zhou, L. Enhancement of Luminescence Intensity and Color Purity of MgxZn1-XMoO4:Eu3+,Bi3+ Phosphors. J. Rare Earths 2015, 33, 1064–1071. [Google Scholar] [CrossRef]
- Cornu, L.; Jubera, V.; Demourgues, A.; Salek, G.; Gaudon, M. Luminescence Properties and Pigment Properties of A-Doped (Zn, Mg)MoO4 Triclinc Oxides (with A=Co, Ni, Cu or Mn). Ceram. Int. 2017, 53, 13377–13378. [Google Scholar] [CrossRef]
- Ran, W.; Wang, L.; Yang, M.; Kong, X.; Qu, D.; Shi, J. Echanced Energy Transfer from Bi3+ to Eu3+ Ions Relying on the Criss-Cross Cluster Structure in MgMoO4 Phosphor. J. Lumin. 2017, 192, 141–147. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Shen, K.; Liu, Y.; Guo, S. Controllable Synthesis of Hierarchical MgMoO4 Nanosheet-Arrays and Nano-Flowers Assembled with Mesoporous Ultrathin Nanosheets. J. Phys. Chem. Solids 2018, 115, 215–220. [Google Scholar] [CrossRef]
- Amberg, M.; Günter, J.R.; Schmalle, H.; Blasse, G. Preparation, Crystal Structure, and Luminescence of Magnesium Molybdate and Tungstate Monohydrates, MgMoO4 · H2O and MgWO4 · H2O. J. Solid. State Chem. 1988, 77, 162–169. [Google Scholar] [CrossRef]
- Yousefipour, K.; Sarraf-Mamoory, R.; Mollayousefi, S. Synthesis of Manganese Molybdate/MWCNT Nanostructure Composite with a Simple Approach for Supercapacitor Applications. RSC Adv. 2022, 12, 27868–27876. [Google Scholar] [CrossRef]
- Haetge, J.; Suchomski, C.; Brezesinski, T. Ordered Mesoporous β-MgMoO4 Thin Films for Lithium-Ion Battery Applications. Small Nanomicro 2013, 9, 2541–2544. [Google Scholar] [CrossRef]
- Lee, E.K.; Jung, K.D.; Joo, O.S.; Shul, Y.G. Catalytic Activity of Mo/MgO Catalyst in the Wet Oxidation of H2S to Sulfur at Room Temperature. Appl. Catal. A Gen. 2004, 268, 83–88. [Google Scholar] [CrossRef]
- Andreichikova, M.P.; Udalova, T.A. Mechanochemical Synthesis of Highly Dispersed Molybdenum from Magnesium Molybdate. Mater. Today Proc. 2020, 31, 526–528. [Google Scholar] [CrossRef]
- Bian, W.; Lu, X.; Wang, Y.; Zhu, H.; Chen, T.; Ta, S.; Zhang, Q. Correlations between Structure and Microwave Dielectric Properties of Co Doped MgMoO4 Ceramics. Ceram. Int. 2020, 46, 22024–22029. [Google Scholar] [CrossRef]
- Braziulis, G.; Janulevicius, G.; Stankeviciute, R.; Zalga, A. Aqueous Sol-Gel Synthesis and Thermoanalytical Study of the Alkaline Earth Molybdate Precursors. J. Therm. Anal. Calorim. 2013, 118, 613–621. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of Pyrolyzed Biochar and Its Application for Dye Removal: Batch, Kinetic and Isotherm with Linear and Non-Linear Mathematical Analysis. Surf. Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Abboudi, M.; Mohmoud, A.; AlWadaani, F. Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules 2018, 23, 3390. [Google Scholar] [CrossRef]
- Kim, J.R.; Santiano, B.; Kim, H.; Kan, E. Heterogeneous Oxidation of Methylene Blue with Surface-Modified Iron-Amended Activated Carbon. Am. J. Anal. Chem. 2013, 04, 115–122. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Ching, O.Y.; Al-Faze, R. Characterization of Organo-Kenyaites: Thermal Stability and Their Effects on Eosin Removal Characteristics. Clay Min. 2018, 53, 91–104. [Google Scholar] [CrossRef]
- Popoola, S.A.; Al Dmour, H.; Rakass, S.; Fatimah, I.; Liu, Y.; Mohmoud, A.; Kooli, F. Enhancement Properties of Zr Modified Porous Clay Heterostructures for Adsorption of Basic-Blue 41 Dye: Equilibrium, Regeneration, and Single Batch Design Adsorber. Materials 2022, 15, 5567. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch Adsorption of Basic Dye Using Acid Treated Kenaf Fibre Char: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Anoop Krishnan, K.; Anirudhan, T.S. A Preliminary Examination of the Adsorption Characteristics of Pb(II) Ions Using Sulphurised Activated Carbon Prepared from Bagasse Pith. Indian. J. Chem. Technol. 2002, 9, 32–40. [Google Scholar]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of Methylene Blue on Chemically Modified Lychee Seed Biochar: Dynamic, Equilibrium, and Thermodynamic Study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Patil, S.; Renukdas, S.; Patel, N. Removal of Methylene Blue, a Basic Dye from Aqueous Solutions by Adsorption Using Teak Tree (Tectona Grandis) Bark Powder. J. Environ. Sci. Vol. 2011, 1, 711–726. [Google Scholar]
- Vadivelan, V.; Vasanth Kumar, K. Equilibrium, Kinetics, Mechanism, and Process Design for the Sorption of Methylene Blue onto Rice Husk. J. Colloid. Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hai Nguyen, T. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. [Google Scholar] [CrossRef]
- Furusawa, T.; Smith, J.M. Intraparticle Mass Transport in Slurries by Dynamic Adsorption Studies. AIChE J. 1974, 20, 88–93. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order Rate Equations from Langmuir and Freundlich Isotherms for Adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M. Dada Langmuir, Freundlich, Temkin and Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn 2+ Unto Phosphoric Acid Modified Rice Husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Zhang, Y.R.; Wang, S.Q.; Shen, S.L.; Zhao, B.X. A Novel Water Treatment Magnetic Nanomaterial for Removal of Anionic and Cationic Dyes under Severe Condition. Chem. Eng. J. 2013, 233, 258–264. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption Behavior of Methylene Blue onto Titanate Nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Rakass, S.; Oudghiri Hassani, H.; Abboudi, M.; Kooli, F.; Mohmoud, A.; Aljuhani, A.; Al Wadaani, F. Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions. Molecules 2018, 23, 2295. [Google Scholar] [CrossRef]
- Sadatshojaei, E.; Esmaeilzadeh, F.; Fathikaljahi, J.; Hosseynian Barzi, S.E.; Wood, D.A. Regeneration of the Midrex Reformer Catalysts Using Supercritical Carbon Dioxide. Chem. Eng. J. 2018, 343, 748–758. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Zheng, T.; Wang, P. Regeneration of Activated Carbon Saturated with Chloramphenicol by Microwave and Ultraviolet Irradiation. Chem. Eng. J. 2017, 320, 264–270. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-Regeneration of Activated Carbon: A Comprehensive Review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]



| Adsorbent | Adsorbate | ∆H° (KJ·mol−1) | ∆S° (KJ·mol−1·K) | ∆G° (KJ·mol−1) | |||
|---|---|---|---|---|---|---|---|
| β-MgMoO4 | MB | −32.463 | −0.017 | 293 K | 313 | 323 K | 333 K |
| −27.425 | −25.649 | −24.699 | −23.422 | ||||
| Model | Equation | Parameters |
|---|---|---|
| Pseudo-first-order (PFD) [58]. | (4) | qt stands for the removal capacity at time t (mg/g); qe stands for the removal capacity at equilibrium (mg/g); K1 stands for the rate constant of pseudo-first-order adsorption (1/min). |
| Pseudo-second-order (PSD) [75]. | (The plot of 1/qt vs.1/t) (5) | qt stands for the removal capacity at time t (mg/g); qe stands for the removal capacity at equilibrium (mg/g); K2 stands for the pseudo-second-order rate constant (g·mg−1·min−1). |
| Intra-particle diffusion (IPD) [76]. | (6) | I (mg/g) and KI (mg/(g·min0.5)) are the intra-particle diffusion constants; qt stands for the removal capacity (mg/g) at time t; t stands for the contact time (min). |
| Dye Cimg/L | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qe (mg/g) | k1 (1/min) | R12 | qe (mg/g) | k2 (g/mg min) | R22 | I (mg/g) | ki (mg/g min0.5) | R32 | |
| 160 | 319 | 145 | 0.341 | 0.975 | 319 | 2.20 | 0.979 | 190 | 41.17 | 0.958 |
| 170 | 318 | 110 | 0.146 | 0.749 | 305 | 1.64 | 0.987 | 165 | 43.71 | 0.810 |
| 180 | 330 | 129 | 0.184 | 0.927 | 316 | 1.65 | 0.990 | 165 | 49.04 | 0.884 |
| 200 | 360 | 167 | 0.162 | 0.939 | 341 | 1.24 | 0.998 | 149 | 59.63 | 0.904 |
| Model | Equation | Parameters |
|---|---|---|
| Freundlich [78]. | (7) | qF stands for the Freundlich constant (mg(1−1/n)L1/ng−1), n: stands for the heterogeneity factor (g/L); qe stands for the amount of MB cationic dye adsorbed by β-MgMoO4 at equilibrium (mg/g); Ce stands for MB the cationic dye concentration at equilibrium (ppm). |
| Langmuir [78]. | (8) | qe stands for the amount of MB cationic dye adsorbed by β-MgMoO4 at equilibrium (mg/g); Ce stands for the MB cationic dye concentration at equilibrium (ppm); qm stands for the highest amount of MB cationic dye removed by β-MgMoO4 (mg/g); KL stands for the Langmuir adsorption constant (L/mg). |
(9) | Ci stands for the initial cationic MB dye concentration; KL stands for the Langmuir constant; RL stands for the values that indicate that the removal of MB cationic dye may be either linear (RL = 1), irreversible (RL = 0), favorable (0 < RL< 1), or unfavorable (RL > 1). | |
| Dubinin–Radushkevich (D-R) [79]. | (10) (11) | K stands for the sorption energy constant (mol2/kJ2); ε stands for the Polanyi potential; T stands for the temperature (K); R stands for the universal gas constant (8.314 J·mol−1 K−1); qm stands for the theoretical saturation capacity; Ce stands for MB cationic dye concentration at equilibrium (ppm). |
| Temkin [80]. | (12) | bT stands for the Temkin constant associated with the heat of sorption (J/mol); BT = RT/bT; R stands for the gas constant (8.314 J/mol K); AT stands for the Temkin isotherm constant (L/g); T stands for the absolute temperature (K). |
| Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | Range RL | qF (mg(1−1/n)L1/ng−1) | 1/n | R2 | AT (L/g) | BT | R2 | qm (mg/g) | R2 | E (Kj/mol) |
| 356 | 1.63 | 0.991 | 0.0031–0.0038 | 315 | 0.025 | 0.343 | 3 × 1016 | 8.306 | 0.338 | 343 | 0.210 | 12.8 |
| Nanosorbent | qm (mg/g) | Reference |
|---|---|---|
| Magnetic iron oxide nanosorbent | 25.54 | [26] |
| Fe3O4 magnetic nanoparticles modified with 3-glycidoxypropyltrimethoxysilane and glycine | 158.00 | [81] |
| Zinc molybdate nanoparticles | 217.86 | [66] |
| Calcined titanate nanotubes | 133.33 | [82] |
| Molybdenum trioxide nanorods and stacked nanoplates | 152.00 | [83] |
| Magnesium molybdate (β-MgMoO4) | 356.00 | This current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohmoud, A.; Rakass, S.; Oudghiri Hassani, H.; Popoola, S.A.; Kooli, F.; Assirey, E.; Abboudi, M. Magnesium Molybdate: An Efficient Nanosorbent for Methylene Blue Cationic Dye Removal from Aqueous Solutions. Molecules 2025, 30, 1606. https://doi.org/10.3390/molecules30071606
Mohmoud A, Rakass S, Oudghiri Hassani H, Popoola SA, Kooli F, Assirey E, Abboudi M. Magnesium Molybdate: An Efficient Nanosorbent for Methylene Blue Cationic Dye Removal from Aqueous Solutions. Molecules. 2025; 30(7):1606. https://doi.org/10.3390/molecules30071606
Chicago/Turabian StyleMohmoud, Ahmed, Souad Rakass, Hicham Oudghiri Hassani, Saheed A. Popoola, Fethi Kooli, Eman Assirey, and Mostafa Abboudi. 2025. "Magnesium Molybdate: An Efficient Nanosorbent for Methylene Blue Cationic Dye Removal from Aqueous Solutions" Molecules 30, no. 7: 1606. https://doi.org/10.3390/molecules30071606
APA StyleMohmoud, A., Rakass, S., Oudghiri Hassani, H., Popoola, S. A., Kooli, F., Assirey, E., & Abboudi, M. (2025). Magnesium Molybdate: An Efficient Nanosorbent for Methylene Blue Cationic Dye Removal from Aqueous Solutions. Molecules, 30(7), 1606. https://doi.org/10.3390/molecules30071606








