Visible Light Active Natural Rutile Photocatalyst Obtained via Nano Milling
Abstract
1. Introduction
2. Results
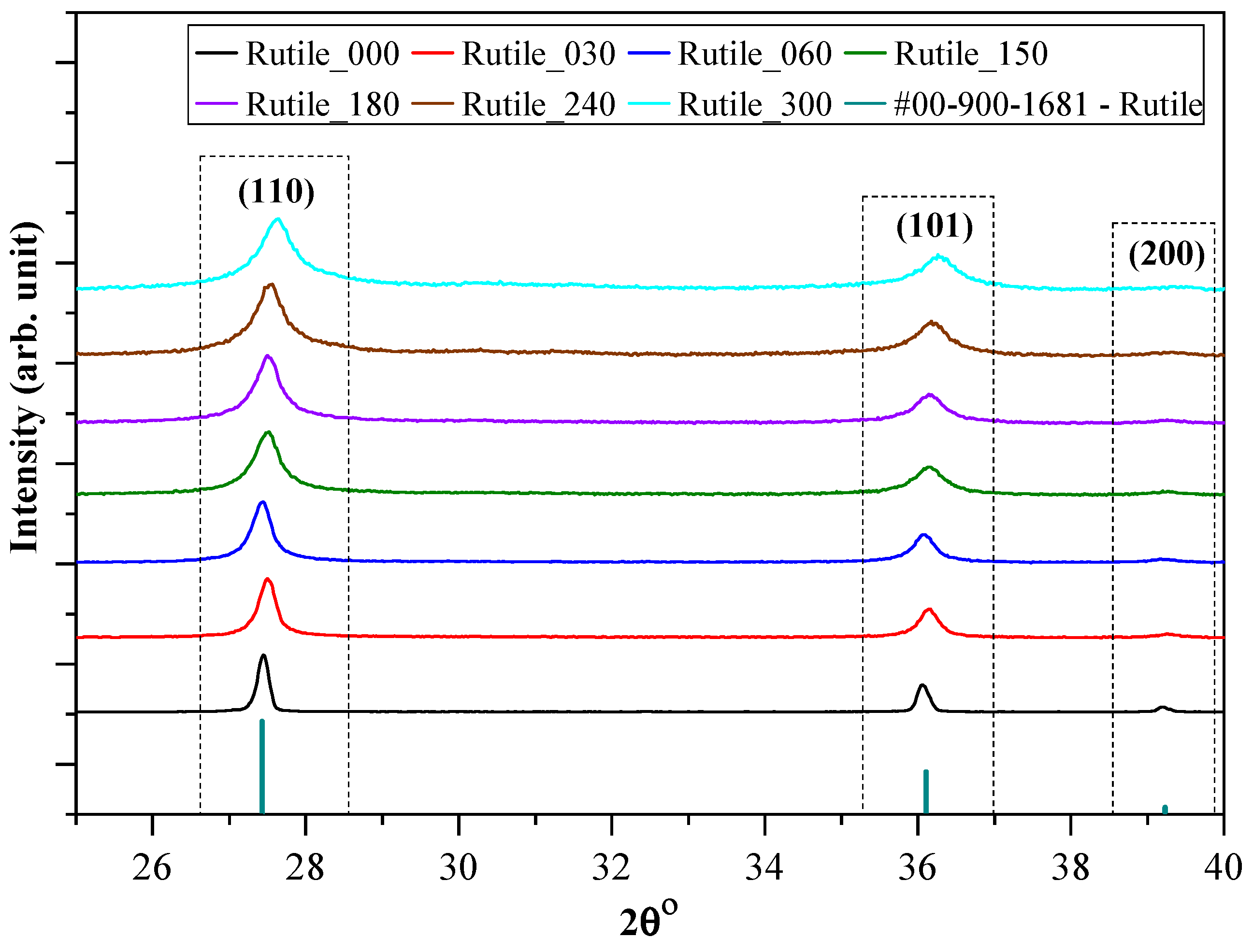
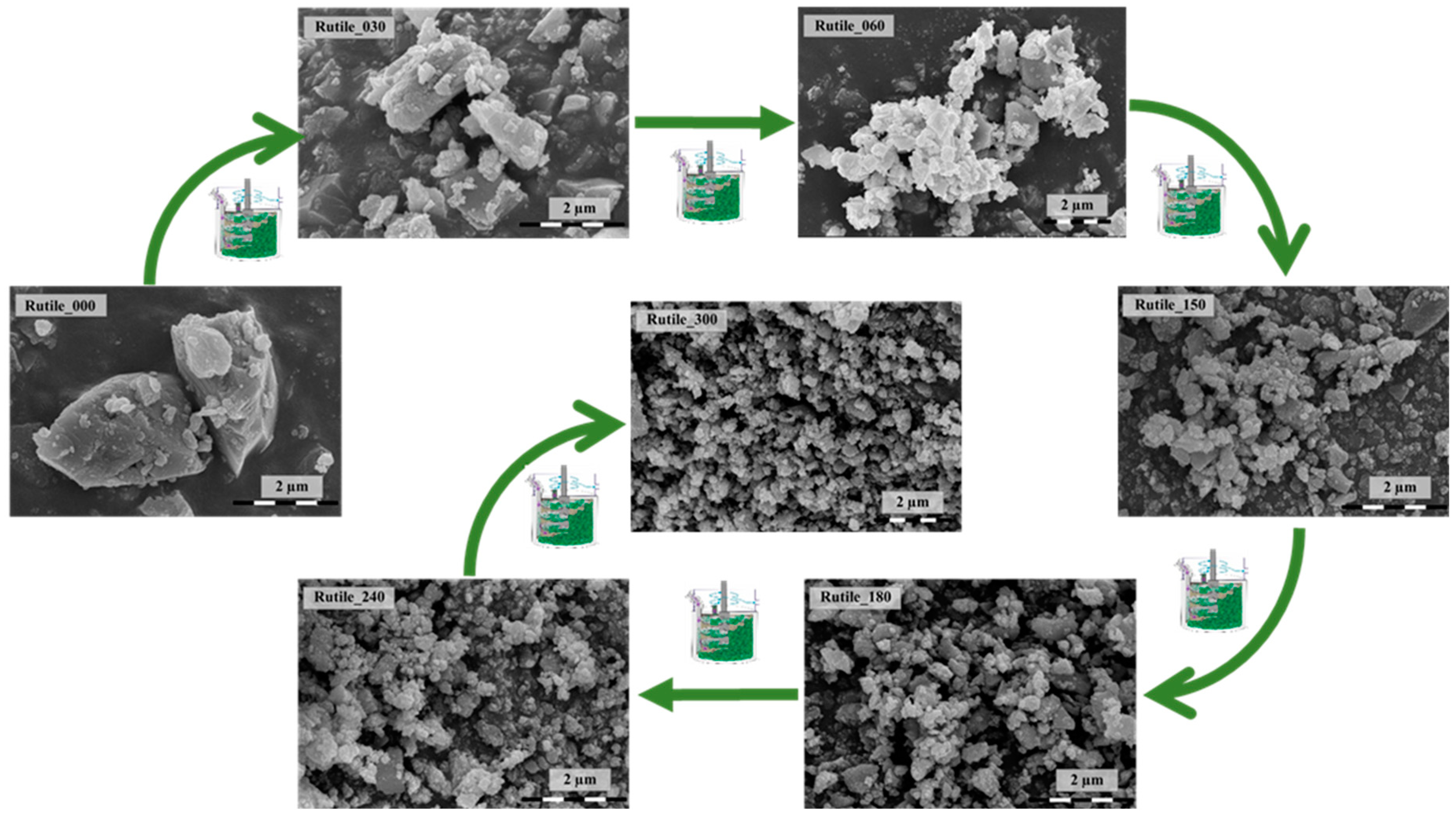
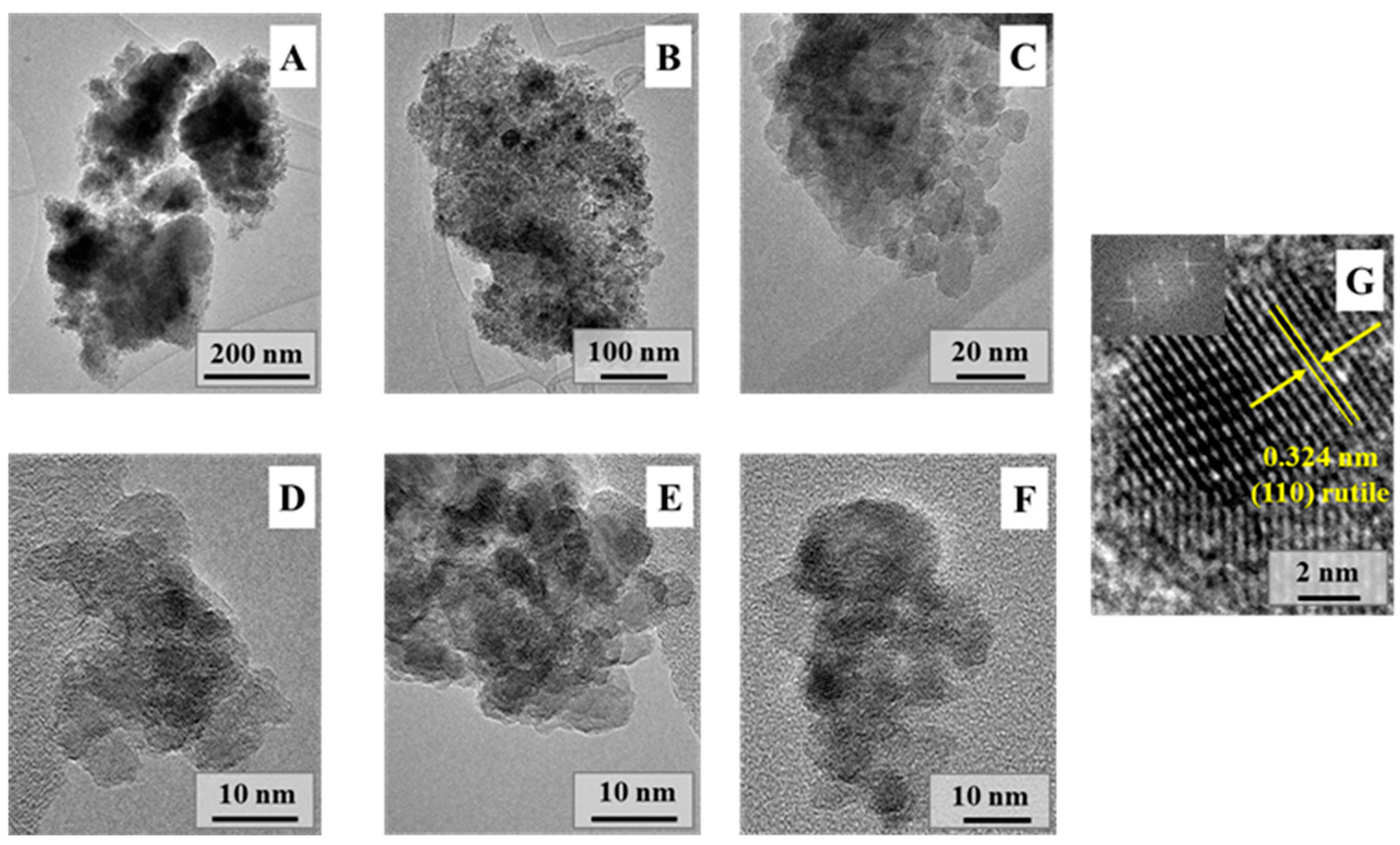
2.1. Structural and Morphological Characterization of the Ground Rutile
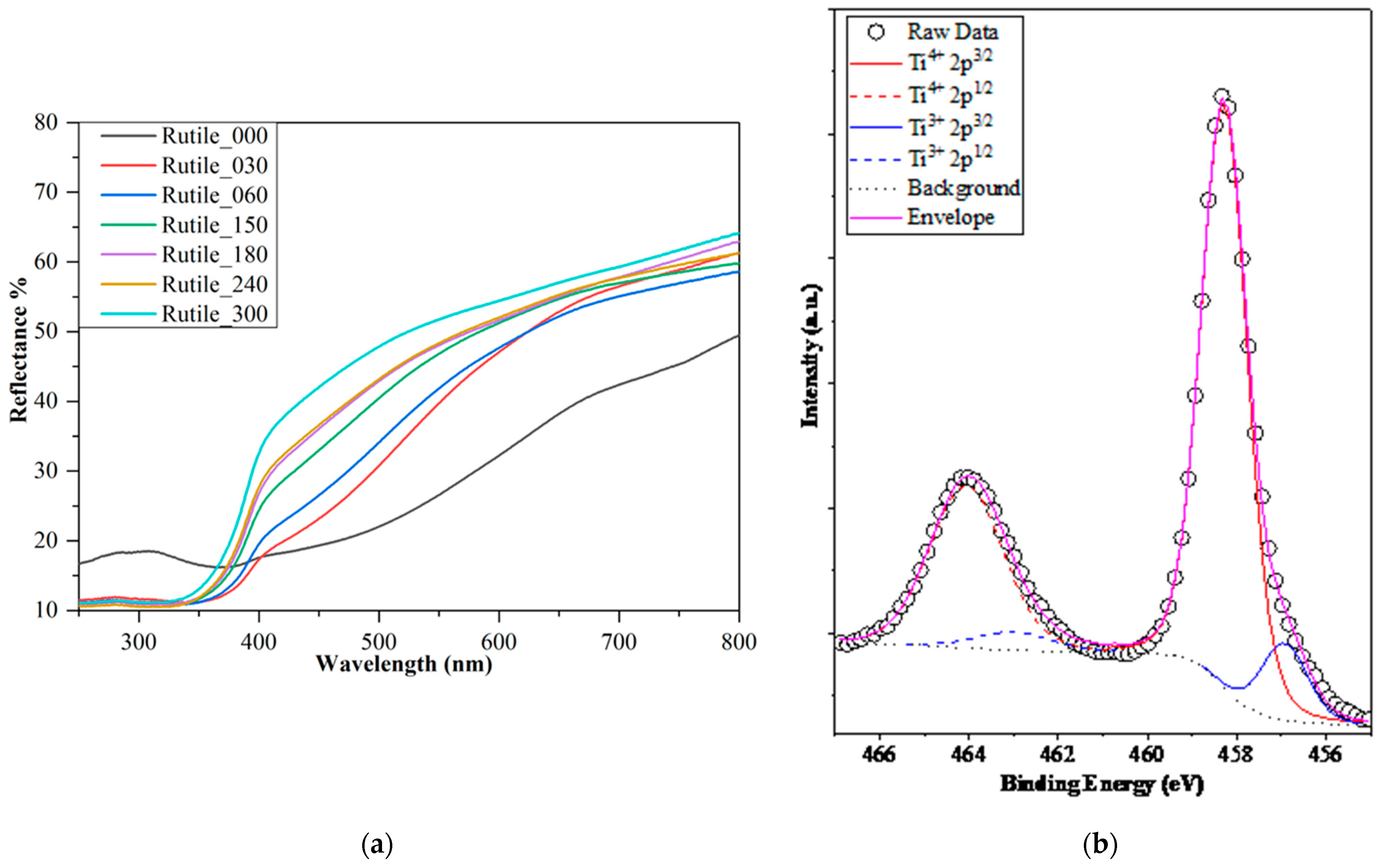
2.2. Photocatalytic Performance of the Samples
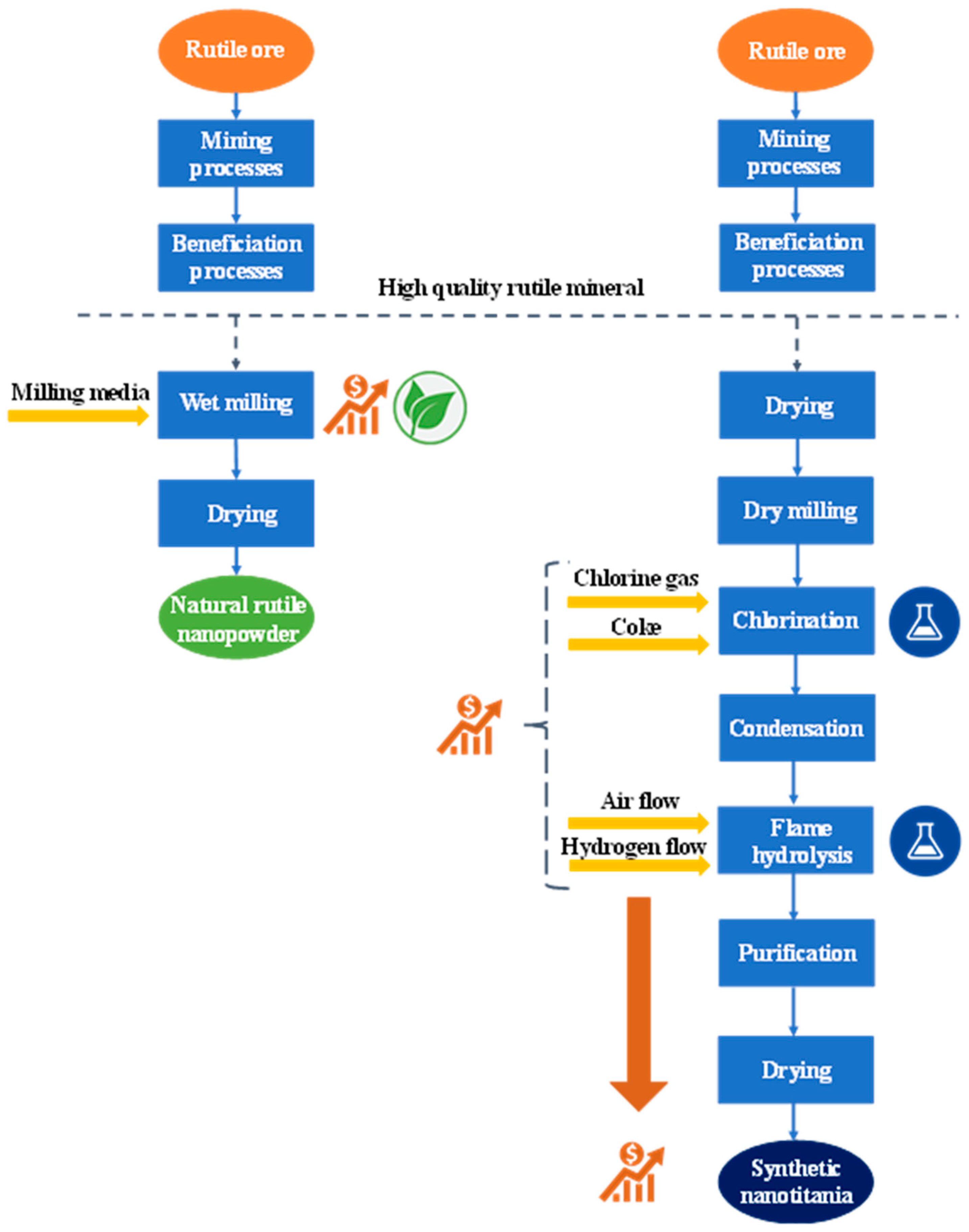
2.3. Comparison of Production Method of Natural and Synthetic TiO2
3. Materials and Methods
3.1. Materials
3.2. Applied Methods
3.3. The Photocatalytic Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–Gel Preparation and Photocatalysis of Titanium Dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Synthesis of Photocatalytic TiO2 Nanoparticles: Optimization of the Preparation Conditions. J. Photochem. Photobiol. A Chem. 2003, 157, 47–53. [Google Scholar] [CrossRef]
- Nachit, W.; Touhtouh, S.; Ramzi, Z.; Zbair, M.; Eddiai, A.; Rguiti, M.; Bouchikhi, A.; Hajjaji, A.; Benkhouja, K. Synthesis of Nanosized TiO2 Powder by Sol Gel Method at Low Temperature. Mol. Cryst. Liq. Cryst. 2016, 627, 170–175. [Google Scholar] [CrossRef]
- Gomathi Thanga Keerthana, B.; Solaiyammal, T.; Muniyappan, S.; Murugakoothan, P. Hydrothermal Synthesis and Characterization of TiO2 Nanostructures Prepared Using Different Solvents. Mater. Lett. 2018, 220, 20–23. [Google Scholar] [CrossRef]
- Nam, C.T.; Yang, W.-D.; Duc, L.M. Solvothermal Synthesis of TiO2 Photocatalysts in Ketone Solvents with Low Boiling Points. J. Nanomater. 2013, 2013, 627385. [Google Scholar] [CrossRef]
- Malekshahi Byranvand, M.; Nemati Kharat, A.; Fatholahi, L.; Malekshahi Beiranvand, Z. A Review on Synthesis of Nano-TiO2 via Different Methods. J. Nanostructures 2013, 3, 1–9. [Google Scholar]
- Xiong, L.; Xu, Y.; Lei, P.; Tao, T.; Xiao, X. Synthesis and Characterization of TiO2/C by a Simple Thermal Decomposition Method. Solid. State Ion. 2014, 268, 265–267. [Google Scholar] [CrossRef]
- Mahajan, J.; Jeevanandam, P. Novel Thermal Decomposition Approach for the Synthesis of TiO2@Ag Core-Shell Nanocomposites and Their Application for Catalytic Reduction of 4-Nitrophenol. J. Nanoparticle Res. 2019, 21, 66. [Google Scholar] [CrossRef]
- Buraso, W.; Lachom, V.; Siriya, P.; Laokul, P. Synthesis of TiO2 Nanoparticles via a Simple Precipitation Method and Photocatalytic Performance. Mater. Res. Express 2018, 5, 115003. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-Temperature Synthesis of Anatase TiO2 Nanoparticles with Tunable Surface Charges for Enhancing Photocatalytic Activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Nan, J. Hydrothermal-Hydrolysis Synthesis and Photocatalytic Properties of Nano-TiO2 with an Adjustable Crystalline Structure. J. Hazard. Mater. 2010, 176, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 Nanoparticles by Hydrolysis and Peptization of Titanium Isopropoxide Solution. J. Mater. Process Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Bahadur, N.M.; Chowdhury, F.; Obaidullah, M.; Hossain, M.S.; Rashid, R.; Akter, Y.; Furusawa, T.; Sato, M.; Suzuki, N. Ultrasonic-Assisted Synthesis, Characterization, and Photocatalytic Application of SiO2@TiO2 Core-Shell Nanocomposite Particles. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Arami, H.; Mazloumi, M.; Khalifehzadeh, R.; Sadrnezhaad, S.K. Sonochemical Preparation of TiO2 Nanoparticles. Mater. Lett. 2007, 61, 4559–4561. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.J.; Lanceros-Méndez, S.; Teixeira, V. Synthesis of Iron-Doped TiO2 Nanoparticles by Ball-Milling Process: The Influence of Process Parameters on the Structural, Optical, Magnetic, and Photocatalytic Properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.B.; Roy, D. Development of Nano-TiO2 by Mechanical Milling. Int. J. Sci. Eng. Res. 2016, 4, 67–69. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of Anatase TiO2 Supported on Porous Solids by Chemical Vapor Deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.Y.; Jurng, J.; Park, Y.-K. The Synthesis and Coating Process of TiO2 Nanoparticles Using CVD Process. Powder Technol. 2011, 214, 64–68. [Google Scholar] [CrossRef]
- Rozenberga-Voska, L.; Grabis, J. Synthesis and Photocatalytic Activity of Modified TiO2; Thin Films Prepared by Spray Pyrolysis. Solid State Phenom. 2017, 267, 3–6. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, K.S.; Kim, J. Spray Pyrolysis Synthesis of Mesoporous TiO2 Microspheres and Their Post Modification for Improved Photocatalytic Activity. Korean J. Chem. Eng. 2018, 35, 2480–2486. [Google Scholar] [CrossRef]
- Inaba, R.; Fukahori, T.; Hamamoto, M.; Ohno, T. Synthesis of Nanosized TiO2 Particles in Reverse Micelle Systems and Their Photocatalytic Activity for Degradation of Toluene in Gas Phase. J. Mol. Catal. A Chem. 2006, 260, 247–254. [Google Scholar] [CrossRef]
- Kluson, P.; Luskova, H.; Solcova, O.; Matejova, L.; Cajthaml, T. Lamellar Micelles-Mediated Synthesis of Nanoscale Thick Sheets of Titania. Mater. Lett. 2007, 61, 2931–2934. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Jardiel, T.; Peiteado, M.; Caballero, A.C.; Fernández-Hevia, D. Microwave-Induced Fast Crystallization of Amorphous Hierarchical Anatase Microspheres. Nanoscale Res. Lett. 2014, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamaguchi, H.; Kageyama, H.; Oaki, Y.; Imai, H. Microwave-assisted rapid synthesis of anatase TiO2 nanosized particles in an ionic liquid-water system. J. Ceram. Soc. Jpn. 2015, 123, 79–82. [Google Scholar] [CrossRef][Green Version]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef]
- Luo, L.; Miao, L.; Tanemura, S.; Tanemura, M. Photocatalytic Sterilization of TiO2 Films Coated on Al Fiber. Mater. Sci. Eng. B 2008, 148, 183–186. [Google Scholar] [CrossRef]
- Singh, T.; Srivastava, N.; Mishra, P.K.; Bhatiya, A.K.; Singh, N.L. Application of TiO2; Nanoparticle in Photocatalytic Degradation of Organic Pollutants. Mater. Sci. Forum 2016, 855, 20–32. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Bassi, A.L.; Bottani, C.E.; Ducati, C. Raman Spectroscopy Characterization of TiO2 Rutile Nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B.F. High Purity Anatase TiO2 Nanocrystals: Near Room-Temperature Synthesis, Grain Growth Kinetics, and Surface Hydration Chemistry. J. Am. Chem. Soc. 2005, 127, 8659–8666. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, M.; Feng, Z.; Li, C. UV Raman Spectroscopic Study on TiO2. II. Effect of Nanoparticle Size on the Outer/Inner Phase Transformations. J. Phys. Chem. C 2009, 113, 1698–1704. [Google Scholar] [CrossRef]
- Satoh, N.; Nakashima, T.; Yamamoto, K. Metastability of Anatase: Size Dependent and Irreversible Anatase-Rutile Phase Transition in Atomic-Level Precise Titania. Sci. Rep. 2013, 3, 1959. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and Its Applications in Earth Sciences. Earth Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Banfield, J.F.; Veblen David, R. The Structure and Origin of Fe-Bearing Platelets in Metamorphic Rutile. Am. Mineral. 1991, 76, 113–127. [Google Scholar]
- Scott, K.M.; Radford, N.W. Rutile Compositions at the Big Bell Au Deposit as a Guide for Exploration. Geochem. Explor. Environ. Anal. 2007, 7, 353–361. [Google Scholar] [CrossRef]
- Downes, P.J.; Griffin, B.J.; Griffin, W.L. Mineral Chemistry and Zircon Geochronology of Xenocrysts and Altered Mantle and Crustal Xenoliths from the Aries Micaceous Kimberlite: Constraints on the Composition and Age of the Central Kimberley Craton, Western Australia. Lithos 2007, 93, 175–198. [Google Scholar] [CrossRef]
- Raman, K.V.; Jackson, M.L. Rutile and Anatase Determination in Soils and Sediments. Am. Mineral. 1965, 50, 1086–1092. [Google Scholar]
- Zack, T.; von Eynatten, H.; Kronz, A. Rutile Geochemistry and Its Potential Use in Quantitative Provenance Studies. Sediment. Geol. 2004, 171, 37–58. [Google Scholar] [CrossRef]
- Luvizotto, G.L.; Zack, T.; Triebold, S.; von Eynatten, H. Rutile Occurrence and Trace Element Behavior in Medium-Grade Metasedimentary Rocks: Example from the Erzgebirge, Germany. Miner. Petrol. 2009, 97, 233–249. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Sand Supported Mixed TiO2 Photocatalysts for Water Decontamination Applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Nagle, J.; Piper, D.J.W.; McFarlane, C.R.M. Geochronology and Trace Element Mobility in Rutile from a Carboniferous Syenite Pegmatite and the Role of Halogens. Am. Mineral. 2019, 104, 501–513. [Google Scholar] [CrossRef]
- Şengün, F.; Zack, T. Trace Element Composition of Rutile and Zr-in-Rutile Thermometry in Meta-Ophiolitic Rocks from the Kazdağ Massif, NW Turkey. Miner. Petrol. 2016, 110, 547–560. [Google Scholar] [CrossRef]
- Luvizotto, G.L.; Zack, T.; Meyer, H.P.; Ludwig, T.; Triebold, S.; Kronz, A.; Münker, C.; Stockli, D.F.; Prowatke, S.; Klemme, S.; et al. Rutile Crystals as Potential Trace Element and Isotope Mineral Standards for Microanalysis. Chem. Geol. 2009, 261, 346–369. [Google Scholar] [CrossRef]
- Arunmetha, S.; Manivasakan, P.; Karthik, A.; Dhinesh Babu, N.R.; Srither, S.R.; Rajendran, V. Effect of Processing Methods on Physicochemical Properties of Titania Nanoparticles Produced from Natural Rutile Sand. Adv. Powder Technol. 2013, 24, 972–979. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Li, Y.; Ding, C.; Wu, J.; Lu, A.; Ding, H.; Qin, S.; Wang, C. Absolute Band Structure Determination on Naturally Occurring Rutile with Complex Chemistry: Implications for Mineral Photocatalysis on Both Earth and Mars. Appl. Surf. Sci. 2018, 439, 660–671. [Google Scholar] [CrossRef]
- Hu, W.; Dong, F.; Zhang, J.; Liu, M.; He, H.; Yang, D.; Deng, H. A High-Efficiency Photocatalyst, Flaky Anatase@natural Rutile Composite Using One-Step Microwave Hydrothermal Synthesis. Res. Chem. Intermed. 2018, 44, 705–720. [Google Scholar] [CrossRef]
- Lu, A.; Liu, J.; Zhao, D.; Guo, Y.; Li, Q.; Li, N. Photocatalysis of V-Bearing Rutile on Degradation of Halohydrocarbons. Catal. Today 2004, 90, 337–342. [Google Scholar] [CrossRef]
- Chuan, X.-Y.; Lu, A.H.; Chen, J.; Li, N.; Guo, Y.J. Microstructure and Photocatalytic Activity of Natural Rutile from China for Oxidation of Methylene Blue in Water. Miner. Petrol. 2008, 93, 143–152. [Google Scholar] [CrossRef]
- Yilleng, M.T.; Gimba, E.C.; Ndukwe, G.I. Assessing the Photo Catalytic Activity of Rutile Ore from the Middle Belt Region of Nigeria on the Degradation of Phenol in Water. IOSR J. Appl. Chem. 2015, 8, 36–43. [Google Scholar]
- Retamoso, C.; Escalona, N.; González, M.; Barrientos, L.; Allende-González, P.; Stancovich, S.; Serpell, R.; Fierro, J.L.G.; Lopez, M. Effect of Particle Size on the Photocatalytic Activity of Modified Rutile Sand (TiO2) for the Discoloration of Methylene Blue in Water. J. Photochem. Photobiol. A Chem. 2019, 378, 136–141. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Lv, M.; Wang, C.; Yang, L.; Liu, J.; Wang, Y.; Wong, K.-H.; Wong, P.-K. Photocatalytic Oxidation of Methyl Orange by Natural V-Bearing Rutile Under Visible Light. Sol. Energy Mater. Sol. Cells 2007, 91, 1849–1855. [Google Scholar] [CrossRef]
- Sajan, C.P.; Basavalingu, B.; Ananda, S.; Byrappa, K. Comparative Study on the Photodegradation of Indigo Caramine Dye Using Commercial TiO2 and Natural Rutile. J. Geol. Soc. India 2011, 77, 82–88. [Google Scholar] [CrossRef]
- Almasi, E.; Pap, Z.; Racz, A.; Bohacs, K.; Mucsi, G.; Rakhely, G. Morpho-structural Properties of Nanogrinded Rutile as Potential Photocatalyst for Water Treatment. Geosci. Eng. 2020, 8, 217–237. [Google Scholar]
- Guo, Z.; Ma, R.; Li, G. Degradation of Phenol by Nanomaterial TiO2 in Wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nag, S.; Ray, A.K. Degradation of Phenolic Compounds Through UV and Visible-Light-Driven Photocatalysis: Technical and Economic Aspects. In Phenolic Compounds-Natural Sources, Importance and Applications; InTech: London, UK, 2017. [Google Scholar]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Jiang, W.; Mkaouar, A.R.; Xu, P. Comparison Study on Photocatalytic Oxidation of Pharmaceuticals by TiO2-Fe and TiO2-Reduced Graphene Oxide Nanocomposites Immobilized on Optical Fibers. J. Hazard. Mater. 2017, 333, 162–168. [Google Scholar] [CrossRef]
- Mehrabadi, Z.; Faghihian, H. Clinoptilolite Modified with TiO2 for Simultaneous Elimination of Two Herbicides; 2,4-D and MCPA by UV and Sunlight-Assisted Photocatalytic Degradation. Mater. Res. Bull. 2019, 119, 110569. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Sakellarides, T.M.; Sakkas, V.A.; Albanis, T.A. Photocatalytic Degradation of Selected S-Triazine Herbicides and Organophosphorus Insecticides over Aqueous TiO2 Suspensions. Environ. Sci. Technol. 2001, 35, 398–405. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, C.; Bao, Y.; Yang, J.; Wu, Y. Photocatalytic Degradation of Pesticide Pyridaben on TiO2 Particles. J. Mol. Catal. A Chem. 2005, 229, 95–105. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Zeng, Z.; Wang, A.; Liu, G.; Cui, H. The Application of Nano-TiO2 Photo Semiconductors in Agriculture. Nanoscale Res. Lett. 2016, 11, 529. [Google Scholar] [CrossRef]
- Gami, A.A.; Shukor, M.Y.; Khalil, K.A.; Dahalan, F.A.; Khalid, A.; Ahmad, S.A. Phenol and Its Toxicity. J. Environ. Microbiol. Toxicol. 2014, 2, 11–23. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T.; Abbas, G. Photocatalytic Degradation of Ibuprofen in Water Using TiO2 and ZnO under Artificial UV and Solar Irradiation. Water Environ. Res. 2019, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and Solar Photodegradation of Ibuprofen and Carbamazepine Catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of Ibuprofen, Diclofenac, and Naproxen from Water Using Chitosan-Modified Waste Tire Crumb Rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Aboualigaledari, N.; Rahmani, M. A Review on the Synthesis of the TiO2-Based Photocatalyst for the Environmental Purification. J. Compos. Compd. 2021, 2, 25–42. [Google Scholar] [CrossRef]
- Eddy, D.R.; Rahmawati, D.; Permana, M.D.; Takei, T.; Solihudin; Suryana; Noviyanti, A.R.; Rahayu, I. A Review of Recent Developments in Green Synthesis of TiO2 Nanoparticles Using Plant Extract: Synthesis, Characterization and Photocatalytic Activity. Inorg. Chem. Commun. 2024, 165, 112531. [Google Scholar] [CrossRef]
- Rajaram, P.; Jeice, A.R.; Jayakumar, K. Review of Green Synthesized TiO2 Nanoparticles for Diverse Applications. Surf. Interfaces 2023, 39, 102912. [Google Scholar] [CrossRef]
- Rathod, S.; Preetam, S.; Pandey, C.; Bera, S.P. Exploring Synthesis and Applications of Green Nanoparticles and the Role of Nanotechnology in Wastewater Treatment. Biotechnol. Rep. 2024, 41, e00830. [Google Scholar] [CrossRef]
- Taheri, B. Iron Removal from Kaolin by Oxalic Acid Using a Novel Pre-Agitating and High-Pressure Washing Technique. Clay Miner. 2023, 58, 224–233. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Liu, T.; Huang, J.; Cai, Z.; Zhang, R. Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid. Minerals 2022, 12, 748. [Google Scholar] [CrossRef]
- Santawaja, P.; Kudo, S.; Tahara, A.; Asano, S.; Hayashi, J. Dissolution of Iron Oxides Highly Loaded in Oxalic Acid Aqueous Solution for a Potential Application in Iron-Making. ISIJ Int. 2022, 62, 2466–2475. [Google Scholar] [CrossRef]
- Kim, E.J.; Baek, K. Selective Recovery of Ferrous Oxalate and Removal of Arsenic and Other Metals from Soil-Washing Wastewater Using a Reduction Reaction. J. Clean. Prod. 2019, 221, 635–643. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Irannajad, M.; Mehdilo, A. Effect of Surface Dissolution by Oxalic Acid on Flotation Behavior of Minerals. J. Mater. Res. Technol. 2019, 8, 2336–2349. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of Metal-Doped Titanium Dioxide Photocatalysts. Appl. Catal. B 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Song, K.; Han, X.; Shao, G. Electronic Properties of Rutile TiO2 Doped with 4d Transition Metals: First-Principles Study. J. Alloys Compd. 2013, 551, 118–124. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Lv, C.; Lan, X.; Wang, L.; Yu, Q.; Zhang, M.; Sun, H.; Shi, J. Alkaline-Earth-Metal-Doped TiO2 for Enhanced Photodegradation and H2 Evolution: Insights into the Mechanisms. Catal. Sci. Technol. 2019, 9, 6124–6135. [Google Scholar] [CrossRef]
- Pap, Z.; Mogyorósi, K.; Veréb, G.; Dombi, A.; Hernádi, K.; Danciu, V.; Baia, L. Commercial and Home-Made Nitrogen Modified Titanias. A Short Reflection about the Advantageous/Disadvantageous Properties of Nitrogen Doping in the Frame of Their Applicability. J. Mol. Struct. 2014, 1073, 157–163. [Google Scholar] [CrossRef]
- Wu, M.-C.; Lin, T.-H.; Chih, J.-S.; Hsiao, K.-C.; Wu, P.-Y. Niobium Doping Induced Morphological Changes and Enhanced Photocatalytic Performance of Anatase TiO2. Jpn. J. Appl. Phys. 2017, 56, 04CP07. [Google Scholar] [CrossRef]
- Kou, Y.; Yang, J.; Li, B.; Fu, S. Solar Photocatalytic Activities of Porous Nb-Doped TiO2 Microspheres by Coupling with Tungsten Oxide. Mater. Res. Bull. 2015, 63, 105–111. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; de Jesus, M.A.M.L.; Ferlauto, A.S.; Viana, M.M.; Mohallem, N.D.S. Characterization and Application of Niobium-Doped Titanium Dioxide Thin Films Prepared by Sol–Gel Process. Appl. Phys. A 2021, 127, 641. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Song, K.; Wang, X.; Yu, K.; Liang, C. Enhanced Photocatalytic Activities of Nd-Doped TiO2 Under Visible Light Using a Facile Sol-Gel Method. J. Rare Earths 2020, 38, 148–156. [Google Scholar] [CrossRef]
- Yeoh, J.Z.; Chan, P.L.; Pung, S.Y.; Ramakrishnan, S.; Joseph, C.G.; Chen, C.Y. Designing A Visible Light Driven TiO2-Based Photocatalyst by Doping and Co-Doping with Niobium (Nb) and Boron (B). Bull. Chem. React. Eng. Catal. 2024, 19, 285–299. [Google Scholar] [CrossRef]
- Garay-Rodríguez, L.F.; Alfaro Cruz, M.R.; González-Ibarra, J.; Torres-Martínez, L.M.; Kim, J.H. Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films. Surfaces 2024, 7, 560–570. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Sushnikova, A.A.; Rempel, A.A. Phase Composition Tuning by High-Energy Ball Milling of Titanium Dioxide Powders. Inorg. Chem. Commun. 2024, 159, 111727. [Google Scholar] [CrossRef]
- Ullah, I.; Haider, A.; Khalid, N.; Ali, S.; Ahmed, S.; Khan, Y.; Ahmed, N.; Zubair, M. Tuning the Band Gap of TiO2 by Tungsten Doping for Efficient UV and Visible Photodegradation of Congo Red Dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 150–157. [Google Scholar] [CrossRef]
- Manikandan, K.; Kesavan, M.P.; Thirugnanasundar, A.; Abdul Khader Jailani, N.M.; Jafar Ahamed, A. Facile Synthesis and Characterization of W-Doped TiO2 Nanoparticles: Promising Ancticancer Activity with High Selectivity. Inorg. Chem. Commun. 2021, 132, 108855. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mozdianfard, M.; Jouypazadeh, H.; Es’hagh-Davatgar, M. Improving Photocatalytic Activity of (100) and (111) TiO2 Nanosheets by Coupling with ZrO2 and HfO2 Nanosheets; A DFT-U Study. J. Phys. Chem. Solids 2024, 189, 111952. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.; Ding, Y.; Zhu, X. First Principle Study of the Electronic Structure of Hafnium-Doped Anatase TiO2. J. Semicond. 2012, 33, 012002. [Google Scholar] [CrossRef]
- Kuruoğlu, N.A.; Choi, F.P.G.; Özdemir, O. Indium and Hafnium Chloride Modified Titanium Oxide Thin Films. Optik 2023, 283, 170921. [Google Scholar] [CrossRef]
- Eglītis, R.; Kraukle, A.; Käämbre, T.; Šmits, K.; Ignatāns, R.; Rubenis, K.; Šutka, A. Nb, Ta and Hf—The Tri-Dopant Tournament for the Enhancement of TiO2 Photochromism. J. Photochem. Photobiol. A Chem. 2023, 439, 114620. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.-H. Size Dependency of Nanocrystalline TiO2 on Its Optical Property and Photocatalytic Reactivity Exemplified by 2-Chlorophenol. Appl. Catal. B 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Karkare, M.M. Estimation of Band Gap and Particle Size of TiO2 Nanoparticle Synthesized Using Sol Gel Technique. In Proceedings of the 2014 International Conference on Advances in Communication and Computing Technologies (ICACACT 2014), Mumbai, India, 10-11 August 2014; pp. 1–5. [Google Scholar]
- Ugur, E.; Ledinský, M.; Allen, T.G.; Holovský, J.; Vlk, A.; De Wolf, S. Life on the Urbach Edge. J. Phys. Chem. Lett. 2022, 13, 7702–7711. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Mabhouti, K.; Golzan, M.M.; Naderali, R. Investigation of Structural, Morphological and Optical Characteristics of Mn Substituted Al-Doped ZnO NPs: A Urbach Energy and Kramers-Kronig Study. Optik 2020, 204, 164227. [Google Scholar] [CrossRef]
- Nassar, Z.M.; Yükselici, M.H. The Effect of Strain and Grain Size on Phonon and Electron Confinements in TiO2 Thin Films. Phys. Status Solidi B 2018, 255, 1700636. [Google Scholar] [CrossRef]
- Brumberg, A.; Kuklinski, O.; Kent, G.T.; Morgan, E.E.; Mikhailovsky, A.A.; Strom, T.A.; Chabinyc, M.L.; Seshadri, R. Tuning the Optical Absorption Edge of Vacancy-Ordered Double Perovskites through Metal Precursor and Solvent Selection. Chem. Mater. 2024, 36, 9625–9635. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Sharma, S.; Singh, R.C. Effect of Tungsten Doping on Structural and Optical Properties of Rutile TiO2 and Band Gap Narrowing. Optik 2019, 182, 538–547. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect Generation, d-d Transition, and Band Gap Reduction in Cu-Doped TiO2 Nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Vasundhara, M. Visible Range Optical Absorption, Urbach Energy Estimation and Paramagnetic Response in Cr-Doped TiO2 Nanocrystals Derived by a Sol–Gel Method. Phys. Chem. Chem. Phys. 2019, 21, 12991–13004. [Google Scholar] [CrossRef]
- Pap, Z.; Karácsonyi, É.; Cegléd, Z.; Dombi, A.; Danciu, V.; Popescu, I.; Baia, L.; Oszkó, A.; Mogyorósi, K. Dynamic Changes on the Surface during the Calcination of Rapid Heat Treated TiO2 Photocatalysts. Appl. Catal. B Environ. 2012, 111–112, 595–604. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic Effect of Ti3+ and Oxygen Doping on Enhancing Photoelectrochemical and Photocatalytic Properties of TiO2/g-C3N Heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef]
- Vajda, K.; Kása, Z.; Dombi, A.; Németh, Z.; Kovács, G.; Danciu, V.; Radu, T.; Ghica, C.; Baia, L.; Hernádi, K.; et al. “Crystallographic” Holes: New Insights for a Beneficial Structural Feature for Photocatalytic Applications. Nanoscale 2015, 7, 5776–5786. [Google Scholar] [CrossRef]
- Székely, I.; Baia, M.; Magyari, K.; Boga, B.; Pap, Z. The Effect of the pH Adjustment upon the WO3-WO3·0.33H2O-TiO2 Ternary Composite Systems’ Photocatalytic Activity. Appl. Surf. Sci. 2019, 490, 469–480. [Google Scholar] [CrossRef]
- Székely, I.; Kovács, Z.; Rusu, M.; Gyulavári, T.; Todea, M.; Focșan, M.; Baia, M.; Pap, Z. Tungsten Oxide Morphology-Dependent Au/TiO2/WO3 Heterostructures with Applications in Heterogenous Photocatalysis and Surface-Enhanced Raman Spectroscopy. Catalysts 2023, 13, 1015. [Google Scholar] [CrossRef]
- Ma, F.; Yang, Q.; Wang, Z.; Liu, Y.; Xin, J.; Zhang, J.; Hao, Y.; Li, L. Enhanced Visible-Light Photocatalytic Activity and Photostability of Ag3PO4/Bi2WO6 Heterostructures Toward Organic Pollutant Degradation and Plasmonic Z-Scheme Mechanism. RSC Adv. 2018, 8, 15853–15862. [Google Scholar] [CrossRef]
- Murakami, N.; Kawakami, S.; Tsubota, T.; Ohno, T. Dependence of Photocatalytic Activity on Particle Size of a Shape-Controlled Anatase Titanium(IV) Oxide Nanocrystal. J. Mol. Catal. A Chem. 2012, 358, 106–111. [Google Scholar] [CrossRef]
- Du, L.; Liu, X.; Huang, W.; Wang, E. A Study on the Interaction between Ibuprofen and Bilayer Lipid Membrane. Electrochim. Acta 2006, 51, 5754–5760. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Parian, M.; Parapari, P.S.; Ghorbani, Y.; Rosenkranz, J. A Comparative Study on the Effects of Dry and Wet Grinding on Mineral Flotation Separation—A Review. J. Mater. Res. Technol. 2019, 8, 5004–5011. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and Rutile in Evonik Aeroxide P25: Heterojunctioned or Individual Nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Balázs, N.; Srankó, D.F.; Dombi, A.; Sipos, P.; Mogyorósi, K. The Effect of Particle Shape on the Activity of Nanocrystalline TiO2 Photocatalysts in Phenol Decomposition. Part 2: The Key Synthesis Parameters Influencing the Particle Shape and Activity. Appl. Catal. B 2010, 96, 569–576. [Google Scholar] [CrossRef]
- Sampath, A.H.J.; Wickramasinghe, N.D.; de Silva, K.M.N.; de Silva, R.M. Methods of Extracting TiO2 and Other Related Compounds from Ilmenite. Minerals 2023, 13, 662. [Google Scholar] [CrossRef]
- Nanografi. Nano Technology. Available online: https://Nanografi.Com/Nanoparticles/P25-Titanium-Dioxide-Nanopowder-TiO2-Purity-99-5-Size-20-Nm (accessed on 1 February 2025).
- 117. Loman Chem Co., Ltd. Available online: http://lomanchem.Com (accessed on 1 February 2025).
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the Debye-Scherrer Equation. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Statista. Available online: https://www.statista.com/statistics/1394503/Global-Price-of-Titanium-Minerals-by-Type (accessed on 1 February 2025).
- Equip X-Netzsch Minicer Technical Data. Available online: https://www.equipx.net/uploads/Netsch/NetzschMinicerTechnicalData.pdf (accessed on 1 February 2025).
- EU Energy. Available online: https://euenergy.live (accessed on 1 February 2025).
- Somes Water Company S.A. Available online: https://casomes.ro (accessed on 1 February 2025).
- Labstac Analytical & Lab Equipment, Drying Oven Data Sheet. Available online: https://labstac.com/Catalog/Cp/OVE074-150/Drying-Oven-OVE074-150-Catalog-Labstac.pdf (accessed on 1 February 2025).

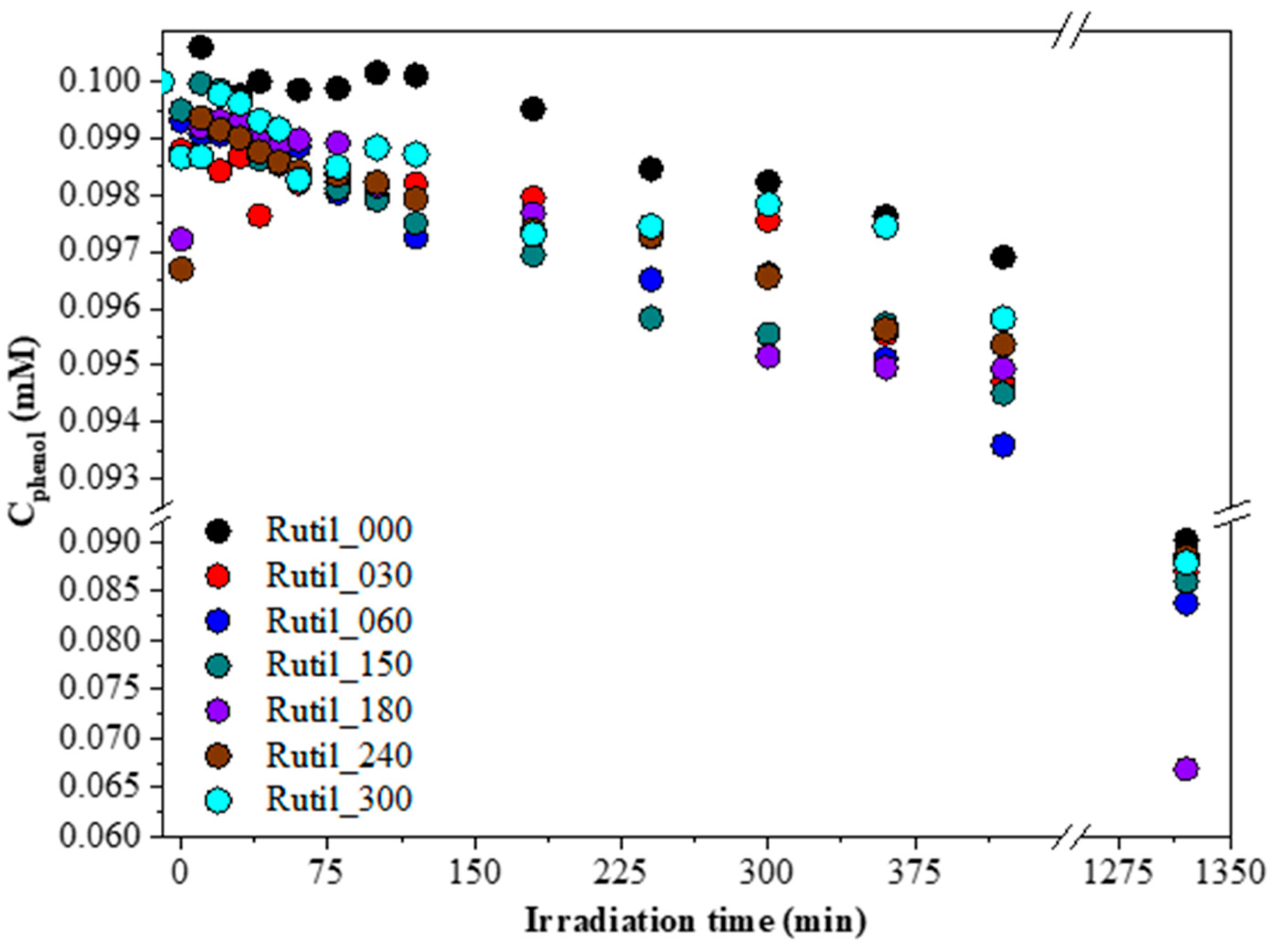
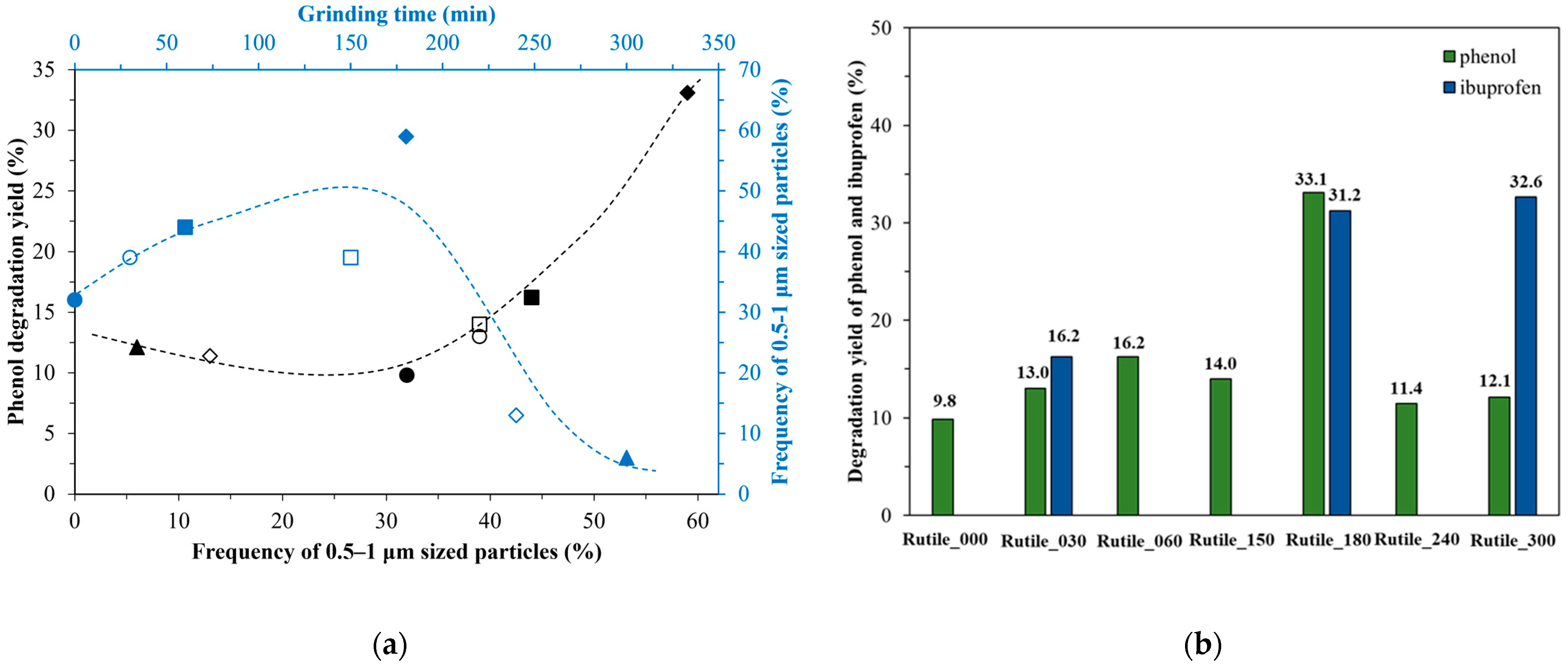
| Sample | Grinding Time (min) | Median Crystal Size (µm) from SEM Micrograph | Grinding Energy (kJ/kW) | Bandgap Energy (eV) | Urbach Energy (meV) | Primary Crystallite Size (nm) | Specific Surface Area (m2∙g−1) | Phenol Degradation (%) | Surface Normalized Transformed Phenol Concentration (mM/m2)∙10−5 | Ibuprofen Degradation (%) | Surface Normalized Ibuprofen Degradation (mM/m2) 10−5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rutile_000 | 0 | 1.9 | 0.00 | - | - | 132.0 | 1 | 9.8 | 983.6 | - | - |
| Rutile_030 | 30 | 0.9 | 3782.7 | 2.42 | 386 | 53.8 | 11 | 13.0 | 118.3 | 16.2 | 7.61 |
| Rutile_060 | 60 | 0.8 | 7565.3 | 2.47 | 369 | 44.1 | 20 | 16.2 | 81.2 | - | - |
| Rutile_150 | 150 | 0.7 | 18,913.3 | 2.62 | 333 | 31.3 | 41 | 14.0 | 34.1 | - | - |
| Rutile_180 | 180 | 0.7 | 22,696 | 2.68 | 316 | 31.4 | 49 | 33.1 | 67.6 | 31.2 | 1.40 |
| Rutile_240 | 240 | 0.6 | 29,854.3 | 2.71 | 313 | 25.3 | 59 | 11.4 | 19.4 | - | - |
| Rutile_300 | 300 | 0.5 | 37,012.6 | 2.78 | 285 | 20.1 | 80 | 12.1 | 15.1 | 32.6 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saszet, K.; Almási, E.E.; Rácz, Á.; Bohács, K.; Todea, M.; Hernádi, K.; Pap, Z.; Baia, L. Visible Light Active Natural Rutile Photocatalyst Obtained via Nano Milling. Molecules 2025, 30, 1600. https://doi.org/10.3390/molecules30071600
Saszet K, Almási EE, Rácz Á, Bohács K, Todea M, Hernádi K, Pap Z, Baia L. Visible Light Active Natural Rutile Photocatalyst Obtained via Nano Milling. Molecules. 2025; 30(7):1600. https://doi.org/10.3390/molecules30071600
Chicago/Turabian StyleSaszet, Kata, Enikő Eszter Almási, Ádám Rácz, Katalin Bohács, Milica Todea, Klára Hernádi, Zsolt Pap, and Lucian Baia. 2025. "Visible Light Active Natural Rutile Photocatalyst Obtained via Nano Milling" Molecules 30, no. 7: 1600. https://doi.org/10.3390/molecules30071600
APA StyleSaszet, K., Almási, E. E., Rácz, Á., Bohács, K., Todea, M., Hernádi, K., Pap, Z., & Baia, L. (2025). Visible Light Active Natural Rutile Photocatalyst Obtained via Nano Milling. Molecules, 30(7), 1600. https://doi.org/10.3390/molecules30071600












