Iridium-Catalyzed Hydrocarboxylation of Olefins with CO2 and H2
Abstract
1. Introduction
2. Results and Discussion
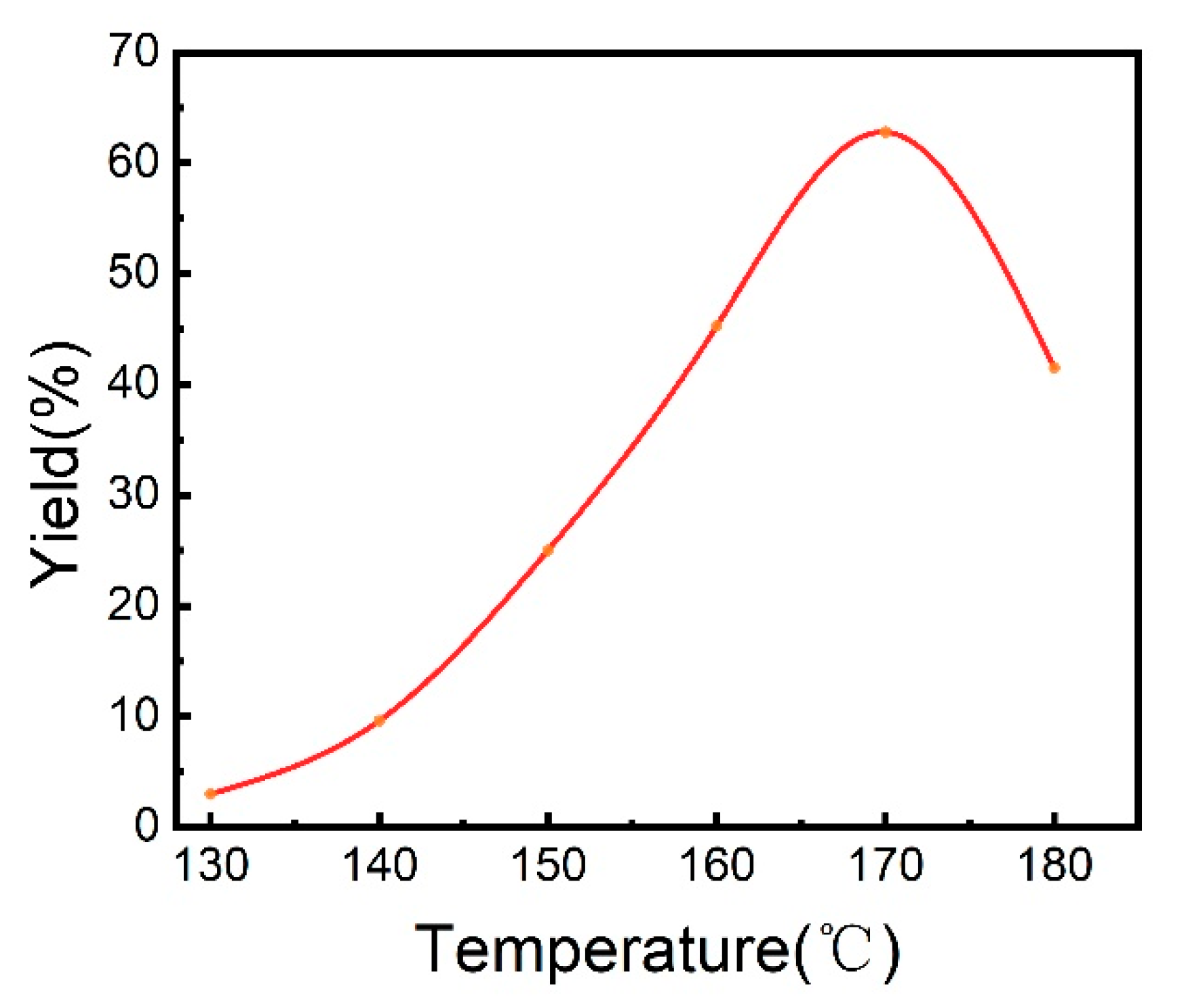
2.1. The Catalytic System
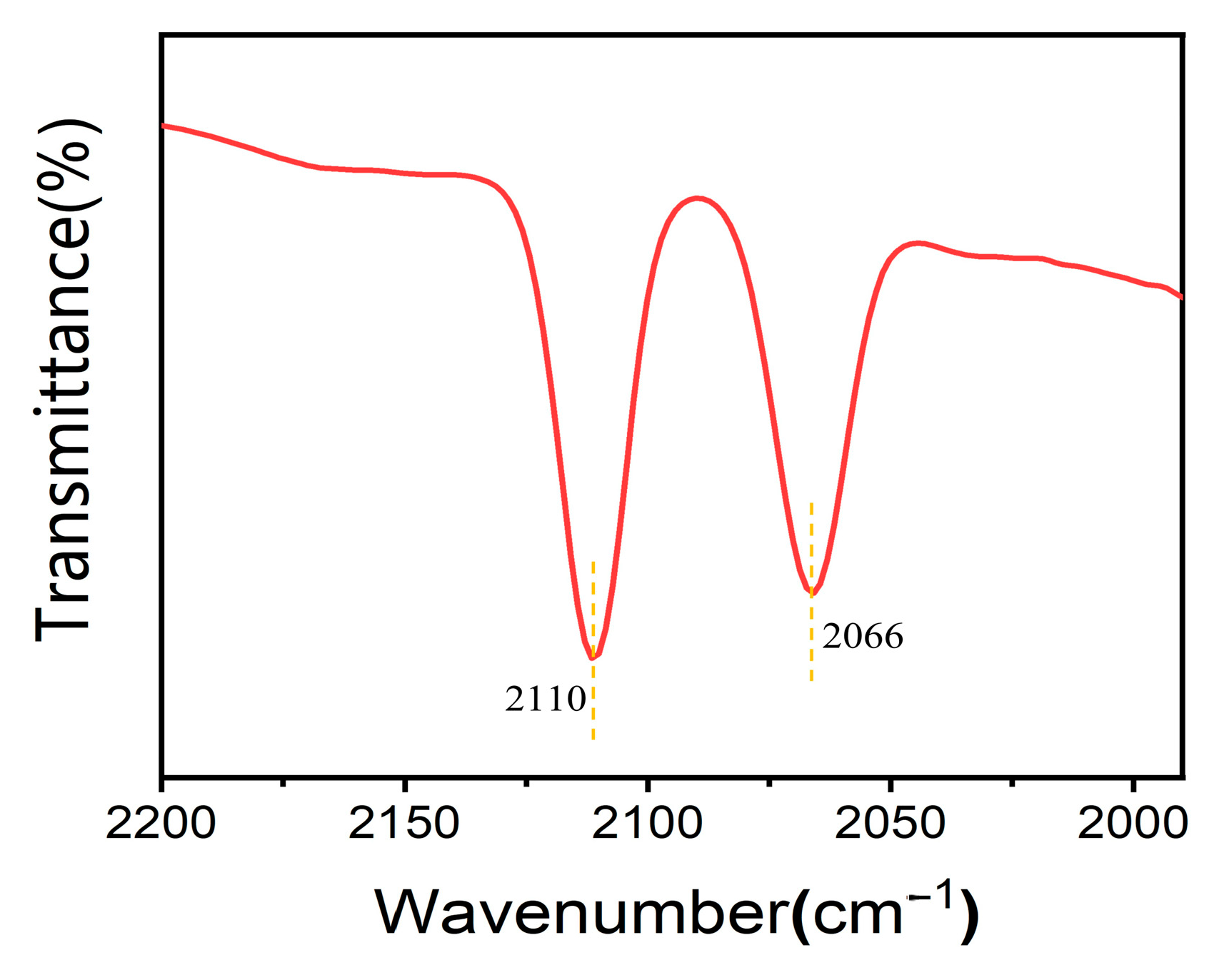
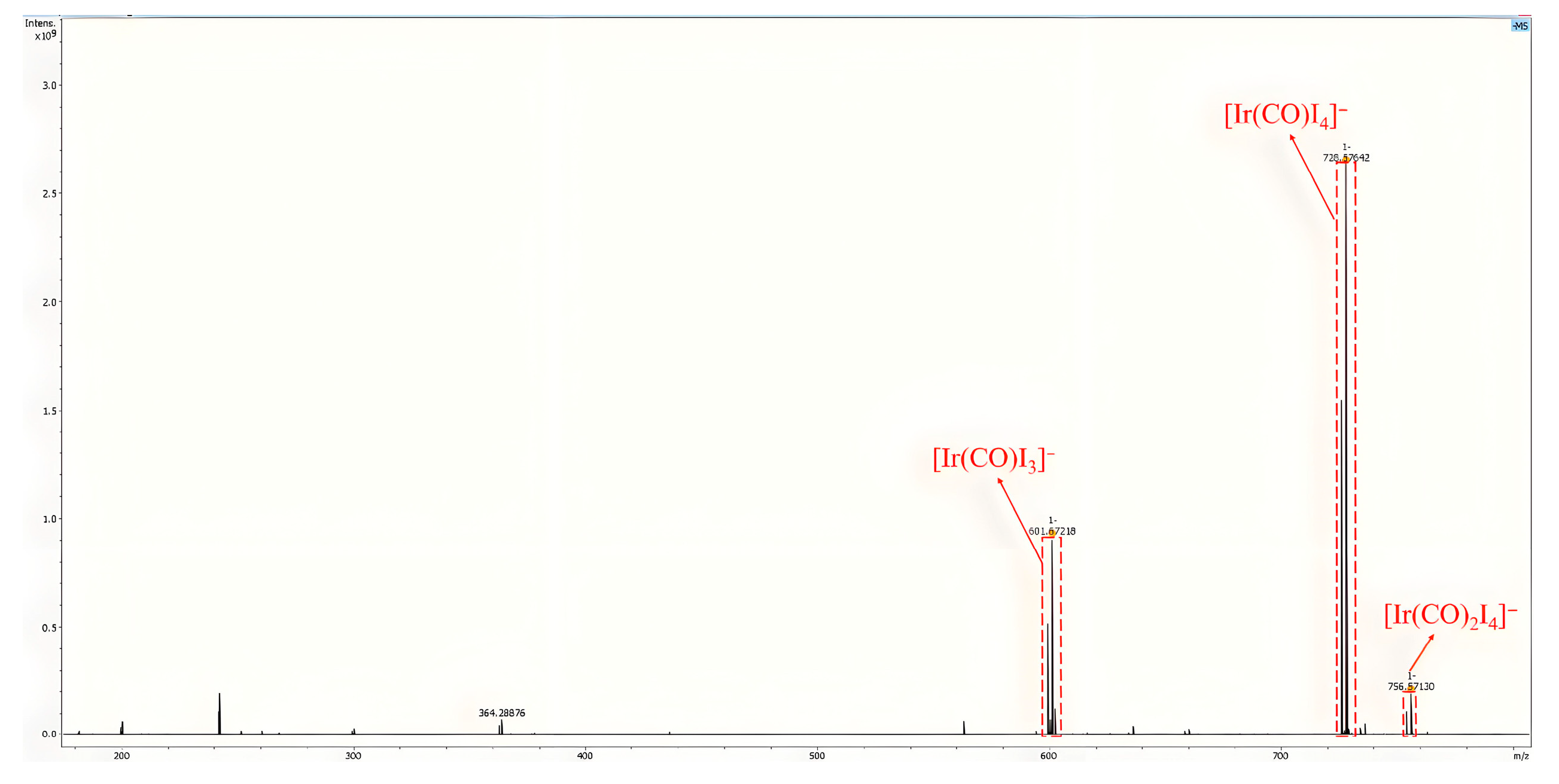
2.2. The Mechanistic Study
2.3. The Extension of the Olefin Feedstocks
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. The Catalytic Reaction
3.3. Analysis Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leclerc, H.O.; Erythropel, H.C.; Backhaus, A.; Lee, D.S.; Judd, D.R.; Paulsen, M.M.; Ishii, M.; Long, A.; Ratjen, L.; Bertho, G.G.; et al. The CO2 Tree: The Potential for Carbon Dioxide Utilization Pathways. ACS Sustain. Chem. Eng. 2025, 13, 5–29. [Google Scholar]
- Gulati, S.; Vijayan, S.; Mansi; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent advances in the application of metal-organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar]
- Qian, Q.; Han, B. Transformation of CO2 and H2 to C2+ chemicals and fuels. Natl. Sci. Rev. 2023, 10, nwad160. [Google Scholar]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar]
- He, Z.; Cui, M.; Qian, Q.; Zhang, J.; Liu, H.; Han, B. Synthesis of liquid fuel via direct hydrogenation of CO2. Proc. Natl. Acad. Sci. USA 2019, 116, 12654–12659. [Google Scholar]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar]
- Kim, C.; Yoo, C.; Oh, H.; Min, B.K.; Lee, U. Review of carbon dioxide utilization technologies and their potential for industrial application. J. CO2 Util. 2022, 65, 102239. [Google Scholar]
- Wang, P.; Wang, R. Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals. Molecules 2024, 29, 3805. [Google Scholar] [CrossRef]
- Ochonma, P.; Gao, X.; Gadikota, G. Tuning Reactive Crystallization Pathways for Integrated CO2 Capture, Conversion, and Storage via Mineralization. Acc. Chem. Res. 2024, 57, 267–274. [Google Scholar] [PubMed]
- Ma, H.; Liu, S.; Wang, H.; Li, G.; Zhao, K.; Cui, X.; Shi, F. In situ CO2 capture and transformation into cyclic carbonates using flue gas. Green Chem. 2023, 25, 2293–2298. [Google Scholar]
- Wu, Q.-J.; Liang, J.; Huang, Y.-B.; Cao, R. Thermo-, Electro-, and Photocatalytic CO2 Conversion to Value-Added Products over Porous Metal/Covalent Organic Frameworks. Acc. Chem. Res. 2022, 55, 2978–2997. [Google Scholar] [PubMed]
- Park, K.; Park, H.; Yoon, H.; Lee, K.R.; Ahn, S.; Kim, C.; Lee, U.; Jung, K.; Yoon, S. Recent progress in heterogeneous CO2 hydrogenation to formic acid towards practical application. Catal. Sci. Technol. 2024, 14, 5811–5832. [Google Scholar]
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Selective catalytic synthesis using the combination of carbon dioxide and hydrogen: Catalytic chess at the interface of energy and chemistry. Angew. Chem. Int. Ed. 2016, 55, 7296–7343. [Google Scholar]
- Rohmann, K.; Kothe, J.; Haenel, M.W.; Englert, U.; Hölscher, M.; Leitner, W. Hydrogenation of CO2 to formic acid with a highly active ruthenium acriphos complex in DMSO and DMSO/water. Angew. Chem. Int. Ed. 2016, 55, 8966–8969. [Google Scholar]
- Jessop, P.G.; Joó, F.; Tai, C.C. Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord. Chem. Rev. 2004, 248, 2425–2442. [Google Scholar]
- Jessop, P.G.; Ikariya, T.; Noyori, R. Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 1994, 368, 231–233. [Google Scholar]
- Tortajada, A.; Juliá-Hernández, F.; Börjesson, M.; Moragas, T.; Martin, R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed. 2018, 57, 15948–15982. [Google Scholar]
- Wu, X.-F.; Zheng, F. Synthesis of Carboxylic Acids and Esters from CO2. Top. Curr. Chem. 2016, 375, 4. [Google Scholar]
- Yu, D.; Teong, S.P.; Zhang, Y. Transition metal complex catalyzed carboxylation reactions with CO2. Coord. Chem. Rev. 2015, 293–294, 279–291. [Google Scholar]
- Qiao, C.; Cao, Y.; He, L.N. Transition Metal-Catalyzed Carboxylation of Terminal Alkynes with CO2. Mini-Rev. Org. Chem. 2018, 15, 283–290. [Google Scholar] [CrossRef]
- Gui, Y.-Y.; Chen, X.-W.; Mo, X.-Y.; Yue, J.-P.; Yuan, R.; Liu, Y.; Liao, L.-L.; Ye, J.-H.; Yu, D.-G. Cu-Catalyzed Asymmetric Dicarboxylation of 1,3-Dienes with CO2. J. Am. Chem. Soc. 2024, 146, 2919–2927. [Google Scholar] [PubMed]
- Zhou, L.; Li, L.; Zhang, S.; Kuang, X.-k.; Zhou, Y.-Y.; Tang, Y. Catalytic Regio- and Enantioselective Remote Hydrocarboxylation of Unactivated Alkenes with CO2. J. Am. Chem. Soc. 2024, 146, 18823–18830. [Google Scholar]
- Xiong, W.; Shi, F.; Cheng, R.; Zhu, B.; Wang, L.; Chen, P.; Lou, H.; Wu, W.; Qi, C.; Lei, M.; et al. Palladium-Catalyzed Highly Regioselective Hydrocarboxylation of Alkynes with Carbon Dioxide. ACS Catal. 2020, 10, 7968–7978. [Google Scholar]
- Tani, Y.; Kuga, K.; Fujihara, T.; Terao, J.; Tsuji, Y. Copper-catalyzed C-C bond-forming transformation of CO2 to alcohol oxidation level: Selective synthesis of homoallylic alcohols from allenes, CO2, and hydrosilanes. Chem. Commun. 2015, 51, 13020–13023. [Google Scholar]
- Wang, X.; Nakajima, M.; Martin, R. Ni-Catalyzed Regioselective Hydrocarboxylation of Alkynes with CO2 by Using Simple Alcohols as Proton Sources. J. Am. Chem. Soc. 2015, 137, 8924–8927. [Google Scholar]
- Greenhalgh, M.D.; Thomas, S.P. Iron-Catalyzed, Highly Regioselective Synthesis of α-Aryl Carboxylic Acids from Styrene Derivatives and CO2. J. Am. Chem. Soc. 2012, 134, 11900–11903. [Google Scholar] [CrossRef]
- Correa, A.; Martin, R. Palladium-Catalyzed Direct Carboxylation of Aryl Bromides with Carbon Dioxide. J. Am. Chem. Soc. 2009, 131, 15974–15975. [Google Scholar]
- Asare Bediako, B.B.; Qian, Q.; Han, B. Synthesis of C2+ Chemicals from CO2 and H2 via C–C Bond Formation. Acc. Chem. Res. 2021, 54, 2467–2476. [Google Scholar]
- Ostapowicz, T.G.; Schmitz, M.; Krystof, M.; Klankermayer, J.; Leitner, W. Carbon Dioxide as a C1 Building Block for the Formation of Carboxylic Acids by Formal Catalytic Hydrocarboxylation. Angew. Chem. Int. Ed. 2013, 52, 12119–12123. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhang, J.; Cui, M.; Han, B. Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 2016, 7, 11481. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Qian, Q.; Zhang, J.; Chen, C.; Han, B. Efficient synthesis of acetic acid via Rh catalyzed methanol hydrocarboxylation with CO2 and H2 under milder conditions. Green Chem. 2017, 19, 3558–3565. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, Q.; Zhang, J.; Bediako, B.B.A.; Wang, Z.; Liu, H.; Han, B. Synthesis of higher carboxylic acids from ethers, CO2 and H2. Nat. Commun. 2019, 10, 5395. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qian, Q.; Li, Y.; Asare Bediako, B.B.; Zhang, J.; Yang, J.; Li, Z.; Han, B. Synthesis of carboxylic acids via the hydrocarboxylation of alcohols with CO2 and H2. Green Chem. 2022, 24, 1973–1977. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, Y.; Li, Y.; He, J.; Wang, Y.; Zhang, L.; Wang, Z.; Qian, Q.; Han, B. Synthesis of Carboxylic Acids via Reaction of Ketones/Aldehydes with CO2 and H2. Organometallics 2023, 42, 2312–2318. [Google Scholar] [CrossRef]
- Asare Bediako, B.B.; Qian, Q.; Wang, Y.; Zhang, J.; Wang, Z.; Li, S.; Liu, H.; Wu, T.; Ge, M.; Han, B. Synthesis of higher carboxylic acids via reaction of polyols with CO2 and H2. Chem. Catal. 2022, 2, 114–124. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Y.; Wang, Z.; Xiang, J.; Han, J.; He, J.; Zhang, L.; Wang, Y.; Meng, Q.; et al. Synthesis of Carboxylic Acids from Saccharides, CO2, and H2. ACS Catal. 2023, 13, 8025–8030. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Y.; Zhang, L.; He, J.; Yu, C.; Li, Y.; Zhang, P.; Ma, J.; Sun, X.; et al. Catalyst-Green Solvent System for Highly Efficient Production of Carboxylic Acids from Light Oxygenates, CO2 and H2. ChemCatChem 2024, 16, e202400077. [Google Scholar] [CrossRef]
- Haynes, A.; Maitlis, P.M.; Morris, G.E. Promotion of iridium-catalyzed methanol carbonylation: Mechanistic studies of the cativa process. J. Am. Chem. Soc. 2004, 126, 2847–2861. [Google Scholar] [CrossRef]
- Maitlis, P.M.; Haynes, A.; James, B.R.; Catellani, M.; Chiusoli, G.P. Iodide effects in transition metal catalyzed reactions. Dalton. Trans. 2004, 3409–3419. [Google Scholar] [CrossRef]


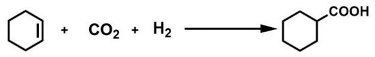 | ||||
| Entry | Catalyst | Promoter | Solvent | Yield (%) a |
| 1 | Ir(acac)(CO)2 | LiI | AcOH | 62.8 |
| 2 | Ir(OAc)3 | LiI | AcOH | 42.3 |
| 3 | IrI4 | LiI | AcOH | 32.7 |
| 4 | Ir4(CO)12 | LiI | AcOH | 43.0 |
| 5 | Ir(acac)3 | LiI | AcOH | 44.5 |
| 6 | IrCl3 | LiI | AcOH | 37.6 |
| 7 | IrO2·2H2O | LiI | AcOH | 0 |
| 8 | IrCl(CO)(PPh3)2 | LiI | AcOH | 0 |
| 9 | Ir(acac)(CO)2 | I2 | AcOH | 0 |
| 10 | Ir(acac)(CO)2 | LiCl | AcOH | 0 |
| 11 | Ir(acac)(CO)2 | LiBr | AcOH | 0 |
| 12 | Ir(acac)(CO)2 | NaI | AcOH | 10.3 |
| 13 | Ir(acac)(CO)2 | KI | AcOH | 0 |
| 14 | Ir(acac)(CO)2 | CH3I | AcOH | 7.2 |
| 15 | Ir(acac)(CO)2 | LiI | CH3CH2COOH | 45.3 |
| Entry | CO [MPa] | H2 [MPa] | Yield (%) |
|---|---|---|---|
| 1 | 0.5 | 0 | 72.3 |
| 2 | 1 | 0 | 59.4 |
| 3 | 2 | 0 | 36.5 |
| Entry | Intermediate | Product | Yield (%) |
|---|---|---|---|
| 1 |  | 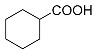 | 36.6 a |
| 34.5 b | |||
| 2 | 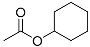 | 45.0 a | |
| 42.7 b |
| Entry | Substrate | Product | Yield (%) [b] |
|---|---|---|---|
| 1 | 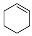 | 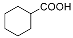 | 62.8 |
| 2 | 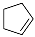 | 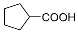 | 32.0 |
| 3 |  | 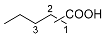 | 49.8 [c] |
| 4 |  | 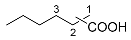 | 45.1 [d] |
| 5 |  |  | 40.7 [e] |
| 6 | 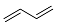 | 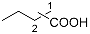  | 36.7 [f] |
| 7 | 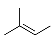 | 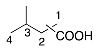 | 17 [g] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Zhang, L.; Zhang, Y.; Guo, J.; Wang, Y.; Yu, C.; He, J.; Wang, Z.; Han, J.; et al. Iridium-Catalyzed Hydrocarboxylation of Olefins with CO2 and H2. Molecules 2025, 30, 1599. https://doi.org/10.3390/molecules30071599
Li Y, Wang Y, Zhang L, Zhang Y, Guo J, Wang Y, Yu C, He J, Wang Z, Han J, et al. Iridium-Catalyzed Hydrocarboxylation of Olefins with CO2 and H2. Molecules. 2025; 30(7):1599. https://doi.org/10.3390/molecules30071599
Chicago/Turabian StyleLi, Yang, Ying Wang, Longbo Zhang, Yanru Zhang, Jia Guo, Yanyan Wang, Chenglong Yu, Jun He, Zhenpeng Wang, Juanjuan Han, and et al. 2025. "Iridium-Catalyzed Hydrocarboxylation of Olefins with CO2 and H2" Molecules 30, no. 7: 1599. https://doi.org/10.3390/molecules30071599
APA StyleLi, Y., Wang, Y., Zhang, L., Zhang, Y., Guo, J., Wang, Y., Yu, C., He, J., Wang, Z., Han, J., Li, Q., Wu, T., Qian, Q., & Han, B. (2025). Iridium-Catalyzed Hydrocarboxylation of Olefins with CO2 and H2. Molecules, 30(7), 1599. https://doi.org/10.3390/molecules30071599








