Ultrasound-Assisted Synthesis of Glycerol Carbonate Using Potassium-Modified Silicalite-1 as a Catalyst
Abstract
1. Introduction
2. Results and Discussion
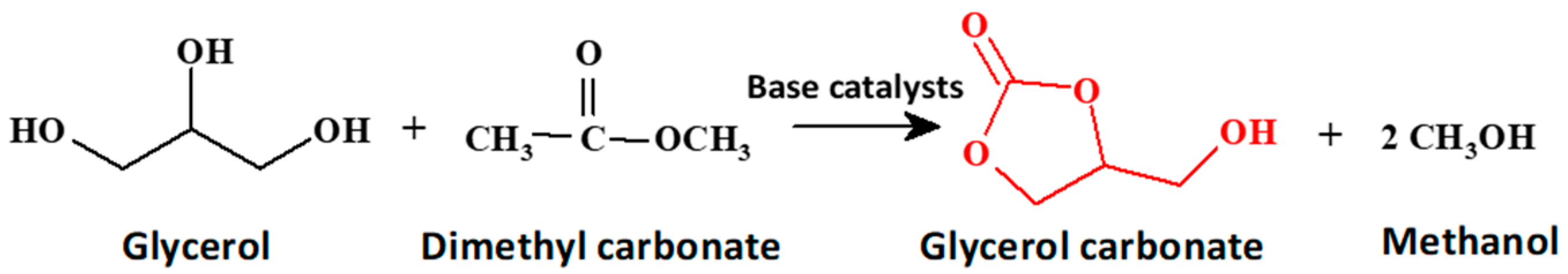
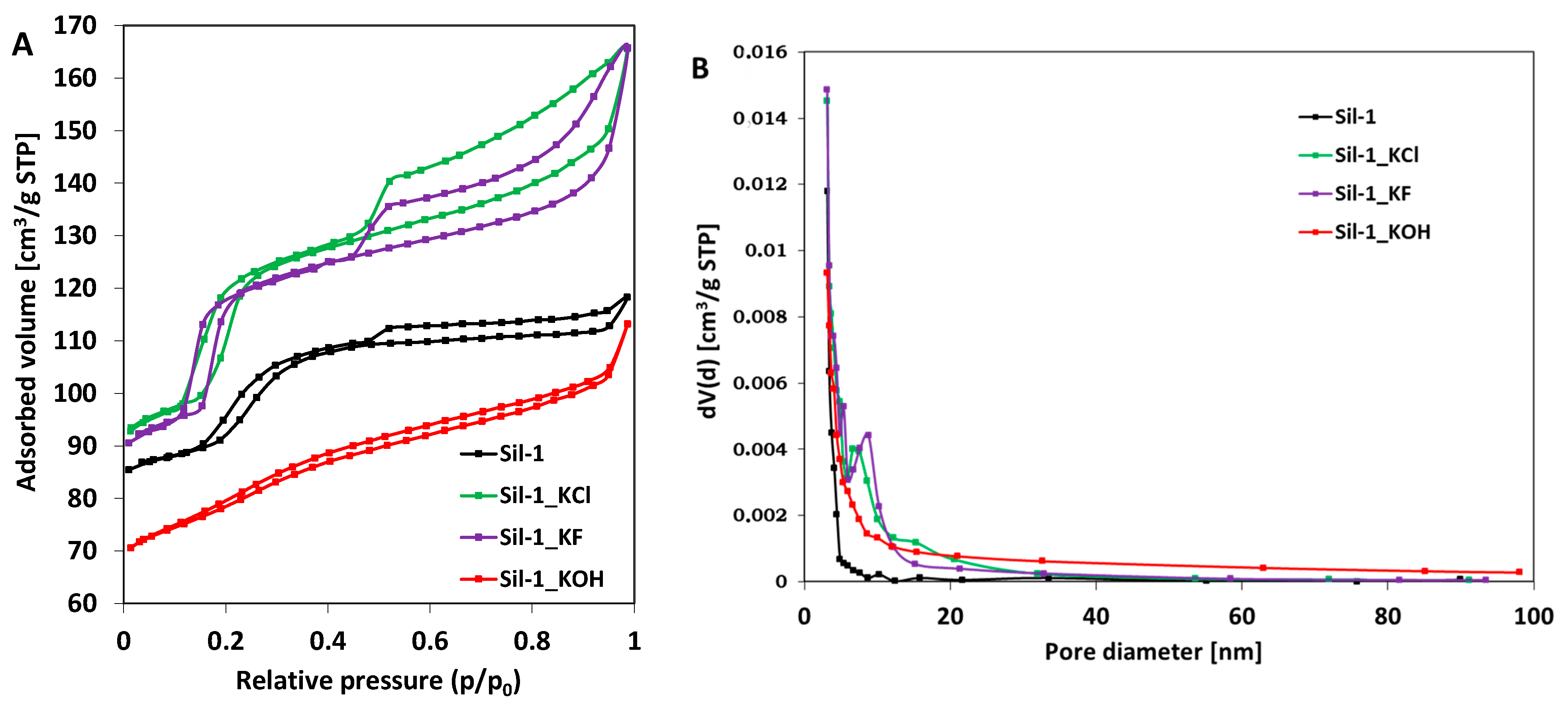
2.1. Characterization of Catalysts
2.2. Catalytic Activity in Batch Reactor
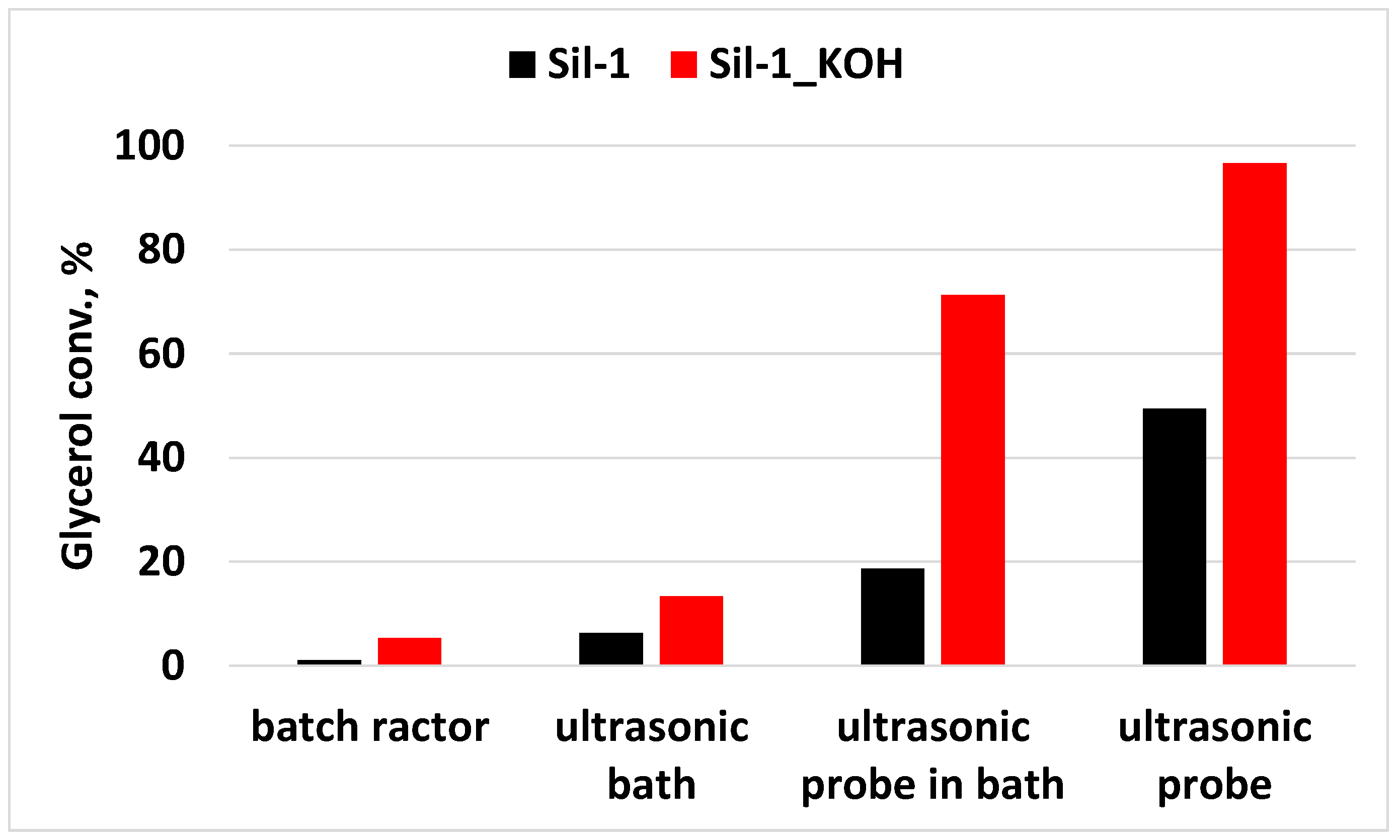
2.3. Comparison Between Ultrasound-Assisted Transesterification of Glycerol and Conventional Heating
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Silicalite-1
4.2. Modification of Silicalite-1 with Potassium Compounds Solutions
4.3. Characterization
4.4. Catalytic Activity in Transesterification Reaction of Glycerol with Dimethyl Carbonate
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as Feasible Petrol Fuel Replacement: A Multidisciplinary Overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of Biodiesel on Engine Performances and Emissions. Renew. Sustain. Energy Rev. 2010, 15, 1098–1116. [Google Scholar] [CrossRef]
- Hazimah, A.H.; Ooi, T.L.; Salmiah, A. Recovery of Glycerol and Diglycerol from Glycerol Pitch. J. Oil Palm Res. 2003, 15, 1–5. [Google Scholar]
- Kolesárová, N.; Hutňan, M.; Bodík, I.; Spalková, V. Utilization of Biodiesel By-Products for Biogas Production. J. Biomed. Biotech. 2011, 2011, 126798. [Google Scholar] [CrossRef]
- Galusnyak, S.C.; Petrescu, L.; Arpad, I.; Cormos, C. Towards Value-Added Chemicals: Technical and Environmental Life Cycle Assessment Evaluation of Different Glycerol Valorisation Pathways. Sustain. Energy Technol. Assess. 2024, 72, 104043. [Google Scholar] [CrossRef]
- Asopa, R.P.; Bhoi, R.; Saharan, V.K. Valorization of Glycerol into Value-Added Products: A Comprehensive Review on Biochemical Route. Bioresour. Technol. Rep. 2022, 20, 101290. [Google Scholar] [CrossRef]
- Ciriminna, R.; Della Pina, C.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Magniont, C.; Escadeillas, G.; Oms-Multon, C.; De Caro, P. The Benefits of Incorporating Glycerol Carbonate into an Innovative Pozzolanic Matrix. Cem. Concr. Res. 2010, 40, 1072–1080. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; Taffin de Givenchy, E.P.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol Carbonate as a Versatile Building Block for Tomorrow: Overview Synthesis, Reactivity, Properties, and Applications. Green Chem. 2013, 1, 283–306. [Google Scholar] [CrossRef]
- Salari, M.; Varela, J.C.; Zhang, H.; Grinstaff, M.W. Sustainable Glycerol Carbonate Electrolytes for Li-Ion Supercapacitors: Performance Evaluation of Butyl, Benzyl, and Ethyl Glycerol Carbonates. Adv. Mater. 2021, 2, 6049. [Google Scholar] [CrossRef]
- Gade, S.M.; Saptal, V.B.; Bhanage, B.M. Perception of Glycerol Carbonate as Green Chemical: Synthesis and Applications. Catal. Commun. 2022, 172, 106542. [Google Scholar] [CrossRef]
- Galletti, G.; Prete, P.; Vanzini, S.; Cucciniello, R.; Fasolini, A.; De Maron, J.; Cavani, F.; Tabanelli, T. Glycerol Carbonate as a Versatile Alkylating Agent for the Synthesis of β-Aryloxy Alcohols. ACS Sustain. Chem. Eng. 2022, 10, 10922–10933. [Google Scholar] [CrossRef]
- Szabó, Y.; Nagy, S.B.; Ádám, A.; Mészáros, R.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Szabados, M. Valorization of Glycerol to Glycerol Carbonate and Glycidol by Different Dialkyl Carbonates Utilizing Tricalcium Aluminate Hexahydrate as Transesterification Catalyst. ChemCatChem 2025, 17, e202401217. [Google Scholar] [CrossRef]
- Sahani, S.; Upadhyay, S.N.; Sharma, Y.C. Critical Review on Production of Glycerol Carbonate from Byproduct Glycerol through Transesterification. Ind. Eng. Chem. Res. 2021, 60, 67–88. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.A.; Nieto-Mestre, J.; Maestro-Madurga, B.; Belsué, M. Synthesis of Glycerol Carbonate from 3-Chloro-1,2-Propanediol and Carbon Dioxide Using Triethylamine as Both Solvent and CO2 Fixation–Activation Agent. Chem. Eng. J. 2011, 175, 505–511. [Google Scholar] [CrossRef]
- Hammond, C.; Lopez-Sanchez, J.A.; Ab Rahim, M.H.; Dimitratos, N.; Jenkins, R.L.; Carley, A.F.; He, Q.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Synthesis of Glycerol Carbonate from Glycerol and Urea with Gold-Based Catalysts. Dalton Trans. 2011, 40, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T. Coupling Reaction and Azeotropic Distillation for the Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate. Chem. Eng. Process. Process Intensif. 2010, 49, 530–535. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Hameed, B.H. Synthesis of Glycerol Carbonate by Transesterification of Glycerol with Dimethyl Carbonate over K-Zeolite Derived from Coal Fly Ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Alvarez, M.; Segarra, A.; Contreras, S.; Sueiras, J.; Medina, F.; Figueras, F. Enhanced Use of Renewable Resources: Transesterification of Glycerol Catalyzed by Hydrotalcite-Like Compounds. Chem. Eng. J. 2010, 161, 340–345. [Google Scholar] [CrossRef]
- Lu, P.; Wang, H.; Hu, K. Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate over the Extruded CaO-Based Catalyst. Chem. Eng. J. 2013, 228, 147–154. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Mg1+xCa1−xO2 as Reusable and Efficient Heterogeneous Catalyst for the Synthesis of Glycerol Carbonate via the Transesterification of Glycerol with Dimethyl Carbonate. Appl. Catal. A Gen. 2013, 466, 272–281. [Google Scholar] [CrossRef]
- Liu, P.; Derchi, M.; Hensen, E. Synthesis of Glycerol Carbonate by Transesterification of Glycerol with Dimethyl Carbonate over MgAl Mixed Oxide Catalysts. Appl. Catal. A Gen. 2013, 467, 124–131. [Google Scholar] [CrossRef]
- Yadav, G.D.; Chandan, P.A. A Green Process for Glycerol Valorization to Glycerol Carbonate over Heterogeneous Hydrotalcite Catalyst. Catal. Today 2014, 237, 47–53. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, D. Transition Metal-Promoted Hierarchical ETS-10 Solid Base for Glycerol Transesterification. RSC Adv. 2018, 8, 33473. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K.; Góra-Marek, K. LTA Zeolites as Catalysts for Transesterification of Glycerol with Dimethyl Carbonate. Fuel 2024, 362, 130757. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Subbaramaiah, V.; Hameed, B.H. Lithium Loaded Coal Fly Ash as Sustainable and Effective Catalyst for the Synthesis of Glycerol Carbonate from Glycerol. J. Environ. Chem. Eng. 2021, 9, 105999. [Google Scholar] [CrossRef]
- Manikandan, M.; Sangeetha, P. Optimizing the Surface Properties of MgO Nanoparticles Towards the Transesterification of Glycerol to Glycerol Carbonate. Chem. Select 2019, 4, 6672–6678. [Google Scholar] [CrossRef]
- Malyaadri, M.; Jagadeeswaraiah, K.; Sai Prasad, P.S.; Lingaiah, N. Synthesis of Glycerol Carbonate by Transesterification of Glycerol with Dimethyl Carbonate over Mg/Al/Zr Catalysts. Appl. Catal. A Gen. 2011, 401, 153–157. [Google Scholar] [CrossRef]
- Liu, P.; Derchi, M.; Hensen, E.J.M. Promotional Effect of Transition Metal Doping on the Basicity and Activity of Calcined Hydrotalcite Catalysts for Glycerol Carbonate Synthesis. Appl. Catal. B Environ. 2014, 144, 135–143. [Google Scholar] [CrossRef]
- Praikaew, W.; Kiatkittipong, W.; Aiouache, F.; Najdanovic-Visak, V.; Termtanun, M.; Lim, J.W.; Lam, S.S.; Kiatkittipong, K.; Laosiripojana, N.; Boonyasuwat, S.; et al. Mechanism of CaO Catalyst Deactivation with Unconventional Monitoring Method for Glycerol Carbonate Production via Transesterification of Glycerol with Dimethyl Carbonate. Int. J. Energy Res. 2022, 46, 1646–1658. [Google Scholar] [CrossRef]
- Chotchuang, A.; Kunsuk, P.; Phanpitakkul, A.; Chanklang, S.; Chareonpanich, M.; Seubsai, A. Production of Glycerol Carbonate from Glycerol over Modified Sodium-Aluminate-Doped Calcium Oxide Catalysts. Catal. Today 2022, 388–389, 351–359. [Google Scholar] [CrossRef]
- Kosawatthanakun, S.; Clatworthy, E.B.; Ghojavand, S.; Sosa, N.; Wittayakun, J.; Mintova, S. Application of a BPH zeolite for the transesterification of glycerol to glycerol carbonate: Effect of morphology, cation type and reaction conditions. Inorg. Chem. Front. 2023, 10, 579–590. [Google Scholar] [CrossRef]
- Delesma, C.; Okoye, P.; Castellanos-López, M.; Longoria, A.; Muñiz, J. Understanding the Heterogeneous Catalytic Mechanisms of Glycerol Carbonate Synthesis on Oil Palm Ash Surface: A Density Functional Theory Approach. Fuel 2022, 307, 121874. [Google Scholar] [CrossRef]
- Souza Júnior, R.L.; Eira, L.C.; Detoni, C.; Souza, M.M.V.M. Glycerol Carbonate Production via Transesterification: The Effect of Support Porosity and Catalyst Basicity. Processes 2024, 12, 2256. [Google Scholar] [CrossRef]
- Zapelini, I.W.; de Paula, G.M.; Cardoso, D. Crystallization Kinetics as a Tool to Fine-Tune the Catalytic Activity of Na-LTA Zeolite Precursors in the Transesterification of Glycerol to Glycerol Carbonate. Catal. Today 2025, 444, 115013. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K. Enhancement of the catalytic activity of H-ZSM-5 zeolites for glycerol acetalization by mechanical grinding. Reac. Kinet. Mech. Catal. 2016, 117, 341–352. [Google Scholar] [CrossRef]
- Lo, P.K.; Leong, S.Y.; Tan, C.Y. Investigation on the Effect of Ultrasonic-Assisted Transesterification for Green Synthesis of Glycerol Carbonate from Crude Glycerol. IOP Conf. Ser. Mater. Sci. Eng. 2020, 943, 012011. [Google Scholar] [CrossRef]
- Janiszewska, E.; Macario, A.; Wilk, J.; Aloise, A.; Kowalak, S.; Nagy, J.B.; Giordano, G. The role of the defect groups on the Silicalite-1 zeolite catalytic behavior. Micropor. Mesopor. Mat. 2013, 182, 220–228. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kowalska-Kuś, J.; Góra-Marek, K.; Szymocha, A.; Nowińska, K.; Kowalak, S. Modification of silicalite-1 with ammonium compounds aimed at preparation of acidic catalyst for acetalization of glycerol with acetone. Appl. Catal. A 2019, 581, 1–10. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Janiszewska, E.; Góra-Marek, K.; Jankowska, A.; Held, A. Enhancing the Catalytic Properties of Silicalite-1 through Ammonium Fluoride Modification for Waste Glycerol Acetalization. Dalton Trans. 2024, 53, 13537–13549. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites; Structure Commission of the IZA, Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Koekkoek, A.J.J.; Xin, H.; Yang, Q.; Li, C.; Hensen, E.J.M. Hierarchically structured Fe/ZSM-5 as catalysts for the oxidation of benzene to phenol. Micropor. Mesopor. Mat. 2011, 145, 172–181. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. Adv. Chem. 1974, 101, 201–229. [Google Scholar] [CrossRef]
- Göhlich, M.; Reschetilowski, W.; Paasch, S. Spectroscopic Study of Phosphorus-Modified H-ZSM-5. Molecules 2011, 16, 178–183. [Google Scholar] [CrossRef]
- Abelló, S.; Bonilla, A.; Pérez-Ramírez, J. Mesoporous ZSM-5 Zeolite Catalysts Prepared by Desilication with Organic Hydroxides and Comparison with NaOH Leaching. Appl. Catal. A Gen. 2009, 364, 191–198. [Google Scholar] [CrossRef]
- Thommes, M. Chapter 15-Textural Characterization of Zeolites and Ordered Mesoporous Materials by Physical Adsorption. Stud. Surf. Sci. Catal. 2007, 168, 495. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Palkovits, R.; Schmidt, W.; Spliethoff, B.; Schüth, F. Hierarchically Structured Monolithic Silicalite-1 Consisting of Crystallized Nanoparticles and Its Performance in the Beckmann Rearrangement of Cyclohexanone Oxime. J. Am. Chem. Soc. 2005, 127, 12595–12600. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Comment: Questions Concerning the Nitrogen Adsorption Data Analysis for Formation of Supermicropores in ZSM-5 Zeolites. Adv. Mater. 2005, 17, 2789–2792. [Google Scholar] [CrossRef]
- Viswanadham, N.; Kamble, R.; Singh, M.; Kumar, M.; Dhar, G.M. Catalytic properties of nano-sized ZSM-5 aggregates. Catal. Today 2009, 141, 182–186. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Panpa, W.; Jinawath, S. Synthesis of ZSM-5 Zeolite and Silicalite from Rice Husk Ash. Appl. Catal. B Environ. 2009, 90, 389–394. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A Review on the Performance of Glycerol Carbonate Production via Catalytic Transesterification: Effects of Influencing Parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Wang, S.; Hao, P.; Li, S.; Zhang, A.; Guan, Y.; Zhang, L. Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate Catalyzed by Calcined Silicates. Appl. Catal. A Gen. 2017, 542, 174–181. [Google Scholar] [CrossRef]
- Jansen, J.; van der Gaag, F.; van Bekkum, H. Identification of ZSM-type and other 5-ring containing zeolites by i.r. spectroscopy. Zeolites 1984, 4, 369–372. [Google Scholar] [CrossRef]







| Sample | CXRD 1 [%] | SBET 2 [m2/g] | Smicro 3 [m2/g] | Sext 4 [m2/g] | Vtot 5 [cm3/g] | Vmicro 6 [cm3/g] | Vmeso 7 [cm3/g] | Smicro/Sext |
|---|---|---|---|---|---|---|---|---|
| Sil-1 | 100 | 293 | 251 | 42 | 0.19 | 0.12 | 0.07 | 6.0 |
| Sil-1_KCl | 99 | 322 | 250 | 72 | 0.24 | 0.12 | 0.12 | 3.5 |
| Sil-1_KF | 93 | 344 | 248 | 96 | 0.26 | 0.12 | 0.14 | 2.6 |
| Sil-1_KOH | 55 | 231 | 160 | 70 | 0.18 | 0.09 | 0.09 | 2.3 |
| Sample | Concentration of Basic Sites, μmol/g | |||
|---|---|---|---|---|
| LT-BS 1 T ≤ 200 °C | MT-BS 2 200–350 °C | HT-BS 3 T ≥ 350 °C | Total | |
| Sil-1 | 1.2 | 0.7 | 2.1 | 4.0 |
| Sil-1_KCl | 1.6 | 3.2 | 3.8 | 8.6 |
| Sil-1_KF | 2.6 | 5.5 | 6.3 | 14.2 |
| Sil-1_KOH | 4.3 | 27.2 | 6.2 | 37.7 |
| Glycerol conv. R2 factor 4 | 0.8652 | 0.992 | 0.3414 | 0.9558 |
| Sample | Si [at. %] | O [at. %] | K [at. %] | Na [at. %] | Si/O Ratio |
|---|---|---|---|---|---|
| Sil-1 | 42.00 | 57.54 | - | 0.46 | 0.73 |
| Sil-1_KCl | 41.03 | 58.57 | 0.40 | - | 0.70 |
| Sil-1_KF | 38.40 | 60.88 | 0.72 | - | 0.63 |
| Sil-1_KOH | 35.83 | 61.10 | 3.07 | - | 0.58 |
| Sample | Method | Reaction Conditions | Glycerol Conversion [%] | TOF [s−1] |
|---|---|---|---|---|
| Sil-1 | batch reactor | 90 °C, 4 h | 2.3 | 0.06 |
| Sil-1_KCl | batch reactor | 90 °C, 4 h | 7.9 | 0.2 |
| Sil-1_KF | batch reactor | 90 °C, 4 h | 9.9 | 0.3 |
| Sil-1_KOH | batch reactor | 90 °C, 4 h | 90.9 | 2.3 |
| Sil-1 | batch reactor | 70 °C, 15 min | 0.7 | 0.3 |
| Sil-1_KOH | batch reactor | 70 °C, 15 min | 5.3 | 2.1 |
| Sil-1 | ultrasonic bath | 70 °C, 15 min | 6.3 | 2.6 |
| Sil-1_KOH | ultrasonic bath | 70 °C, 15 min | 13.4 | 5.4 |
| Sil-1 | ultrasonic probe in bath | 70 °C, 15 min | 18.6 | 7.5 |
| Sil-1_1KOH | ultrasonic probe in bath | 70 °C, 15 min | 71.3 | 28.9 |
| Sil-1 | ultrasonic probe in the reaction mixture | 70 °C, 15 min | 49.4 | 20.0 |
| Sil-1_1KOH | ultrasonic probe in the reaction mixture | 70 °C, 15 min | 96.6 | 39.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska-Kuś, J.; Janiszewska, E.; Held, A.; Jankowska, A.; Hanć, A.; Kowalak, S. Ultrasound-Assisted Synthesis of Glycerol Carbonate Using Potassium-Modified Silicalite-1 as a Catalyst. Molecules 2025, 30, 1590. https://doi.org/10.3390/molecules30071590
Kowalska-Kuś J, Janiszewska E, Held A, Jankowska A, Hanć A, Kowalak S. Ultrasound-Assisted Synthesis of Glycerol Carbonate Using Potassium-Modified Silicalite-1 as a Catalyst. Molecules. 2025; 30(7):1590. https://doi.org/10.3390/molecules30071590
Chicago/Turabian StyleKowalska-Kuś, Jolanta, Ewa Janiszewska, Agnieszka Held, Aldona Jankowska, Anetta Hanć, and Stanisław Kowalak. 2025. "Ultrasound-Assisted Synthesis of Glycerol Carbonate Using Potassium-Modified Silicalite-1 as a Catalyst" Molecules 30, no. 7: 1590. https://doi.org/10.3390/molecules30071590
APA StyleKowalska-Kuś, J., Janiszewska, E., Held, A., Jankowska, A., Hanć, A., & Kowalak, S. (2025). Ultrasound-Assisted Synthesis of Glycerol Carbonate Using Potassium-Modified Silicalite-1 as a Catalyst. Molecules, 30(7), 1590. https://doi.org/10.3390/molecules30071590






