Solvent-Driven Enrichment and Multiplex Analysis of Local Anesthetics by Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. TLC-SERS Strategy by Solvent-Driven Enrichment
2.2. Selection of Developing Solvent
2.3. Selection of Ag NPs as SERS Enhancement Substrate
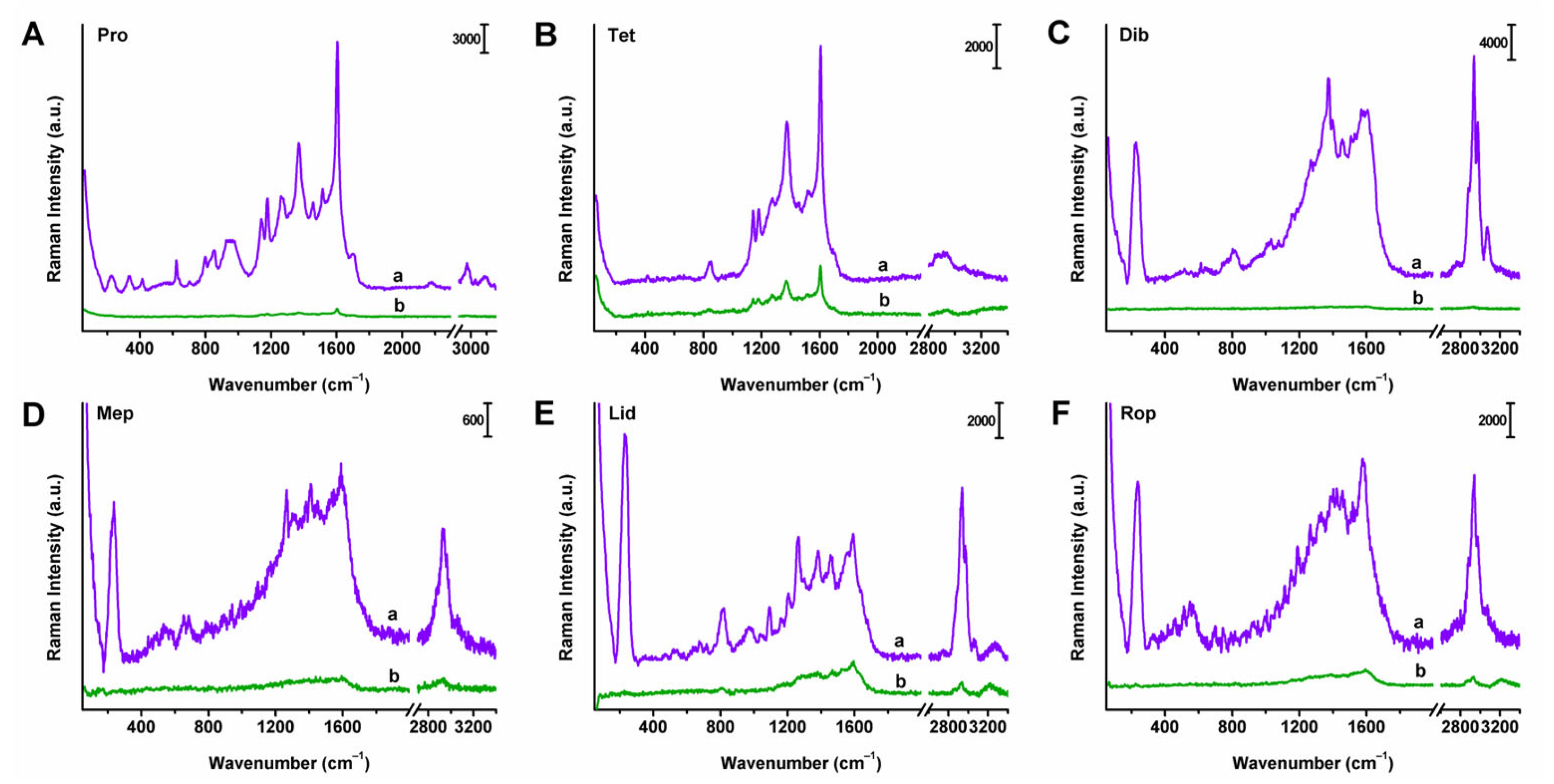
2.4. Raman and SERS Spectra
2.5. Spotting Amount-Dependent SERS Spectra
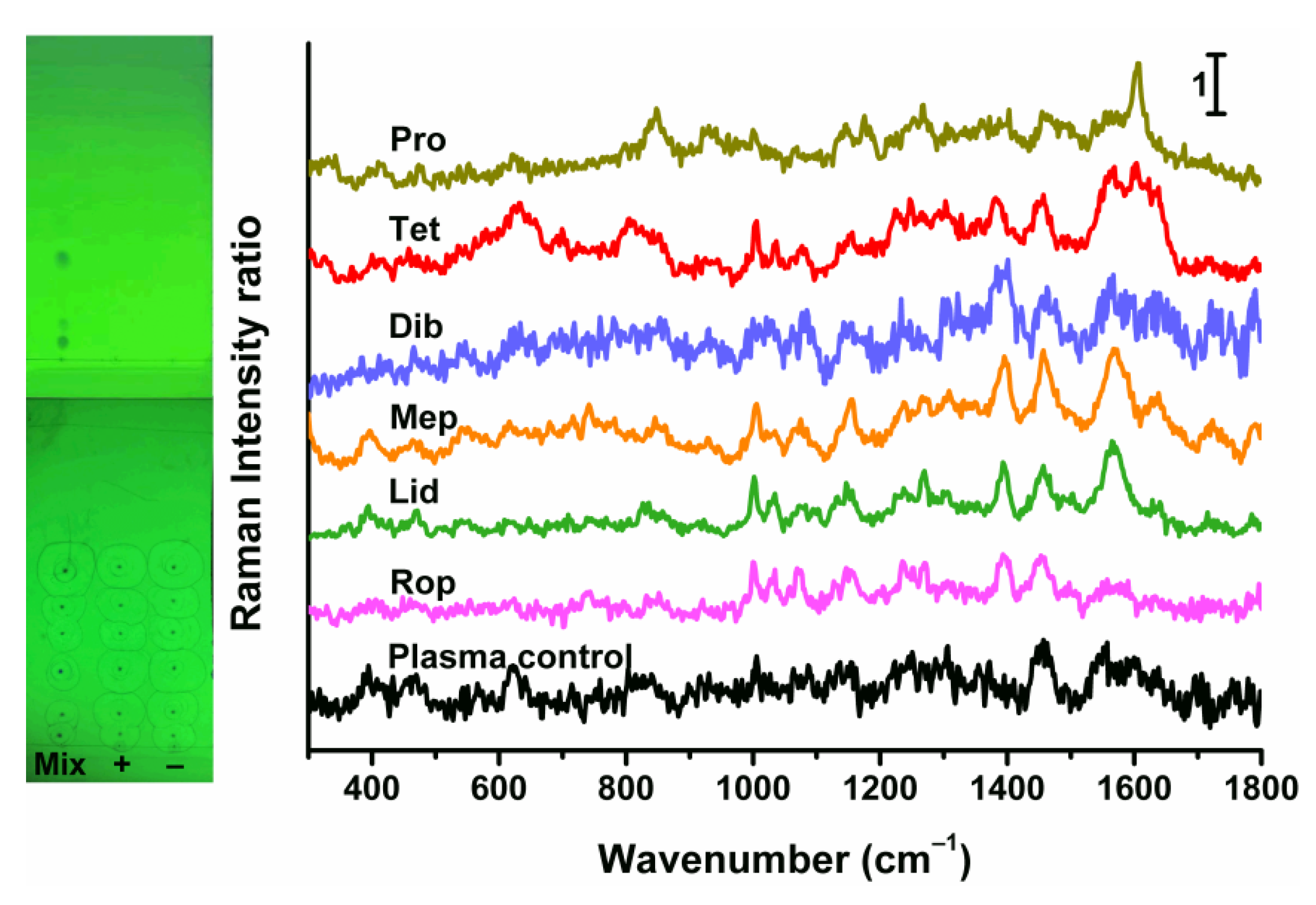
2.6. Determination of Human Plasma Samples and Verification by HPLC
2.7. Stability
3. Materials and Methods
3.1. Chemical Reagents
3.2. Apparatus and Measurement
3.3. Preparation of Ag NPs
3.4. Preparation of Standard Solutions
3.5. Preparation of Human Plasma Samples
3.6. TLC Separation, Solvent-Driven Enrichment, and SERS Measurement for Anesthetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tetzlaff, J.E. The pharmacology of local anesthetics. Anesthesiol. Clin. N. Am. 2000, 18, 217–233. [Google Scholar]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anaesthesia 2021, 76 (Suppl. S1), 27–39. [Google Scholar] [PubMed]
- Lin, S.; Qiu, W.; Hua, Y.; Yang, Y. Rapid determination of caine-based anesthetics and their metabolite residues in fish using a modified QuEChERS method coupled with UPLC-MS/MS. Food Chem. X 2024, 24, 102032. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lu, Q.; Zhao, X.; Yan, X.; Zenobi, R. Spatio-temporal analysis of anesthetics in mice by solid-phase microextraction: Dielectric barrier discharge ionization mass spectrometry. Anal. Chem. 2023, 95, 12470–12477. [Google Scholar]
- Li, F.; Wang, Z.; Zhu, B.; Xu, X.; Liu, Z. Development and application of a rapid screening SPE-LC-QTOF method for the quantification of 14 anesthetics in aquatic products. Food Anal. Methods 2023, 16, 633–649. [Google Scholar]
- Hong, S.; Kwon, N.; Kang, H.-S.; Jang, E.; Kim, H.; Han, E. Development of an analytical method for detection of anesthetics and sedatives in fish. J. AOAC Int. 2022, 105, 774–783. [Google Scholar]
- Di Rago, M.; Pantatan, S.; Hargreaves, M.; Wong, K.; Mantinieks, D.; Kotsos, A.; Glowacki, L.; Drummer, O.H.; Gerostamoulos, D. High throughput detection of 327 drugs in blood by LC–MS-MS with automated data processing. J. Anal. Toxicol. 2021, 45, 154–183. [Google Scholar] [CrossRef]
- Tonooka, K.; Naruki, N.; Honma, K.; Agei, K.; Okutsu, M.; Hosono, T.; Kunisue, Y.; Terada, M.; Tomobe, K.; Shinozuka, T. Sensitive liquid chromatography/tandem mass spectrometry method for the simultaneous determination of nine local anesthetic drugs. Forensic Sci. Int. 2016, 265, 182–185. [Google Scholar]
- del Mar Ĺopez Guerrero, M.; Hernández-Mesa, M.; Cruces-Blanco, C.; García-Campaña, A.M. On-line preconcentration strategy for the simultaneous quantification of three local anesthetics in human urine using CZE. Electrophoresis 2015, 36, 2961–2967. [Google Scholar]
- Turyshev, E.S.; Kopytin, A.V.; Zhizhin, K.Y.; Kubasov, A.S.; Shpigun, L.K.; Kuznetsov, N.T. Potentiometric quantitation of general local anesthetics with a new highly sensitive membrane sensor. Talanta 2022, 241, 123239. [Google Scholar]
- Yu, Y.; He, Z.; Lee, A.A.M.; Gao, J.; Tan, S.C.L.; Goh, W.P.; Jiang, C.; Zheng, X.T.; Ke, K.L.; Suwardi, A.; et al. Electrochemiluminescence drug detection with nanoparticleencapsulated luminophore on screen-printed electrodes. Mater. Today Chem. 2023, 29, 101442. [Google Scholar] [CrossRef]
- Özbek, O.; Altunoluk, O.C. Potentiometric determination of the local anesthetic procaine in pharmaceutical samples. Anal. Biochem. 2024, 695, 115657. [Google Scholar] [CrossRef] [PubMed]
- Karishma, B.R.; Manasa, G.; Bhakta, A.K.; Maiyalagan, T.; Mascarenhas, R.J.; Shetti, N.P. Electrocatalytic barium-oxide decorated MWCNT amperometric sensor for the quantification of anesthetic drug Procaine. Colloids Surf. B Biointerfaces 2023, 227, 113363. [Google Scholar] [CrossRef] [PubMed]
- Bottelli, E.D.; de Lima, L.F.; Paixão, T.R.L.C.; de Araujo, W.R. Laser-scribed graphene toward scalable fabrication of electrochemical paper-based devices for lidocaine detection in forensic and pharmaceutical samples. Electrochim. Acta 2024, 507, 145162. [Google Scholar] [CrossRef]
- Frag, E.Y.; Elashery, S.E.A.; Mohamed, G.G.; Sleim, A.A.E. Elucidating the performance of thick film screen printed electrodes in detection of a local anesthetic. J. Anal. Chem. 2023, 78, 1426–1436. [Google Scholar] [CrossRef]
- Demir, E.; Silah, H.; Erkmen, C.; Uslu, B. Electrochemical sensor design based on silver nanoparticles modified multiwalled carbon nanotubes paste electrode for the sensitive determination of mepivacaine. Comb. Chem. High Throughput Screen. 2023, 26, 2644–2655. [Google Scholar] [CrossRef]
- Ahmadpour, H.; Hosseini, S.M.M. A molecularly imprinted modified CdSeS/ZnS core–shell quantum dot embedded glass slide for highly selective and sensitive solid phase optosensing of trace amounts of lidocaine in biological samples. Anal. Methods 2019, 11, 851. [Google Scholar] [CrossRef]
- Meng, L.F.; Wu, H.Z. Carbon quantum dots from carrot as fluorescence probes for high-sensitivity detection of lidocaine. Appl. Ecol. Environ. Res. 2023, 21, 4531–4544. [Google Scholar] [CrossRef]
- Wiberg, K.; Hagman, A.; Burén, P.; Jacobsson, S.P. Determination of the content and identity of lidocaine solutions with UV-visible spectroscopy and multivariate calibration. Analyst 2001, 126, 1142–1148. [Google Scholar] [CrossRef]
- Sinha, S.S.; Jones, S.; Pramanik, A.; Ray, P.C. Nanoarchitecture based SERS for biomolecular fingerprinting and label-free disease markers diagnosis. Acc. Chem. Res. 2016, 49, 2725–2735. [Google Scholar] [CrossRef]
- Xie, L.; Gong, K.; Liu, Y.; Zhang, L. Strategies and challenges of identifying nanoplastics in environment by surface-enhanced Raman spectroscopy. Environ. Sci. Technol. 2023, 57, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, D.W.; Pu, H.; Wei, Q. Rapid nondestructive detection of mixed pesticides residues on fruit surface using SERS combined with self-modeling mixture analysis method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Shin, H.; Oh, S.; Joo, J.H.; Choi, Y.; Lee, J.S. Wrapping AgCl nanostructures with trimetallic nanomeshes for plasmon-enhanced catalysis and in situ SERS monitoring of chemical reactions. ACS Appl. Mater. Interfaces 2020, 12, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Ma, X.; Zang, J.; Liu, T.; Wang, H.; Fu, S.; Han, C.; Sui, H. In situ enrichment and determination of 6 kinds of caine-type anesthetics in cosmetics and rat serum by thin layer chromatography-Raman spectroscopy. Arab. J. Chem. 2023, 16, 105121. [Google Scholar] [CrossRef]
- Sherlin, Y.S.; Vijayakumar, T.; Binoy, J.; Roy, S.D.D.; Jayakumar, V.S. Buchi’s model based analysis of local anesthetic action in procaine hydrochloride: Vibrational spectroscopic approach. Spectrochim. Acta A 2018, 205, 55–65. [Google Scholar] [CrossRef]
- Badawi, H.M.; Forner, W.; Ali, S.A. The conformational stability, solvation and the assignments of the experimental infrared, Raman, 1H and 13C NMR spectra of the local anesthetic drug lidocaine. Spectrochim. Acta A 2015, 142, 382–391. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]




| Analytes | Linear Equation | Linear Range | R2 | Lowest Detectable Amounts | Lowest Detectable Amounts by TLC-Raman [24] |
|---|---|---|---|---|---|
| Pro | y = 4.886139 − 1.178272x | 0.1 μg–1 ng | 0.9961 | 1 ng | 0.4 μg |
| Tet | y = 3.93642 − 0.824827x | 1 μg–0.1 ng | 0.9742 | 10 pg | 0.2 μg |
| Dib | y = 4.828164 − 1.806599x | 5 μg–10 ng | 0.9920 | 10 ng | 1.2 μg |
| Mep | y = 2.897006 − 1.975927x | 5–0.1 μg | 0.9984 | 50 ng | 1 μg |
| Lid | y = 2.484772 − 1.492415x | 5–0.1 μg | 0.9714 | 50 ng | 1 μg |
| Rop | y = 3.369356 − 2.664862x | 5–0.1 μg | 0.9981 | 0.1 μg | 1 μg |
| Analytes | Added Amounts (ng) | Measured Amounts (ng) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Pro | 6 | 6.12 ± 0.33 | 102.1 | 5.5 |
| Tet | 6 | 5.87 ± 0.38 | 97.9 | 6.5 |
| Dib | 16 | 15.75 ± 0.83 | 98.4 | 5.3 |
| Mep | 120 | 122.15 ± 5.28 | 101.8 | 4.4 |
| Lid | 120 | 127.21 ± 9.03 | 106.0 | 7.1 |
| Rop | 120 | 113.21 ± 6.51 | 94.3 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, H.; Li, M.; Gao, Y.; Luo, J.; Ban, F.; Xu, T.; Fu, S.; Zhao, C.-Y.; Wen, H.; Han, C. Solvent-Driven Enrichment and Multiplex Analysis of Local Anesthetics by Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopy. Molecules 2025, 30, 1585. https://doi.org/10.3390/molecules30071585
Sui H, Li M, Gao Y, Luo J, Ban F, Xu T, Fu S, Zhao C-Y, Wen H, Han C. Solvent-Driven Enrichment and Multiplex Analysis of Local Anesthetics by Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopy. Molecules. 2025; 30(7):1585. https://doi.org/10.3390/molecules30071585
Chicago/Turabian StyleSui, Huimin, Miao Li, Yangyang Gao, Jie Luo, Fangyuan Ban, Tao Xu, Shuang Fu, Chao-Yang Zhao, Hailin Wen, and Cuiyan Han. 2025. "Solvent-Driven Enrichment and Multiplex Analysis of Local Anesthetics by Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopy" Molecules 30, no. 7: 1585. https://doi.org/10.3390/molecules30071585
APA StyleSui, H., Li, M., Gao, Y., Luo, J., Ban, F., Xu, T., Fu, S., Zhao, C.-Y., Wen, H., & Han, C. (2025). Solvent-Driven Enrichment and Multiplex Analysis of Local Anesthetics by Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopy. Molecules, 30(7), 1585. https://doi.org/10.3390/molecules30071585







