Nanoparticles for Photodynamic Therapy of Breast Cancer: A Review of Recent Studies
Abstract
1. Introduction
2. Nanoparticles for Photodynamic Therapy of Breast Cancer
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding Breast Cancer as a Global Health Concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.-H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast Cancer: Presentation, Investigation and Management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M. Panelists of the St Gallen Consensus Conference Customizing Local and Systemic Therapies for Women with Early Breast Cancer: The St. Gallen International Consensus Guidelines for Treatment of Early Breast Cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy–Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagnosis Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Q.; Liu, J.; Wu, M.; Ji, H.; Qin, Y.; Zhou, X.; Wu, L. Innovative Strategies for Enhanced Tumor Photodynamic Therapy. J. Mater. Chem. B 2021, 9, 7347–7370. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Kruger, C.A.; Kadanyo, S.; Mishra, A. Nanoparticles for Advanced Photodynamic Therapy of Cancer. Photomed. Laser Surg. 2017, 35, 581–588. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, S.; Jang, S.; Park, E.; Lee, Y.; Koo, H. Overcoming the Obstacles of Current Photodynamic Therapy in Tumors Using Nanoparticles. Bioact. Mater. 2022, 8, 20–34. [Google Scholar] [CrossRef]
- Yi, G.; Hong, S.H.; Son, J.; Yoo, J.; Park, C.; Choi, Y.; Koo, H. Recent Advances in Nanoparticle Carriers for Photodynamic Therapy. Quant. Imaging Med. Surg. 2018, 8, 433–443. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Satalkar, P.; Elger, B.S.; Shaw, D.M. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci. Eng. Ethics 2016, 22, 1255–1276. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional Nanoparticles--Properties and Prospects for Their Use in Human Medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Silva, L.B.; Castro, K.A.D.F.; Botteon, C.E.A.; Oliveira, C.L.P.; da Silva, R.S.; Marcato, P.D. Hybrid Nanoparticles as an Efficient Porphyrin Delivery System for Cancer Cells to Enhance Photodynamic Therapy. Front. Bioeng. Biotechnol. 2021, 9, 679128. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, J.; Xu, W.; Zhang, M.; Cui, P.; Guo, Z.; Deng, Y.; Chen, H.; Sun, W. Highly Efficient Far-Red/NIR-Absorbing Neutral Ir(III) Complex Micelles for Potent Photodynamic/Photothermal Therapy. Adv. Mater. 2021, 33, e2100795. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ji, J.; Liu, Z. Multifunctional MnO2 Nanoparticles for Tumor Microenvironment Modulation and Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1720. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, L.; Sun, J.; Sun, Y.; Gong, L.; Ge, S.; Zheng, Y.; Gao, W.; Wei, X. Hypoxia Reversion by Low-Immunogenic Ultra-Acid-Sensitive Comicelles of Protein-Polymer Conjugates Sensitizes Tumors to Photodynamic Therapy. J. Am. Chem. Soc. 2024, 146, 7543–7554. [Google Scholar] [CrossRef]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of Hypoxia-Induced Exosomes in Tumor Biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating Tumor Hypoxia toward Enhanced Photodynamic Therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Yi, M.; Xiong, B.; Li, Y.; Guo, W.; Huang, Y.; Lu, B. Manipulate Tumor Hypoxia for Improved Photodynamic Therapy Using Nanomaterials. Eur. J. Med. Chem. 2023, 247, 115084. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and Its Application in Drug Delivery. Expert. Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The Role of Albumin Receptors in Regulation of Albumin Homeostasis: Implications for Drug Delivery. J. Control. Release 2015, 211, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic Acid and Albumin Based Nanoparticles for Drug Delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef]
- Marconi, A.; Mattioli, E.J.; Ingargiola, F.; Giugliano, G.; Marforio, T.D.; Prodi, L.; Di Giosia, M.; Calvaresi, M. Dissecting the Interactions between Chlorin E6 and Human Serum Albumin. Molecules 2023, 28, 2348. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent Advances in Strategies for Overcoming Hypoxia in Photodynamic Therapy of Cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Keykhaee, M.; Khorramdelazad, H.; Karimi, M.Y.; Nejatbakhsh Samimi, L.; Aghamohamadi, N.; Karimi, M.; Falak, R.; Khoobi, M. Catalase Application in Cancer Therapy: Simultaneous Focusing on Hypoxia Attenuation and Macrophage Reprogramming. Biomed. Pharmacother. 2022, 153, 113483. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent Advances in Nanomedicines for Photodynamic Therapy (PDT)-Driven Cancer Immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. The Latest Look at PDT and Immune Checkpoints. Curr. Issues Mol. Biol. 2024, 46, 7239–7257. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Gurung, P.; Lim, J.; Shrestha, R.; Kim, Y.-W. Chlorin E6-Associated Photodynamic Therapy Enhances Abscopal Antitumor Effects via Inhibition of PD-1/PD-L1 Immune Checkpoint. Sci. Rep. 2023, 13, 4647. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Delos Santos, G.B.; Chiu, S.; Xue, L.; Berlin, J.C.; Peng, X.; Wu, H.; Lam, M.; et al. Structural Factors and Mechanisms Underlying the Improved Photodynamic Cell Killing with Silicon Phthalocyanine Photosensitizers Directed to Lysosomes Versus Mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Tao, J.; Su, X.; Zhu, F.; Lu, W.; Han, X.; Dang, M.; Weng, L. Mitochondria-Targeting and Oxygen Self-Supplying Eccentric Hollow Nanoplatform for Enhanced Breast Cancer Photodynamic Therapy. Bioinorg. Chem. Appl. 2024, 2024, 6618388. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Nader, N.D.; Aghanejad, A. Targeting Mitochondria in Cancer Therapy: Insight into Photodynamic and Photothermal Therapies. Life Sci. 2022, 307, 120898. [Google Scholar] [CrossRef]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting Mitochondria for Cancer Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef]
- Yue, C.; Yang, Y.; Zhang, C.; Alfranca, G.; Cheng, S.; Ma, L.; Liu, Y.; Zhi, X.; Ni, J.; Jiang, W.; et al. ROS-Responsive Mitochondria-Targeting Blended Nanoparticles: Chemo- and Photodynamic Synergistic Therapy for Lung Cancer with On-Demand Drug Release upon Irradiation with a Single Light Source. Theranostics 2016, 6, 2352–2366. [Google Scholar] [CrossRef]
- Yue, C.; Yang, Y.; Song, J.; Alfranca, G.; Zhang, C.; Zhang, Q.; Yin, T.; Pan, F.; de la Fuente, J.M.; Cui, D. Mitochondria-Targeting near-Infrared Light-Triggered Thermosensitive Liposomes for Localized Photothermal and Photodynamic Ablation of Tumors Combined with Chemotherapy. Nanoscale 2017, 9, 11103–11118. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Drug Delivery to Mitochondria: The Key to Mitochondrial Medicine. Adv. Drug Deliv. Rev. 2000, 41, 235–250. [Google Scholar] [CrossRef]
- Marrache, S.; Pathak, R.K.; Dhar, S. Detouring of Cisplatin to Access Mitochondrial Genome for Overcoming Resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 10444–10449. [Google Scholar] [CrossRef]
- Kang, L.; Sun, T.; Liu, S.; Zhao, H.; Zhao, Y. Porphyrin Derivative with Binary Properties of Photodynamic Therapy and Water-Dependent Reversible Photoacidity Therapy for Treating Hypoxic Tumor. Adv. Healthc. Mater. 2024, 13, e2303856. [Google Scholar] [CrossRef]
- Yang, F.; Xu, M.; Chen, X.; Luo, Y. Spotlight on Porphyrins: Classifications, Mechanisms and Medical Applications. Biomed. Pharmacother. 2023, 164, 114933. [Google Scholar] [CrossRef]
- Ishihara, H. Current Status and Prospects of Polyethyleneglycol-Modified Medicines. Biol. Pharm. Bull. 2013, 36, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kiwada, H. Anti-Polyethyleneglycol Antibody Response to PEGylated Substances. Biol. Pharm. Bull. 2013, 36, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Gdovin, M.J.; Kadri, N.; Rios, L.; Holliday, S.; Jordan, Z. Focal Photodynamic Intracellular Acidification as a Cancer Therapeutic. Semin. Cancer Biol. 2017, 43, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhao, B.; Zhang, J.; Miao, G.; Wei, S.; Tang, Y.; Liu, X.; Qian, H.; Huang, D.; Chen, W.; et al. ROS-Responsive Core-Shell Nano-Inhibitor Impedes Pyruvate Metabolism for Reinforced Photodynamic Therapy and Interrupted Pre-Metastatic Niche Formation. Acta Biomater. 2024, 182, 288–300. [Google Scholar] [CrossRef]
- Buchanan, J.L.; Taylor, E.B. Mitochondrial Pyruvate Carrier Function in Health and Disease across the Lifespan. Biomolecules 2020, 10, 1162. [Google Scholar] [CrossRef]
- Tavoulari, S.; Sichrovsky, M.; Kunji, E.R.S. Fifty Years of the Mitochondrial Pyruvate Carrier: New Insights into Its Structure, Function, and Inhibition. Acta Physiol. 2023, 238, e14016. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A Potential Carrier for Anti-Tumor Targeted Delivery-Hyaluronic Acid Nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-Triggered Size Shrink and Laser-Enhanced NO Release Nanoparticles for Deep Tumor Penetration and Combination Therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.-N.; Lu, J.; Zubair Iqbal, M.; Zhang, Q.; Kong, X. Synergistic Photodynamic and Photothermal Therapy of BODIPY-Conjugated Hyaluronic Acid Nanoparticles. J. Biomater. Sci. Polym. Ed. 2021, 32, 2028–2045. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Liao, X.; Xiao, Q.; Huang, Q.; Huang, X. Photostabilities and Anti-Tumor Effects of Curcumin and Curcumin-Loaded Polydopamine Nanoparticles. RSC Adv. 2024, 14, 13694–13702. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at Biological Interfaces. Adv. Colloid. Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. Natural Photosensitizers in Clinical Trials. Appl. Sci. 2024, 14, 8436. [Google Scholar] [CrossRef]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tønnesen, H.H. Formulation and Characterization of Lyophilized Curcumin Solid Dispersions for Antimicrobial Photodynamic Therapy (aPDT): Studies on Curcumin and Curcuminoids LII. Drug Dev. Ind. Pharm. 2015, 41, 969–977. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Q.-L.; Yao, Y.; Huang, Y.-L.; Zheng, Z.; Li, M.; Xu, H.; Tan, L.; Liao, X.; Xia, B.; et al. Alkyl Chain Length-Regulated in Situ Intelligent Nano-Assemblies with AIE-Active Photosensitizers for Photodynamic Cancer Therapy. Asian J. Pharm. Sci. 2024, 19, 100967. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, H.; Liu, Y.; Zhang, L.; Feng, G.; Tang, B.Z. Recent Progress in Type I Aggregation-Induced Emission Photosensitizers for Photodynamic Therapy. Molecules 2022, 28, 332. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, Y.; Zhou, M.; Shi, X.; Pu, X.; He, Z.; Zhang, S.; Qin, F.; Luo, C. Small Molecule-Engineered Nanoassembly for Lipid Peroxidation-Amplified Photodynamic Therapy. Drug Deliv. Transl. Res. 2024, 14, 1860–1871. [Google Scholar] [CrossRef]
- Delanaye, L.; Bahri, M.A.; Tfibel, F.; Fontaine-Aupart, M.-P.; Mouithys-Mickalad, A.; Heine, B.; Piette, J.; Hoebeke, M. Physical and Chemical Properties of Pyropheophorbide-a Methyl Ester in Ethanol, Phosphate Buffer and Aqueous Dispersion of Small Unilamellar Dimyristoyl-L-Alpha-Phosphatidylcholine Vesicles. Photochem. Photobiol. Sci. 2006, 5, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Eichwurzel, I.; Stiel, H.; Röder, B. Photophysical Studies of the Pheophorbide a Dimer. J. Photochem. Photobiol. B 2000, 54, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Adriouach, S.; Vorobiev, V.; Trefalt, G.; Allémann, E.; Lange, N.; Babič, A. Squalene-PEG: Pyropheophorbide-a Nanoconstructs for Tumor Theranostics. Nanomedicine 2019, 15, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Li, Z.; Wang, W.; Wu, Y.; Pan, D.; Gu, Z.; Sheng, R.; Tomás, H.; Zhang, H.; et al. Glycodendron/Pyropheophorbide-a (Ppa)-Functionalized Hyaluronic Acid as a Nanosystem for Tumor Photodynamic Therapy. Carbohydr. Polym. 2020, 247, 116749. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Li, Z.; Zhang, J.; Pan, D.; Wang, B.; Zhu, H.; Zhang, H.; Gu, Z.; Luo, K. Dendron-Functionalized Polyglutamate-Pyropheophorbide-a Conjugates as Nanomedicines for Breast Cancer Photodynamic Therapy. Macromol. Rapid Commun. 2021, 42, e2100013. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zhang, X.; Li, Z.; Zhang, Q.; Wang, W.; Pan, D.; Zheng, X.; Gu, Z.; Zhang, H.; et al. Tumor Microenvironment-Responsive PEGylated Heparin-Pyropheophorbide-a Nanoconjugates for Photodynamic Therapy. Carbohydr. Polym. 2021, 255, 117490. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Jin, F.; Xu, X.; Fang, T.; Du, Y. Tumor Oxygenation Nanoliposomes Promote Deep Photodynamic Therapy for Triple-Negative Breast Cancer. Biomater. Sci. 2024, 12, 4967–4979. [Google Scholar] [CrossRef]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.J.M.; Kock, L.M. Biomedical Applications of Photo- and Sono-Activated Rose Bengal: A Review. Photobiomodulation Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef]
- Doughty, M.J. Rose Bengal Staining as an Assessment of Ocular Surface Damage and Recovery in Dry Eye Disease-a Review. Contact Lens Anterior Eye 2013, 36, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Stolik, S.; Delgado, J.A.; Pérez, A.; Anasagasti, L. Measurement of the Penetration Depths of Red and near Infrared Light in Human “Ex Vivo” Tissues. J. Photochem. Photobiol. B 2000, 57, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peña, J.; Xing, J. Upconversion Nanoparticle-Assisted Photopolymerization. Photochem. Photobiol. 2020, 96, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ru, B.; Hu, J.; Xu, L.; Wan, Q.; Liu, W.; Cai, W.; Zhu, T.; Ji, Z.; Guo, R.; et al. Recent Advances of CREKA Peptide-Based Nanoplatforms in Biomedical Applications. J. Nanobiotechnol. 2023, 21, 77. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Li, Y.; Zeng, H.; Li, Z.; Wang, C.; Xu, C.; Deng, Q.; Wang, Q.; Yang, X.; et al. Precise Fibrin Decomposition and Tumor Mechanics Modulation with Hydroxyethyl Starch-Based Smart Nanomedicine for Enhanced Antitumor Efficacy. J. Mater. Chem. B 2022, 10, 8193–8210. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhang, Y.; Lu, Y.; Li, J.; Wang, H.; Yao, D.; Wang, D. Fibronectin-Targeting and Cathepsin B-Activatable Theranostic Nanoprobe for MR/Fluorescence Imaging and Enhanced Photodynamic Therapy for Triple Negative Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 33564–33574. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Hong, G.; Du, J.; Song, F.; Peng, X. Self-Assembly-Integrated Tumor Targeting and Electron Transfer Programming towards Boosting Tumor Type I Photodynamic Therapy. Chem. Sci. 2024, 15, 10945–10953. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I Photodynamic Therapy by Organic–Inorganic Hybrid Materials: From Strategies to Applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef]
- Rezuchova, I.; Bartosova, M.; Belvoncikova, P.; Takacova, M.; Zatovicova, M.; Jelenska, L.; Csaderova, L.; Meciarova, I.; Pohlodek, K. Carbonic Anhydrase IX in Tumor Tissue and Plasma of Breast Cancer Patients: Reliable Biomarker of Hypoxia and Prognosis. Int. J. Mol. Sci. 2023, 24, 4325. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Jin, Y.-T.; Zhang, W.; Yu, J.; Yang, H.-P.; Wang, H.-Y.; Zhang, Z.-J.; Liu, X.-P.; Zou, Q. CA IX Is Upregulated in CoCl2-Induced Hypoxia and Associated with Cell Invasive Potential and a Poor Prognosis of Breast Cancer. Int. J. Oncol. 2016, 48, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Jirstrom, K.; Kronblad, A.; Millikan, R.C.; Landberg, G.; Duffy, M.J.; Rydén, L.; Gallagher, W.M.; O’Brien, S.L. CA IX Is an Independent Prognostic Marker in Premenopausal Breast Cancer Patients with One to Three Positive Lymph Nodes and a Putative Marker of Radiation Resistance. Clin. Cancer Res. 2006, 12, 6421–6431. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Liu, S.; Li, B.; Li, Z.; Wu, C.; Xu, D.; Pan, W.; Li, Z.; Liu, X.; Liu, B. Electron Transfer Mediator Modulates Type II Porphyrin-Based Metal-Organic Framework Photosensitizers for Type I Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2025, 64, e202420643. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A Small Molecule from Nature with High Therapeutic Potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Zhuang, J.; Qi, G.; Feng, Y.; Wu, M.; Zhang, H.; Wang, D.; Zhang, X.; Chong, K.C.; Li, B.; Liu, S.; et al. Thymoquinone as an Electron Transfer Mediator to Convert Type II Photosensitizers to Type I Photosensitizers. Nat. Commun. 2024, 15, 4943. [Google Scholar] [CrossRef]
- Cui, Z.; Ji, R.; Xie, J.; Wang, C.; Tian, J.; Zhang, W. Tumor Microenvironment-Triggered Self-Adaptive Polymeric Photosensitizers for Enhanced Photodynamic Therapy. Biomacromolecules 2024, 25, 2302–2311. [Google Scholar] [CrossRef]
- Lutz, J.-F. Thermo-Switchable Materials Prepared Using the OEGMA-Platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Collard, D.M. Tricomponent Amphiphilic Poly(Oligo(Ethylene Glycol) Methacrylate) Brush-Grafted Poly(Lactic Acid): Synthesis, Nanoparticle Formation, and In Vitro Uptake and Release of Hydrophobic Dyes. Macromolecules 2020, 53, 4274–4283. [Google Scholar] [CrossRef]
- Mousavi, M.; Ghaleh, H.; Jalili, K.; Abbasi, F. Multi-Layer PDMS Films Having Antifouling Property for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2020, 32, 678–693. [Google Scholar] [CrossRef]
- Ozer, I.; Slezak, A.; Sirohi, P.; Li, X.; Zakharov, N.; Yao, Y.; Everitt, J.I.; Spasojevic, I.; Craig, S.L.; Collier, J.H.; et al. An Injectable PEG-like Conjugate Forms a Subcutaneous Depot and Enables Sustained Delivery of a Peptide Drug. Biomaterials 2023, 294, 121985. [Google Scholar] [CrossRef] [PubMed]
- Ozer, I.; Kelly, G.; Gu, R.; Li, X.; Zakharov, N.; Sirohi, P.; Nair, S.K.; Collier, J.H.; Hershfield, M.S.; Hucknall, A.M.; et al. Polyethylene Glycol-Like Brush Polymer Conjugate of a Protein Drug Does Not Induce an Antipolymer Immune Response and Has Enhanced Pharmacokinetics than Its Polyethylene Glycol Counterpart. Adv. Sci. 2022, 9, e2103672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, W.; Luo, H.; Zhang, K.; Lv, J.; Jiang, L.; Huang, Y.; Song, J.; Yang, Z.; Huang, W. Toward Type I/II ROS Generation Photoimmunotherapy by Molecular Engineering of Semiconducting Perylene Diimide. Adv. Healthcare Mater. 2024, 13, 2303175. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fang, Z.; Sun, F.; Zhu, C.; Jia, M.; Miao, X.; Huang, L.; Hu, W.; Fan, Q.; Yang, Z.; et al. Deciphering Oxygen-Independent Augmented Photodynamic Oncotherapy by Facilitating the Separation of Electron-Hole Pairs. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401036. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, N.; Wang, Y.; Ling, G.; Zhang, P. Perylene Diimide-Based Treatment and Diagnosis of Diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main Features and Their Applications in Topical and Transdermal Administration of Drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef]

| Inclusion Criteria |
| Articles describing photodynamic therapy |
| Articles describing cancer therapy |
| Articles describing nanoparticles |
| Articles published in 2024 |
| Exclusion criteria |
| Articles describing photodynamic therapy combined with other forms of therapy (chemotherapy, radiation therapy, gene therapy, photothermal therapy, etc.) or imaging |
| Articles describing cancers other than breast cancer |
| Articles other than original research papers |
| Articles in which the results of therapy were described only in vitro |
| Articles in a language other than English and Polish |
| Name of the Nanoparticle | Construction | Results | Quoting |
|---|---|---|---|
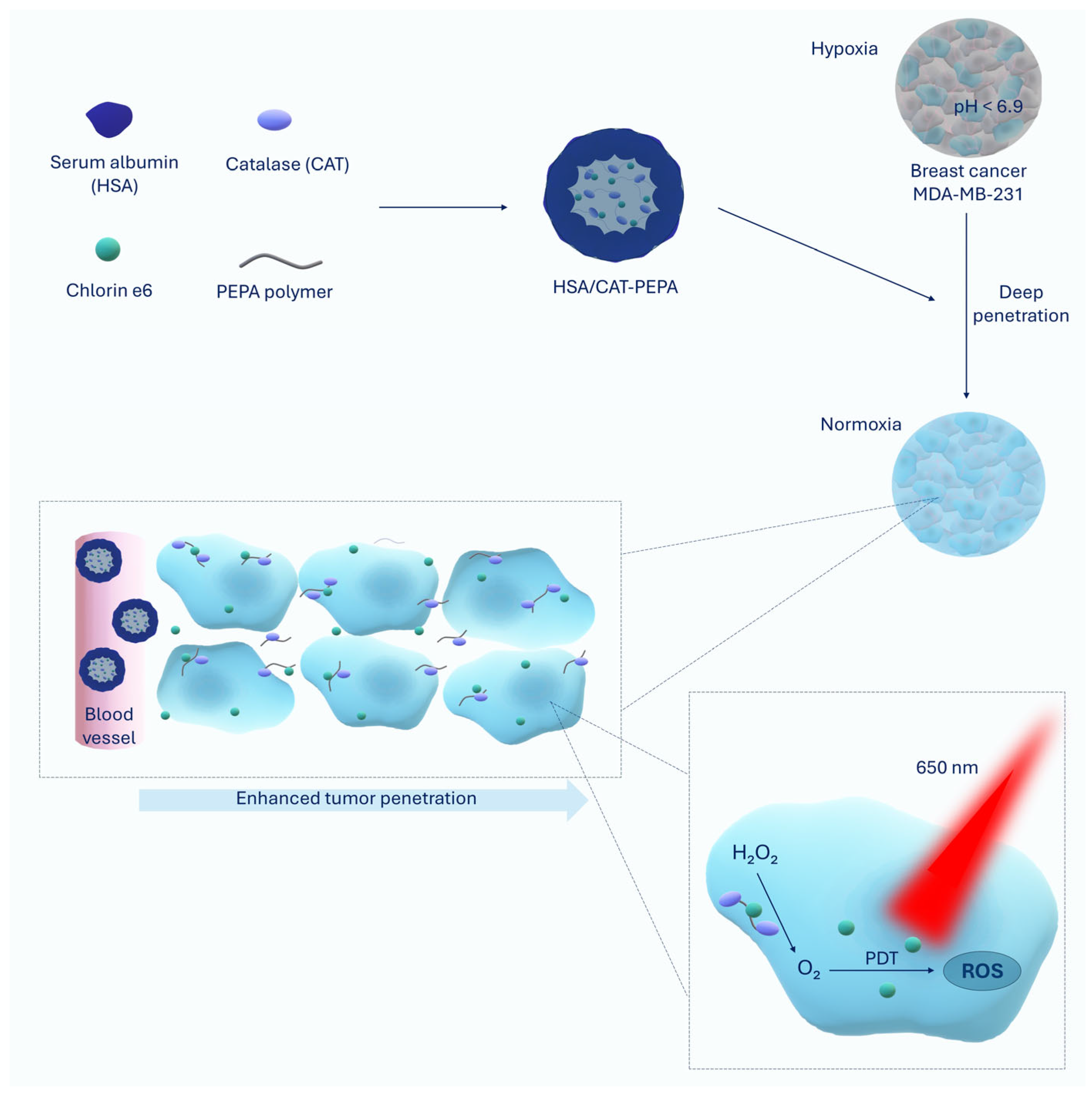
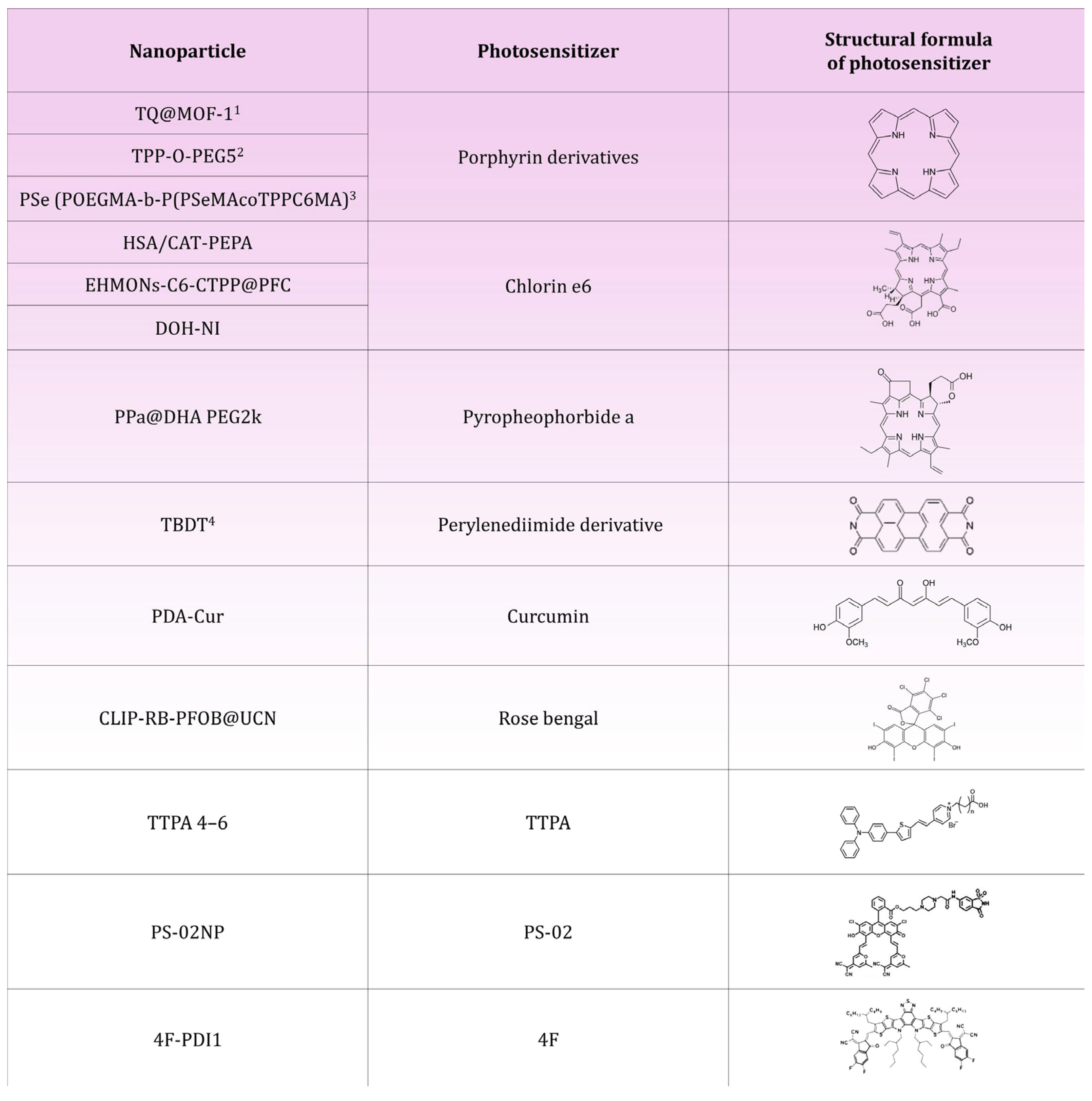
| HSA/CAT-PEPA@Ce6 | Serum albumin (HSA), catalase (CAT), PEPA polymer, Chlorin e6 | Reduction in MDA-MB-231 breast cancer volume by 90% | [23] |
| EHMONs-Ce6-CTPP@PFC | Eccentric hollow mesoporous organic silica nanoparticles (EHMONs), triphenylphosphine (CTPP), Chlorin e6, perfluorocarbons (PFCs) | Reduction in 4T1 breast cancer volume by 80% | [42] |
| TPP-O-PEG5 | Modified porphyrin with four phenyl groups with a polyethyleneglycol (PEG) substituent | Reduction in breast cancer volume of 4T1 and MDA-MB-231o 73% for tumors with a diameter of 7.0–8.0 mm; reduction in breast cancer volume of 4T1 and MDA-MB-231o 89% for tumors with a diameter of 9.0–11.0 mm | [49] |
| DOH-NI | Biodegradable dendritic poly(carbonate) (DPC), mitochondrial pyruvate carrier (MPC) inhibitor UK5099, hyaluronic acid (HA) coating, Chlorin e6 | Reduction in volume of cancer 4T1Luc breast by 89%; reduction in pyruvate uptake in pulmonary metastases by 62% | [54] |
| PDA-Cur | Polydopamine core (PDA), curcumin (Cur) | Reduction in MCF-7 breast cancer volume by 51% | [62] |
| TTPA 4–6 | Triphenylamine (TPA), pyridine fragment, alkyl chains | 4T1 breast cancer volume reduction of 85% for TTPA 4, 92% for TTPA 5 and 88% for TTPA 6 | [68] |
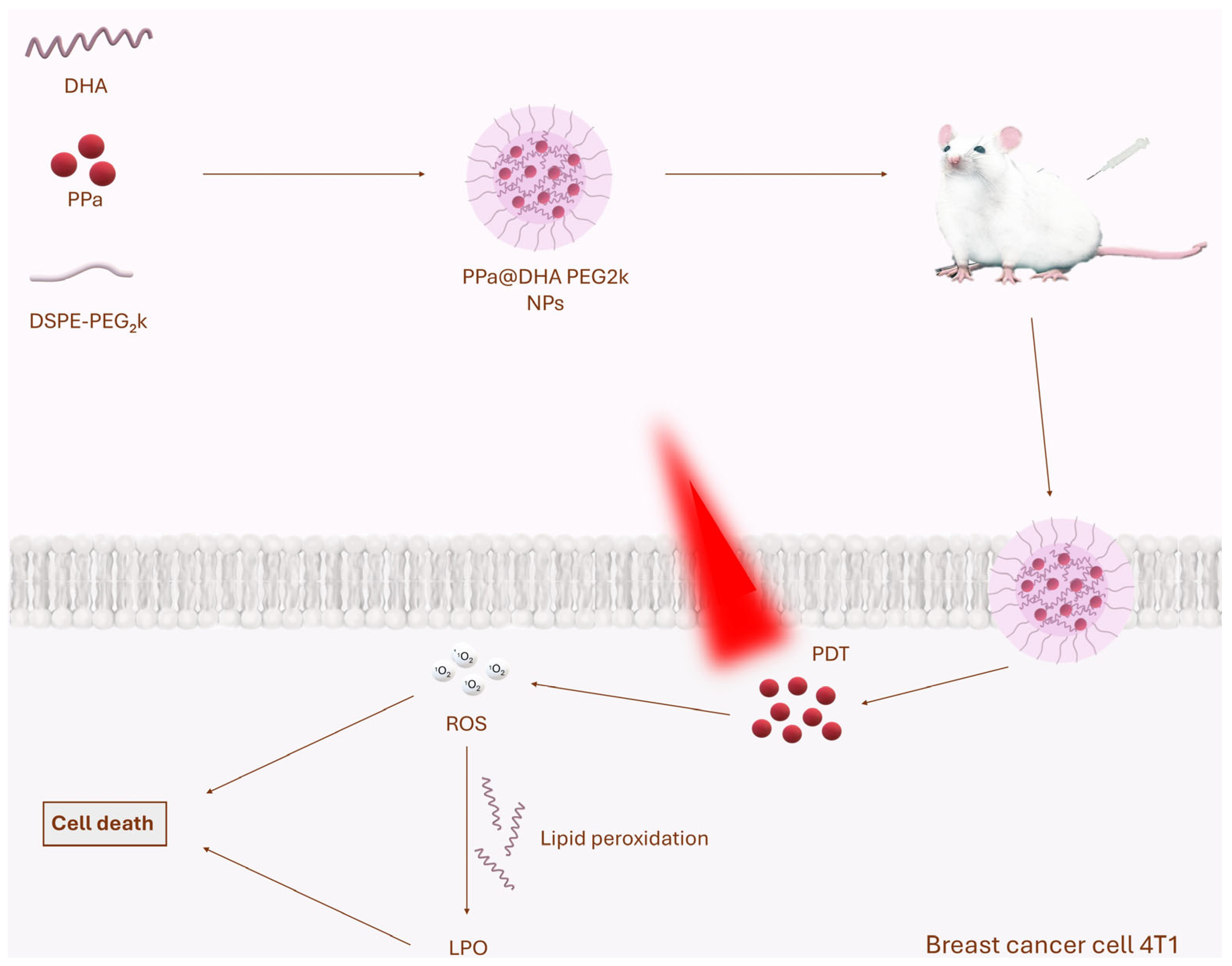
| PPa@DHA PEG2k | Pyropheophorbide-a (PPa), docosahexaenoic acid (DHA), stabiliser DSPE-PEG2k | Reduction in 4T1 breast cancer volume by 80% | [70] |
| CLIP-RB-PFOB@UCNP | UCNP core, CLIP liposomal coating, Rose Bengal, Perfluorooctane (PFOB) | Significant reduction in breast cancer volume of TNBC model; reduction in HIF-1α levels; inhibition of lung metastasis | [79] |
| PS-02 | PS-02 thermally activated delayed fluorescence photosensitizer (TADF), piperazine, 6-NS ligand | Reduction in MDA-MB-231 breast cancer volume by 80% | [87] |
| TQ@MOF-1 | MOF-1 core (PCN-224), TCPP ligand, thymoquinone (TQ), F-127 coating | Reduction in 4T1 breast cancer volume by 85% | [93] |
| PSe (POEGMA-b-P(PSeMA-co-TPPC6MA) | POEGMA (Poly(oligoethylene glycol) methacrylate), selenium units in the form of selenide (PSeMA), tetraphenylporphyrin (TPP) modified with a methacrylate-terminated hexyl side chain (TPPC6MA) | Reduction in 4T1 breast cancer volume by 70% | [97] |
| TBDT | Perylenediimide (PDI) with bromine and pyrrolidine | Reduction in 4T1 breast cancer volume by 90%; increase in activated CD8+ T lymphocytes, dendritic cells and decrease in M2-type macrophages | [103] |
| 4F-PDI1 | L8-BO-EH-4F semiconductor, perylenodiimide (PDI), Pluronic F-127 | Reduction in 4T1 breast cancer volume by 80% | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Przygórzewska, A.; Woźnicki, P.; Aebisher, D. Nanoparticles for Photodynamic Therapy of Breast Cancer: A Review of Recent Studies. Molecules 2025, 30, 1571. https://doi.org/10.3390/molecules30071571
Bartusik-Aebisher D, Przygórzewska A, Woźnicki P, Aebisher D. Nanoparticles for Photodynamic Therapy of Breast Cancer: A Review of Recent Studies. Molecules. 2025; 30(7):1571. https://doi.org/10.3390/molecules30071571
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Agnieszka Przygórzewska, Paweł Woźnicki, and David Aebisher. 2025. "Nanoparticles for Photodynamic Therapy of Breast Cancer: A Review of Recent Studies" Molecules 30, no. 7: 1571. https://doi.org/10.3390/molecules30071571
APA StyleBartusik-Aebisher, D., Przygórzewska, A., Woźnicki, P., & Aebisher, D. (2025). Nanoparticles for Photodynamic Therapy of Breast Cancer: A Review of Recent Studies. Molecules, 30(7), 1571. https://doi.org/10.3390/molecules30071571








