Abstract
Sugarcane bagasse (SCB) was transformed into polyaminocarboxylated modified hydrochar (ACHC) by hydrothermal carbonization (HTC), which was then followed by activation, etherification, amination, and carboxylation successively. ACHC was systematically characterized, and batch adsorption studies were used to assess its methylene blue (MB) adsorption capacity. Adsorption was analyzed by adsorption isotherm models, the adsorption mass transfer model, and the adsorption thermodynamics model. Density functional theory (DFT) was utilized to explain adsorption mechanisms. The findings demonstrated the adsorption was one type of endothermic, spontaneous, and homogenous monolayer adsorption with intra-particle diffusion, containing both chemical and physical adsorption, involving electrostatic attraction, hydrogen bonding, and π-π interaction. At 303 and 323 K, the highest adsorption capacity was 1017.29 and 1060.45 mg·g−1, respectively. Furthermore, when the recycle time was 4, the equilibrium adsorption capacity remained at 665.43 mg·g−1, which implied fairly good regeneration performance. The modification provided a simple, environmentally friendly, and economical solution for converting sugarcane bagasse into an efficient adsorbent for MB treatment.
1. Introduction
Dyes are utilized in textile, leather, plastics, printing, food, cosmetics, and other industries, while the toxicity and carcinogenicity of dyes in wastewater present a huge challenge to the world [1,2,3,4]. Methylene blue (MB) is one type of phenothiazine cationic dye that is widely utilized in many areas; however, its toxicity, carcinogenicity, and non-biodegradability can harm the human nervous system and eyes and cause gastritis, breathing difficulties, and other illnesses [5,6,7]. Thus, investigating the method of removing MB from wastewater is really crucial.
Physical, chemical, and biological techniques can be employed to remove MB, and the commonly used physical methods are adsorption, nanofiltration, reverse osmosis, ion exchange, and so on [8]. The low cost and ease of application of adsorption make it a promising technology [9]. This process requires an adsorbent material to which contaminants such as MB can be adsorbed. Hydrochar is a common carbon-rich adsorbent prepared by hydrothermal carbonization (HTC) at comparatively moderate temperatures (180–350 °C) and pressure from waste biomass such as sugarcane bagasse [10,11]. Sugarcane bagasse (SCB) is one type of significant residual product in the cane sugar industry, with an estimated 5.26 million tonnes produced worldwide in 2019 [12]. SCB mainly contains cellulose (40–45%), hemicellulose (30–35%), and lignin (20–30%), and polysaccharide constituents are important for framing the carbon skeleton of hydrochar with functional groups [8,13,14,15]. Hydrochar has been reported to have excellent chemical stability, biocompatibility, electrical conductivity, and thermal conductivity, so research about the use of hydrochar as an adsorbent has attracted increasing attention [10,11]. However, hydrochar still requires improvement owing to its low porosity, low specific surface area, and strong binding site deficiency [16,17]. Hydrochar contains abundant hydroxyl groups, which are conducive to offering chemical reactive sites for further covalent functionalization with organic reagents; moreover, adding more oxygen-containing functional groups to hydrochar can increase its adsorption capability [17,18].
Diethylenetriamine contains numerous primary and secondary amino groups, which can react with hydroxyl groups of hydrochar, thereby introducing amino groups. The strong reactivity of amino groups allows them to form reactions with chloroacetic acid to produce carboxylate groups. Introducing amino groups and carboxylate groups is conducive to providing adsorption sites. And the carboxylate groups are negatively charged; hydrochar with carboxylate groups could adsorb MB through electrostatic attraction. Furthermore, introducing polyaminocarboxylate groups through adding these two reagents is simple and economical. Li et al. prepared polyaminocarboxylated modified bamboo hydrochar successfully through adopting these two reagents and confirmed that this method was feasible [17]. This study provides a simple, environmentally friendly, and economic method to transform sugarcane bagasse into polyaminocarboxylated modified hydrochar (ACHC), which has excellent adsorption capacity for MB. The primary goals are (i) to prepare ACHC by introducing polyaminocarboxylate groups through diethylenetriamine and chloroacetic acid; (ii) to characterize ACHC by multiple methods and evaluate its adsorption capacity; and (iii) to explore the adsorption mechanism.
2. Results
2.1. Characterization
2.1.1. Morphology Analysis
By utilizing scanning electron microscopy (SEM), the morphology of sugarcane bagasse (SCB), sugarcane bagasse hydrochar (HC), polyaminocarboxylated modified hydrochar (ACHC), and polyaminocarboxylated modified hydrochar/methylene blue (ACHC/MB) was examined, and findings are demonstrated in Figure 1. Less porosity and a comparatively smooth and flat surface were seen on SCB (Figure 1a), while HC had a rough surface with open pores evenly distributed (Figure 1b), and there were numerous carbon microspheres of varying sizes randomly distributed on the surface, leading to partial blockage of the pores. The pore blockages were brought on by the hydrocarbon condensation that occurred on the surface during the hydrothermal carbonization (HTC) process [19]. The formation of the spherical structure could be due to the condensation of the solubilized hemicellulose, cellulose, and a tiny amount of lignin during the HTC process with the presence of phosphoric acid [20]. ACHC presented a fluffy and porous surface structure. The pore shape slightly was altered compared with the HC, with round openings rather than elliptical ones observed (Figure 1c), which indicated the modification might further corrode the hydrochar through volatilization and activation [21]. Furthermore, the morphology of the ACHC after the adsorption of MB was visualized (Figure 1d). The external and inner structures of ACHC were occupied by MB to some degree, demonstrating the effective adsorption of MB onto ACHC.
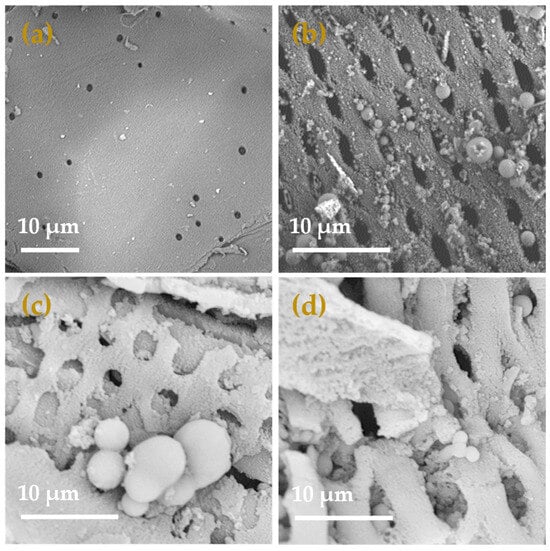
Figure 1.
SEM spectra of (a) SCB; (b) HC; (c) ACHC; (d) ACHC/MB.
2.1.2. Pore Structure Analysis
Porosity of HC and ACHC was analyzed by specific surface area and porosity meter (BET). Figure 2 displays N2 adsorption-desorption isotherms together with relevant pore size distribution curves. Type IV isotherms and H3 type of hysteresis were displayed (Figure 2a), illuminating the existence of mesopores and irregular slits in HC and ACHC (Figure 2b) [22]. While ACHC showed less N2 adsorption capacity, indicating that the modification process impacted surface area and total pore volume, pore size did not significantly change, nevertheless. The results were in agreement with the above-mentioned pore types, and this was further confirmed by calculation in Table 1. Since ACHC had a smaller capacity than HC, its rate of N2 adsorption with relative pressure increment increased at a slower pace, which might suggest that the polyaminocarboxylated modification had no improvement in pore structure. The mesoporous structure was not entirely blocked by the grafting of amino and carboxylate groups, which would have been better for the MB adsorption process [16,23].
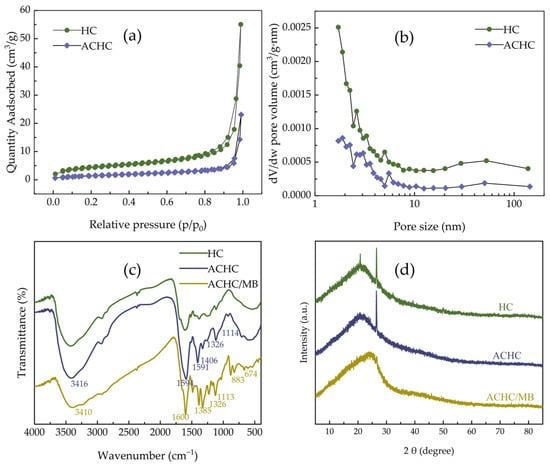
Figure 2.
(a) N2 adsorption-desorption isotherms of HC and ACHC; (b) pore size distributions of HC and ACHC; (c) FTIR spectrum of HC, ACHC, and ACHC/MB; (d) XRD patterns of HC, ACHC, and ACHC/MB.

Table 1.
BET surface areas, total pore volumes, and average pore sizes of HC and ACHC.
2.1.3. Functional Group Analysis
HC, ACHC, and ACHC/MB were examined by Fourier transform infrared spectroscopy (FTIR), and the resulting spectra are displayed in Figure 2c. A significant portion of peaks identified in HC was also observed in ACHC, including the wide and strong characteristic band around 3416 cm−1 representing the O-H stretching vibrations of hydroxyl or carboxyl groups, which revealed that O-H groups primitively in cellulose, hemicellulose, and lignin were not entirely destroyed in the HTC process [18,24]. Characteristic bands occurring in the region of 1300~1000 cm−1 are ascribed to C-O single bonds [25], and distinctive bands at 1594 and 1406 cm−1 match with symmetric and asymmetric stretching vibrations in -COOH [26,27]. These characteristic peaks show that both HC and ACHC included a significant number of functional groups that contained oxygen. Because these oxygen-containing functional groups may interact with cationic MB and act as proton donors, ACHC’s adsorption ability may increase with the number increment of oxygen-containing functional groups it contained [18]. It was noteworthy that three unique phenomena occurred following the modification: first, characteristic peak intensity at 3416 cm−1 was increased; second, strong peaks emerged at 1594 and 1406 cm−1, matching with -COOH groups; third, a new characteristic peak coordinating with C-N groups showed up at 1114 cm−1, supposing an N atom had been successfully incorporated on ACHC [28]. The appearance of these different characteristic peaks indicates that the hydroxyl groups of HC had undergone modification with reagents, and the amino groups and carboxylate groups had been successfully introduced. These oxygen-containing groups could provide sufficient adsorption sites for adsorption.
After adsorption of MB by ACHC, the characteristic peaks migrated to 3410, 1591, 1385, 1326, and 883 cm−1, indicating that ACHC successfully adsorbed MB [17]. The O-H characteristic peak migrated from 3416 to 3410 cm−1, confirming that the hydroxyl groups of ACHC were hydrogen bonded with the N atom in the phenothiazine ring of MB [9,29]. The characteristic peak of C=N migrated from 1589 to 1591 cm−1, and C-H stretching vibration shifted from 826 to 883 cm−1, suggesting π-π interaction between adsorbent and adsorbate [17]. C-N and symmetric -CH3 stretching vibrations of -N(CH3)2+ migrated from 1389 and 1319 to 1385 and 1326 cm−1 respectively, confirming electrostatic interaction between ACHC and MB [9].
2.1.4. Structure Property Analysis
Figure 2d displays X-ray diffractometer (XRD) patterns of HC, ACHC, and ACHC/MB. In HC and ACHC, an obvious diffraction peak around 2θ of 23.8° corresponding to the (002) crystal plane was observed, supposing the production of graphitic carbon structures [30,31]. Modification merely modulated the structural properties and did not affect the crystallinity, as evidenced by the fact that the position and intensity of the diffraction peaks did not vary appreciably [32]. After adsorption of MB onto ACHC, the diffraction peak at 2θ = 23.8° shifted to the right, and the peak width increased, supposing the crystallinity was changed after adsorption of MB by ACHC, which meant that MB was successfully adsorbed by ACHC.
2.1.5. Chemical Composition Analysis
Chemical compositions of ACHC were analyzed by X-ray photoelectron spectroscopy (XPS) (Figure 3a). Three peaks at 284.80, 286.25, and 288.25 eV were identified in the C1s spectrum of ACHC, coordinating with C-C/C=C, C-O, and C=O [33]. ACHC was rich in oxygen-containing functional groups, as evidenced by the O1s spectrum, which could be deconvoluted into two peaks at 531.42 and 533.08 eV, reflecting O-H and C=O [34]. This observation aligned with the FTIR analysis conclusion. Groups that concluded oxygen were able to serve as negatively charged active adsorption sites, and higher adsorption was produced by electrostatic interaction between oxygen-containing groups and MB [35]. The spectrum of N1s can be fitted to three peaks, namely -NH (399.44 eV), -NH2 (400.36 eV), and CH3CO-NH (401.96 eV), demonstrating that the N atom had been successfully incorporated on ACHC [36], which conformed well with the results shown in FTIR analysis. The addition of groups containing nitrogen may be responsible for the increase in adsorption capability. Furthermore, due to the high content of C=O in O1s and the high content of -NH in N1s (Figure 3), these two functional groups were chosen as the representative functional groups in the density functional theory (DFT) analysis later.
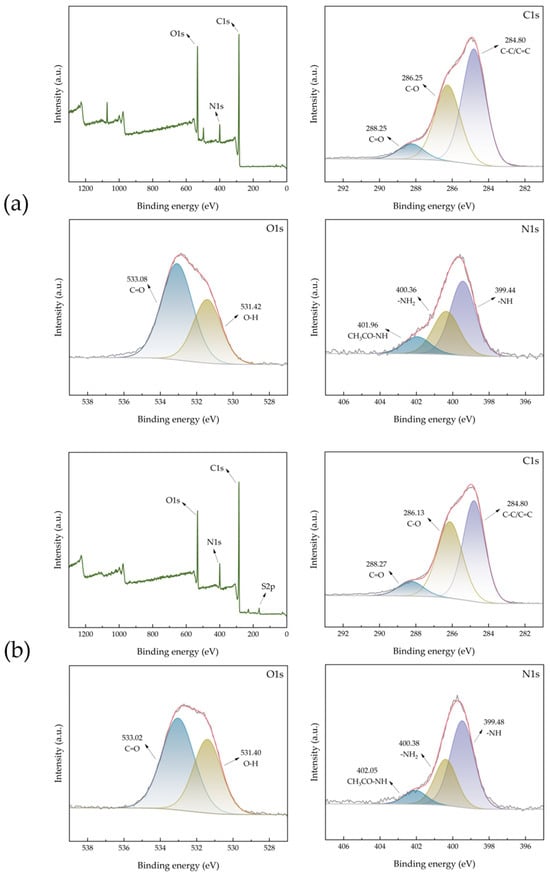
Figure 3.
The XPS spectra of (a) ACHC and (b) ACHC/MB.
As shown in Figure 3b, after the adsorption of MB, a new elemental peak emerged at 164.94 eV (S2p), which demonstrated that ACHC had successfully adsorbed MB [37]. And after the adsorption of MB, there were changes in the binding energies of C1s and O1s, suggesting that the oxygen-containing functional groups had participated in the adsorption, which was consistent with the results of FTIR [37].
2.2. Effects of pH
Figure 4 illustrates how the initial pH value affects the zeta potential and ACHC’s adsorption capacity for MB. ACHC’s adsorption capacity was relatively low between pH of 1.0–3.0; it increased sharply when pH increased to 5.0; it increased slowly between pH of 7.0–11.0; and it reached its maximum when pH was 11.0. With the increment of pH value, the zeta potential of ACHC decreased continuously, and the electronegativity of ACHC increased constantly. In aqueous solution, MB may exist in the cationic form (MB+) or exist as undissociated molecules (MB0). According to experimental results, the pHpzc of ACHC was about 1.5. When pH < pHpzc, MB0 was the main part of MB and the ACHC surface was positively charged, which would generate electrostatic repulsion with tiny dissociated MB+, hindering the adsorption of MB by ACHC, and excess H+ would compete with MB+ for adsorption sites, which was unfavorable to the adsorption of MB by ACHC [38]. Meanwhile, phenothiazine’s N atoms in MB were easily protonated at low pH levels, which hindered adsorption by hydrogen bonding [29]. When pH > pHpzc, functional groups on the ACHC surface were prone to deprotonation so that ACHC was negatively charged and MB almost existed as MB+, which promoted the electrostatic attraction between ACHC and MB, promoting ACHC to adsorb MB [38]. Simultaneously, there was a higher probability of hydrogen bonding between phenothiazine’s N atoms in MB and the C-OH in ACHC [5].
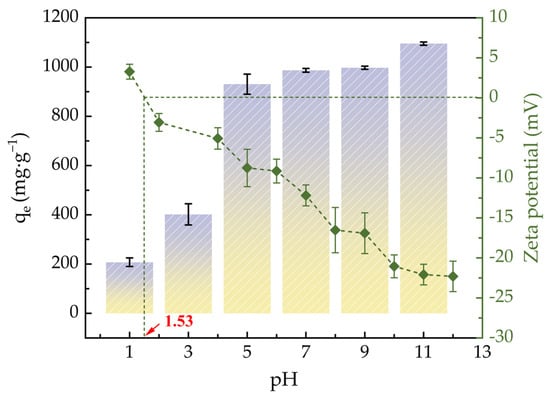
Figure 4.
The pH’s effects on zeta potential and adsorption capacity (qe means adsorption amount at adsorption equilibrium) of ACHC (bar graph refers to left axis, line graph refers to right axis).
2.3. Adsorption Capacity Comparison
Figure 5 shows the MB adsorption capacity comparison of SCB, HC, activated hydrochar (AHC), ACHC, and activated carbon (AC). AC is a kind of commercial adsorbent, and we used AC to compare adsorption capacity with our materials. The adsorption capacities for MB were 51.36, 77.23, 130.68, 986.73, and 310.87 mg·g−1, respectively. The MB adsorption ability of HC was slightly higher than SCB because the aromaticity and porosity of HC were improved during the HTC process in a phosphoric acid medium. The MB adsorption ability of AHC was slightly higher than HC due to the NaOH activation. And the adsorption capacity of ACHC was much higher than HC, mainly because of the introduction of functional groups, demonstrating that modification was greatly necessary. Furthermore, the adsorption capacity of ACHC was also much higher than AC, confirming that adsorbent ACHC can outperform commercial activated carbon. Table 2 summarizes MB’s qm (the maximum equilibrium adsorption capacity) on different adsorbents, and ACHC’s maximum adsorption capability was top-ranked.
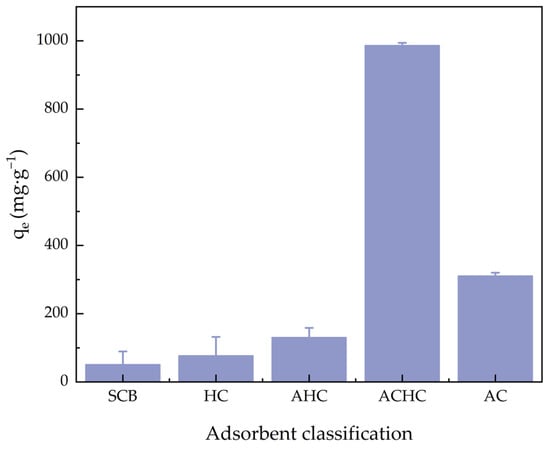
Figure 5.
Comparison of adsorption capacity for MB of SCB, HC, AHC, ACHC, and AC.

Table 2.
Comparison of adsorption capacity for MB with reported results in literature.
Figure 6 shows the comparison of ACHC adsorption capacity on different dyes. In the multiple dye system, which contained three different dyes at the same time, the adsorption capacity of methylene blue (MB), methyl orange (MO), and malachite green (MG) was 1080.46, 1227.29, and 174.20 mg·g−1, respectively. The π-π stacking between MB and MO may participate in the adsorption of MB, and the MB molecules adsorbed by the adsorbent may become new adsorption sites for MO adsorption due to the synergistic effect [43,44]. Furthermore, our modification introduced many carboxylate groups and amino groups, and a predecessor’s study showed that carboxylate groups were beneficial for the adsorption of MB and the amino groups were beneficial for the adsorption of MO [45,46]. And the poor adsorption capacity of MG may be relevant to its big molecular weight, because small dye molecules were more easily able to enter the pores of the adsorbent [47].
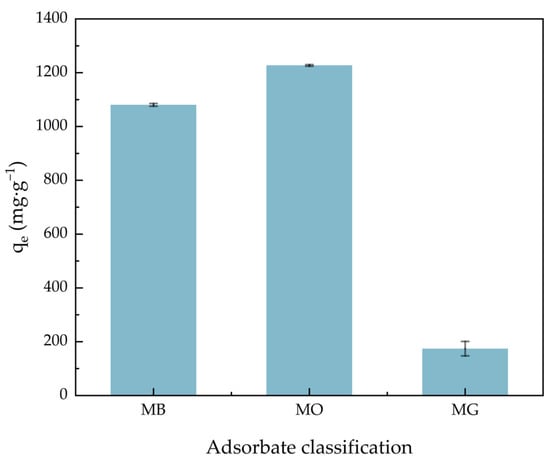
Figure 6.
Comparison of ACHC adsorption ability for MB, MO, and MG.
2.4. Adsorption Isotherm Models
Fitting curves of adsorption at three distinct temperatures are depicted in Figure 7a. As the value of Ce increased, qe increased rapidly, then slowly, and eventually reached a plateau. As temperature rose, qm rose as well, indicating that adsorption was essentially endothermic. Whereas the Freundlich model simulated heterogeneous multilayer adsorption behavior, the Langmuir model imitated homogeneous monolayer adsorption behavior [48].
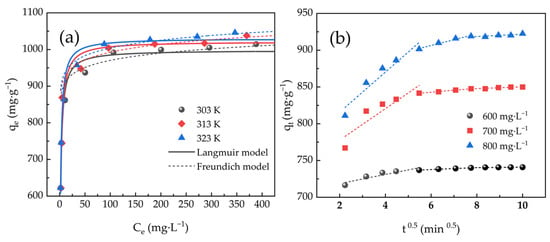
Figure 7.
(a) Langmuir and Freundlich fitting curves of ACHC/MB and (b) linearity of IPD model for adsorption.
The equation of the Langmuir isotherm model is [49]:
where qe (mg·g−1) means adsorption amount at adsorption equilibrium; qm (mg·g−1) means the maximum equilibrium adsorption capacity; KL (L·mg−1) stands for the adsorption constant of the Langmuir isotherm model; Ce (mg·g−1) represents residual dye concentration at equilibrium adsorption.
The equation of the Freundlich isotherm model is [49]:
where KF (mg·g−1) means constant of Freundlich isotherm model; n stands for constant that is used to assess how adsorbent and adsorbate interact.
Table 3 displays parameters of the adsorption isotherm model, and parameters were calculated from fitting curves. The Langmuir model’s correlation coefficients (R2) were greater than the Freundlich model’s R2, and qm of MB adsorbed by ACHC corresponding to the Langmuir model were 1017.29, 1048.22, and 1060.45 mg·g−1 respectively when at 303, 313, and 323 K, which were consistent with actual experimental data. Therefore, the Langmuir model characterized adsorption more accurately than Freundlich did, supposing it was homogeneous monolayer adsorption [29].

Table 3.
Isotherm model parameters.
2.5. Adsorption Mass Transfer Model
Intra-particle diffusion (IPD) model of MB adsorbed by ACHC was presented in Figure 7b, which describes a multi-level linear relationship deviating from the origin, supposing adsorption was involved in multiple mechanisms.
The IPD model derived by Weber and Morris is [50]:
where qt (mg·g−1) represents adsorption capacity at time t; KIPD (mg·g−1·min−0.5) means IPD rate constant; c (mg·g−1) stands for linear graph intercept of qt versus t0.5.
Table 4 displays parameters of the IPD model used for adsorption. At the three distinct MB initial concentrations, the IPD model’s R2 were all high (R2 > 0.644), indicating that IPD would affect adsorption rate. Multiple stages governed the mass transfer process, as evidenced by the multilinear relationship deviating from the origin that the IPD model exhibited. The concentration difference between the substrate solution and the adsorbent surface drove the first step of external diffusion, where the adsorbate penetrated the liquid layer around the adsorbent. Adsorbent dispersed inside its pores during the second step, known as internal diffusion. Adsorbate bonded to its active site in the third step, which was the last equilibrium stage. The rate-limiting process was not limited to internal diffusion alone [38].

Table 4.
IPD model parameters.
2.6. Adsorption Thermodynamics Model
Thermodynamic parameters include Gibbs free energy (ΔG), enthalpy (ΔH), entropy (ΔS), and so on. Van’t Hoff’s equation is used to calculate thermodynamic parameters.
Van’t Hoff’s equation is [51]:
where K means adsorption distribution coefficient; ∆G (kJ·mol−1) stands for Gibbs free energy; R (8.314 J·mol−1·K−1) represents universal gas constant; T (K) denotes absolute temperature; ∆H (kJ·mol−1) is enthalpy; and ∆S (kJ·mol−1·K−1) is entropy.
Thermodynamic parameters are summarized in Table 5. A positive ∆H value (5.81 kJ·mol−1) suggested adsorption was facilitated by rising temperature; that is, adsorption of MB by ACHC was endothermic, which was consistent with the isotherm model conclusion; a positive ∆S value (13.38 J·mol−1·K−1) suggested strong randomness of the solid-liquid interface in adsorption; and a negative ∆G value (−4.05~4.70 kJ·mol−1) suggested adsorption was spontaneous [16]. Physical adsorption occurs when ΔG is between −20 and 0 kJ·mol−1, and chemical adsorption occurs between −400 and −80 kJ·mol−1 [52]. The ∆G value obtained from the adsorption thermodynamic analysis was between −4.70 and −4.05 kJ·mol−1, indicating that adsorption was physical. We noticed that the nonlinear form of the thermodynamic model was better than its linear form when it was used for adsorption analysis; however, the difference in the magnitude of the thermodynamic parameters (∆H and ∆S) calculated between these two forms was not remarkable [53].

Table 5.
Thermodynamic model parameters.
2.7. Density Functional Theory (DFT) Calculations
To see how the molecules of MB and ACHC interacted, DFT calculations were performed. The 24-ring graphene structure was employed to represent HC [54], and functional groups on the graphene structure were proposed based on FTIR and XPS study results as discussed earlier. Oxygen-containing functional group C=O and nitrogen-containing group pyrrolic N were the two main functional groups identified; therefore, the pristine 24-ring graphene and that with C=O and pyrrolic N groups were used in this study. A 24-ring graphene represented HC, while both 24-ring graphene with C=O and 24-ring graphene with pyrrolic N represented ACHC. And the surface electrostatic potential (ESP) of the models was provided based on the geometry-optimized structures (Figure 8). As shown in Figure 8, the electrical distribution of molecules was represented and reaction sites were predicted, reflecting the electrostatic interactions between molecules [42,55]. Electrostatic potential (ESP) values increased from −60 to 60 kcal/mol while the color changed from blue to red, signifying positively and negatively charged areas of the molecule [42,56]. The negatively charged C=O site and the slightly positively charged pyrrolic N site on the surface of the graphene ring could be the active sites for adsorption of the ionized MB molecules (MB+) and the unionized MB molecules (MB0), respectively. MB ionization in aqueous solution was a spontaneous process that arose from the electrophilic -N(CH3)2 groups that existed in the MB0 and MB+, was the dominant form in our batch adsorption experiments; hence, the positively charged MB+ was used in the following calculations [42,55,57]. There was an electrostatic attraction between MB and pristine graphene/modified graphene, because the electron distribution in MB molecule created a positively charged molecular surface and pristine graphene/modified graphene formed a negatively charged molecular surface [54,58]. Notably, the oxygenated functional group (C=O) formed a preferentially negative surface, which enhanced the electrostatic attraction. FTIR analysis mentioned above also demonstrated electrostatic attraction.
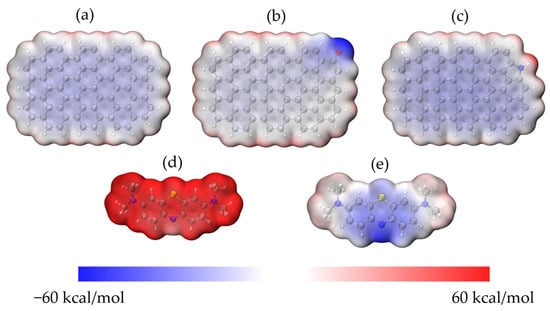
Figure 8.
ESP surfaces of (a) graphene; (b) carbonyl-graphene; (c) pyrrolic nitrogen-graphene; (d) MB+ and (e) MB0. Grey: carbon, white: hydrogen, red: oxygen, blue: nitrogen, and yellow: sulfur.
Optimized configurations are listed in Figure 9, with binding energy or adsorption energy calculated. With an affinity of only −171.77 kJ/mol, MB had the lowest affinity to pristine graphene. Graphene grafted with the two functional groups showed improved interactions with MB in different degrees [54], with the pyrrolic N functionalized graphene providing the best adsorption of MB, of which the adsorption energy was −183.39 kJ/mol. The improved adsorption performance of oxygen-containing and nitrogen-containing functional groups modified graphene explained that the polyaminocarboxylated modification had improved adsorption capacity [49]. And even the pristine graphene structure also showed satisfactory adsorption energy. The strong polarization effect of π-electron density may be the cause of the strong interaction between pristine graphene/modified graphene [59,60]. If adsorption energy was lower than −30 kJ/mol, the interactions between the pristine graphene/modified graphene and MB can be classified as physical adsorption, and the interactions can be regarded as chemisorption behavior if adsorption energy was higher than −50 kJ/mol [49,61]. Adsorption energy was completely negative and lower than −50 kJ/mol for pristine and modified graphene, indicating that the interactions were physical adsorption, which was consistent with adsorption thermodynamics analysis.
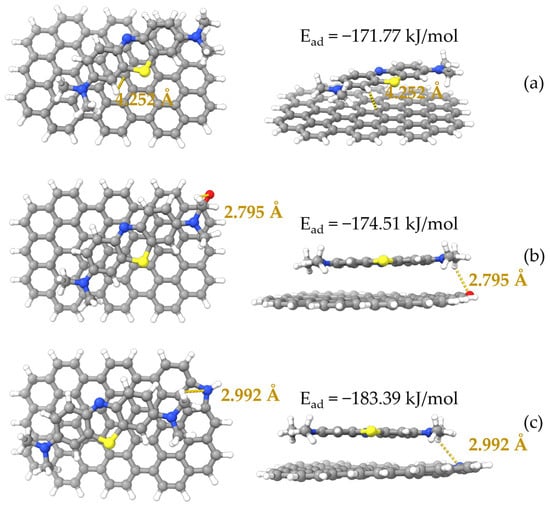
Figure 9.
Optimized geometries of MB adsorbed on different graphene surfaces. (a) MB/graphene; (b) MB/carbonyl-graphene; and (c) MB/pyrrolic nitrogen-graphene. Grey: carbon, white: hydrogen, red: oxygen, blue: nitrogen and yellow: sulfur.
Figure 9 also shows the results of hydrogen bonding calculation for the model after MB adsorption. Functional groups (C=O, pyrrolic N) of graphene had hydrogen bond distances of 2.795 Å and 2.992 Å, supposing adsorbate could be adsorbed onto modified graphene because of hydrogen bonding [42]. The FTIR analysis showed that there was hydrogen bonding between ACHC’s hydroxyl groups and the N atom in the phenothiazine ring of MB [9,29]. It is worthwhile to mention that graphite phases may be generated by HTC of glucose, even if π-π stacking interactions between MB and graphene were not calculated [58,59,60,61,62]. It is possible to anticipate the occurrence of π-π stacking between aromatic phases in ACHC and MB based on characteristics of ACHC precursor material [62], agreeing with the FTIR analysis above.
2.8. Adsorption Mechanisms
SEM analysis displayed that MB could be successfully adsorbed by ACHC. Pollutant removal performance may be improved by the rough surface, adequate cracks, channels, and pores, as well as the loaded functional groups [63]. The BET study showed that polyaminocarboxylated modification had no improvement on the pore structure. But the mesoporous structure was not completely clogged by grafting functional groups, which implied that modification might lead to higher adsorption capacity. FTIR analysis showed that adsorption of MB by ACHC involved hydrogen bonding and π-π interaction together with electrostatic attraction. After adsorption, migration of the C-H characteristic peak indicated the existence of hydrogen bond association. The migration of the C=N characteristic peak and the migration of the C-H stretching vibration indicated the existence of π-π interaction, and the stretching vibrations of C-N and symmetric CH3 indicated the presence of electrostatic attraction. XRD analysis showed that crystallinity changed after adsorption of MB onto ACHC, illustrating that ACHC had successfully adsorbed MB. XPS analysis demonstrated that ACHC revealed a high concentration of oxygen-containing groups and nitrogen-containing groups; it also revealed the successful adsorption for MB.
Adsorption isotherm model analysis revealed that adsorption followed the Langmuir model, which supposed adsorption was a homogeneous monolayer. And the adsorption was affected by the rate of IPD. Thermodynamic analysis suggested adsorption was physical, endothermic, and spontaneous, and randomness of the solid-liquid interface was strong. The pH analysis showed that electrostatic repulsion between tiny dissociated MB+ and positively charged ACHC and competition between tiny dissociated MB+ and excessive H+ at adsorption sites would hinder the adsorption of MB by ACHC, while electrostatic attraction between MB+ and negatively charged ACHC would facilitate adsorption. And DFT analysis showed that there was both physical and chemical adsorption between ACHC and MB.
2.9. Adsorbent Regeneration
Figure 10 shows how the quantity of ACHC regeneration cycles affected MBs adsorption efficiency. Adsorption capacity was still high after the adsorption-desorption cycle, and it remained high at 665.43 mg·g−1 after five adsorption-desorption cycles, indicating that ACHC could be used to remove MB repeatedly and it had a great recovery capacity. Perhaps it was because MB did not bind to the strongest binding site, and the porous structure of ACHC provided space for MB desorption diffusion [64].
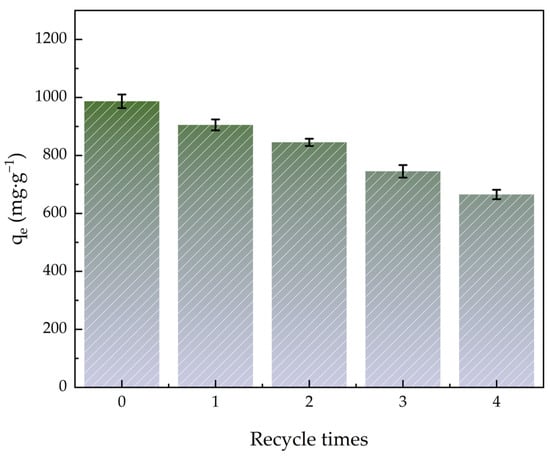
Figure 10.
Effect of quantity of regeneration cycles on adsorption efficiency.
3. Materials and Methods
3.1. Materials
Sugarcane bagasse (SCB) was sourced from Guangxi Fengtang Biochemical Co., Ltd. (Liuzhou, China). Phosphoric acid, sodium hydroxide, hydrochloric acid, methylene blue (MB), and malachite green (MG) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Anhydrous ethanol was procured from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). Epichlorohydrin, diethylenetriamine, and chloroacetic acid were supplied by Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Activated carbon (AC) was sourced from Dongguan Hongsheng Activated Carbon Co., Ltd. (Dongguan, China). Methyl orange (MO) was obtained from Tianjin Damao Chemical Reagent Factory (Tianjin, China).
3.2. Preparation of Sugarcane Bagasse Hydrochar (HC)
SCB powder and a 1.5 mol·L−1 phosphoric acid solution were mixed in a Teflon-lined autoclave with a mass ratio of 1:19. The reactor was placed in a muffle furnace and reacted at 513 K for 10 h. After reaction, the reactor was taken out and cooled to room temperature, then the mixture was filtered and washed with deionized water and anhydrous ethanol until the extracted filtrate was nearly colorless; finally, the solid was dried to constant weight at 378 K and was denoted as HC. The HC was further ground and screened by a 100-mesh sieve (diameter = 0.15 mm) and was stored in a brown glass bottle for further use.
3.3. Preparation of Polyaminocarboxylated Modified Hydrochar (ACHC)
ACHC was synthesized referring to the method described in previous reports from predecessors’ literature, as Figure 11 [17]. HC (2.5 g) was put in 50 mL of 1.5 M NaOH solution and then agitated at 150 rpm for 2 h at 298 K, followed by vacuum filtration, repeated washing, and drying at 353 K overnight to constant weight. The obtained product was ground using an agate mortar, passed through a 100-mesh sieve, sealed, and stored in a brown bottle, and was denoted as Activated hydrochar (AHC). Three steps, including etherification, amination, and carboxylation, were then performed to obtain ACHC. Specifically, 20 g AHC was mixed with 100 mL epichlorohydrin and then reacted under vigorous stirring at 353 K for 4 h, filtered, washed, and dried at 333 K to steady mass. The obtained product was denoted as etherified hydrochar. This was further subjected to the amination treatment, where etherified hydrochar was added to the mixture of water/diethylenetriamine (v/v of 1:1) and agitated at 333 K for 4 h, filtered, washed, and dried at 333 K to stable mass and designated as aminated hydrochar. The aminated hydrochar was then further subjected to the carboxylation procedure, where 15 g of aminated hydrochar and 25 g of sodium hydroxide were mixed with 50 mL of deionized water and 200 mL of anhydrous ethanol, stirred for 10 min at ambient temperature. This was followed by adding a solution containing 60 g chloroacetic acid and 100 mL anhydrous ethanol dropwise within 60 min and reacting under stirring at 333 K for 4 h. The reaction system’s pH was kept above 9.0 by adding 0.1 M sodium hydroxide. After reaction, the product was filtered, washed, and dried at 333 K to a consistent mass, thereby obtaining polyaminocarboxylated hydrochar (ACHC).
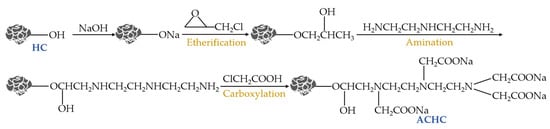
Figure 11.
Synthetic route of ACHC.
3.4. Characterization of Carbon Materials
Structural properties were measured by scanning electron microscopy (SEM; Tescan Mira Lms, Tescan, China) at an acceleration voltage of 5.00 kV. A specific surface area and porosity meter (BET; ASAP 2460, Micromeritics, USA) was employed to generate N2 adsorption-desorption isotherms to study specific surface area, pore volume, as well as pore size. Surface functional groups were characterized by using Fourier transform infrared spectroscopy (FTIR; TENSOR II, Bruker, Germany) at resolution of 6 cm−1. Crystallinity was analyzed by X-ray diffractometer (XRD) with Cu-Kα as the radiation source in a scanning range of 2θ = 5–85°. Composition and chemical state were studied by X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, ThermoFisher, USA). The data obtained was calibrated against the standard C1s binding energy set at 284.80 eV. Zeta potential at different pH was determined by using a zeta potential analyzer (zeta potential; NanoBrook Omni, Brookhaven, USA).
3.5. Batch Adsorption Experiments
To a 100 mL conical flask, 50 mL of MB of specified concentration was added. The dosage of ACHC in each bottle was 40 mg. An orbital shaker was used to shake the sealed conical flasks at a fixed temperature for a set amount of time while maintaining an invariable speed of 150 rpm in an incubator. After adsorption, ACHC was filtered with a 0.45 μm microporous filter membrane. Adsorption efficiency was determined by recording absorbance at 665 nm of filtrate, and MB concentration in filtrate was calculated based on the MB standard curve.
Effects of pH: Ten portions of 50 mL of 1000 mg·L−1 MB were fabricated, and the pH was reset to 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 using 0.1 M sodium hydroxide or hydrochloric acid. To the flasks, 40 mg ACHC was added, and the flask was oscillated at 303 K for 24 h, followed by determining MB concentration in the filtrate. A zeta potential analyzer was used to determine the zeta potential of the adsorbent. Three parallel experiments were conducted.
Adsorption comparison among adsorbents of SCB, HC, AHC, ACHC, and AC: To 100 mL conical flasks, 50 mL MB solution of 1000 mg·L−1 was added, respectively. The pH was reset to 7.0 using 0.1 M sodium hydroxide or hydrochloric acid. The dosage of adsorbent in each bottle was 40 mg. An orbital shaker was used to shake the sealed conical flasks at 303 K for 24 h at 150 rpm, followed by filtering the solution with a 0.45 μm filter membrane. Adsorption efficiency was determined by recording absorbance at 665 nm of filtrate, and concentration was calculated based on the standard curve. Three parallel experiments were conducted.
Adsorption comparison among adsorbates of MB, MO, and MG: To a 100 mL conical flask, 50 mL solution was added. The MB, MO, and MG concentrations in the solution were 1000 mg·L−1, respectively. The pH was reset to 7.0 using 0.1 M sodium hydroxide or hydrochloric acid. The dosage of adsorbent ACHC in each bottle was 40 mg. An orbital shaker was used to shake the sealed conical flasks at 303 K for 24 h at 150 rpm, followed by filtering the solution with a 0.45 μm filter membrane. Adsorption efficiency was determined by recording absorbance at 665 nm (MB), 464 nm (MO), and 620 nm (MG) of filtrate, and concentration was calculated based on standard curves. Three parallel experiments were conducted.
Adsorption performance: The two following formulas were used to calculate adsorption capacity at adsorption equilibrium and time t [49].
where qe (mg·g−1) means adsorption amount at adsorption equilibrium; C0 (mg·g−1) stands for dye starting concentration; Ce (mg·g−1) means residual dye concentration at equilibrium adsorption; V (L) denotes dye volume; m (g) is adsorbent mass; qt (mg·g−1) means adsorption capacity at time t; and Ct (mg·g−1) is residual dye concentration at time t (min).
Adsorption isotherm study: 50 mL MB of 500, 600, 700, 800, 900, 1000, 1100, and 1200 mg·L−1 was placed in a flask. The pH was reset to 7.0, and 40 mg ACHC was added. The flask was oscillated at 303, 313, and 323 K for 24 h and filtered. MB concentration in filtrate was measured. Adsorption capacity was calculated when the adsorption reached equilibrium.
The adsorbent regeneration behavior was also observed. For 24 h, at 303 K, ACHC (40 mg) was added to MB (50 mL), which had an initial pH of 7.0 and a starting concentration of 1 mg·mL−1. Adsorbent was repeatedly eluted with a 0.1 M hydrochloric acid solution and then dried after being repeatedly washed and filtered with 0.1 M sodium hydroxide and deionized water. Three parallel experiments were conducted.
3.6. Density Functional Theory (DFT) Calculations
Gaussian 16, A03, was the software program used for all DFT computations [65]. With the PCM solvation model of water and the B3LYP functional and 6–31 G* base, geometry optimization calculations were carried out with Grimme dispersion corrections (GD3BJ). The B3LYP functional and a bigger 6–311 G* base with the SMD solvation model of water, containing Grimme dispersion corrections (GD3BJ), were then used as the foundation for singlet point energy calculations. The Multiwfn 3.8 (dev) program assisted in achieving the ESP [66].
4. Conclusions
The ACHC prepared by activation, etherification, amination, and carboxylation of SCB after HTC treatment had extraordinary ability to adsorb MB. SEM analysis demonstrated that ACHC provided more adsorption sites for MB adsorption when compared with HC. Although BET analysis demonstrated that modification did not improve the pore structure, FTIR and XPS analysis showed that modification introduced plenty of oxygen- and nitrogen-containing groups, which were very beneficial for improving adsorption capacity. XRD analysis confirmed that MB was successfully adsorbed by ACHC. According to adsorption models, the adsorption was endothermic spontaneous homogeneous monolayer adsorption dominated by both chemical adsorption and physical adsorption with intra-particle diffusion. DFT calculations revealed that C=O and pyrrolic N modification improved adsorption energy and the adsorption involved electrostatic attraction, hydrogen bonding, and π-π interaction, conforming with characterization results. ACHC not only had high adsorption capacity but also displayed good regeneration performance, indicating that ACHC might be utilized as an excellent adsorbent for adsorbing MB in wastewater.
Author Contributions
Conceptualization, X.L. and F.Z.; data curation, X.L. and F.Z.; formal analysis, X.L.; funding acquisition, C.X.; investigation, F.Z.; methodology, X.L.; project administration, C.X.; software, X.L., F.Z., C.S., and J.R.; supervision, X.L., F.Z., C.S., J.R., F.H., and C.X.; validation, X.L.; visualization, X.L. and Z.L.; writing—original draft, F.Z.; writing—review and editing, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Program of Agriculture Research System of China (CARS-17), the Natural Science Foundation of China (no. 32160570), and the Research Programs of the Science and Technology of Guangxi Zhuang Autonomous (AA22117015-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors extend their gratitude to funding supports and they also extend their gratitude to the Theoretical and Computational Chemistry Team from Shiyanjia Lab (www.shiyanjia.com) for providing invaluable assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, F. Effect of Oxygen-Containing Functional Groups on the Adsorption of Cationic Dye by Magnetic Graphene Nanosheets. Chem. Eng. Res. Des. 2017, 128, 155–161. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Ma, G.; Kong, D.; Xiong, W.; Wang, J.; Zhu, Y.; Xia, Y. Heteroatom-Doped Porous Carbons with Enhanced Carbon Dioxide Uptake and Excellent Methylene Blue Adsorption Capacities. Microporous Mesoporous Mater. 2018, 257, 1–8. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of Methylene Blue by Adsorption onto Activated Carbon Developed from Ficus Carica Bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Liu, J.-L.; Qian, W.-C.; Guo, J.-Z.; Shen, Y.; Li, B. Selective Removal of Anionic and Cationic Dyes by Magnetic Fe3O4-Loaded Amine-Modified Hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Li, H.-Z.; Zhang, Y.-N.; Guo, J.-Z.; Lv, J.-Q.; Huan, W.-W.; Li, B. Preparation of Hydrochar with High Adsorption Performance for Methylene Blue by Co-Hydrothermal Carbonization of Polyvinyl Chloride and Bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Li, X.; Bonjour, E.; Jame, P.; Kuzhir, P.; Hurel, C. Production of Hydrochar from Biomass Waste as Economical Adsorbents for Methylene Blue—Insight of Occurring Adsorption Phenomena. Biomass Convers. Biorefinery 2024, 14, 19723–19737. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and Photocatalysis of Methylene Blue Dye Using Various Nanocatalytic Assemblies—A Critical Review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A Review on Modified Sugarcane Bagasse Biosorbent for Removal of Dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Lv, B.-W.; Xu, H.; Guo, J.-Z.; Bai, L.-Q.; Li, B. Efficient Adsorption of Methylene Blue on Carboxylate-Rich Hydrochar Prepared by One-Step Hydrothermal Carbonization of Bamboo and Acrylic Acid with Ammonium Persulphate. J. Hazard. Mater. 2022, 421, 126741. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and Characterization of Hydrochar from Waste Eucalyptus Bark by Hydrothermal Carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Peng, J.; Kang, X.; Zhao, S.; Zhao, P.; Ragauskas, A.J.; Si, C.; Xu, T.; Song, X. Growth Mechanism of Glucose-Based Hydrochar under the Effects of Acid and Temperature Regulation. J. Colloid Interface Sci. 2023, 630, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane Bagasse Pyrolysis: A Review of Operating Conditions and Products Properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with Excellent Pb(II) Adsorption Property Produced from Fresh and Dehydrated Banana Peels via Hydrothermal Carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Buapeth, P.; Watcharin, W.; Dechtrirat, D.; Chuenchom, L. Carbon Adsorbents from Sugarcane Bagasse Prepared through Hydrothermal Carbonization for Adsorption of Methylene Blue: Effect of Heat Treatment on Adsorption Efficiency. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012003. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S.; Ismail, H. Sugarcane Bagasse Fiber and Its Cellulose Nanocrystals for Polymer Reinforcement and Heavy Metal Adsorbent: A Review. Cellulose 2018, 25, 4303–4330. [Google Scholar] [CrossRef]
- Qian, W.-C.; Luo, X.-P.; Wang, X.; Guo, M.; Li, B. Removal of Methylene Blue from Aqueous Solution by Modified Bamboo Hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.-Q.; Guo, J.-Z.; Fu, S.-Y.; Guo, M.; Yang, P. The Polyaminocarboxylated Modified Hydrochar for Efficient Capturing Methylene Blue and Cu(II) from Water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Zhao, Q.; Liu, X.; Rinklebe, J.; Ren, H. Ammonium Nitrogen Recovery from Digestate by Hydrothermal Pretreatment Followed by Activated Hydrochar Sorption. Chem. Eng. J. 2020, 379, 122254. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Optimized Production and Characterization of Highly Porous Activated Carbon from Algal-Derived Hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, H.; Sheng, K.; Mustafa, A.M.; Yu, Y. Evaluation of Engineered Hydrochar from KMnO4 Treated Bamboo Residues: Physicochemical Properties, Hygroscopic Dynamics, and Morphology. Bioresour. Technol. 2018, 250, 806–811. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper Removal from Aqueous Solution Using Biochar: Effect of Chemical Activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Wang, R.-F.; Deng, L.-G.; Li, K.; Fan, X.-J.; Li, W.; Lu, H.-Q. Fabrication and Characterization of Sugarcane Bagasse–Calcium Carbonate Composite for the Efficient Removal of Crystal Violet Dye from Wastewater. Ceram. Int. 2020, 46, 27484–27492. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Guo, J.-Z.; Wu, C.; Huan, W.-W.; Chen, L.; Li, B. Enhanced Removal of Cr(VI) by Cation Functionalized Bamboo Hydrochar. Bioresour. Technol. 2022, 347, 126703. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, B.; Olivares-Marín, M.; Álvarez-Murillo, A.; Roman, S.; Nabais, J.M.V. Method for Promoting in-Situ Hydrochar Porosity in Hydrothermal Carbonization of Almond Shells with Air Activation. J. Supercrit. Fluids 2018, 138, 187–192. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Opanasenko, M.; Čejka, J. A Novel Nickel Metal–Organic Framework with Fluorite-like Structure: Gas Adsorption Properties and Catalytic Activity in Knoevenagel Condensation. Dalton Trans. 2014, 43, 3730–3738. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Xie, X.; Gao, H.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z. Polyethyleneimine Modified Activated Carbon for Adsorption of Cd(II) in Aqueous Solution. J. Environ. Chem. Eng. 2019, 7, 103183. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of Methylene Blue and Cd(II) onto Maleylated Modified Hydrochar from Water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Li, H.; Li, S.; Cheng, K.; Li, J.-S.; Tsang, D.C.W. Corn Straw-Derived Biochar Impregnated with α-FeOOH Nanorods for Highly Effective Copper Removal. Chem. Eng. J. 2018, 348, 191–201. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Al-Muhtaseb, A.H.; Khan, A.A.; Khan, A. Effective and Fast Adsorptive Removal of Toxic Cationic Dye (MB) from Aqueous Medium Using Amino-Functionalized Magnetic Multiwall Carbon Nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Quan, X.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. Polyethyleneimine (PEI) Incorporated Cu-BTC Composites: Extended Applications in Ultra-High Efficient Removal of Congo Red. J. Solid State Chem. 2019, 270, 231–241. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A Green Biochar/Iron Oxide Composite for Methylene Blue Removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Liu, Y. Structural Properties and Adsorption Performance Relationship towards Three Categories of Lignin and Their Derived Biochar. Bioresour. Technol. 2024, 401, 130712. [Google Scholar] [CrossRef]
- Karimi, H.; Heidari, M.A.; Emrooz, H.B.M.; Shokouhimehr, M. Carbonization Temperature Effects on Adsorption Performance of Metal-Organic Framework Derived Nanoporous Carbon for Removal of Methylene Blue from Wastewater; Experimental and Spectrometry Study. Diam. Relat. Mater. 2020, 108, 107999. [Google Scholar] [CrossRef]
- Su, X.; Wang, X.; Ge, Z.; Bao, Z.; Lin, L.; Chen, Y.; Dai, W.; Sun, Y.; Yuan, H.; Yang, W.; et al. KOH-Activated Biochar and Chitosan Composites for Efficient Adsorption of Industrial Dye Pollutants. Chem. Eng. J. 2024, 486, 150387. [Google Scholar] [CrossRef]
- Zhou, F.; Li, K.; Hang, F.; Zhang, Z.; Chen, P.; Wei, L.; Xie, C. Efficient Removal of Methylene Blue by Activated Hydrochar Prepared by Hydrothermal Carbonization and NaOH Activation of Sugarcane Bagasse and Phosphoric Acid. RSC Adv. 2022, 12, 1885–1896. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Yang, R.; Wang, C. Branched Polyethylenimine Grafted Electrospun Polyacrylonitrile Fiber Membrane: A Novel and Effective Adsorbent for Cr(VI) Remediation in Wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
- Ayedi, N.; Rzig, B.; Bellakhal, N. Catalytic Hydrothermal Carbonization of Olive Wood Charcoal for Methylene Blue Adsorption from Wastewater: Optimization, Isotherm, Kinetic and Thermodynamic Studies. Chem. Afr. 2023, 6, 765–778. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Yu, X. Fabricating Porous Carbon Materials by One-Step Hydrothermal Carbonization of Glucose. Processes 2023, 11, 1923. [Google Scholar] [CrossRef]
- Hessien, M. Methylene Blue Dye Adsorption on Iron Oxide-Hydrochar Composite Synthesized via a Facile Microwave-Assisted Hydrothermal Carbonization of Pomegranate Peels’ Waste. Molecules 2023, 28, 4526. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Gao, L.; Li, S.; Ma, Z.; Li, L.; Hu, M. Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue. Sustainability 2023, 15, 11867. [Google Scholar] [CrossRef]
- An, Y.; Zheng, H.; Yu, Z.; Sun, Y.; Wang, Y.; Zhao, C.; Ding, W. Functioned Hollow Glass Microsphere as a Self-Floating Adsorbent: Rapid and High-Efficient Removal of Anionic Dye. J. Hazard. Mater. 2020, 381, 120971. [Google Scholar] [CrossRef]
- Wang, S.; Ng, C.W.; Wang, W.; Li, Q.; Hao, Z. Synergistic and Competitive Adsorption of Organic Dyes on Multiwalled Carbon Nanotubes. Chem. Eng. J. 2012, 197, 34–40. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Rajendra Kumar, R.T. Adsorption Behaviour of Reduced Graphene Oxide towards Cationic and Anionic Dyes: Co-Action of Electrostatic and π-π Interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective Adsorption and Separation of Organic Dyes Using Functionalized Cellulose Nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- Hadi, P.; Guo, J.; Barford, J.; McKay, G. Multilayer Dye Adsorption in Activated Carbons—Facile Approach to Exploit Vacant Sites and Interlayer Charge Interaction. Environ. Sci. Technol. 2016, 50, 5041–5049. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced Adsorption of Pb(II) onto Modified Hydrochar: Modeling and Mechanism Analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef]
- Wang, S.; Xie, C.; Wang, S.; Hang, F.; Li, W.; Li, K.; Mann, A.; Sarina, S.; Doherty, W.; Shi, C. Facile Ultrasonic-Assisted One-Step Preparation of Sugarcane Bagasse Carbon Sorbent for Bio-Based Odor Removal Cat Litter Formulation. Ind. Crops Prod. 2022, 187, 115493. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Kong, L.; Zhang, J.; Zuo, W.; Li, Y.; Cai, G. A Novel 3D Superelastic Polyethyleneimine Functionalized Chitosan Aerogels for Selective Removal of Cr(VI) from Aqueous Solution: Performance and Mechanisms. Chem. Eng. J. 2021, 425, 131722. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.; Hou, L.; Shi, C.; Li, K.; Hang, F.; Xie, C. Amine-Functionalized MOF-Derived Carbon Materials for Efficient Removal of Congo Red Dye from Aqueous Solutions: Simulation and Adsorption Studies. RSC Adv. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an Anionic Dye from Aqueous Medium by Organoclays: Equilibrium Modeling, Kinetic and Thermodynamic Exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the Nonlinear and Linear Forms of the van’t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Shi, C.; Yan, B.; Gong, L.; Chen, J.; Xiang, L.; Xu, H.; Liu, Q.; Zeng, H. Unraveling the Molecular Interaction Mechanism between Graphene Oxide and Aromatic Organic Compounds with Implications on Wastewater Treatment. Chem. Eng. J. 2019, 358, 842–849. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.; Cheng, X.; Ling, Q.; Chen, H.; Barati, B.; Yao, Q.; Abomohra, A.; Hu, X.; Bartocci, P.; et al. A Mechanism Study of Methylene Blue Adsorption on Seaweed Biomass Derived Carbon: From Macroscopic to Microscopic Scale. Process Saf. Environ. Prot. 2023, 172, 1132–1143. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, L.; Yu, H.; Ren, J.; Peng, D.; Zhang, L.; Meng, P. Multiple Adsorption Systems and Electron-Scale Insights into the High Efficiency Coadsorption of a Novel Assembled Cellulose via Experiments and DFT Calculations. J. Hazard. Mater. 2021, 416, 125748. [Google Scholar] [CrossRef] [PubMed]
- Allangawi, A.; Aziz Aljar, M.A.; Ayub, K.; El-Fattah, A.A.; Mahmood, T. Removal of Methylene Blue by Using Sodium Alginate-Based Hydrogel; Validation of Experimental Findings via DFT Calculations. J. Mol. Graph. Model. 2023, 122, 108468. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.B.; Tompsett, G.A.; Partopour, B.; Deskins, N.A.; Timko, M.T. Hydrochar Structural Determination from Artifact-Free Raman Analysis. Carbon 2020, 167, 378–387. [Google Scholar] [CrossRef]
- Reynosa-Martínez, A.C.; Tovar, G.N.; Gallegos, W.R.; Rodríguez-Meléndez, H.; Torres-Cadena, R.; Mondragón-Solórzano, G.; Barroso-Flores, J.; Alvarez-Lemus, M.A.; Montalvo, V.G.; López-Honorato, E. Effect of the Degree of Oxidation of Graphene Oxide on As(III) Adsorption. J. Hazard. Mater. 2020, 384, 121440. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, Y.; Liu, X.; Mu, L.; Zhu, J. Sorption Mechanism of Organic Dyes on a Novel Self-Nitrogen-Doped Porous Graphite Biochar: Coupling DFT Calculations with Experiments. Chem. Eng. Sci. 2021, 242, 116739. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Yang, Y.; Zhao, Y.; Zheng, Y. The Role of SO2 in Arsenic Removal by Carbon-Based Sorbents: A DFT Study. Chem. Eng. J. 2021, 410, 128439. [Google Scholar] [CrossRef]
- Santana, M.S.; Alves, R.P.; Santana, L.S.; Gonçalves, M.A.; Guerreiro, M.C. Structural, Inorganic, and Adsorptive Properties of Hydrochars Obtained by Hydrothermal Carbonization of Coffee Waste. J. Environ. Manag. 2022, 302, 114021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, J.; Liang, T.; Chen, T.; Han, X.; Hu, G.; Li, B. Efficient Removal of Cr(VI) by Protonated Amino-Bamboo Char Prepared via Radiation Grafting: Behavior and Mechanism. Sustainability 2023, 15, 13560. [Google Scholar] [CrossRef]
- Maimaiti, T.; Hu, R.; Yuan, H.; Liang, C.; Liu, F.; Li, Q.; Lan, S.; Yu, B.; Yang, S.-T. Magnetic Fe3O4/TiO2/Graphene Sponge for the Adsorption of Methylene Blue in Aqueous Solution. Diam. Relat. Mater. 2022, 123, 108811. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).