Manipulating Electronic Effect of Nitrogen Donor-Based Ligands for Efficient Complexation and Separation of Palladium from Highly Acidic Solution
Abstract
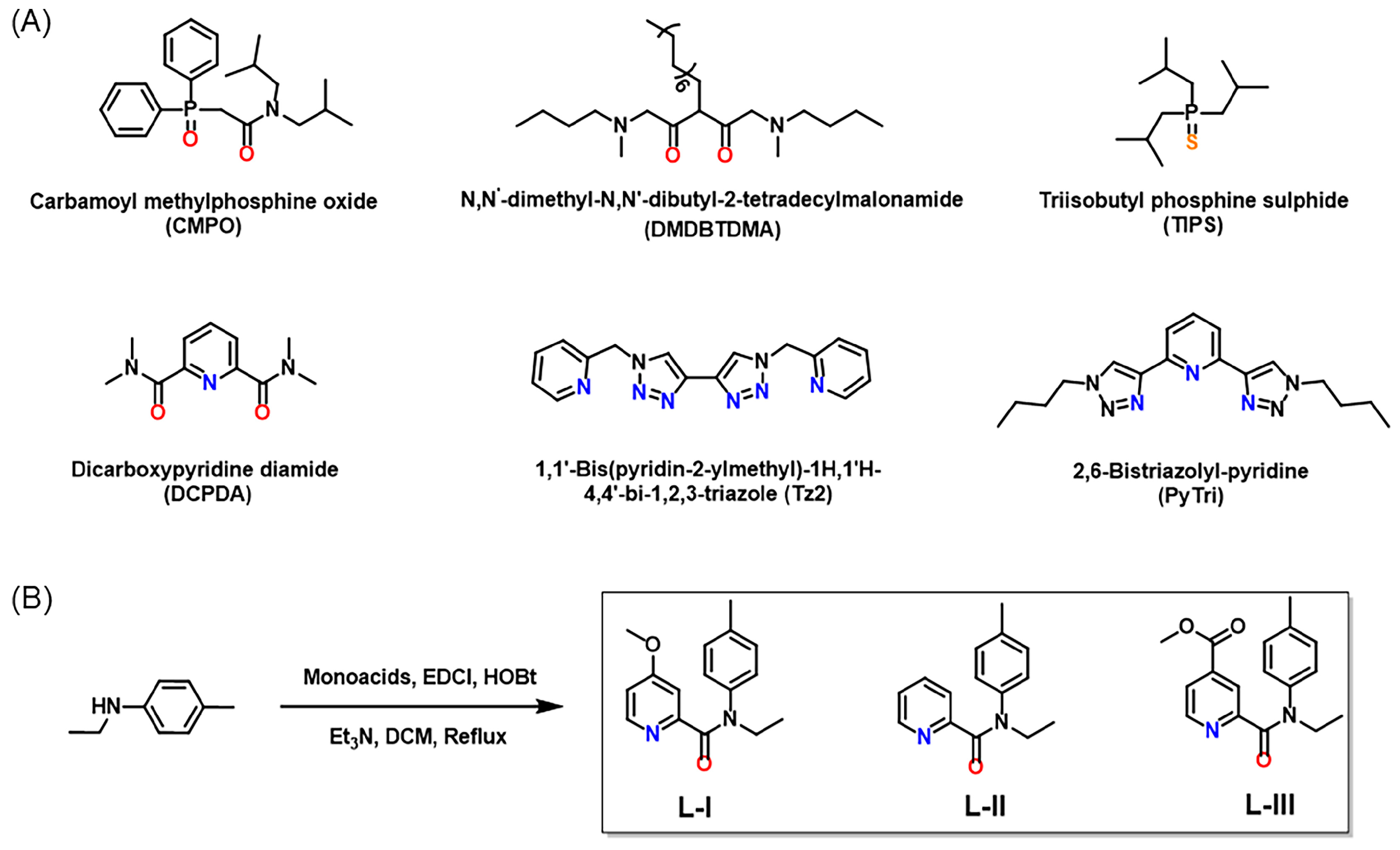
1. Introduction
2. Results and Discussion
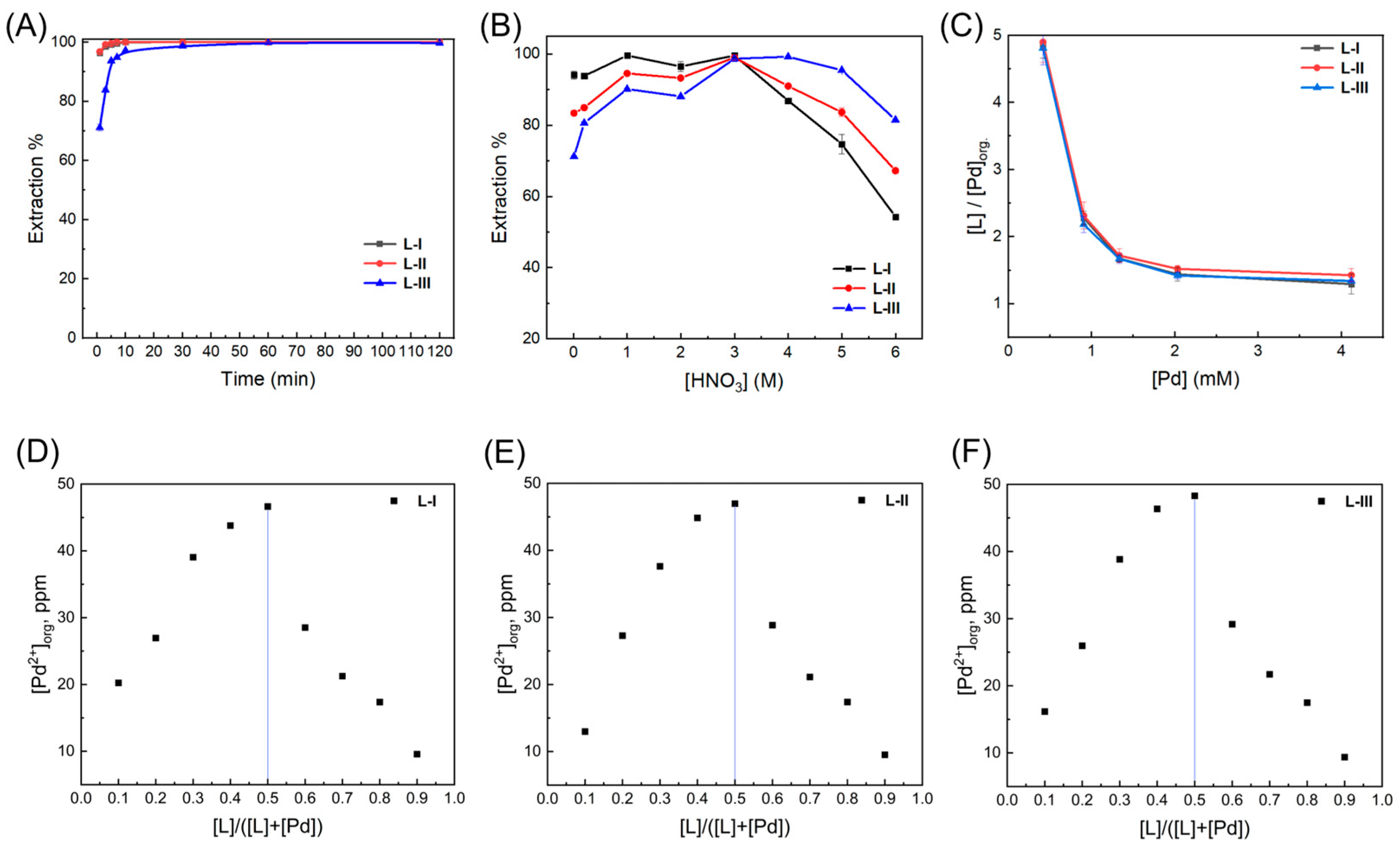
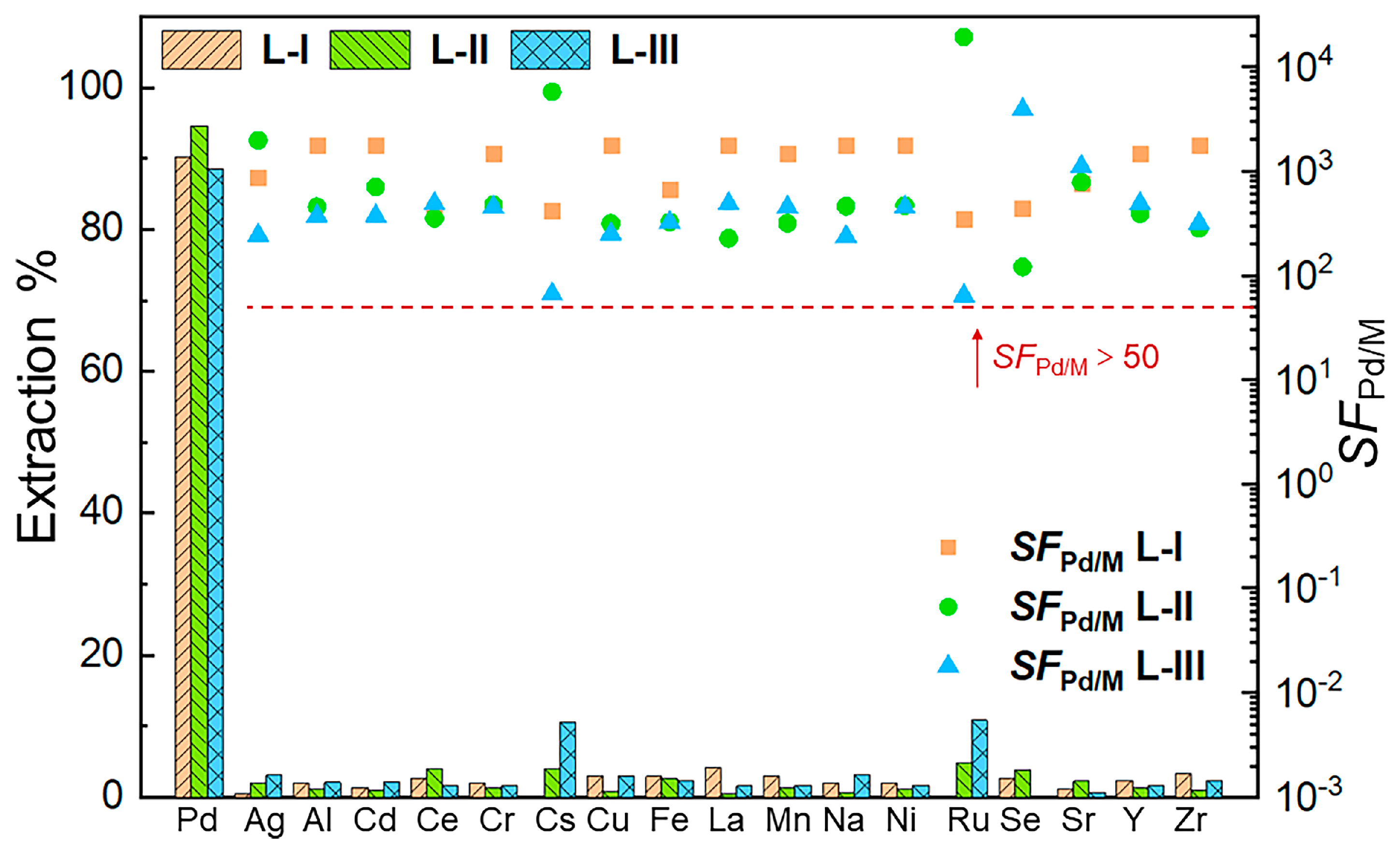
2.1. Solvent Extraction
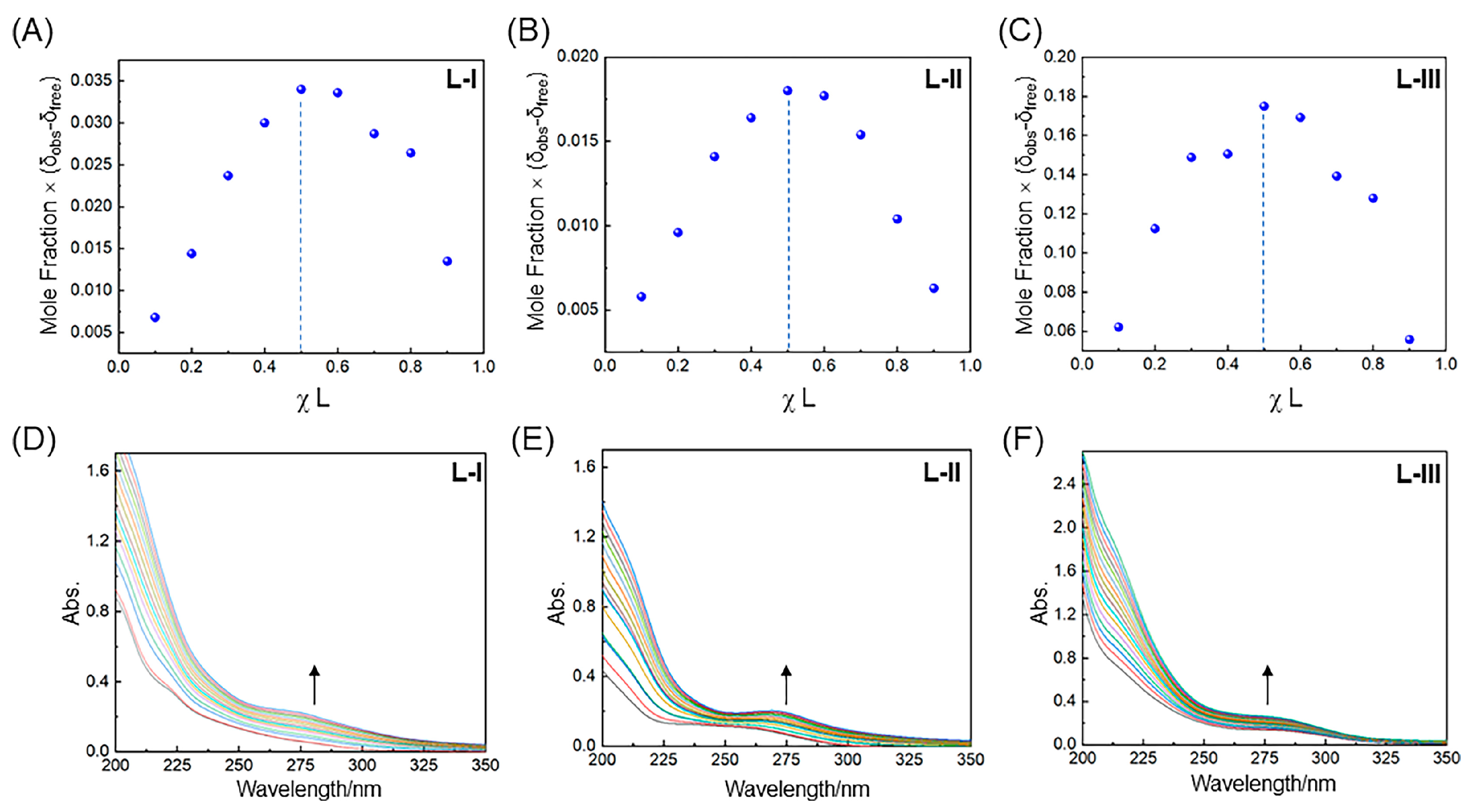
2.2. UV-Vis Spectrophotometric Studies
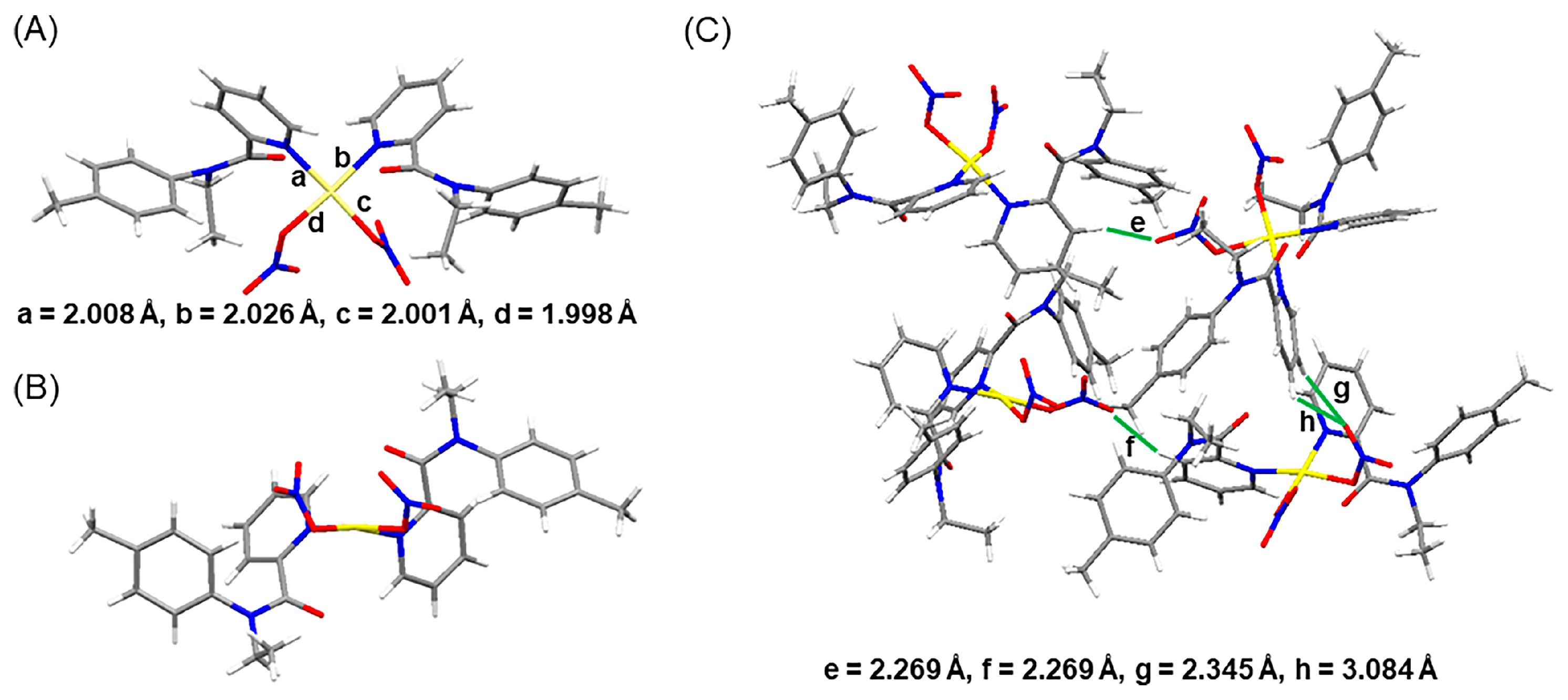
2.3. X-Ray Crystallographic Studies
2.4. Structures Simulations and Binding Energies Calculations
3. Materials and Methods
3.1. General
3.2. Synthesis of Ligands
3.3. Extraction Procedure
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamantiades, A.; Kessides, I. Nuclear Power for Sustainable Development: Current Status and Future Prospects. Energy Policy 2009, 37, 5149. [Google Scholar] [CrossRef]
- Naimoğlu, M. The Impact of Nuclear Energy Use, Energy Prices and Energy Imports on CO2 Emissions: Evidence from Energy Importer Emerging Economies Which Use Nuclear Energy. J. Clean. Prod. 2022, 373, 133937. [Google Scholar] [CrossRef]
- Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive Wastewater Treatment Technologies: A Review. Molecules 2023, 28, 1935. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for F-Element Extraction Used in the Nuclear Fuel Cycle. Chem. Soc. Rev. 2017, 46, 7229. [Google Scholar] [CrossRef]
- Zhang, H.; Li, A.; Li, K.; Wang, Z.; Xu, X.; Wang, Y.; Sheridan, M.V.; Hu, H.S.; Xu, C.; Alekseev, E.V.; et al. Ultrafiltration Separation of Am(VI)-Polyoxometalate from Lanthanides. Nature 2023, 616, 482. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Dong, X.; Wu, T.; Qing, Q.; Chen, J.; Lu, Y.; Xu, C. Ion Sieving in Graphene Oxide Membrane Enables Efficient Actinides/Lanthanides Separation. Nat. Commun. 2023, 14, 261. [Google Scholar] [CrossRef]
- Yang, X.; Wu, W.; Xie, Y.; Hao, M.; Liu, X.; Chen, Z.; Yang, H.; Waterhouse, G.I.N.; Ma, S.; Wang, X. Modulating Anion Nanotraps via Halogenation for High-Efficiency 99TcO4−/ReO4− Removal under Wide-Ranging pH Conditions. Environ. Sci. Technol. 2023, 57, 10870. [Google Scholar] [CrossRef]
- Tian, P.; Ai, Z.; Hu, H.; Wang, M.; Li, Y.; Gao, X.; Qian, J.; Su, X.; Xiao, S.; Xu, H.; et al. Synthesis of Electron-Rich Porous Organic Polymers via Schiff-Base Chemistry for Efficient Iodine Capture. Molecules 2022, 27, 5161. [Google Scholar] [CrossRef]
- Bao, L.; Cai, Y.; Liu, Z.; Li, B.; Bian, Q.; Hu, B.; Wang, X. High Sorption and Selective Extraction of Actinides from Aqueous Solutions. Molecules 2021, 26, 7101. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, J.; Li, Q.; Yang, P.; Conradson, S.D.; Cai, Y.; Feng, W.; Yuan, L. Ultra-selective and efficient static/dynamic palladium capture from highly acidic solution with robust macrocycle-based polymers. Adv. Funct. Mater. 2023, 2304051. [Google Scholar] [CrossRef]
- Fujii, T.; Egusa, S.; Uehara, A.; Kirishima, A.; Yamagishi, I.; Morita, Y.; Yamana, H. Electronic absorption spectra of palladium(II) in concentrated nitric acid solutions. J. Radioanal. Nucl. Chem. 2011, 290, 475. [Google Scholar] [CrossRef]
- Wang, J.; Dong, G. Palladium/Norbornene Cooperative Catalysis. Chem Rev. 2019, 119, 7478. [Google Scholar] [CrossRef] [PubMed]
- Uehling, M.; King, R.; Krska, S.; Cernak, T.; Buchwald, S. Pharmaceutical Diversification via Palladium Oxidative Addition Complexes. Science 2019, 363, 405. [Google Scholar] [CrossRef] [PubMed]
- Barson, S.; Skeldon, P.; Thompson, G.; Piekoszewski, J.; Chemielewski, A.; Werner, Z.; Grötzschel, R.; Wieser, E. Corrosion Protection of Titanium by Pulsed Plasma Deposition of Palladium. Corros. Sci. 2000, 42, 1213. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, J.; Cheng, Q.; Han, L.; Li, Y.; Meng, X.; Ibarlucea, B.; Zhao, H.; Yang, F.; Liu, H.; et al. Applications of 2D-Layered Palladium Diselenide and Its Van Der Waals Heterostructures in Electronics and Optoelectronics. Nano-Micro Lett. 2021, 13, 143. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, L.; He, L.; Li, B.; Chen, L.; Wu, F.; Chen, L.; Zhang, M.; Liu, Z.; Chai, Z.; et al. Precise Recognition of Palladium through Interlaminar Chelation in a Covalent Organic Framework. Chem 2022, 8, 1442. [Google Scholar] [CrossRef]
- Parajuli, D.; Hirota, K.; Seko, N. Effective separation of palladium from simulated high level radioactive waste. J. Radioanal. Nucl. Chem. 2011, 288, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wu, Y.; Li, J.; Hu, B.; Cai, Y.; Yuan, L.; Feng, W. Selective Complexation and Separation of Uranium(VI) from Thorium(IV) with New Tetradentate N,O-Hybrid Diamide Ligands: Synthesis, Extraction, Spectroscopy, and Crystallographic Studies. Inorg. Chem. 2023, 62, 4922. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Egberink, R.J.M.; Mohapatra, P.K.; Verma, P.K.; Kanekar, A.S.; Yadav, A.K.; Jha, S.N.; Bhatacharyya, D.; Huskens, J.; Verboom, W. Remarkable Enhancement in Extraction of Trivalent F-Block Elements Using a Macrocyclic Ligand with Four Diglycolamide Arms: Synthesis, Extraction, and Spectroscopic and Density Functional Theory Studies. Inorg. Chem. 2019, 58, 14885. [Google Scholar] [CrossRef]
- Ruhela, R.; Singh, A.K.; Tomar, B.S.; Hubli, R.C. Separation of Palladium from High Level Liquid Waste—A Review. RSC Adv. 2014, 4, 24344. [Google Scholar] [CrossRef]
- Li, S.; Chu, N.; Li, X.; Dong, F.; Shen, Y. Recovery of Palladium from Acidic Nitrate Media with Triazole Type Extractants in Ionic Liquid. Hydrometallurgy 2019, 189, 105148. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, M.; Yuan, L.; Feng, W. Complexation and Separation of Palladium with Tridentate 2,6-Bis-Triazolyl-Pyridine ligands: Synthesis, Solvent extraction, Spectroscopy, Crystallography, and DFT Calculations. Inorg. Chem. 2023, 62, 9168. [Google Scholar] [CrossRef] [PubMed]
- Courson, O.; Lebrun, M.; Malmbeck, R.; Pagliosa, G.; Römer, K.; Sätmark, B.; Glatz, J. Partitioning of Minor Actinides from HLLW Using the DIAMEX Process. Part 1—Demonstration of Extraction Performances and Hydraulic Behaviour of the Solvent in a Continuous Process. Radiochim. Acta 2000, 88, 857. [Google Scholar] [CrossRef]
- Zhu, Z.; Sasaki, Y.; Suzuki, H.; Suzuki, S.; Kimura, T. Cumulative Study on Solvent Extraction of Elements by N,N,N’,N’-Tetraoctyl-3-Oxapentanediamide (TODGA) from Nitric Acid into N-Dodecane. Anal. Chim. Acta 2004, 527, 163. [Google Scholar] [CrossRef]
- Rizvi, G.H.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Recovery of Fission Product Palladium from Acidic High Level Waste Solutions. Sep. Sci. Technol. 1996, 31, 1805. [Google Scholar] [CrossRef]
- Hudson, M.; Harwood, L.; Laventine, D.; Lewis, F. Use of Soft Heterocyclic N-Donor Ligands to Separate Actinides and Lanthanides. Inorg. Chem. 2013, 52, 3414. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J. Separation of Actinides from Spent Nuclear Fuel: A Review. J. Hazard. Mater. 2016, 318, 266. [Google Scholar] [CrossRef]
- Xu, L.; Ding, W.; Zhang, A.; Liu, Z. Effect of Ligand Initial Conformation and Counteranion on Complexation Behaviors of R-BTBP toward Pd(II) Contained in Highly Active Liquid Waste. Chin. Chem. Lett. 2022, 33, 3565. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Leoncini, A.; Egberink, R.J.M.; Mohapatra, P.K.; Verma, P.K.; Kanekar, A.S.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Huskens, J.; et al. First Report on the Complexation of Actinides and Lanthanides Using 2,2′,2″-(((1,4,7-Triazonane-1,4,7-Triyl)tris(2-Oxoethane-2,1-Diyl)) tris(oxy)) tris( N, N-Dioctylacetamide): Synthesis, Extraction, Luminescence, EXAFS, and DFT Studies. Inorg. Chem. 2018, 57, 12987. [Google Scholar] [CrossRef]
- Ansari, S.; Mohapatra, P.K.; Leoncini, A.; Ali, S.M.; Huskens, J.; Verboom, W. Highly Efficient N-Pivot Tripodal Diglycolamide Ligands for Trivalent F-Cations: Synthesis, Extraction, Spectroscopy, and Density Functional Theory Studies. Inorg. Chem. 2019, 58, 8633. [Google Scholar] [CrossRef]
- Sun, M.; Xu, L.; Yang, X.; Wang, S.; Lei, L.; Xiao, C. Complexation Behaviors of a Tridentate Phenanthroline Carboxamide Ligand with Trivalent F-Block Elements in Different Anion Systems: A Thermodynamic and Crystallographic Perspective. Inorg. Chem. 2022, 61, 2824. [Google Scholar] [CrossRef] [PubMed]
- Fleck, M.; Wieder, M.; Boresch, S. Dummy Atoms in Alchemical Free Energy Calculations. J. Chem. Theory Comput. 2021, 17, 4403. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Cai, Y.; Huang, S.; Li, X.; Feng, W.; Yuan, L. Manipulating Electronic Effect of Nitrogen Donor-Based Ligands for Efficient Complexation and Separation of Palladium from Highly Acidic Solution. Molecules 2025, 30, 1533. https://doi.org/10.3390/molecules30071533
Gan Y, Cai Y, Huang S, Li X, Feng W, Yuan L. Manipulating Electronic Effect of Nitrogen Donor-Based Ligands for Efficient Complexation and Separation of Palladium from Highly Acidic Solution. Molecules. 2025; 30(7):1533. https://doi.org/10.3390/molecules30071533
Chicago/Turabian StyleGan, Yuyang, Yimin Cai, Song Huang, Xiaowei Li, Wen Feng, and Lihua Yuan. 2025. "Manipulating Electronic Effect of Nitrogen Donor-Based Ligands for Efficient Complexation and Separation of Palladium from Highly Acidic Solution" Molecules 30, no. 7: 1533. https://doi.org/10.3390/molecules30071533
APA StyleGan, Y., Cai, Y., Huang, S., Li, X., Feng, W., & Yuan, L. (2025). Manipulating Electronic Effect of Nitrogen Donor-Based Ligands for Efficient Complexation and Separation of Palladium from Highly Acidic Solution. Molecules, 30(7), 1533. https://doi.org/10.3390/molecules30071533









