TiO2/LaFeO3 Composites for the Efficient Degradation of Benzoic Acid and Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
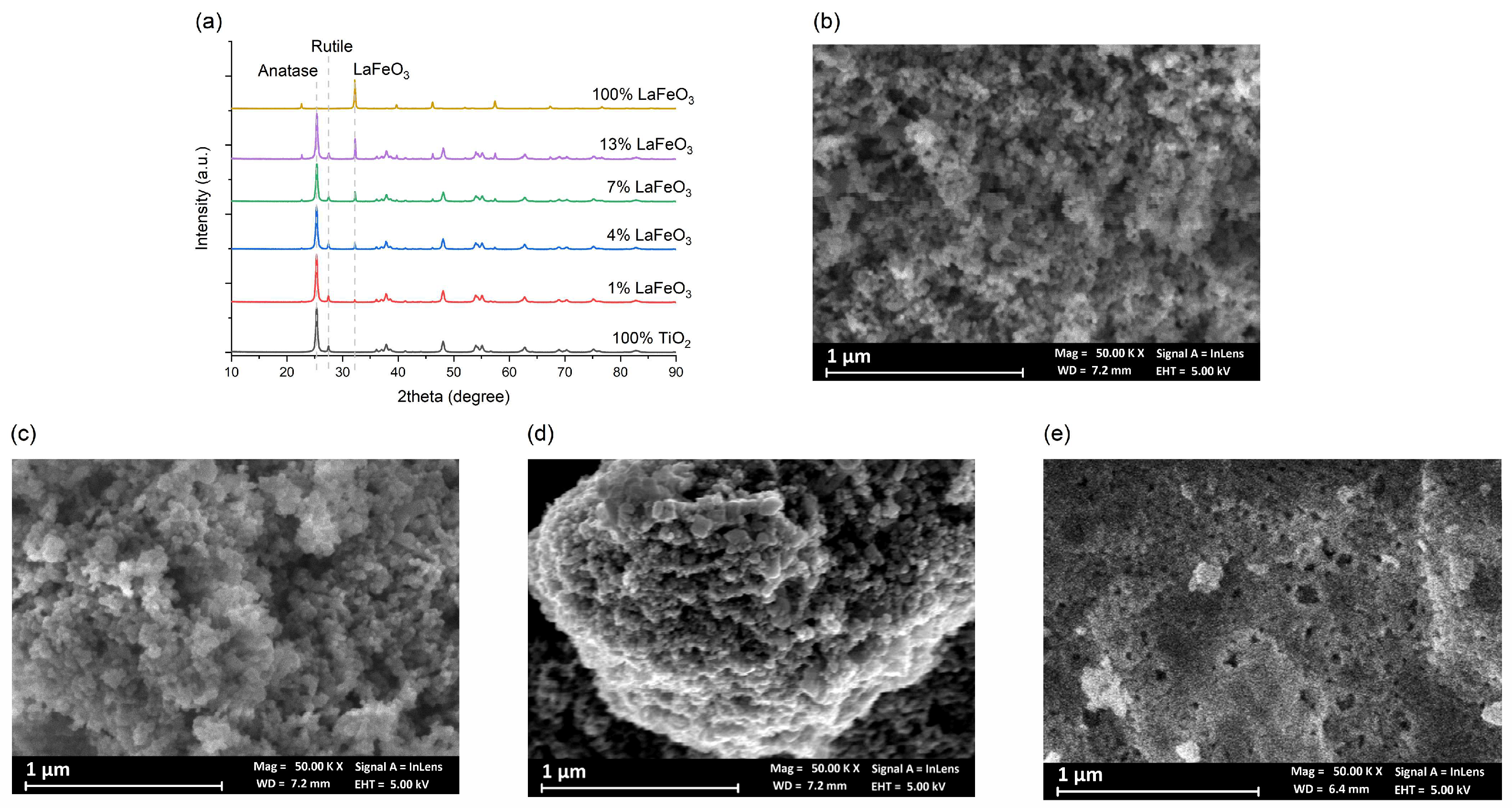
2.1. Phase Composition and Microstructure
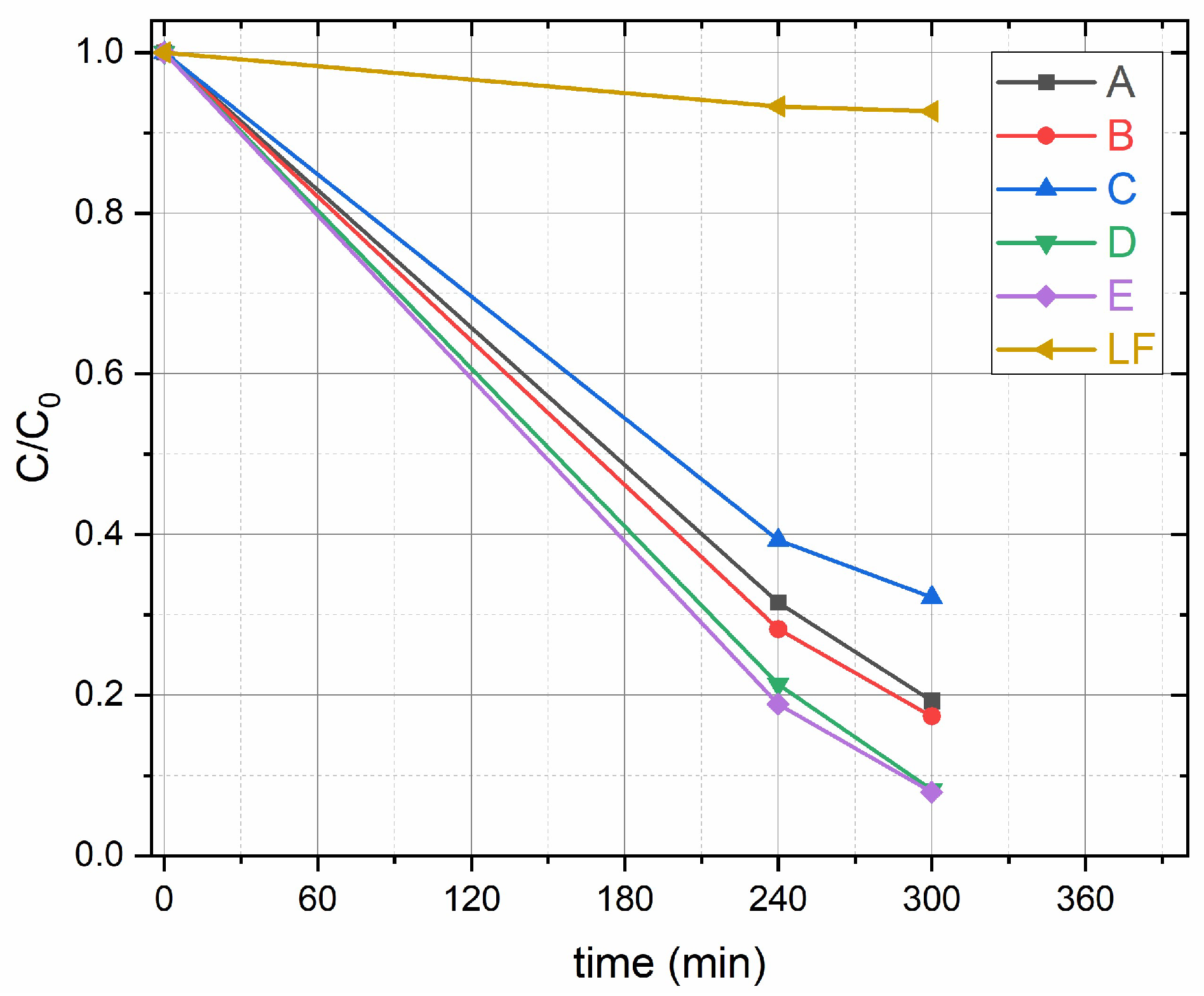
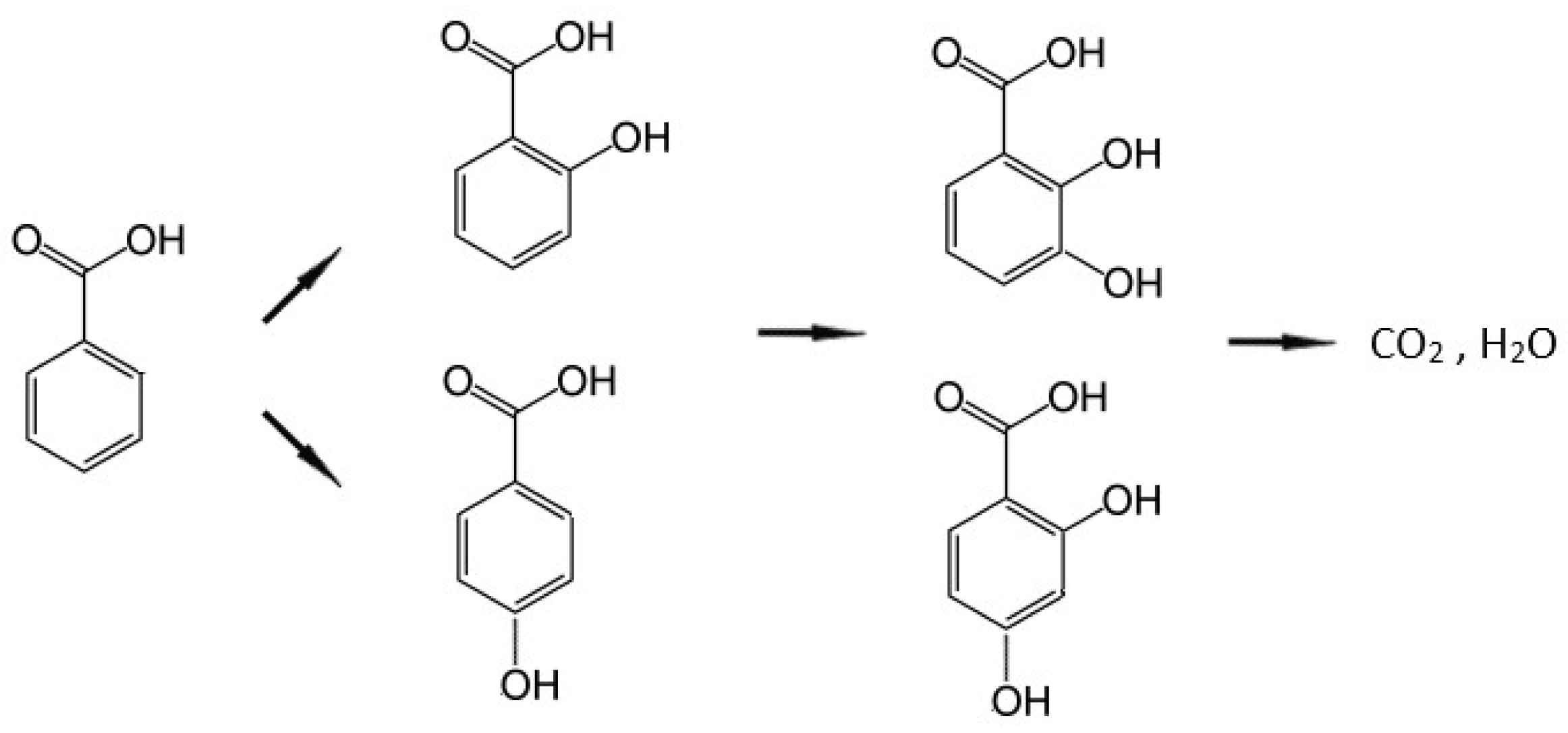
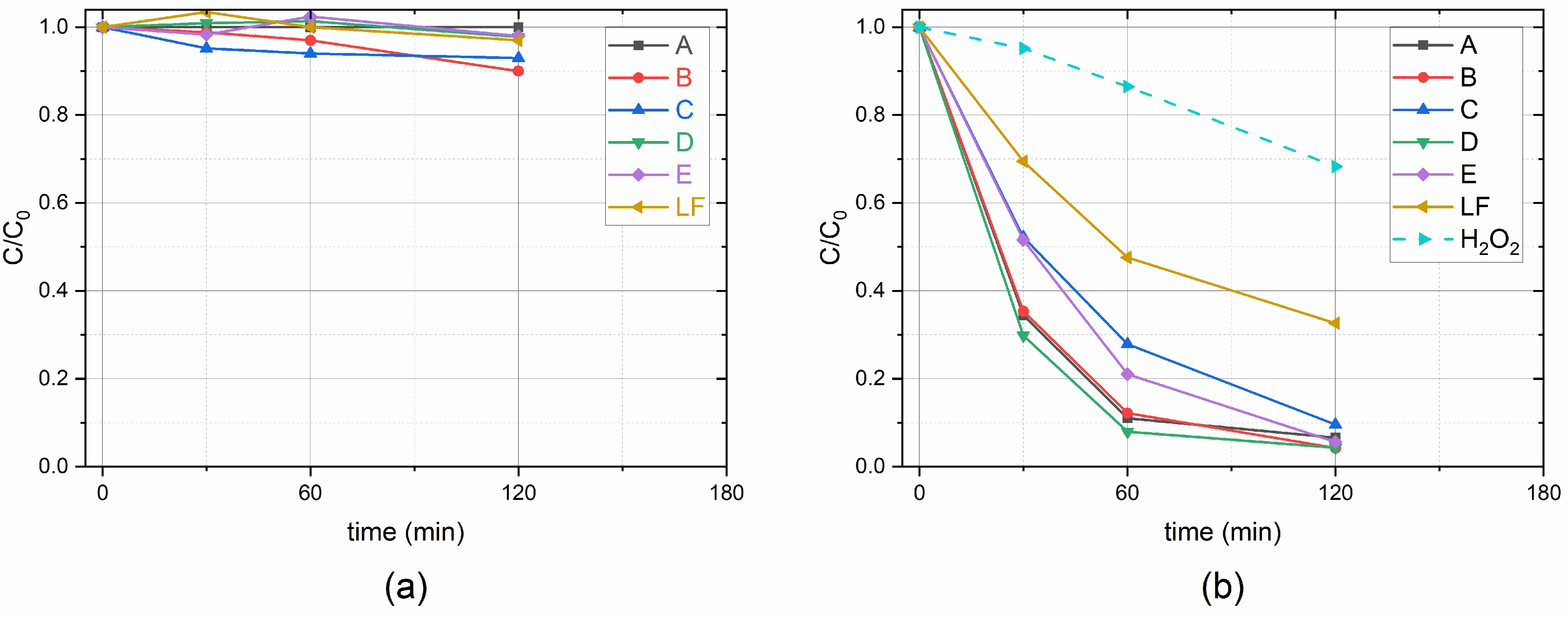
2.2. Benzoic Acid Degradation Using Daylight Lamps in LaFeO3/TiO2 Water Suspensions
2.3. Benzoic Acid Degradation Under 450 nm Light in LaFeO3/TiO2 Water Suspensions With and Without H2O2
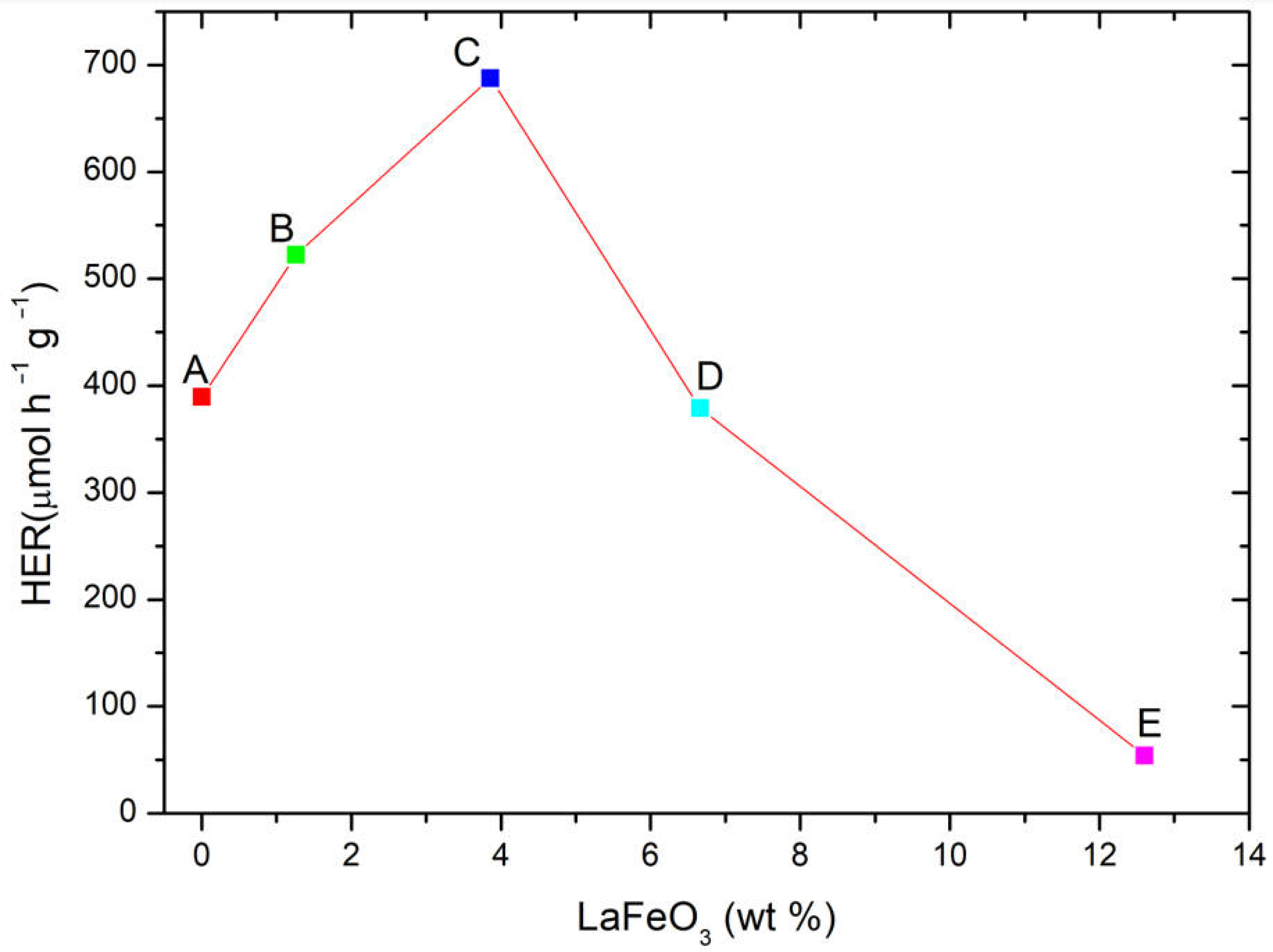
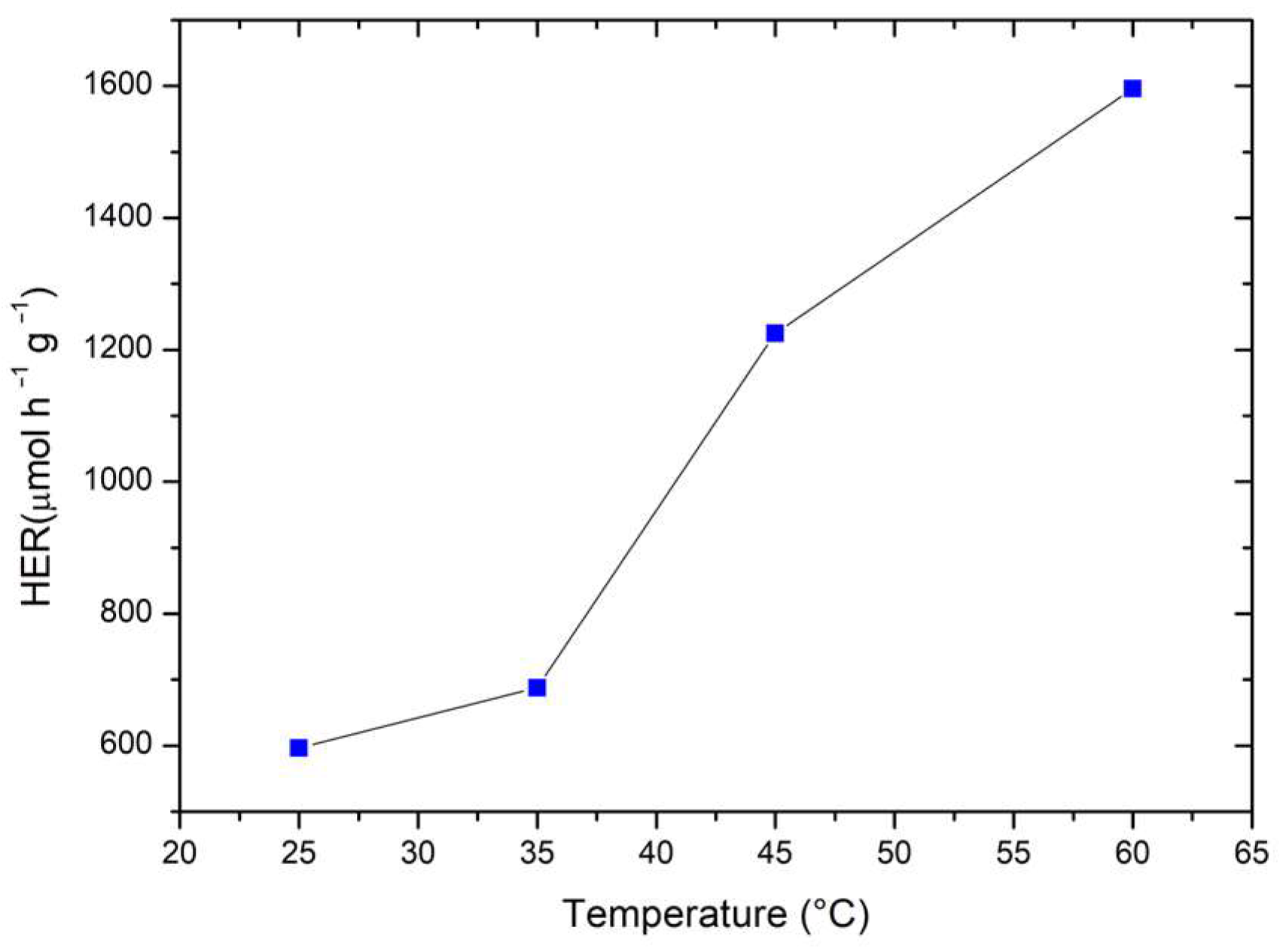
2.4. Hydrogen Evolution
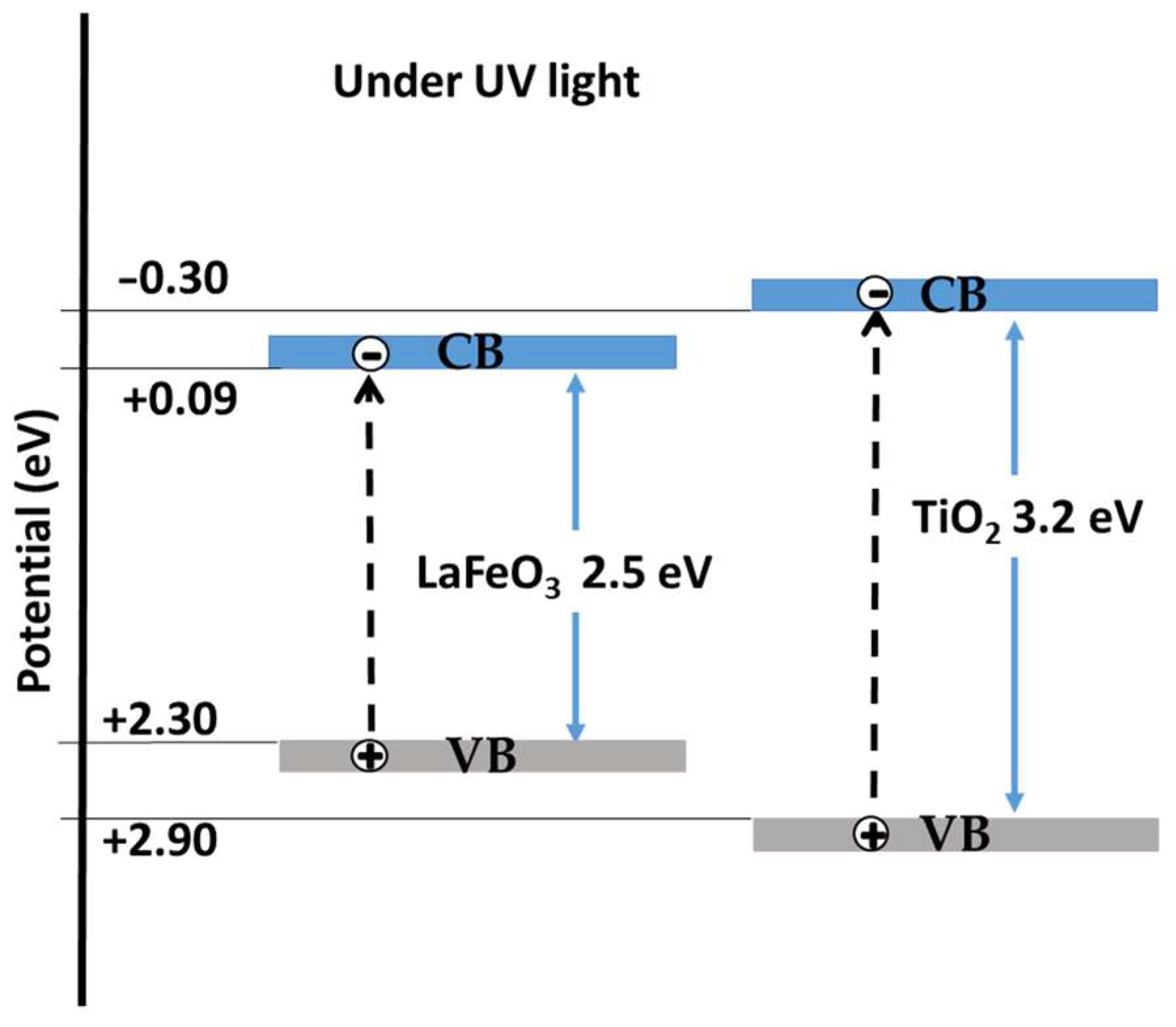
2.5. Activation of LaFeO3/TiO2 Nanocomposites
3. Materials and Methods
3.1. Chemicals and Preparations
3.2. Microstructural Characterization
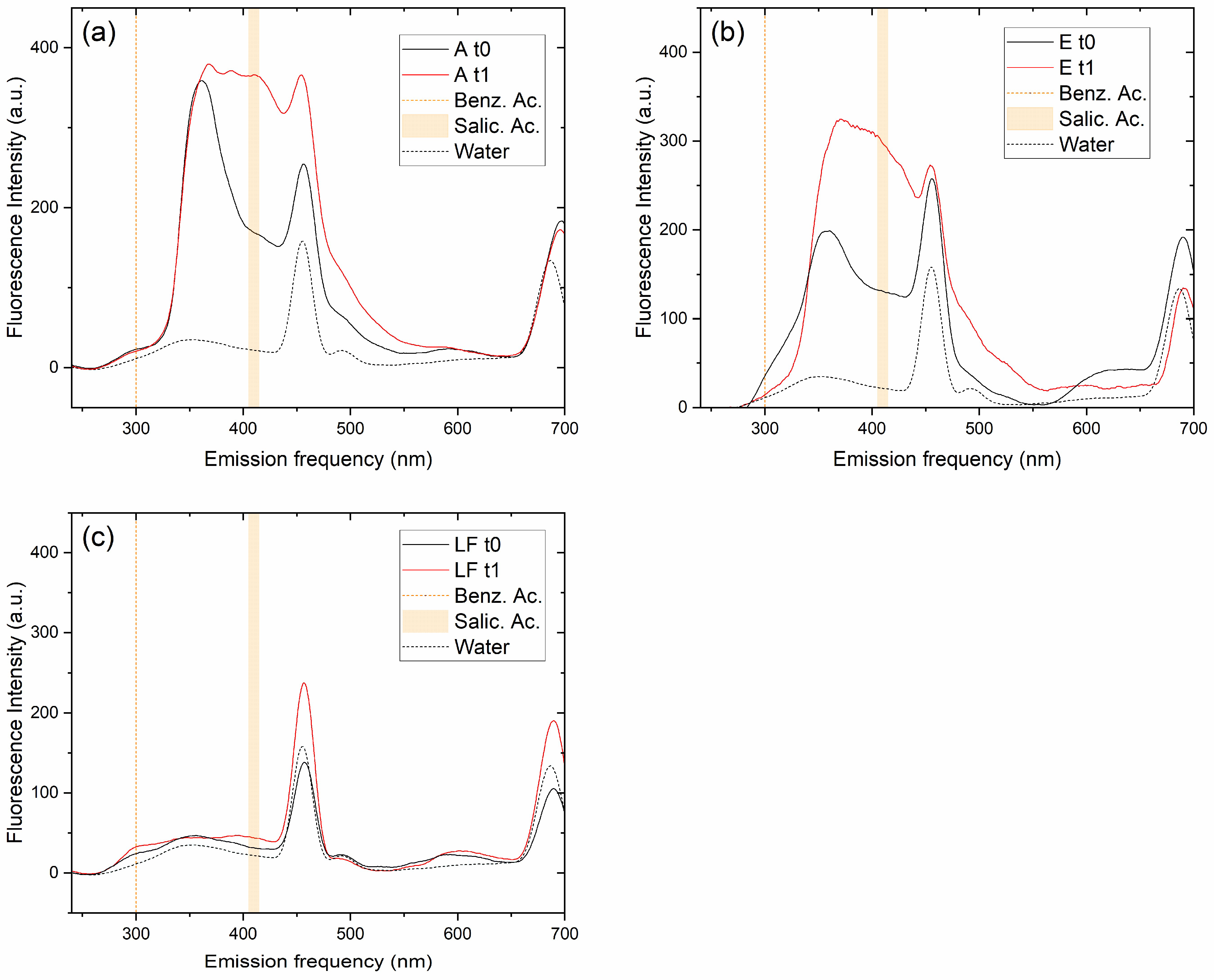
3.3. Florescence Spectroscopy
3.4. Photocatalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, H.; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; Kumar, A.; Parmar, R. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A Review on Heterogeneous Photocatalysis for Environmental Remediation: From Semiconductors to Modification Strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent Advances in Semiconductor Metal Oxides with Enhanced Methods for Solar Photocatalytic Applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Gómez, L.D.; Rodríguez-Páez, J.E. Micro/Nanoscale Mesoporous Nb2O5 Particles: Effect of Synthesis Conditions and Doping with N, C, or S on Their Properties. Nano-Struct. Nano-Objects 2019, 17, 43–57. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Henderson, M.A. A Surface Science Perspective on TiO2 Photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Sora, I.N.; Fontana, F.; Passalacqua, R.; Ampelli, C.; Perathoner, S.; Centi, G.; Parrino, F.; Palmisano, L. Photoelectrochemical Properties of Doped Lanthanum Orthoferrites. Electrochim. Acta 2013, 109, 710–715. [Google Scholar] [CrossRef]
- Bolognino, I.; Pelosato, R.; Marcì, G.; Natali Sora, I. Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions. Molecules 2023, 28, 3807. [Google Scholar] [CrossRef]
- Pelosato, R.; Carrara, V.; Sora, I.N. Enhanced Photocatalytic Degradation of Ibuprofen in Aqueous Solution under Visible-Light Irradiation: Effects of LaFeO3 and Cu/LaFeO3. Chem. Eng. Trans. 2019, 73, 181–186. [Google Scholar] [CrossRef]
- Turkten, N.; Sora, I.N.; Tomruk, A.; Bekbolet, M. Photocatalytic Degradation of Humic Acids Using LaFeO3. Catalysts 2018, 8, 630. [Google Scholar] [CrossRef]
- Dhinesh Kumar, R.; Thangappan, R.; Jayavel, R. Synthesis and Characterization of LaFeO3/TiO2 Nanocomposites for Visible Light Photocatalytic Activity. J. Phys. Chem. Solids 2017, 101, 25–33. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Su, H.; Yao, C.; Fu, H. Synthesis of High-Activity TiO2-Based Photocatalysts by Compounding a Small Amount of Porous Nanosized LaFeO3 and the Activity-Enhanced Mechanisms. J. Phys. Chem. C 2011, 115, 12375–12380. [Google Scholar] [CrossRef]
- Humayun, M.; Li, Z.; Sun, L.; Zhang, X.; Raziq, F.; Zada, A.; Qu, Y.; Jing, L. Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants. Nanomaterials 2016, 6, 22. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Albukhari, S.M.; Ismail, A.A. Visible-Light Driven of Heterostructured LaFeO3/TiO2 Photocatalysts for Degradation of Antibiotics: Ciprofloxacin as Case Study. J. Photochem. Photobiol. A Chem. 2022, 432, 114078. [Google Scholar] [CrossRef]
- Hao, P.; Shi, R.; Wang, X.; Zhang, J.; Li, B.; Wang, J.; Liu, B.; Liu, Y.; Qiao, X.; Wang, Z. Efficient Tetracycline Degradation Using Carbon Quantum Dot Modified TiO2@LaFeO3 Hollow Core Shell Photocatalysts. Sci. Rep. 2024, 14, 27057. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Ivanez, J.; Robert, D.; Keller, N. Activity Enhancement Pathways in LaFeO3@TiO2 Heterojunction Photocatalysts for Visible and Solar Light Driven Degradation of Myclobutanil Pesticide in Water. J. Hazard. Mater. 2020, 400, 123099. [Google Scholar] [CrossRef]
- Pandey, V.; Bansal, A.; Toor, A.P. Synthesis and Performance Evaluation of S-Scheme Heterostructured LaFeO3/TiO2 Photocatalyst for the Efficient Degradation of Thiamethoxam. Environ. Sci. Pollut. Res. 2024, 31, 28578–28593. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.-J.; Chan, T.-S.; et al. Single-Phase Perovskite Oxide with Super-Exchange Induced Atomic-Scale Synergistic Active Centers Enables Ultrafast Hydrogen Evolution. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology Control and Electronic Tailoring of CoxAy (A = P, S, Se) Electrocatalysts for Water Splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Lv, T.; Wang, H.; Hong, W.; Wang, P.; Jia, L. In Situ Self-Assembly Synthesis of Sandwich-like TiO2/Reduced Graphene Oxide/LaFeO3 Z-Scheme Ternary Heterostructure towards Enhanced Photocatalytic Hydrogen Production. Mol. Catal. 2019, 475, 110497. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, Q.; Xu, F.; Sun, X.; Ding, Y. Synthesis of TiO2/LaFeO3 Composites for the Photoelectrochemical Hydrogen Evolution. J. Mater. Sci. 2021, 56, 15188–15204. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Wu, F.; Xie, T.; Li, T. Surface Electronic States and Photovoltage Gas-Sensitive Characters of Nanocrystalline LaFeO3. Mater. Chem. Phys. 2000, 64, 269–272. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-Dependent Surface Charging and Points of Zero Charge II. Update. J. Colloid Interface Sci. 2004, 275, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Mrowetz, M.; Selli, E. Photocatalytic Degradation of Formic and Benzoic Acids and Hydrogen Peroxide Evolution in TiO2 and ZnO Water Suspensions. J. Photochem. Photobiol. A Chem. 2006, 180, 15–22. [Google Scholar] [CrossRef]
- Ajmera, A.A.; Sawant, S.B.; Pangarkar, V.G.; Beenackers, A.A.C.M. Solar-Assisted Photocatalytic Degradation of Benzoic Acid Using Titanium Dioxide as a Photocatalyst. Chem. Eng. Technol. 2002, 25, 173–180. [Google Scholar] [CrossRef]
- Hidaka, H.; Honjo, H.; Horikoshi, S.; Serpone, N. Photocatalyzed Degradation on a TiO2-Coated Quartz Crystal Microbalance. Adsorption/Desorption Processes in Real Time in the Degradation of Benzoic Acid and Salicylic Acid. Catal. Commun. 2006, 7, 331–335. [Google Scholar] [CrossRef]
- Smith, G.J.; Miller, I.J. The Effect of Molecular Environment on the Photochemistry of P-Methoxycinnamic Acid and its Esters. J. Photochem. Photobiol. A Chem. 1998, 118, 93–97. [Google Scholar] [CrossRef]
- Bouleghlimat, E.; Bethell, D.; Davies, P.R. The Photocatalytic Destruction of Cinnamic Acid and Cinnamyl Alcohol: Mechanism and the Effect of Aqueous Ions. Chemosphere 2020, 251, 126469. [Google Scholar] [CrossRef] [PubMed]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, C.K.; Jirousek, M.; Grätzel, M. Decomposition of Organophosphorus Compounds on Photoactivated TiO2 Surfaces. J. Mol. Catal. 1990, 60, 375–387. [Google Scholar] [CrossRef]
- Muscetta, M.; Clarizia, L.; Race, M.; Andreozzi, R.; Marotta, R.; Di Somma, I. Visible—Light Driven Systems: Effect of the Parameters Affecting Hydrogen Production through Photoreforming of Organics in Presence of Cu2O/TiO2 Nanocomposite Photocatalyst. Appl. Sci. 2023, 13, 2337. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Muscetta, M.; Jitan, S.A.; Palmisano, G.; Andreozzi, R.; Marotta, R.; Cimino, S.; Di Somma, I. Visible Light—Driven Photocatalytic Hydrogen Production Using Cu2O/TiO2 composites Prepared by Facile Mechanochemical Synthesis. J. Environ. Chem. Eng. 2022, 10, 107735. [Google Scholar] [CrossRef]

| Photocatalyst | LaFeO3:TiO2 | Radiation | Organic Compound | Catalyst | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| LaFeO3/TiO2 rutile calcination 800 °C/6 h | 1.5:1 (m/m) | visible | methyl orange | n.a. | 90 | 180 | [14] |
| LaFeO3@TiO2 heterojunction | 5:100 | 300–800 nm | myclobutanil 20 mg L−1 | 1 g L−1 | 100 | 180 | [19] |
| LaFeO3@TiO2 heterojunction | 12.5:100 | >420 nm | myclobutanil 20 mg L−1 | 1 g L−1 | 37 | 240 | [19] |
| LaFeO3/TiO2 anatase calcination 500 °C/3 h | 3:100 | >420 nm | ciprofloxacin 10 mg L−1 | 2.4 g L−1 | 100 | 90 | [17] |
| LaFeO3/TiO2 (P25) calcination 450 °C/2 h | 0.85:100 | 254 nm | thiamethozam 20 mg L−1 | 0.8 g L−1 | 97 | 120 | [20] |
| LaFeO3/TiO2 (P25) calcination 450 °C/2 h | 0.85:100 | direct sunlight | thiamethozam 20 mg L−1 | 0.8 g L-1 | 79 | 120 | [20] |
| LaFeO3/TiO2 (P25) ball-milling | 6.2:100 | LED450 nm | benzoic acid 13.4 mg L−1 | 0.12 g L−1 H2O2 1 mM | 96 | 120 | this work |
| LaFeO3/TiO2 (P25) ball-milling | 6.2:100 | LED 450 nm | benzoic acid 13.4 mg L−1 | 0.12 g L−1 | 9 | 120 | this work |
| LaFeO3/TiO2 (P25) ball-milling | 6.2:100 | 380–800 nm | benzoic acid 13.4 mg L−1 | 0.12 g L−1 | 90 | 300 | this work |
| Catalyst | k (s−1) | R2 |
|---|---|---|
| A | 4.1 × 10−4 | 0.934 |
| B | 4.4 × 10−4 | 0.969 |
| C | 3.2 × 10−4 | 0.996 |
| D | 4.8 × 10−4 | 0.943 |
| E | 4.0 × 10−4 | 0.995 |
| F | 1.5 × 10−4 | 0.967 |
| Benzoic Acid | 4-Methoxycinnamic Acid | |
|---|---|---|
| N. CAS | 65-85-0 | 943-89-5 |
| Chemical structure | 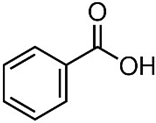 | 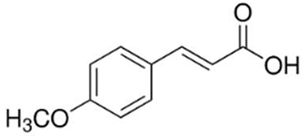 |
| Molecular formula | C6H5COOH | C10H10O3 |
| Molecular mass | 122.12 g/mol | 178.18 g/mol |
| Physical form | White powder | White powder |
| Solubility in water | 3.4 g/L at 25 °C | 0.71 g/L at 25 °C |
| pKa | 4.19 | 4.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natali Sora, I.; Bertolotti, B.; Pelosato, R.; Lucotti, A.; Tommasini, M.; Muscetta, M. TiO2/LaFeO3 Composites for the Efficient Degradation of Benzoic Acid and Hydrogen Production. Molecules 2025, 30, 1526. https://doi.org/10.3390/molecules30071526
Natali Sora I, Bertolotti B, Pelosato R, Lucotti A, Tommasini M, Muscetta M. TiO2/LaFeO3 Composites for the Efficient Degradation of Benzoic Acid and Hydrogen Production. Molecules. 2025; 30(7):1526. https://doi.org/10.3390/molecules30071526
Chicago/Turabian StyleNatali Sora, Isabella, Benedetta Bertolotti, Renato Pelosato, Andrea Lucotti, Matteo Tommasini, and Marica Muscetta. 2025. "TiO2/LaFeO3 Composites for the Efficient Degradation of Benzoic Acid and Hydrogen Production" Molecules 30, no. 7: 1526. https://doi.org/10.3390/molecules30071526
APA StyleNatali Sora, I., Bertolotti, B., Pelosato, R., Lucotti, A., Tommasini, M., & Muscetta, M. (2025). TiO2/LaFeO3 Composites for the Efficient Degradation of Benzoic Acid and Hydrogen Production. Molecules, 30(7), 1526. https://doi.org/10.3390/molecules30071526









