Preparation and Properties of Low-Exothermic Polyurethanes Doped with Modified Hydrated Salt Phase Change Materials
Abstract
:1. Introduction
2. Experiment and Method
2.1. Materials
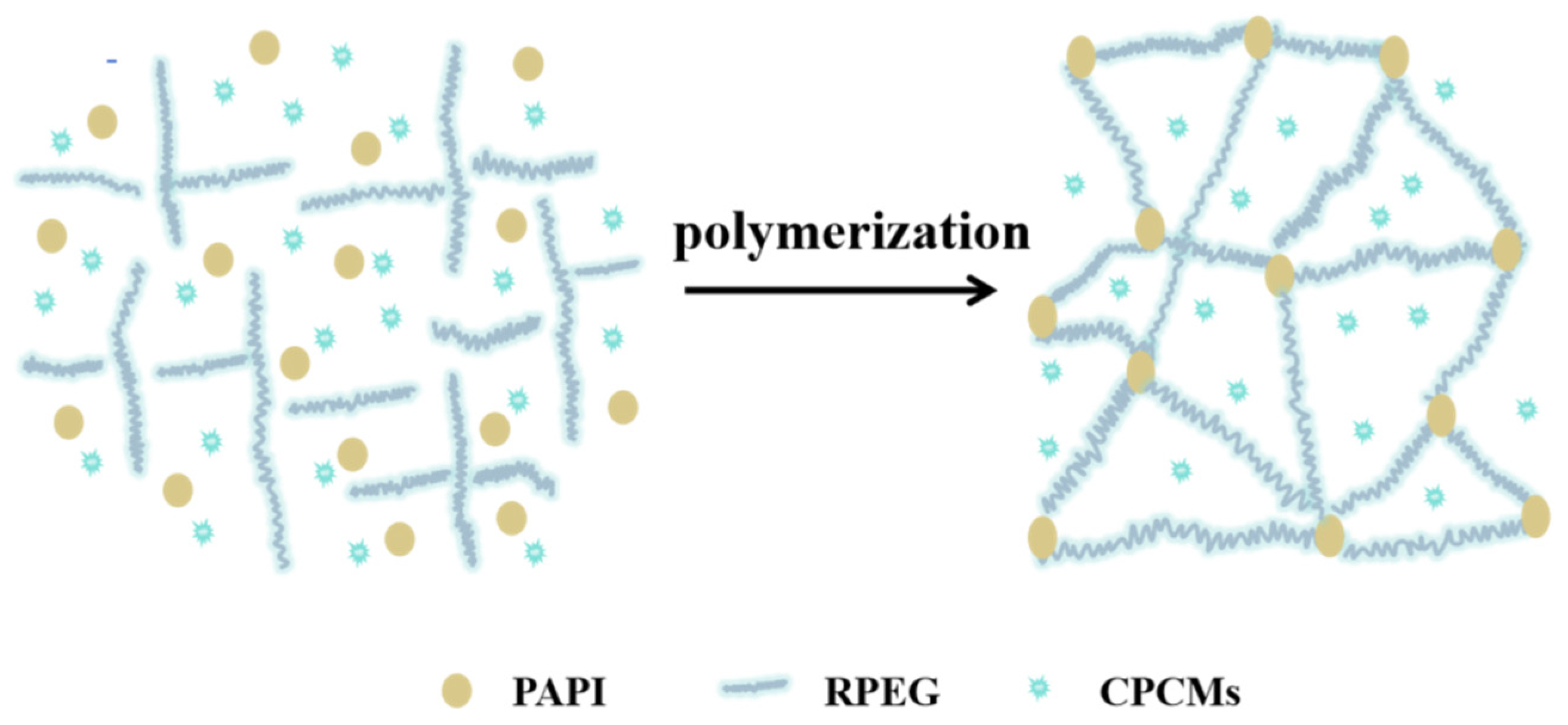
2.2. Preparation of CPCMs
2.3. Formatting of Mathematical Components
2.4. Testing and Characterization
3. Discussion
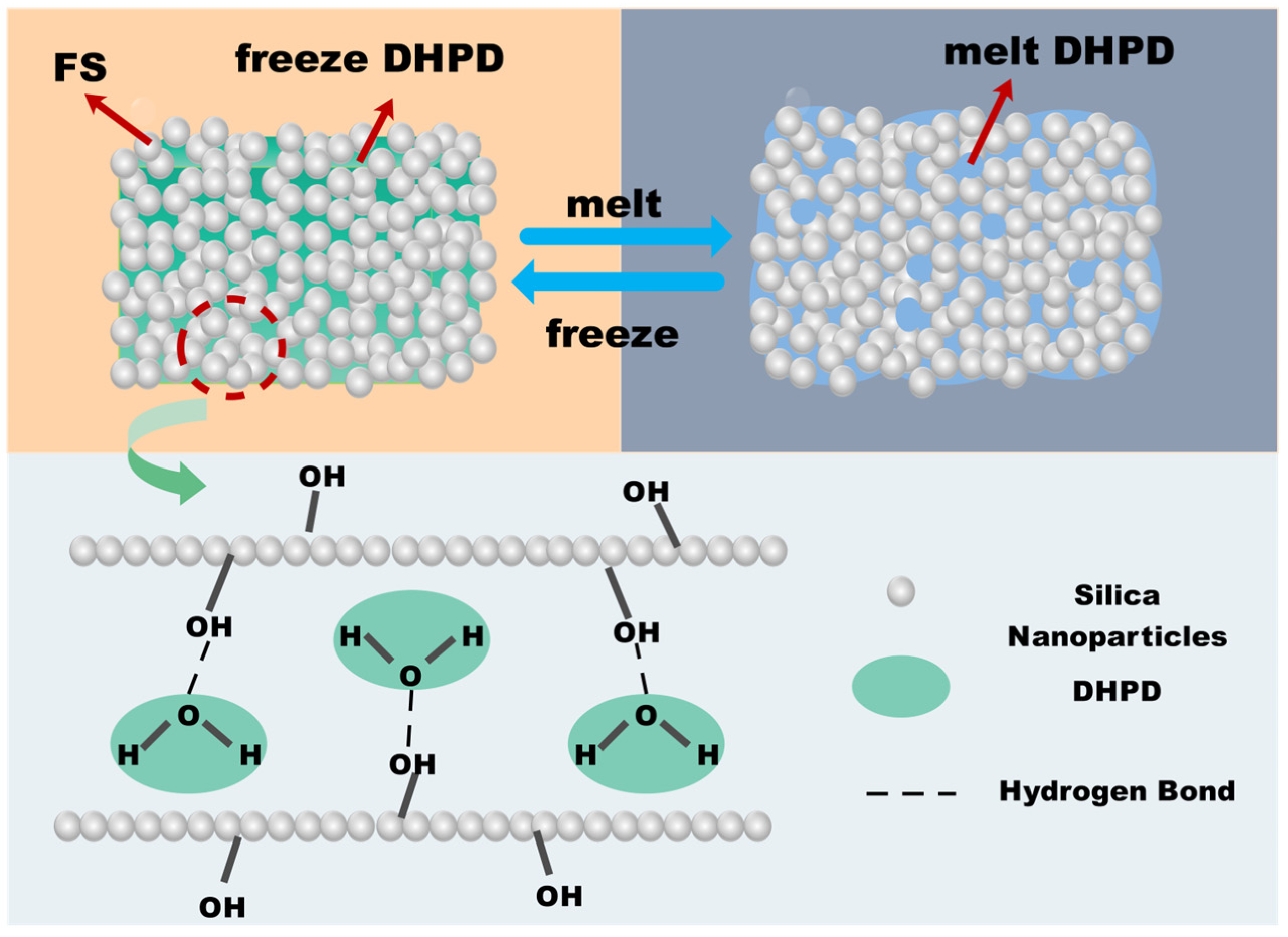
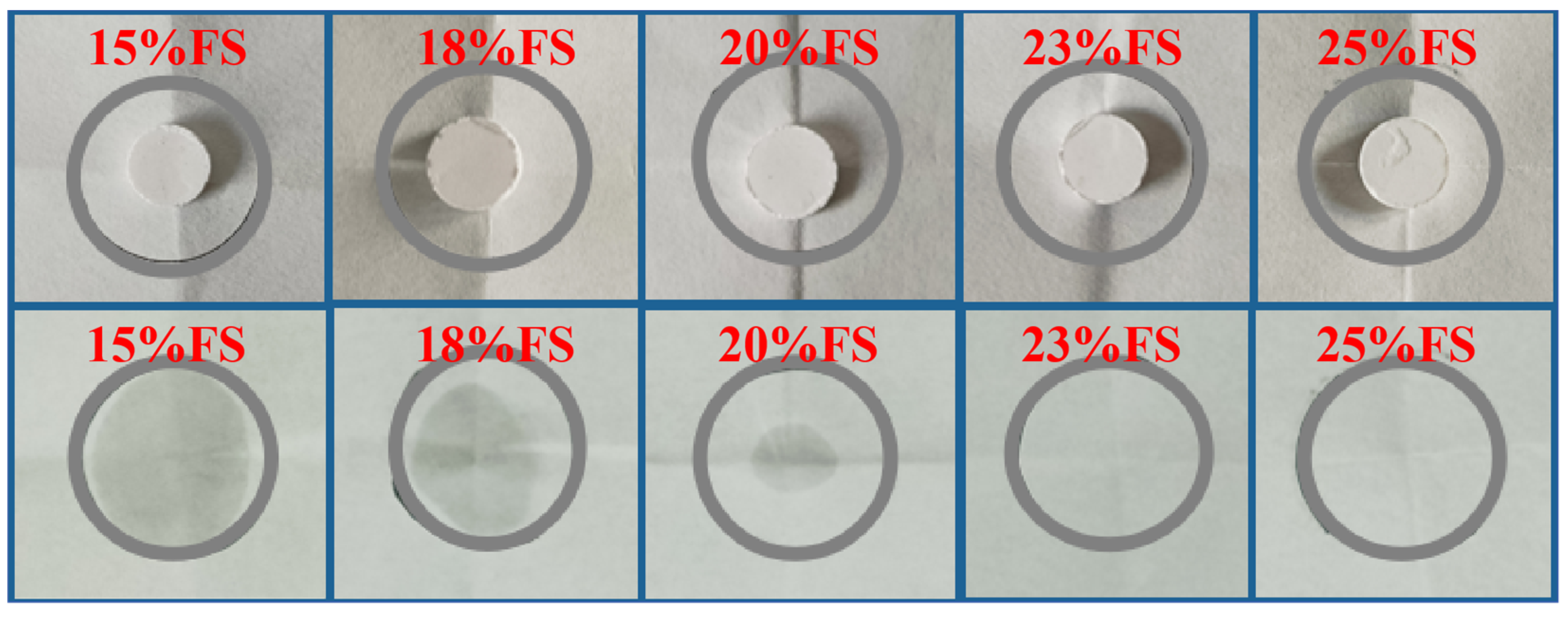
3.1. Shape Stability
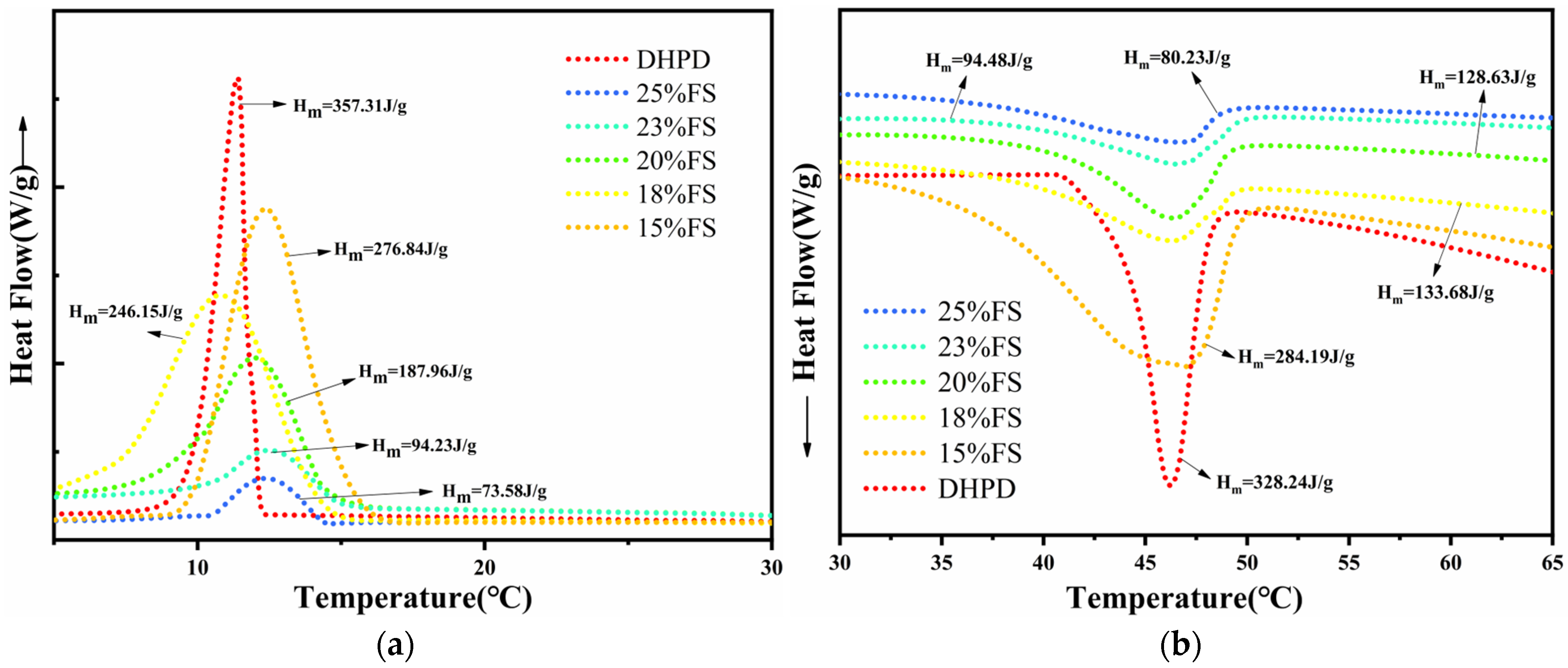
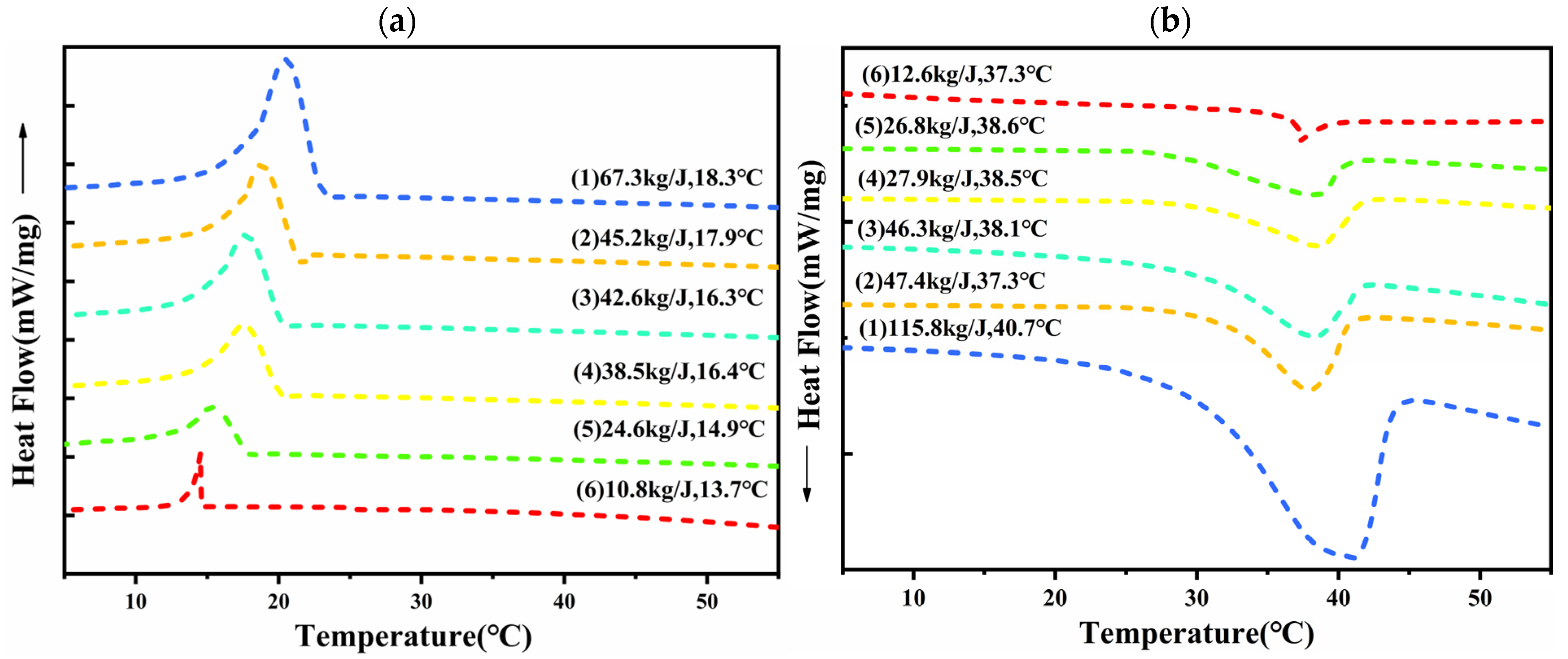
3.2. Thermal Storage Properties of CPCMs
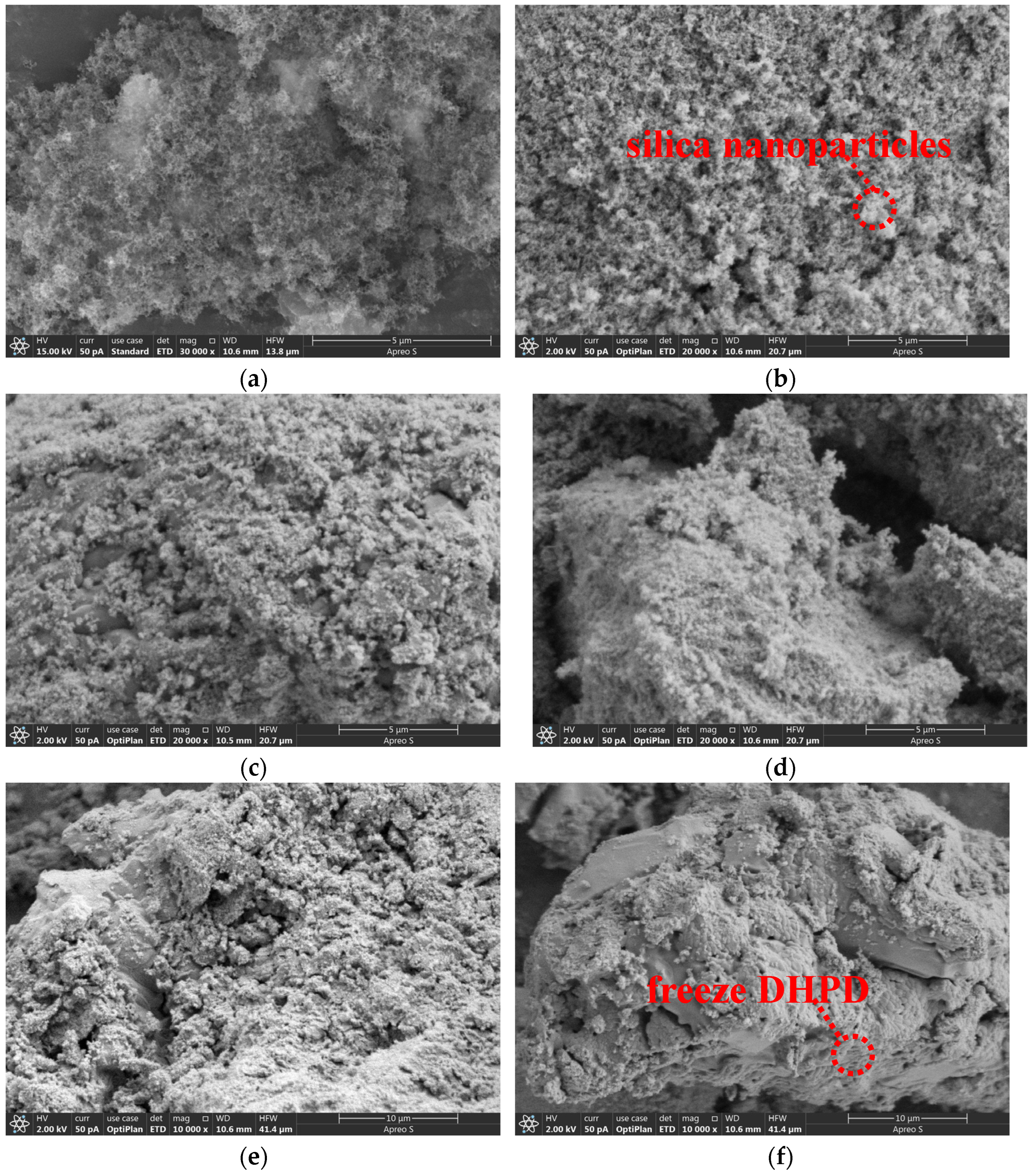
3.3. Microscopic Morphology of CPCMs
3.4. Microscopic Morphology of CPCM-RPUFs
3.5. Chemical Structure of CPCM-RPUFs
3.6. CPCM-RPUF Thermal Stability and Thermal Storage Performance
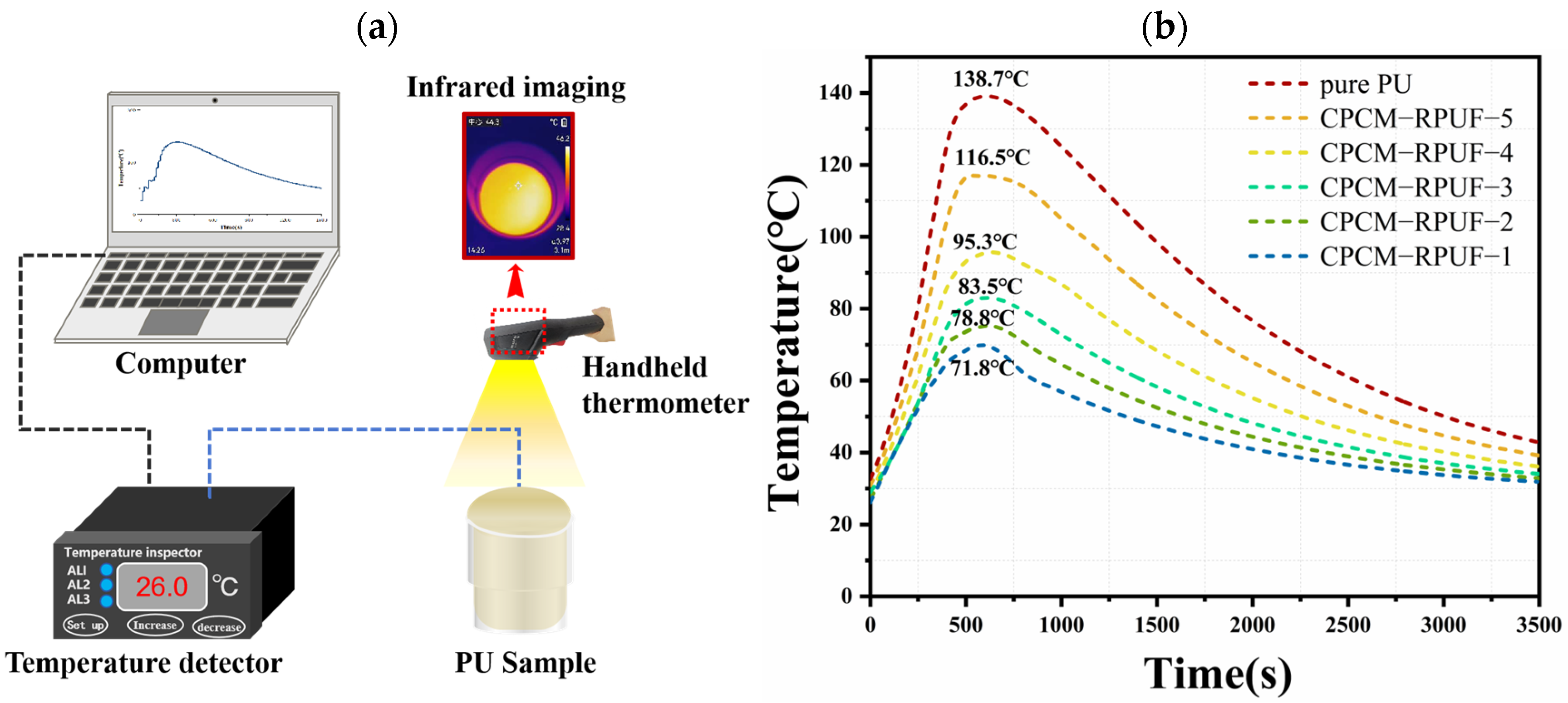
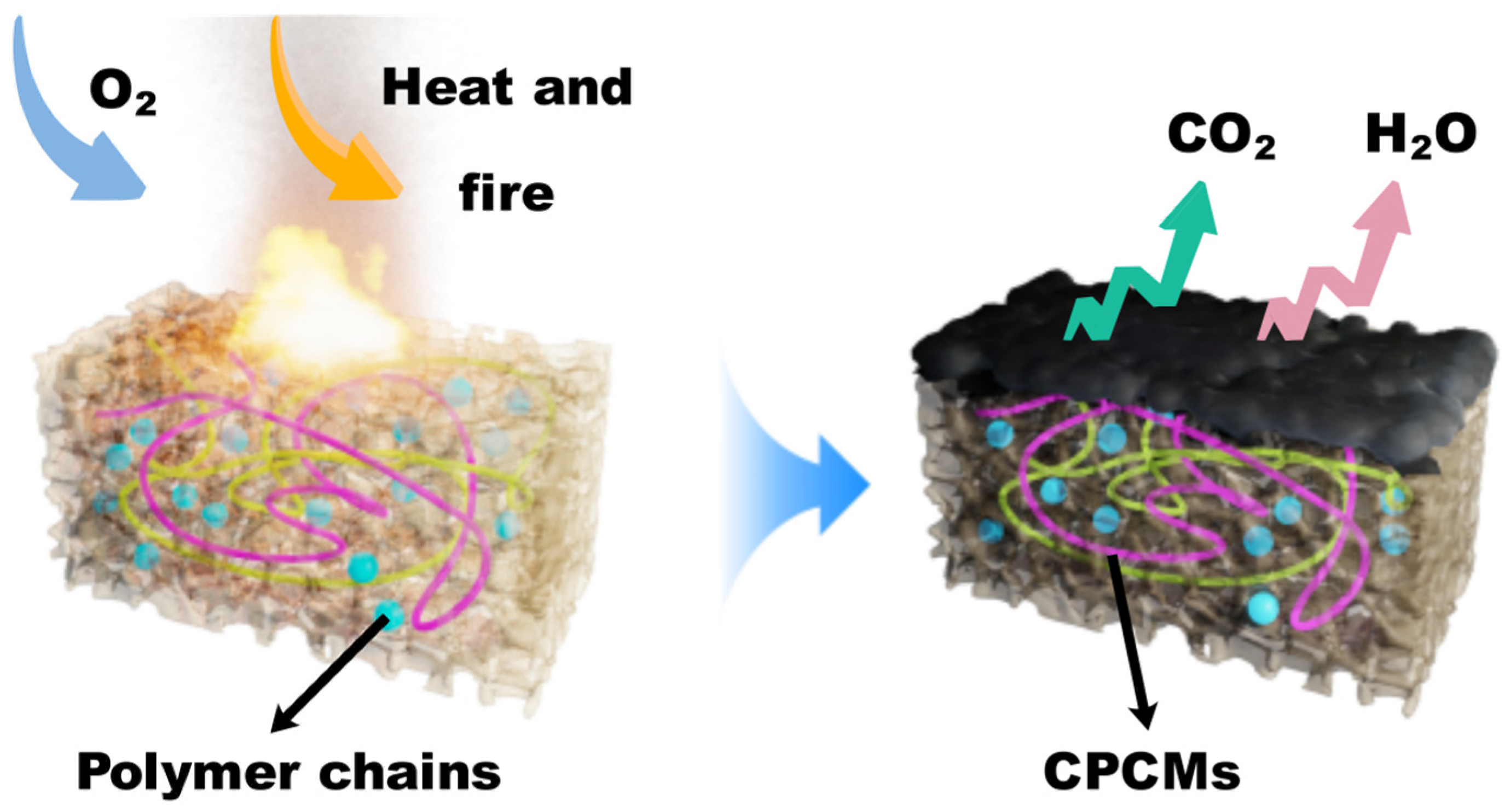
3.7. Research on Temperature Regulation Performance and Flame-Retardant Mechanism of CPCM-RPUF
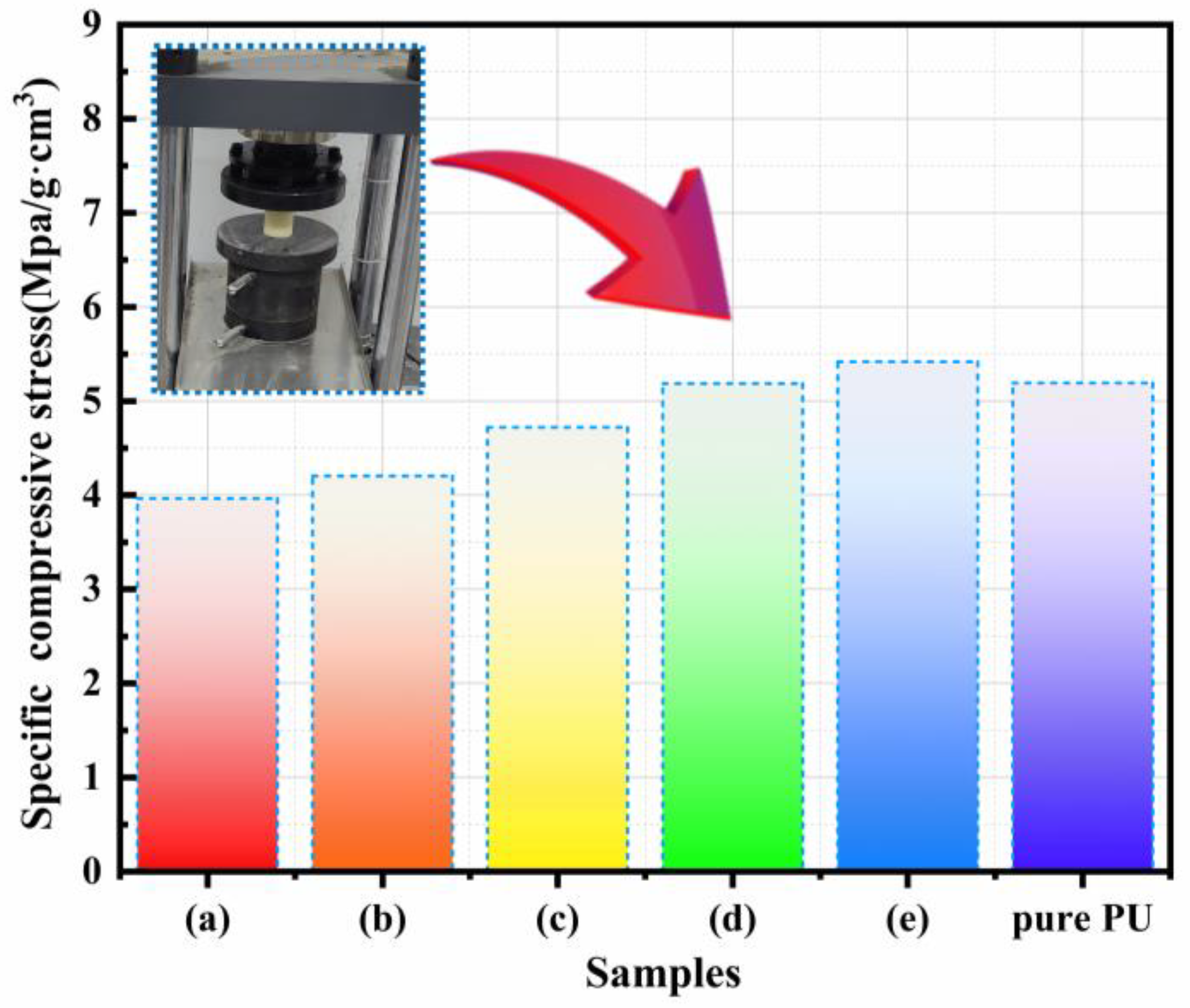
3.8. Compression Properties of CPCM-RPUFs
4. Conclusions
- 1.
- The enthalpy of the melt phase transition of the prepared CPCMs reached the highest value of 152.19 J/g. The FS encapsulated DHPD by 87%, which could effectively support and set DHPD and prevent leakage.
- 2.
- Compared with pure PU, the phase transition temperature and maximum weight loss temperature of CPCM-RPUF moved to higher values, showing better thermal cycling ability and thermal stability. Even under the influence of external ignition sources, the synergistic effect of DHPD and Si-O-Si could enhance the stability of the carbon layer of the foam and improve the flame-retardant property of CPCM-RPUF.
- 3.
- The CPCM-RPUF system has an obvious temperature hysteresis phenomenon, and the significant degree of the temperature hysteresis phenomenon increases with the decrease in the FS content in CPCMs, which demonstrates the good distribution characteristics and thermal buffering effect of CPCMs in the PU matrix.
- 4.
- CPCM-RPUF composites with higher FS content (23% and 25%) prevent PCM leakage and maintain or improve compressive strength compared to pure PUs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.G.; Xu, L.; Chen, N. Thermal expansion of polyurethane foam at low temperature. Energy Convers. Manag. 2007, 48, 481–485. [Google Scholar]
- Lu, W.; Zeng, Z.; He, Z.; Liang, Y.; Sun, Y.; Song, S.; Wang, L.; Liu, R. A highly efficient melamine/red phosphorus flame retardant for polyurethane-based foams. J. Appl. Polym. Sci. 2023, 140, e53546. [Google Scholar]
- Hu, D.; Wang, Z.; Ma, W. Fabrication and characterization of a novel polyurethane microencapsulated phase change material for thermal energy storage. Prog. Org. Coat. 2021, 151, 106006. [Google Scholar] [CrossRef]
- Li, C.; Chang, Y.; Zhang, L.; Wang, B.; Tian, X. Effect of NCO content on properties of latent curing one-component polyurethane. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2023, 42, 85–91. [Google Scholar]
- Zhang, B.; Wang, B.; Zhong, Y.; Wang, S.; Li, X.; Wang, L. Experimental Study on Reducing the Heat of Curing Reaction of Polyurethane Polymer Grouting Material. Adv. Polym. Technol. 2021, 2021, 9954498. [Google Scholar] [CrossRef]
- Amundarain, I.; Miguel-Fernandez, R.; Asueta, A. Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers 2022, 14, 1157. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Xin, S. Preparation and characterization of oligomeric thermal phase change polyurethane foam. J. Energy Storage 2023, 72, 108703. [Google Scholar]
- Qin, C.R.; Lu, W.; Li, J.Y. Analysis of the cooling mechanism of cooling polyurethane/nano fly ash grouting material. J. China Coal Soc. 2019, 44, 178–186. [Google Scholar]
- Chung, Y.-j.; Kim, Y.; Kim, S. Flame retardant properties of polyurethane produced by the addition of phosphorous containing polyurethane oligomers (II). Ind. Eng. Chem. 2009, 15, 888–893. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X. Mechanical Properties and Flame Retardancy of Rigid Polyurethane Foams Containing SiO2 Nanospheres/Graphene Oxide Hybrid and Dimethyl Methylphosphonate. Polym. Plast. Technol. Eng. 2018, 57, 884–892. [Google Scholar]
- Rao, W.-H.; Liao, W.; Wang, H. Flame-retardant and smoke-suppressant flexible polyurethane foams based on reactive phosphorus-containing polyol and expandable graphite. J. Hazard. Mater. 2018, 360, 651–660. [Google Scholar] [PubMed]
- Yu, N.; Wang, T.; Xu, C.-X. In-situ packing self-intumescent aerogel particles in rigid polyurethane foam towards thermal insulation, flame retardance and smoke suppression. Chem. Eng. J. 2025, 503, 158514. [Google Scholar] [CrossRef]
- Efimova, A.; Pinnau, S.; Mischke, M.; Breitkopf, C.; Ruck, M.; Schmidt, P. Development of salt hydrate eutectics as latent heat storage for air conditioning and cooling. Thermochim. Acta 2014, 575, 276–278. [Google Scholar]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Rao, Z.; Zhang, G.; Xu, T.; Hong, K. Experimental study on a novel form-stable phase change materials based on diatomite for solar energy storage. Sol. Energy Mater. Sol. Cells 2018, 182, 52–60. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Preparation and thermal properties of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrate salt as a novel phase change material for energy storage. Appl. Therm. Eng. 2017, 112, 606–609. [Google Scholar]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar]
- Liu, Y.; Yang, Y. Form-stable phase change material based on Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrated salt/expanded graphite oxide composite: The influence of chemical structures of expanded graphite oxide. Renew. Energy 2018, 115, 734–740. [Google Scholar]
- Jeong, S.-G.; Jeon, J.; Cha, J.; Kim, J.; Kim, S. Preparation and evaluation of thermal enhanced silica fume by incorporating organic PCM, for application to concrete. Energy Build. 2013, 62, 190–195. [Google Scholar]
- Canbazoğlu, S.; Şahinaslan, A.; Ekmekyapar, A. Enhancement of solar thermal energy storage performance using sodium thiosulfate pentahydrate of a conventional solar water-heating system. Energy Build. 2005, 37, 235–242. [Google Scholar]
- Tamaru, M.; Suzuki, H.; Hidema, R. Fabrication of hard-shell microcapsules containing inorganic materials. Int. J. Refrig. Revue Int. Du Froid 2017, 82, 97–105. [Google Scholar]
- Lan, X.Z.; Tan, Z.-C.; Shi, Q. A novel gelling method for stabilization of phase change material Na2HPO4·12H2O with sodium alginate grafted sodium acrylate. Thermochim. Acta 2007, 463, 18–20. [Google Scholar]
- Feng, J.; Mo, W.; Ma, S.; Wang, Y.; Yang, J.; Su, X.; Wang, G.; Lin, M. Thermal shrinkage inhibition mechanism of fumed silica based thermal insulating composite. Appl. Therm. Eng. 2017, 113, 749–755. [Google Scholar]
- Kang, Y.; Jeong, S.-G.; Wi, S.; Kim, S. Energy efficient Bio-based PCM with silica fume composites to apply in concrete for energy saving in buildings. Sol. Energy Mater. Sol. Cells 2015, 143, 430–434. [Google Scholar]
- Peng, S.; Huang, J.; Wang, T. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Thermochim. Acta 2019, 675, 1–8. [Google Scholar]
- Du, X.; Qiu, J.; Deng, S. Flame-retardant and solid-solid phase change composites based on dopamine-decorated BP nanosheets/Polyurethane for efficient solar-to-thermal energy storage. Renew. Energy 2021, 164, 1–10. [Google Scholar]
- Morishige, K.; Kataoka, T. Origin of melting point depression for rare gas solids confined in carbon pores. J. Chem. Phys. 2015, 143, 034707. [Google Scholar]
- Nomura, T.; Zhu, C.; Sheng, N. Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol. Energy Mater. Sol. Cells 2015, 143, 424–429. [Google Scholar]
- Gu, X.; Xu, G.; Wang, C.; Gao, Y.; Zhao, P. Preparation of mullite whisker ceramic membrane and its oil-water separation performance. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2023, 42, 70–77. [Google Scholar]
- Mohammadi, A.; Barikani, M.; Barmar, M. Synthesis and investigation of thermal and mechanical properties of in situ prepared biocompatible Fe3O4/polyurethane elastomer nanocomposites. Polym. Bull. 2015, 72, 219–234. [Google Scholar] [CrossRef]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef]
- Gao, N.; Tang, T.; Xiang, H. Preparation and structure-properties of crosslinking organic montmorillonite/polyurethane as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111831. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar]
- Zygmunt-Kowalska, B.; Pielichowska, K.; Trestka, P. The Effect of Ash Silanization on the Selected Properties of Rigid Polyurethane Foam/Coal Fly Ash Composites. Energy 2022, 15, 9725. [Google Scholar]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Producing lignin-based polyols through microwave-assisted liquefaction for rigid polyurethane foam production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, X.; Chen, W.; Zhang, H.; Han, D. Modification of Rigid Polyurethane Foams with the Addition of Nano-SiO2 or Lignocellulosic Biomass. Polymers 2020, 12, 107. [Google Scholar] [CrossRef]

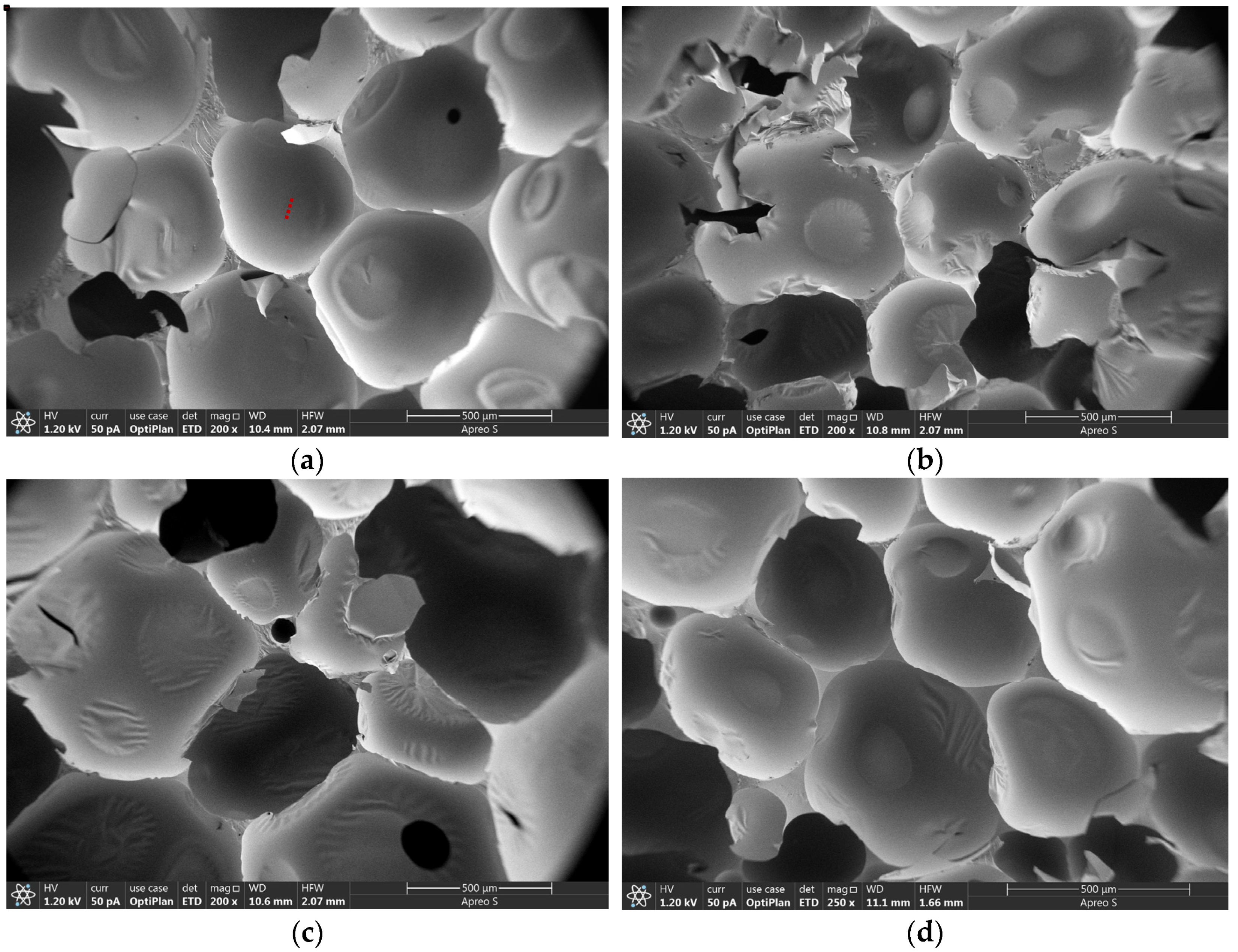
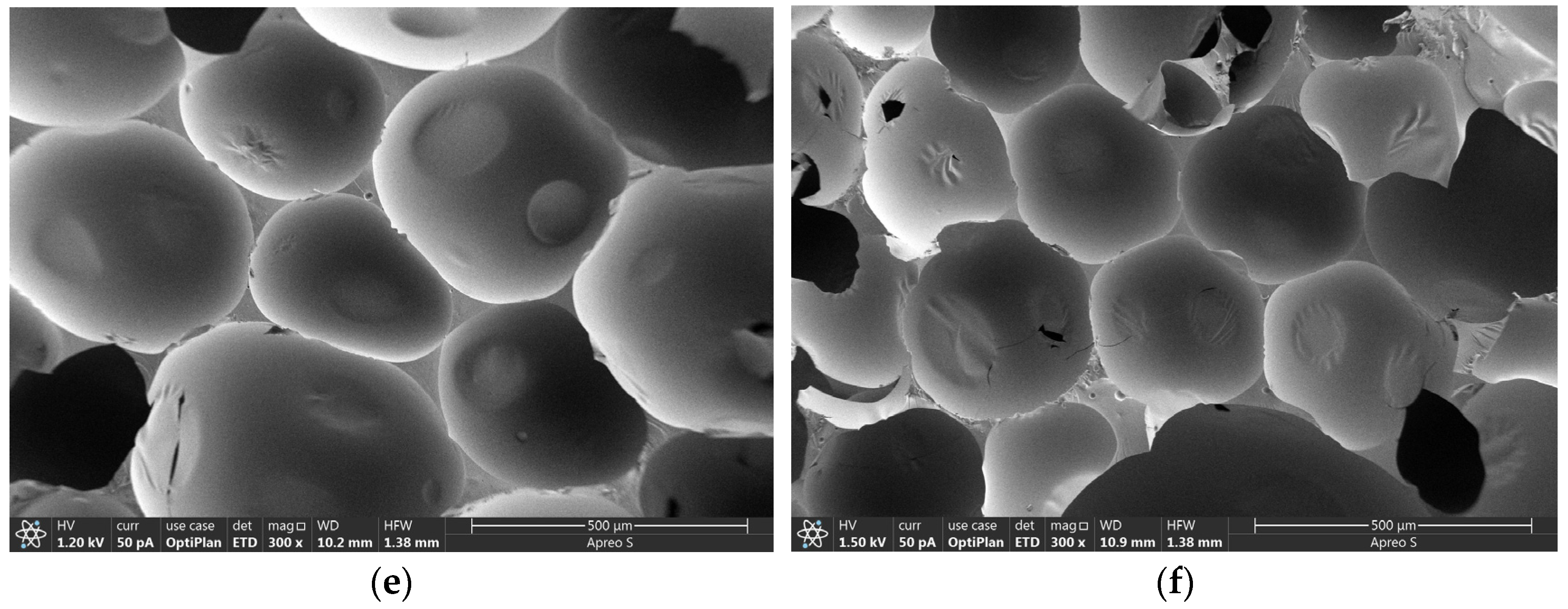
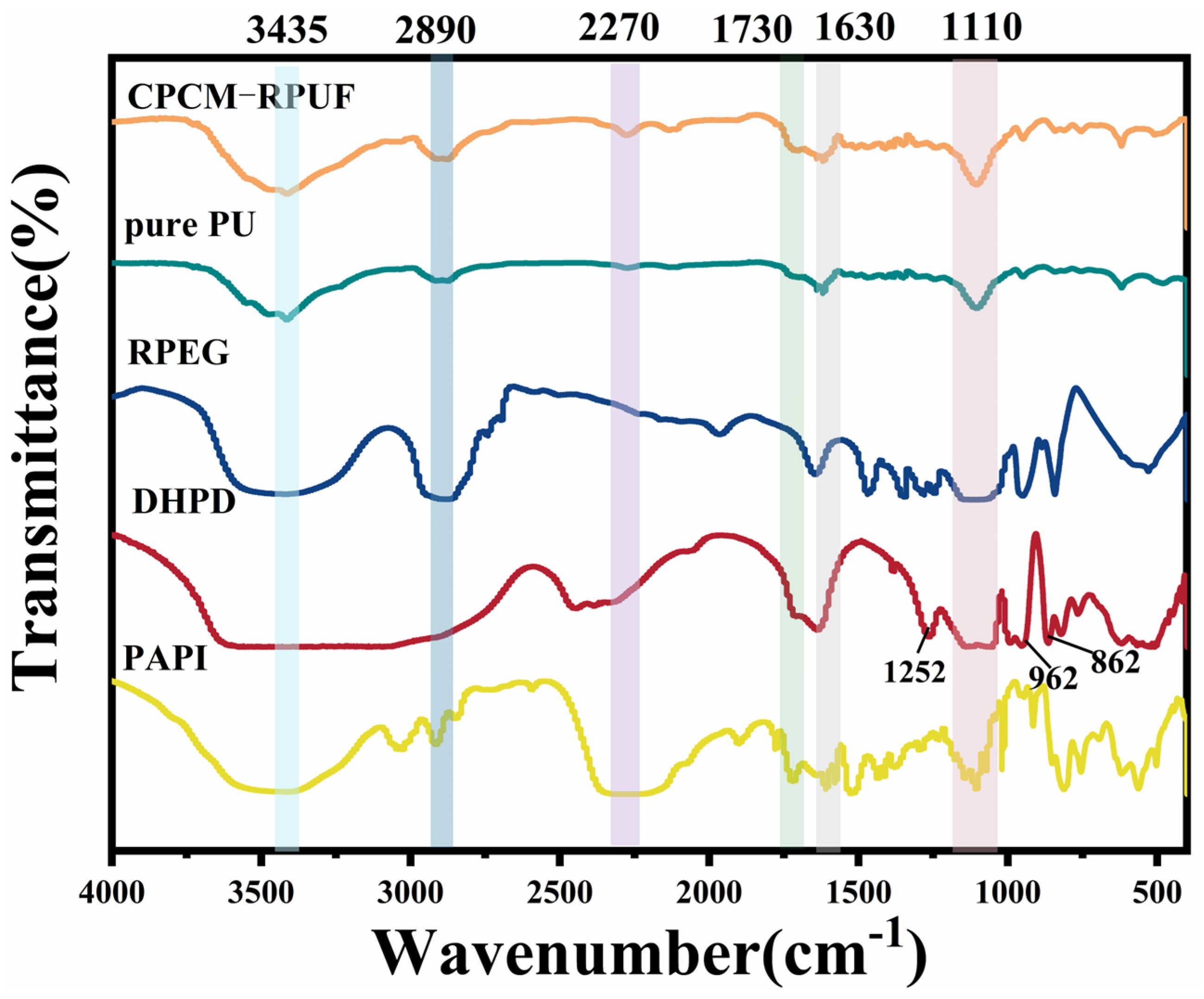
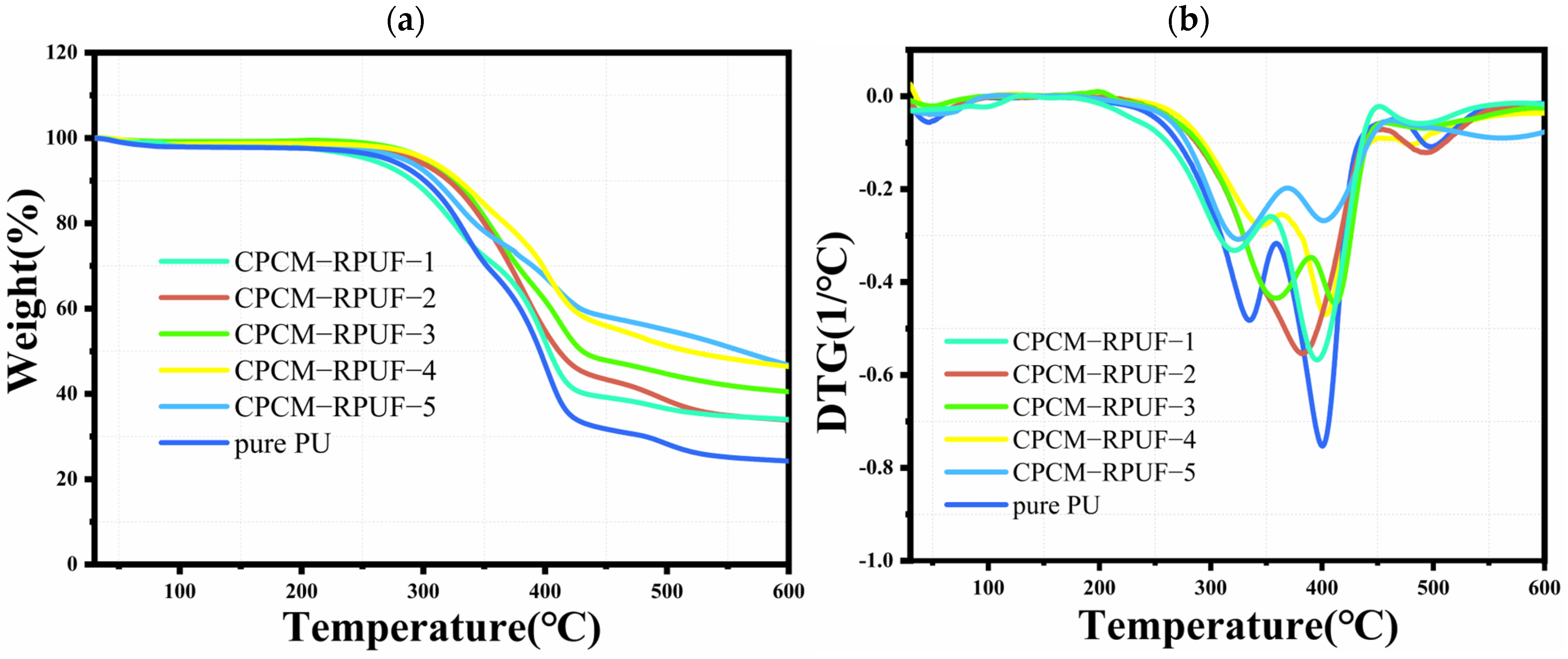

| Sample | T5% (°C) | T10% (°C) | T50% (°C) | TDTGmax1 | TDTGmax2 | TDTGmax3 | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|---|
| pure PU | 270 | 301 | 396 | 334 | 401 | 500 | 23 |
| CPCM-RPUF-1 | 254 | 292 | 404 | 319 | 396 | 484 | 34 |
| CPCM-RPUF-2 | 291 | 327 | 411 | - | 381 | 493 | 34 |
| CPCM-RPUF-3 | 302 | 328 | 430 | 357 | 411 | 493 | 40 |
| CPCM-RPUF-4 | 303 | 329 | 518 | 346 | 403 | 480 | 46 |
| CPCM-RPUF-5 | 285 | 309 | 561 | 303 | 402 | 562 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, S.; Sun, M.; Liu, S.; Zhang, X.; Liu, H. Preparation and Properties of Low-Exothermic Polyurethanes Doped with Modified Hydrated Salt Phase Change Materials. Molecules 2025, 30, 1508. https://doi.org/10.3390/molecules30071508
Xin S, Sun M, Liu S, Zhang X, Liu H. Preparation and Properties of Low-Exothermic Polyurethanes Doped with Modified Hydrated Salt Phase Change Materials. Molecules. 2025; 30(7):1508. https://doi.org/10.3390/molecules30071508
Chicago/Turabian StyleXin, Song, Mengya Sun, Shangxiao Liu, Xuan Zhang, and Han Liu. 2025. "Preparation and Properties of Low-Exothermic Polyurethanes Doped with Modified Hydrated Salt Phase Change Materials" Molecules 30, no. 7: 1508. https://doi.org/10.3390/molecules30071508
APA StyleXin, S., Sun, M., Liu, S., Zhang, X., & Liu, H. (2025). Preparation and Properties of Low-Exothermic Polyurethanes Doped with Modified Hydrated Salt Phase Change Materials. Molecules, 30(7), 1508. https://doi.org/10.3390/molecules30071508




