Effect of the Substitution of the Mesityl Group with Other Bulky Substituents on the Luminescence Performance of [Pt(1,3-bis(4-Mesityl-pyridin-2-yl)-4,6-difluoro-benzene)Cl]
Abstract
1. Introduction
2. Results and Discussion
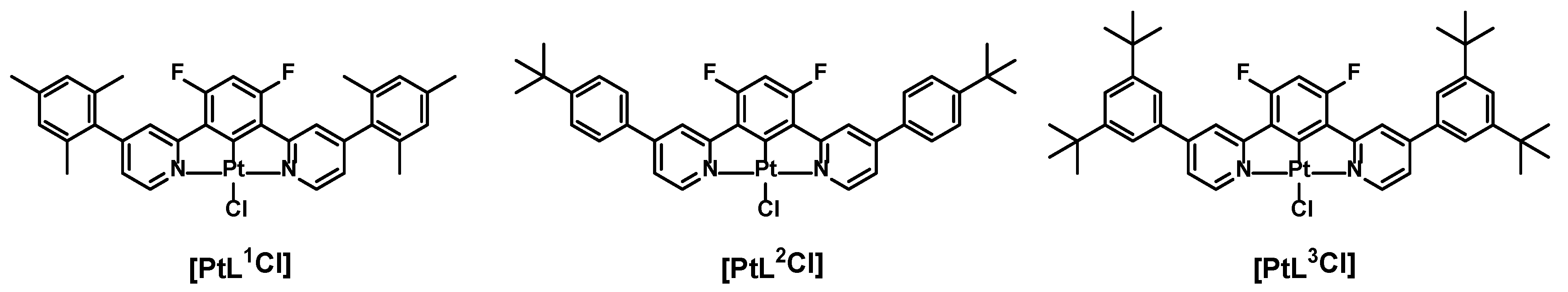
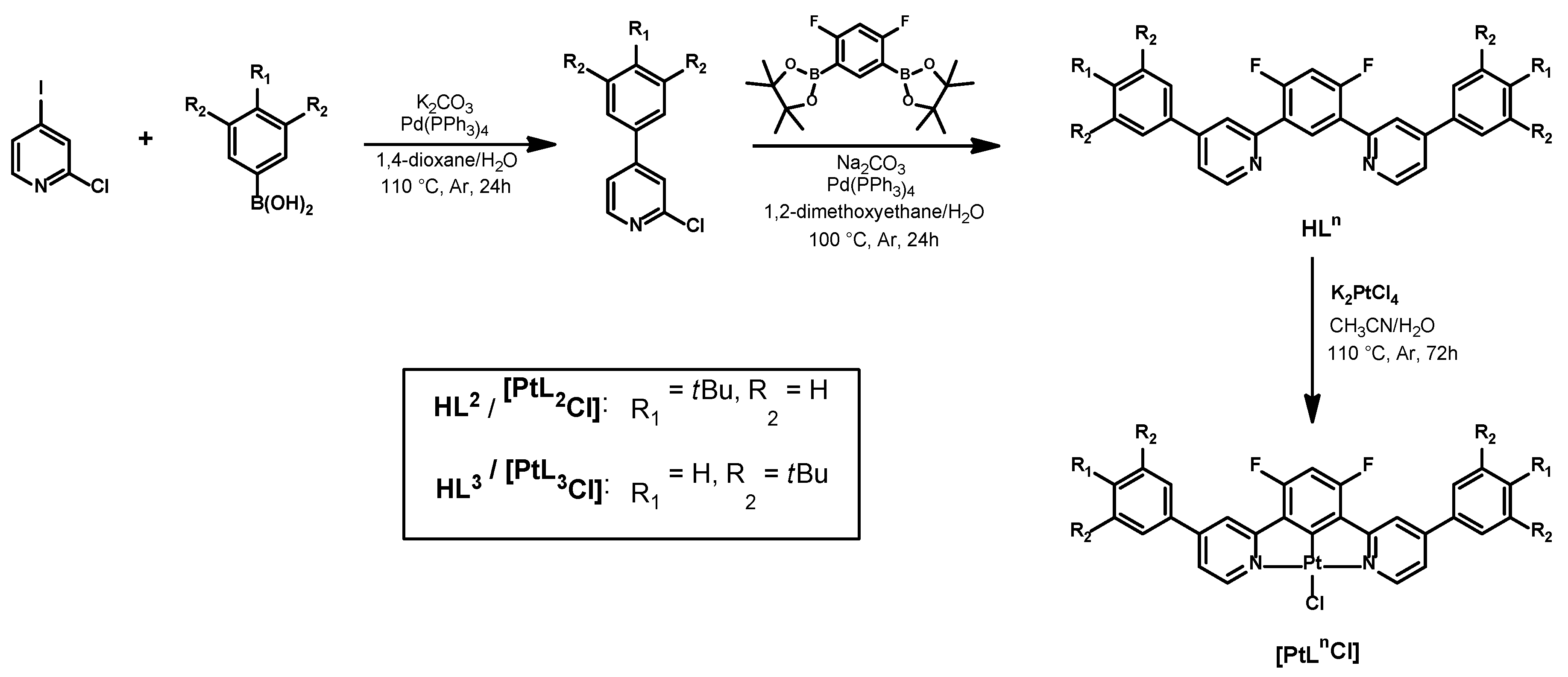
2.1. Preparation of the Complexes
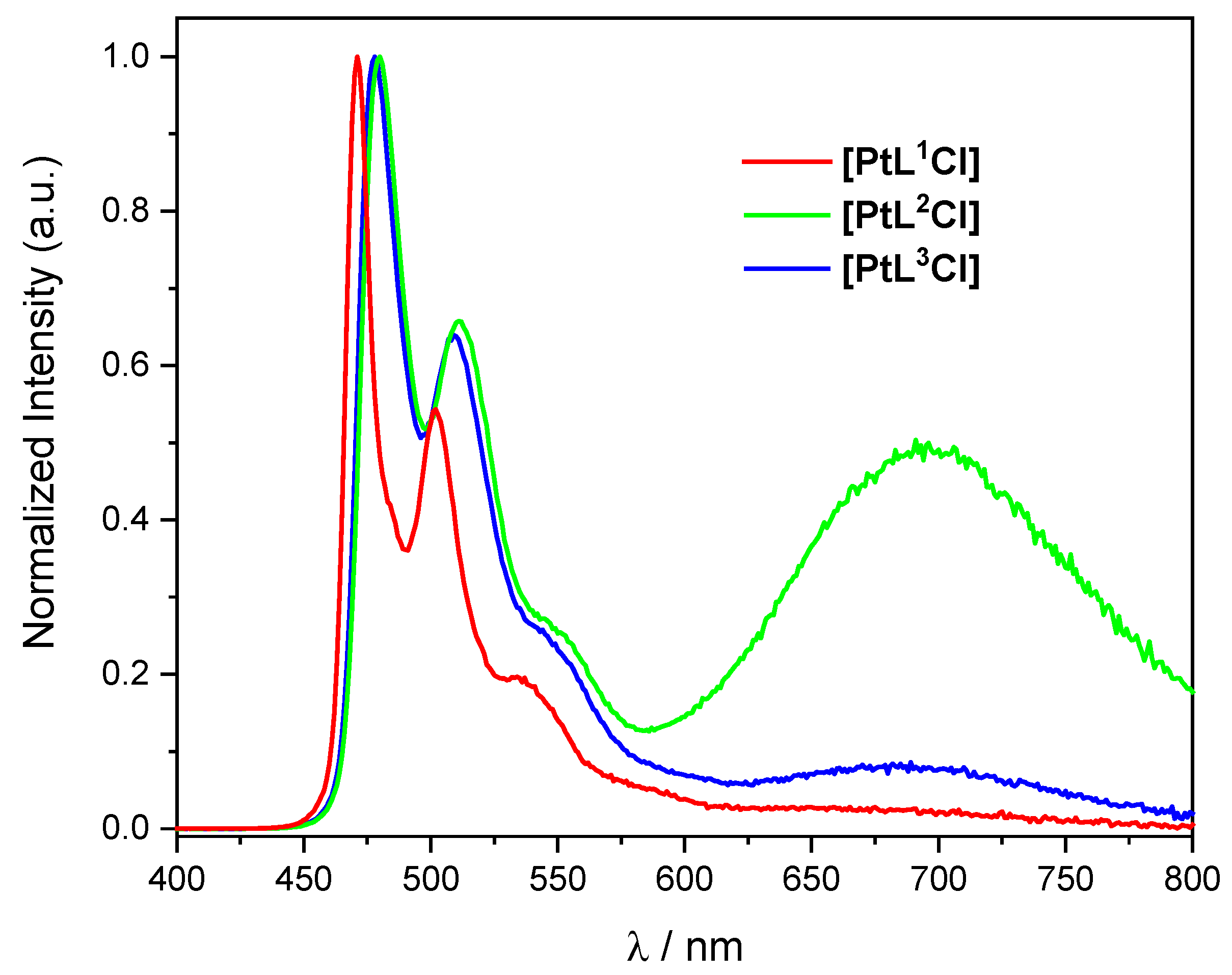
2.2. Photophysical Properties
3. Materials and Methods
3.1. Procedure for the Synthesis of [PtL2Cl]
3.1.1. Synthesis of HL2
- 4-(4-(tert-butyl)phenyl)-2-chloropyridine (1):
- 2,2′-(4,6-difluoro-1,3-phenylene)bis(4-(4-(tert-butyl)phenyl)pyridine) (HL2):
3.1.2. Synthesis of [PtL2Cl]
3.2. Procedure for the Synthesis of [PtL3Cl]
3.2.1. Synthesis of HL3
- 2-chloro-4-(3,5-di-tert-butylphenyl)pyridine (2):
- 2,2′-(4,6-difluoro-1,3-phenylene)bis(4-(3,5-di-tert-butylphenyl)pyridine) (HL3):
3.2.2. Synthesis of [PtL3Cl]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deplano, P.; Pilia, L.; Espa, D.; Mercuri, M.L.; Serpe, A. Square-planar d8 metal mixed-ligand dithiolene complexes as second order nonlinear optical chromophores: Structure/property relationship. Coord. Chem. Rev. 2010, 254, 1434–1447. [Google Scholar]
- Colombo, A.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Roberto, D.; Tavazzi, S.; Escadeillas, M.; Guerchais, V.; Le Bozec, H.; Boucekkine, A.; et al. Cyclometallated 4-styryl-2-phenylpyridine Pt(II) acetylacetonate complexes as second-order NLO building blocks for SHG active polymeric films. Organometallics 2013, 32, 3890–3894. [Google Scholar]
- Espa, D.; Pilia, L.; Marchiò, L.; Mercuri, M.L.; Serpe, A.; Barsella, A.; Fort, A.; Dalgleish, S.J.; Robertson, N.; Deplano, P. Redox-switchable chromophores based on metal (Ni, Pd, Pt) mixed-ligand dithiolene complexes showing molecular second-order nonlinear-optical activity. Inorg. Chem. 2011, 50, 2058–2060. [Google Scholar] [PubMed]
- Espa, D.; Pilia, L.; Marchiò, L.; Pizzotti, M.; Robertson, N.; Tessore, F.; Mercuri, M.L.; Serpe, A.; Deplano, P. Electrochromic second-order NLO chromophores based on MII (M = Ni, Pd, Pt) complexes with diselenolato–dithione (donor–acceptor) ligands. Dalton Trans. 2012, 41, 12106–12113. [Google Scholar]
- Espa, D.; Pilia, L.; Makedonas, C.; Marchiò, L.; Mercuri, M.L.; Serpe, A.; Barsella, A.; Fort, A.; Mitsopoulou, C.A.; Deplano, P. Role of the acceptor in tuning the properties of metal [M(II) = Ni, Pd, Pt] dithiolato/dithione (donor/acceptor) second-order nonlinear chromophores: Combined experimental and theoretical studies. Inorg. Chem. 2014, 53, 1170–1183. [Google Scholar]
- Espa, D.; Pilia, L.; Attar, S.; Serpe, A.; Deplano, P. Molecular engineering of heteroleptic metal-dithiolene complexes with optimized second-order NLO response. Inorg. Chim. Acta 2018, 470, 295–302. [Google Scholar]
- Zhao, H.; Garoni, E.; Roisnel, T.; Colombo, A.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Roberto, D.; Jacquemin, D.; Boixel, J.; et al. Photochromic DTE-substituted-1,3-di(2-pyridyl)benzene platinum(II) complexes: Photomodulation of luminescence and second-order nonlinear optical properties. Inorg. Chem. 2018, 57, 7051–7063. [Google Scholar]
- Fagnani, F.; Colombo, A.; Malandrino, G.; Dragonetti, C.; Pellegrino, A.L. Luminescent 1,10-phenanthroline β-diketonate europium complexes with large second-order nonlinear optical properties. Molecules 2022, 27, 6990. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Guerchais, V.; Roisnel, T.; Marinotto, D.; Fantacci, S. Multifunctional organometallic compounds: An interesting luminescent NLO-active alkynylplatinum (II) complex. Eur. J. Inorg. Chem. 2024, 27, e202400478. [Google Scholar]
- Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Fantacci, S. Dipolar copper(I) complexes: A novel appealing class of highly active second-order NLO-phores. Molecules 2025, 30, 1009. [Google Scholar] [CrossRef]
- Chakraborty, S.; Wadas, T.J.; Hester, H.; Schmehl, R.; Eisenberg, R. Platinum chromophore-based systems for photoinduced charge separation: A molecular design approach for artificial photosynthesis. Inorg. Chem. 2005, 44, 6865–6878. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Saito, K.; Matsukawa, H.; Yanagida, S.; Ebina, M.; Maegawa, Y.; Inagaki, S.; Kobayashi, A.; Kato, M. Immobilization of luminescent Platinum(II) complexes on periodic mesoporous organosilica and their water reduction photocatalysis. J. Photochem. Photobiol. A 2018, 358, 334–344. [Google Scholar] [CrossRef]
- Domingo-Legarda, P.; Casado-Sánchez, A.; Marzo, L.; Alemán, J.; Cabrera, S. Photocatalytic water-soluble cationic platinum(II) complexes bearing quinolinate and phosphine ligands. Inorg. Chem. 2020, 59, 13845–13857. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Segura, D.; Corral-Zorzano, A.; Alcolea, E.; Moreno, M.T.; Lalinde, E. Phenylbenzothiazole-based platinum (II) and diplatinum (II) and (III) complexes with pyrazolate groups: Optical properties and photocatalysis. Inorg. Chem. 2024, 63, 1589–1606. [Google Scholar] [CrossRef]
- Wenger, O.S. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013, 5, 3686–3733. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Vapochromic platinum(II) complexes: Crystal engineering toward intelligent sensing devices. Eur. J. Inorg. Chem. 2014, 27, 4469–4483. [Google Scholar] [CrossRef]
- Rodrigue-Witchel, A.; Rochester, D.L.; Zhao, S.-B.; Lavelle, K.B.; Williams, J.A.G.; Wang, S.; Connick, W.B.; Reber, C. Pressure-induced variations of MLCT and ligand-centered luminescence spectra in square-planar platinum(II) complexes. Polyhedron 2016, 108, 151–155. [Google Scholar]
- Attar, S.; Espa, D.; Artizzu, F.; Mercuri, M.L.; Serpe, A.; Sessini, E.; Concas, G.; Congiu, F.; Marchiò, L.; Deplano, P. Platinum–dithiolene monoanionic salt exhibiting multiproperties, including room-temperature proton-dependent solution luminescence. Inorg. Chem. 2016, 55, 5118–5126. [Google Scholar] [CrossRef]
- Attar, S.; Espa, D.; Artizzu, F.; Pilia, L.; Serpe, A.; Pizzotti, M.; Di Carlo, G.; Marchiò, L.; Deplano, P. Optically multiresponsive heteroleptic platinum dithiolene complex with proton-switchable properties. Inorg. Chem. 2017, 56, 6763–6767. [Google Scholar] [CrossRef]
- Law, A.S.-Y.; Yeung, M.C.-L.; Yam, V.W.-W. Arginine-rich peptide-induced supramolecular self-assembly of water-soluble anionic alkynylplatinum(II) complexes: A continuous and label-free luminescence assay for trypsin and inhibitor screening. ACS Appl. Mater. Interfaces 2017, 9, 41143–41150. [Google Scholar] [CrossRef]
- Attar, S.; Artizzu, F.; Marchiò, L.; Espa, D.; Pilia, L.; Casula, M.F.; Serpe, A.; Pizzotti, M.; Orbelli-Biroli, A.; Deplano, P. Uncommon optical properties and silver-responsive turn-off/on luminescence in a PtII heteroleptic dithiolene complex. Chem. Eur. J. 2018, 24, 10503–10512. [Google Scholar] [PubMed]
- Zheng, Q.; Borsley, S.; Tu, T.; Cockroft, S.L. Reversible stimuli-responsive chromism of a cyclometallated platinum(II) complex. Chem. Commun. 2020, 93, 14705–14708. [Google Scholar] [CrossRef]
- Haque, A.; El Moll, H.; Alenezi, K.M.; Khan, M.S.; Wong, W.Y. Functional materials based on cyclometalated platinum (II) β-diketonate complexes: A Review of structure–property relationships and applications. Materials 2021, 14, 4236–4261. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.C.; Au-Yeung, H.-L.; Chan, A.K.-W.; Hong, E.Y.-H.; Cheng, Y.-H.; Leung, M.-Y.; Lai, S.-L.; Low, K.-H.; Yam, V.W.-W. Cyclometalated platinum(II) complexes with donor-acceptor-containing bidentate ligands and their application studies as organic resistive memories. Chem. Asian J. 2021, 16, 3669–3676. [Google Scholar] [PubMed]
- Yam, V.W.-W.; Cheng, Y.-H. Stimuli-responsive and switchable platinum(II) Complexes and their applications in memory storage. Bull. Chem. Soc. Japan 2022, 95, 846–854. [Google Scholar]
- Ning, Y.Y.; Jin, G.Q.; Wang, M.X.; Gao, S.; Zhang, J.L. Recent progress in metal-based molecular probes for optical bioimaging and biosensing. Curr. Opin. Chem. Biol. 2022, 66, 102097–102107. [Google Scholar]
- Chan, C.W.-T.; Law, A.S.-Y.; Yam, V.W.-W. A luminescence assay in the red for the detection of hydrogen peroxide and glucose based on metal coordination polyelectrolyte-induced supramolecular self-assembly of alkynylplatinum(II) complexes. Chem. Eur. J. 2023, 29, e202300203. [Google Scholar] [CrossRef]
- Chan, C.W.-T.; Chan, K.; Yam, V.W.-W. Induced self-assembly and disassembly of alkynylplatinum(II) 2,6-bis(benzimidazol-2’-yl)pyridine complexes with charge reversal properties: “proof-of-principle” demonstration of ratiometric Förster resonance energy transfer sensing of pH. ACS Appl. Mater. Interfaces 2023, 15, 25122–25133. [Google Scholar]
- Xu, Y.; Leung, M.-Y.; Yan, L.; Chen, Z.; Li, P.; Cheng, Y.-H.; Chan, M.H.-Y.; Yam, V.W.-W. Synthesis, characterization, and resistive memory behaviors of highly strained cyclometalated platinum(II) nanohoops. J. Am. Chem. Soc. 2024, 146, 13226–13235. [Google Scholar]
- Cocchi, M.; Virgili, D.; Fattori, V.; Rochester, D.L.; Williams, J.A.G. N^C^N-coordinated platinum(II) complexes as phosphorescent emitters in high-performance organic light-emitting devices. Adv. Funct. Mater. 2007, 17, 285–289. [Google Scholar]
- Che, C.M.; Kwok, C.C.; Lai, S.W.; Rausch, A.F.; Finkenzeller, W.J.; Zhu, N.Y.; Yersin, H. Photophysical Properties and OLED Applications of Phosphorescent Platinum(II) Schiff Base Complexes. Chem. Eur. J. 2010, 16, 233–247. [Google Scholar] [PubMed]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar]
- Gildea, L.F.; Williams, J.A.G. Iridium and platinum complexes for OLEDs. In Organic Light-Emitting Diodes: Materials, Devices and Applications; Buckley, A., Ed.; Woodhead: Cambridge, UK, 2013. [Google Scholar]
- Cheng, G.; Chow, P.-K.; Kui, S.C.F.; Kwok, C.-C.; Che, C.-M. High-efficiency polymer light-emitting devices with robust phosphorescent platinum(II) emitters containing tetradentate dianionic O^N^C^N ligands. Adv. Mater. 2013, 25, 6765–6770. [Google Scholar]
- Hsu, C.-W.; Ly, K.T.; Lee, W.-K.; Wu, C.-C.; Wu, L.C.; Lee, J.-J.; Lin, T.-C.; Liu, S.-H.; Chou, P.-T.; Lee, G.-H.; et al. Triboluminescence and metal phosphor for organic light-emitting diodes: Functional Pt(II) complexes with both 2-pyridylimidazol-2-ylidene and bipyrazolate chelates. ACS Appl. Mater. Interfaces 2016, 8, 33888–33898. [Google Scholar]
- Cebrian, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar]
- Ly, K.T.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar]
- Chen, W.-C.; Sukpattanacharoen, C.; Chan, W.-H.; Huang, C.-C.; Hsu, H.-F.; Shen, D.; Hung, W.-Y.; Kungwan, N.; Escudero, D.; Lee, C.-S.; et al. Modulation of Solid-State Aggregation of Square-Planar Pt(II) Based Emitters: Enabling Highly Efficient Deep-Red/Near Infrared Electroluminescence. Adv. Funct. Mater. 2020, 30, 2002494. [Google Scholar] [CrossRef]
- Dragonetti, C.; Fagnani, F.; Marinotto, D.; di Biase, A.; Roberto, D.; Cocchi, M.; Fantacci, S.; Colombo, A. First member of an appealing class of cyclometalated 1,3-di-(2-pyridyl)benzene platinum(II) complexes for solution-processable OLEDs. J. Mater Chem. C 2020, 8, 7873–7881. [Google Scholar]
- Shafikov, M.Z.; Pander, P.; Zaytsev, A.V.; Daniels, R.; Martinscroft, R.; Dias, F.B.; Williams, J.A.G.; Kozhevnikov, V.N. Extended ligand conjugation and dinuclearity as a route to efficient platinum-based near-infrared (NIR) triplet emitters and solution-processed NIR-OLEDs. J. Mater. Chem. C 2021, 9, 127–135. [Google Scholar] [CrossRef]
- Colombo, A.; De Soricellis, G.; Fagnani, F.; Dragonetti, C.; Cocchi, M.; Carboni, B.; Guerchais, V.; Marinotto, D. Introduction of a triphenylamine substituent on pyridyl rings as a springboard for a new appealing brightly luminescent 1,3-di-(2-pyridyl)benzene platinum(ii) complex family. Dalton Trans. 2022, 51, 12161–12169. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Marinotto, D. The intriguing effect of thiolates as co-ligands in platinum(II) complexes bearing a cyclometalated 1,3-di(2-pyridyl)benzene. Inorg. Chim. Acta 2022, 532, 120744. [Google Scholar]
- Roberto, D.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Cocchi, M.; Marinotto, D. Novel class of cyclometalated platinum(II) complexes for solution-processable OLEDs. Molecules 2022, 27, 5171. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; De Soricellis, G.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Carboni, B.; Guerchais, V.; Roisnel, T.; Cocchi, M.; Fantacci, S.; et al. Introduction of a mesityl substituent on pyridyl rings as a facile strategy for improving the performance of luminescent 1,3-bis-(2-pyridyl)benzene platinum( ii ) complexes: A springboard for blue OLEDs. J. Mater. Chem. C 2024, 12, 9702–9715. [Google Scholar]
- Zhou, F.; Pan, Y.; Hung, W.-Y.; Chen, C.-F.; Chen, K.-M.; Li, J.-L.; Yiu, S.-M.; Liu, Y.-M.; Chou, P.-T.; Chi, Y.; et al. Tetradentate Pt(II) complexes based on xylenylamino linked dual pyrazolate chelates for organic light emitting diodes. Chem. Eur. J. 2024, 30, e202402636. [Google Scholar]
- Hung, C.-M.; Wang, S.-F.; Chao, W.-C.; Li, J.-L.; Chen, B.-H.; Lu, C.-H.; Tu, K.-Y.; Yang, S.-D.; Hung, W.-Y.; Chi, Y.; et al. High-performance near-infrared OLEDs maximized at 925 nm and 1022 nm through interfacial energy transfer. Nat Commun. 2024, 15, 4664. [Google Scholar]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Baggaley, E.; Botchway, S.W.; Haycock, J.W.; Morris, H.; Sazanovich, I.V.; Williams, J.A.G.; Weinstein, J.A. Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: From FLIM to PLIM and beyond. Chem. Sci. 2014, 5, 879–886. [Google Scholar]
- Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N.S.; De Cola, L. When self-assembly meets biology: Luminescent platinum complexes for imaging applications. Chem. Soc. Rev. 2014, 43, 4144–4166. [Google Scholar]
- Colombo, A.; Fiorini, F.; Septiadi, D.; Dragonetti, C.; Nisic, F.; Valore, A.; Roberto, D.; Mauro, M.; De Cola, L. Neutral N^C^N terdentate luminescent Pt(II) complexes: Their synthesis, photophysical properties, and bio-imaging applications. Dalton Trans. 2015, 44, 8478–8487. [Google Scholar]
- Mitra, K.; Lyons, C.E.; Hartman, M.C.T. A platinum(II) complex of heptamethine cyanine for photoenhanced cytotoxicity and cellular imaging in near-IR light. Angew. Chem. Int. Ed. 2018, 57, 10263–10267. [Google Scholar]
- Yam, V.W.-W.; Law, A.S.-Y. Luminescent d8 metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar]
- Law, A.S.-Y.; Lee, L.C.-C.; Lo, K.K.-W.; Yam, V.W.-W. Aggregation and supramolecular self-assembly of low-energy red luminescent alkynylplatinum(II) complexes for RNA detection, nucleolus imaging, and RNA synthesis inhibitor screening. J. Am. Chem. Soc. 2021, 143, 5396–5405. [Google Scholar] [PubMed]
- Li, B.; Wang, Y.; Chan, M.H.-Y.; Pan, M.; Li, Y.; Yam, V.W.-W. Supramolecular Assembly of Organoplatinum(II) Complexes for Subcellular Distribution and Cell Viability Monitoring with Differentiated Imaging. Angew. Chem. 2022, 61, e202210703. [Google Scholar]
- Ai, Y.; Zhang, Z.; Fei, Y.; Ye, R.; Law, A.S.-Y.; Mao, Z.-W.; Liu, J.; Li, Y.; Yam, V.W.-W. Rational design of platinum(II) complexes with orthogonally oriented triazolyl ligand with emission enhancement characteristics for cancer chemotherapy in vivo. Sci. China 2023, 66, 2878–2884. [Google Scholar]
- Berrones Reyes, J.; Sherin, P.S.; Sarkar, A.; Kuimova, M.K.; Vilar, R. Platinum(II)-based optical probes for imaging quadruplex DNA structures via phosphorescence lifetime imaging microscopy. Angew. Chem. Int. Ed. 2023, 62, e202310402. [Google Scholar]
- Wu, J.; Xu, B.; Xu, Y.; Yue, L.; Chen, J.; Xie, G.; Zhao, J. Reblooming of the cis-bis(2-phenylpyridine) platinum(II) complex: Synthesis updating, aggregation-induced emission, electroluminescence, and cell imaging. Inorg. Chem. 2023, 62, 19142–19152. [Google Scholar]
- Clancy, E.; Ramadurai, S.; Needham, S.R.; Baker, K.; Eastwood, T.A.; Weinstein, J.A.; Mulvihill, D.P.; Botchway, S.W. Fluorescence and phosphorescence lifetime imaging reveals a significant cell nuclear viscosity and refractive index changes upon DNA damage. Sci. Rep. 2023, 13, 422. [Google Scholar]
- Doherty, R.E.; Sazanovich, I.V.; McKenzie, L.K.; Stasheuski, A.S.; Coyle, R.; Baggaley, E.; Bottomley, S.; Weinstein, J.A.; Bryant, H.E. Photodynamic killing of cancer cells by a platinum(II) complex with cyclometallating ligand. Sci. Rep. 2016, 6, 22668. [Google Scholar]
- Chatzisideri, T.; Thysiadis, S.; Katsamakas, S.; Dalezis, P.; Sigala, I.; Lazarides, T.; Nikolakaki, E.; Trafalis, D.; Gederaas, O.A.; Lindgren, M.; et al. Synthesis and biological evaluation of a platinum(II)-c(RGDyK) conjugate for integrin-targeted photodynamic therapy. Eur. J. Med. Chem. 2017, 141, 221–231. [Google Scholar]
- Shi, H.; Clarkson, G.J.; Sadler, P.J. Dual action photosensitive platinum(II) anticancer prodrugs with photoreleasable.azide ligands. Inorg. Chim. Acta 2019, 489, 230–235. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Anticancer activity, DNA binding, and photodynamic properties of a N^C^N-coordinated Pt(II) complex. Inorg. Chem. 2021, 60, 10350–10360. [Google Scholar]
- De Soricellis, G.; Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D. Exploring the potential of N^C^N cyclometalated Pt(II) complexes bearing 1,3-di(2-pyridyl)benzene derivatives for imaging and photodynamic therapy. Inorg. Chim. Acta 2022, 541, 121082. [Google Scholar] [CrossRef]
- Fagnani, F.; De Soricellis, G.; Colombo, A.; Dragonetti, C.; Roberto, D.; di Biase, A.; Fantacci, S.; Marinotto, D. Photophysical investigation of highly phosphorescent N^C^N platinum(II) azido complexes and their triazole derivatives. Dye. Pigment. 2024, 225, 112064. [Google Scholar]
- Upadhyay, A.; Nepalia, A.; Bera, A.; Saini, D.K.; Chakravarty, A.R. A Platinum(II) boron-dipyrromethene complex for cellular imaging and mitochondria-targeted photodynamic therapy in red light. Chem. Asian J. 2023, 18, e202300667. [Google Scholar]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar]
- Chou, P.T.; Chi, Y.; Chung, M.W.; Lin, C.C. Harvesting luminescence via harnessing the photophysical properties of transition metal complexes. Coord. Chem. Rev. 2011, 255, 2653–2665. [Google Scholar] [CrossRef]
- Cocchi, M.; Kalinowski, J.; Fattori, V.; Williams, J.A.G.; Murphy, L. Color-variable highly efficient organic electrophosphorescent diodes manipulating molecular exciton and excimer emissions. Appl. Phys. Lett. 2009, 94, 073309–073311. [Google Scholar]
- Williams, J.A.G. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An alternative route to highly luminescent platinum(II) complexes: cyclometalation with N∧C∧N-coordinating dipyridylbenzene ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.G. Photochemistry and Photophysics of Coordination Compounds: Platinum. Top. Curr. Chem. 2007, 281, 205–268. [Google Scholar]
- Mdleleni, M.M.; Bridgewater, J.S.; Watts, R.J.; Ford, P.C. Synthesis, structure, and spectroscopic properties of ortho-metalated platinum(II) complexes. Inorg. Chem. 1995, 34, 2334–2342. [Google Scholar] [CrossRef]
- Sotoyama, W.; Satoh, T.; Sato, H.; Matsuura, A.; Sawatari, N. Excited states of phosphorescent platinum(II) complexes containing N^C^N-coordinating tridentate ligands: Spectroscopic investigations and time-dependent density functional theory calculations. J. Phys. Chem. A 2005, 109, 9760–9766. [Google Scholar] [CrossRef]
- Cocchi, M.; Kalinowski, J.; Murphy, L.; Williams, J.A.G.; Fattori, V. Mixing of molecular exciton and excimer phosphorescence to tune color and efficiency of organic LEDs. Org. Electron. 2010, 11, 388–396. [Google Scholar] [CrossRef]
- Shen, Y.; Kong, X.; Yang, F.; Bian, H.-D.; Cheng, G.; Cook, T.R.; Zhang, Y. Deep Blue Phosphorescence from Platinum Complexes Featuring Cyclometalated N-Pyridyl Carbazole Ligands with Monocarborane Clusters (CB11H12−). Inorg. Chem. 2022, 61, 16707–16717. [Google Scholar] [CrossRef]
- Maganti, T.; Venkatesan, K. The Search for Efficient True Blue and Deep Blue Emitters: An Overview of Platinum Carbene Acetylide Complexes. ChemPlusChem 2022, 87, e202200014. [Google Scholar] [CrossRef]
- Nguyen, Y.H.; Dang, V.Q.; Soares, J.V.; Wu, J.I.; Teets, T.S. Efficient blue-phosphorescent trans-bis(acyclic diaminocarbene) platinum(ii) acetylide complexes. Chem. Sci. 2023, 14, 4857–4862. [Google Scholar] [CrossRef]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
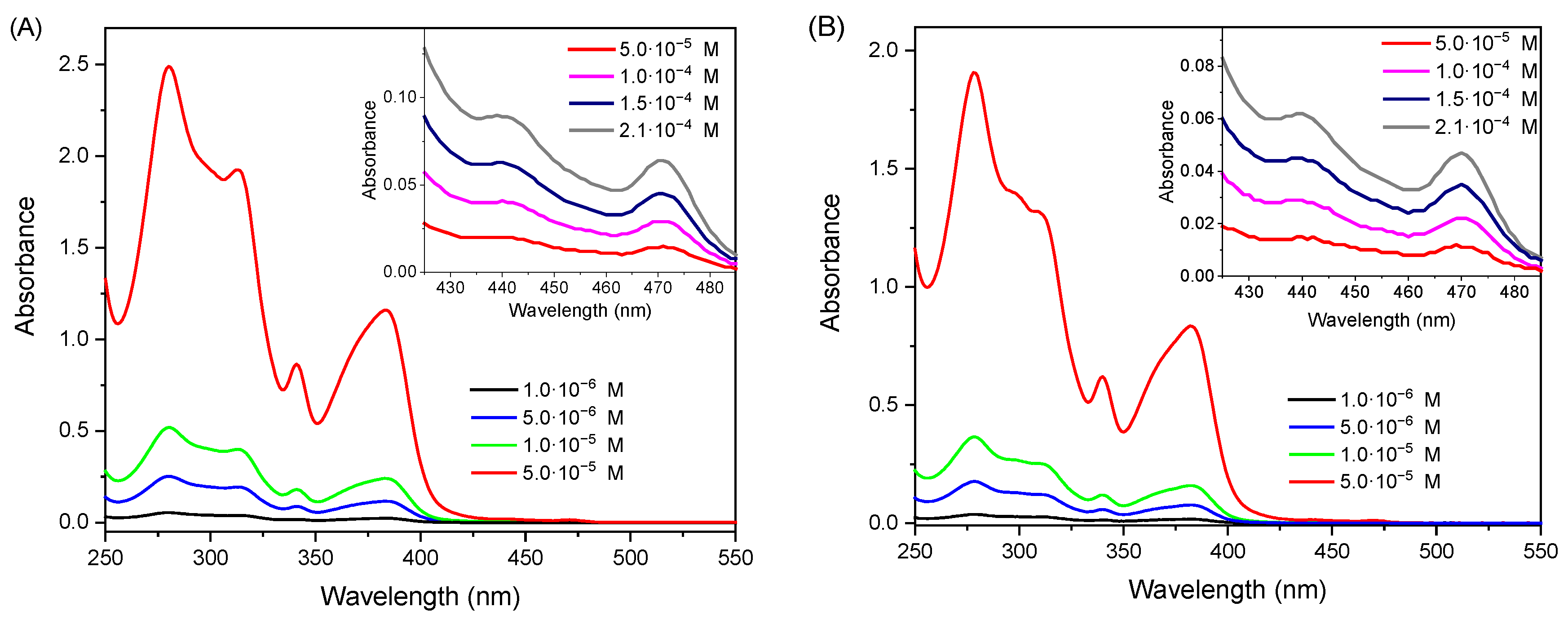
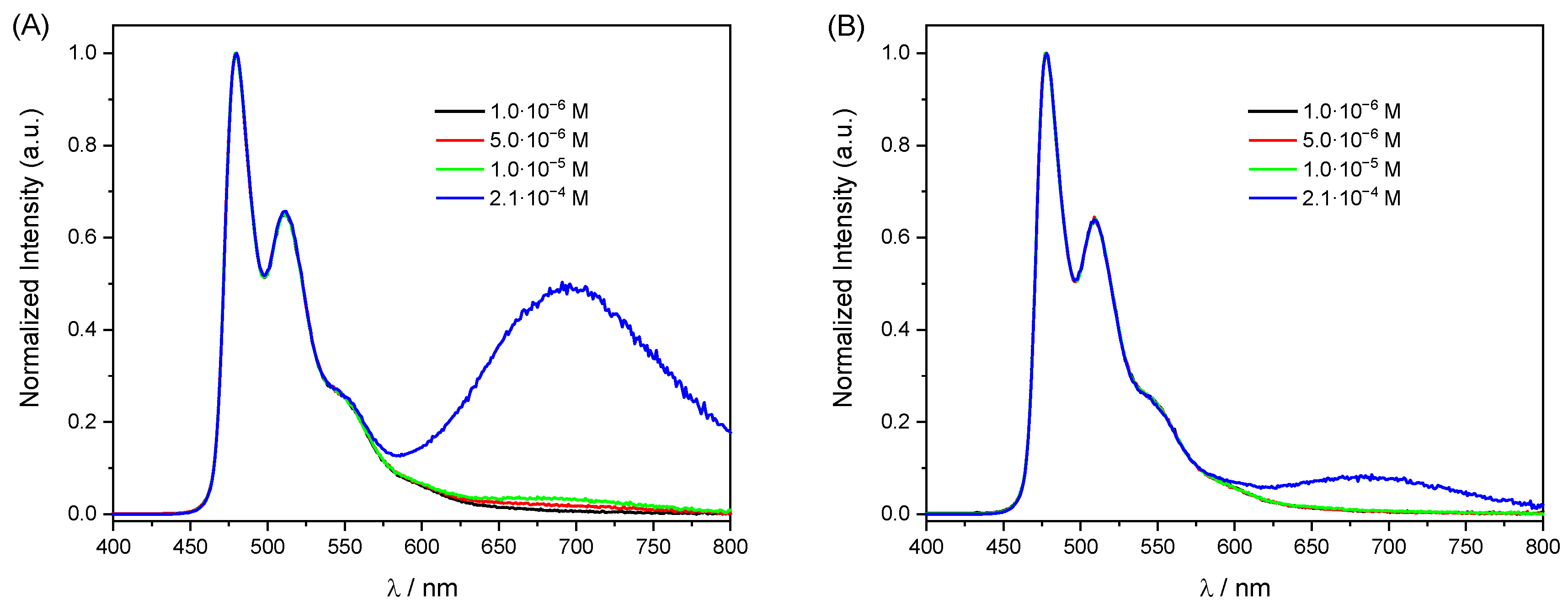
| λmax,abs (S0→T1)/nm [ε/M−1cm−1] | λmax,em/nm b Monomer [excimer/Aggregate] c | Φlum d [Aerated] | τ/µs | kr e/s−1 | knr e/s−1 | |
|---|---|---|---|---|---|---|
| [PtL1Cl] (5·10−6M) | 467 [120] | 471 [680] | 0.97 [0.18] | 4.77 | 2.0·105 | 6.3·103 |
| [PtL1Cl] (2·10−4M) | 0.62 [0.12] | 2.41 | 2.6·105 | 1.6·105 | ||
| [PtL2Cl] (5·10−6M) | 470 [310] | 480 [697] | 0.89 [0.26] | 3.91 | 2.3·105 | 2.8·104 |
| [PtL2Cl] (2·10−4M) | 0.57 [0.23] | 1.17 | 4.9·105 | 3.7·105 | ||
| [PtL3Cl] (5·10−6M) | 470 [225] | 478 [690] | 0.95 [0.21] | 3.96 | 2.4·105 | 1.3·104 |
| [PtL3Cl] (2·10−4M) | 0.54 [0.17] | 2.11 | 2.6·105 | 2.2·105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Soricellis, G.; Guerchais, V.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Marinotto, D. Effect of the Substitution of the Mesityl Group with Other Bulky Substituents on the Luminescence Performance of [Pt(1,3-bis(4-Mesityl-pyridin-2-yl)-4,6-difluoro-benzene)Cl]. Molecules 2025, 30, 1498. https://doi.org/10.3390/molecules30071498
De Soricellis G, Guerchais V, Colombo A, Dragonetti C, Fagnani F, Roberto D, Marinotto D. Effect of the Substitution of the Mesityl Group with Other Bulky Substituents on the Luminescence Performance of [Pt(1,3-bis(4-Mesityl-pyridin-2-yl)-4,6-difluoro-benzene)Cl]. Molecules. 2025; 30(7):1498. https://doi.org/10.3390/molecules30071498
Chicago/Turabian StyleDe Soricellis, Giulia, Véronique Guerchais, Alessia Colombo, Claudia Dragonetti, Francesco Fagnani, Dominique Roberto, and Daniele Marinotto. 2025. "Effect of the Substitution of the Mesityl Group with Other Bulky Substituents on the Luminescence Performance of [Pt(1,3-bis(4-Mesityl-pyridin-2-yl)-4,6-difluoro-benzene)Cl]" Molecules 30, no. 7: 1498. https://doi.org/10.3390/molecules30071498
APA StyleDe Soricellis, G., Guerchais, V., Colombo, A., Dragonetti, C., Fagnani, F., Roberto, D., & Marinotto, D. (2025). Effect of the Substitution of the Mesityl Group with Other Bulky Substituents on the Luminescence Performance of [Pt(1,3-bis(4-Mesityl-pyridin-2-yl)-4,6-difluoro-benzene)Cl]. Molecules, 30(7), 1498. https://doi.org/10.3390/molecules30071498








