Synthesis, Characterization and Application of NNN Pincer Manganese Complexes with Pyrazole Framework in α-Alkylation Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Complexes
2.2. Catalytic Studies
3. Experimental Section
3.1. General
3.2. General Procedure for Synthesis of the NNN Pincer Manganese(I) Complexes 4
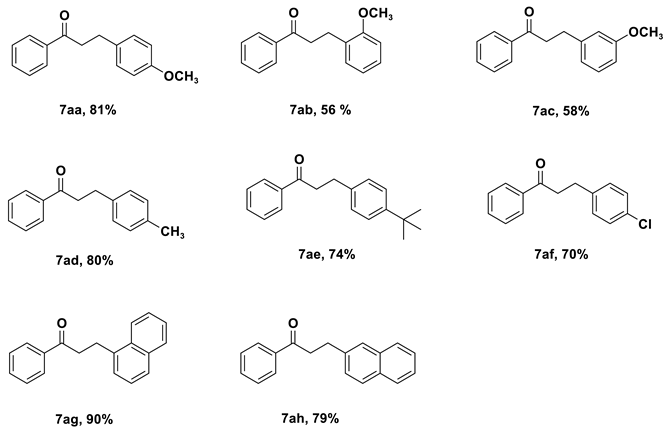
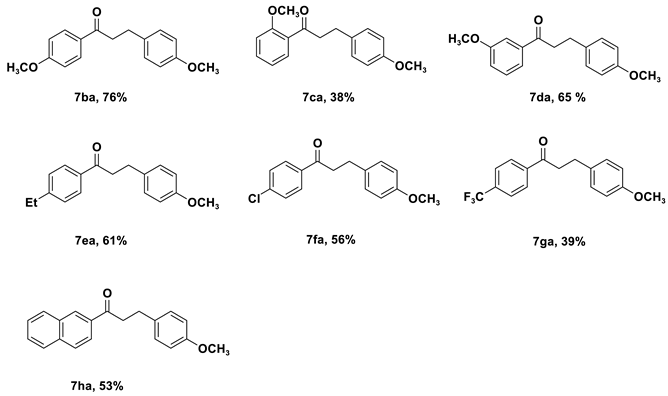
3.3. General Procedure for the α-Alkylation Reaction
3.4. Crystal Structure Determination and Data Collection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esfandiari, M.; Havaei, G.; Zahiri, S.; Mohammadnezhad, G. Pincer complex immobilization onto different supports: Strategies and applications. Coord. Chem. Rev. 2022, 472, 214778. [Google Scholar]
- Wang, H.; Wen, J.; Zhang, X. Chiral tridentate ligands in transition metal-catalyzed asymmetric hydrogenation. Chem. Rev. 2021, 121, 7530. [Google Scholar] [PubMed]
- Wu, S.; Wu, Z.; Ge, Q.; Zheng, X.; Yang, Z. Antitumor activity of tridentate pincer and related metal complexes. Org. Biomol. Chem. 2021, 19, 5254. [Google Scholar] [CrossRef] [PubMed]
- Urgoitia, G.; Herrero, M.T.; Churruca, F.; Conde, N.; SanMartin, R. Direct Arylation in the Presence of Palladium Pincer Complexes. Molecules 2021, 26, 4385. [Google Scholar] [CrossRef]
- Liu, J.-K.; Gong, J.-F.; Song, M.-P. Chiral palladium pincer complexes for asymmetric catalytic reactions. Org. Biomol. Chem. 2019, 17, 6069. [Google Scholar]
- González-Sebastián, L.; Morales-Morales, D. Cross-coupling reactions catalysed by palladium pincer complexes. A review of recent advances. J. Organomet. Chem. 2019, 893, 39. [Google Scholar]
- Selander, N.; Szabo, K.J. Catalysis by palladium pincer complexes. Chem. Rev. 2011, 111, 2048. [Google Scholar]
- Niu, J.-L.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Symmetrical and unsymmetrical pincer complexes with group 10 metals: Synthesis via aryl C–H activation and some catalytic applications. Dalton Trans. 2011, 40, 5135. [Google Scholar]
- Keskin, E.; Arslan, H. Palladium complexes containing NNN pincer type ligands and their activities in Suzuki-Miyaura cross coupling reaction. Heliyon 2023, 9, e17608. [Google Scholar]
- Sudharsan, M.; Nethaji, M.; Bhuvanesh, N.S.; Suresh, D. Heteroleptic palladium(II) complexes of thiazolinyl-picolinamide derived NNN pincer ligand: An efficient catalyst for acylative suzuki coupling reactions. Asian J. Org. Chem. 2021, 10, 2982. [Google Scholar] [CrossRef]
- Lo, P.-C.; Yang, C.-W.; Wu, W.-K.; Chen, C.-T. Synthesis, characterization, and catalytic application of palladium complexes containing indolyl-NNN-type ligands. Molecules 2021, 26, 4426. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.N.; Bhuvanesh, N.; Kumar, A.; Joshi, H. Design and syntheses of palladium complexes of NNN/CNN pincer ligands: Catalyst for cross dehydrogenative coupling reaction of heteroarenes. Organometallics 2020, 39, 324. [Google Scholar]
- Kuyuldar, S.; Burda, C.; Connick, W.B. Halide exchange studies of novel Pd(IIi) NNN-pincer complexes. RSC Adv. 2019, 9, 25703. [Google Scholar]
- Guin, A.K.; Pal, S.; Chakraborty, S.; Chakraborty, S.; Paul, N.D. N-alkylation of amines by C1–C10 aliphatic alcohols using a well-defined Ru(II)-catalyst. a metal–ligand cooperative approach. J. Org. Chem. 2023, 88, 5944. [Google Scholar] [PubMed]
- Guin, A.K.; Mondal, R.; Chakraborty, G.; Pal, S.; Paul, N.D. Ruthenium-catalyzed dehydrogenative functionalization of alcohols to pyrroles: A comparison between metal–ligand cooperative and non-cooperative approaches. J. Org. Chem. 2022, 87, 7106. [Google Scholar]
- Nandi, P.G.; Jadi, P.K.; Das, K.; Prathapa, S.J.; Mandal, B.B.; Kumar, A. Synthesis of NNN chiral ruthenium complexes and their cytotoxicity studies. Inorg. Chem. 2021, 60, 7422. [Google Scholar]
- Bhattacharyya, D.; Sarmah, B.K.; Nandi, S.; Srivastava, H.K.; Das, A. Selective catalytic synthesis of α-alkylated ketones and β-disubstituted ketones via acceptorless dehydrogenative cross-coupling of alcohols. Org. Lett. 2021, 23, 869. [Google Scholar]
- Maji, M.; Chakrabarti, K.; Paul, B.; Roy, B.C.; Kundu, S. Ruthenium(II)-NNN-pincer-complex-catalyzed reactions between various alcohols and amines for sustainable C−N and C−C bond formation. Adv. Synth. Catal. 2018, 360, 722. [Google Scholar]
- Cao, X.-N.; Wan, X.-M.; Yang, F.-L.; Li, K.; Hao, X.-Q.; Shao, T.; Zhu, X.; Song, M.-P. NNN pincer Ru(II)-complex-catalyzed α-alkylation of ketones with alcohols. J. Org. Chem. 2018, 83, 3657. [Google Scholar]
- Teimouri, M.; Raju, S.; Acheampong, E.; Schmittou, A.N.; Donnadieu, B.; Wipf, D.O.; Pierce, B.S.; Stokes, S.L.; Emerson, J.P. Aminoquinoline-based tridentate (NNN)-copper catalyst for C–N bond-forming reactions from aniline and diazo compounds. Molecules 2024, 29, 730. [Google Scholar] [CrossRef]
- Raju, S.; Teimouri, M.; Adhikari, B.; Donnadieu, B.; Stokes, S.L.; Emerson, J.P. Copper complexes for the chemoselective N-arylation of arylamines and sulfanilamides via Chan–Evans–Lam cross-coupling. Dalton Trans. 2023, 52, 15986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Hao, Z.; Han, Z.; Lin, J.; Lu, G.-L. Copper complexes with N,N,N-tridentate quinolinyl anilido-imine ligands: Synthesis and their catalytic application in Chan−Lam reactions. Molecules 2023, 28, 7406. [Google Scholar] [CrossRef] [PubMed]
- Ataie, S.; Baker, R.T. Comparing B-H bond activation in NiIIX(NNN)-catalyzed nitrile dihydroboration (X = Anionic N-, C-, O-, S-, or P-donor). Inorg. Chem. 2022, 61, 19998. [Google Scholar] [CrossRef]
- Subaramanian, M.; Midya, S.P.; Ramar, P.M.; Balaraman, E. General synthesis of N-alkylation of amines with secondary alcohols via hydrogen autotransfer. Org. Lett. 2019, 21, 8899. [Google Scholar] [CrossRef]
- Patel, U.N.; Pandey, D.K.; Gonnade, R.G.; Punji, B. Synthesis of quinoline-based NNN-pincer nickel(II) complexes: A robust and improved catalyst system for C–H bond alkylation of azoles with alkyl halides. Organometallics 2016, 35, 1785. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Zhang, X.-Y.; Sun, Y.; Yu, Q.-L.; Zhang, X.-H.; Lu, Z. Cobalt-catalyzed regiodivergent double hydrosilylation of arylacetylenes. Angew. Chem. Int. Ed. 2023, 62, e202215029. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.; Kumar, A.; Gunanathan, C. Cobalt-catalysed [1,2]-Wittig rearrangement of ethers to secondary alcohols. Chem. Commun. 2023, 59, 1853. [Google Scholar] [CrossRef]
- Chen, J.; Ying, J.; Lu, Z. Cobalt-catalyzed branched selective hydroallylation of terminal alkynes. Nat. Commun. 2022, 13, 4518. [Google Scholar] [CrossRef]
- Banach, Ł.; Brykczynska, D.; Gorczynski, A.; Wyrzykiewicz, B.; Skrodzki, M.; Pawluc, P. Markovnikov-selective double hydrosilylation of challenging terminal aryl alkynes under cobalt and iron catalysis. Chem. Commun. 2022, 58, 13763. [Google Scholar] [CrossRef]
- Pradhan, D.R.; Pattanaik, S.; Kishore, J.; Gunanathan, C. Cobalt-catalyzed acceptorless dehydrogenation of alcohols to carboxylate salts and hydrogen. Org. Lett. 2020, 22, 1852. [Google Scholar] [CrossRef]
- Pal, S.; Das, S.; Chakraborty, S.; Khanra, S.; Paul, N.D. Zn(II)-catalyzed multicomponent sustainable synthesis of pyridines in air. J. Org. Chem. 2023, 88, 3650. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mondal, R.; Chakraborty, G.; Guin, A.K.; Das, A.; Paul, N.D. Zinc stabilized azo-anion radical in dehydrogenative synthesis of N-heterocycles. an exclusively ligand centered redox controlled approach. ACS Catal. 2021, 11, 7498. [Google Scholar]
- Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L.J.W.; Ben David, Y.; Espinosa Jalapa, N.A.; Milstein, D. Manganese-catalyzed environmentally benign dehydrogenative coupling of alcohols and amines to form aldimines and H2: A catalytic and mechanistic study. J. Am. Chem. Soc. 2016, 138, 4298. [Google Scholar]
- Sklyaruk, J.; Borghs, J.C.; El-Sepelgy, O.; Rueping, M. Catalytic C1 alkylation with methanol and isotope-labeled methanol. Angew. Chem. Int. Ed. 2019, 58, 775. [Google Scholar]
- Fu, S.; Shao, Z.; Wang, Y.; Liu, Q. Manganese-catalyzed upgrading of ethanol into 1-butanol. J. Am. Chem. Soc. 2017, 139, 11941. [Google Scholar] [PubMed]
- Espinosa-Jalapa, N.A.; Kumar, A.; Leitus, G.; Diskin-Posner, Y.; Milstein, D. Synthesis of cyclic imides by acceptorless dehydrogenative coupling of diols and amines catalyzed by a manganese pincer complex. J. Am. Chem. Soc. 2017, 139, 11722. [Google Scholar]
- Widegren, M.B.; Harkness, G.J.; Slawin, A.M.Z.; Cordes, D.B.; Clarke, M.L. A highly active manganese catalyst for enantioselective ketone and ester hydrogenation. Angew. Chem. Int. Ed. 2017, 56, 5825. [Google Scholar]
- Kallmeier, F.; Irrgang, T.; Dietel, T.; Kempe, R. Highly active and selective manganese C=O bond hydrogenation catalysts: The importance of the multidentate ligand, the ancillary ligands, and the oxidation state. Angew. Chem. Int. Ed. 2016, 55, 11806. [Google Scholar] [CrossRef]
- Das, U.K.; Janes, T.; Kumara, A.; Milstein, D. Manganese catalyzed selective hydrogenation of cyclic imides to diols and amines. Green Chem. 2020, 22, 3079. [Google Scholar]
- Zhang, L.; Tang, Y.; Han, Z.; Ding, K. Lutidine-based chiral pincer manganese catalysts for enantioselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2019, 58, 4973. [Google Scholar]
- Homberg, L.; Roller, A.; Hultzsch, K.C. A highly active PN3 manganese pincer complex performing N-alkylation of amines under mild conditions. Org. Lett. 2019, 21, 3142. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Hou, H.; Chen, J.; Nian, S.; Yi, X.; Wang, Z.; Song, D.; Zhong, W. Highly enantioselective synthesis of chiral benzhydrols via manganese catalyzed asymmetric hydrogenation of unsymmetrical benzophenones using an imidazole-based chiral PNN tridentate ligand. Org. Lett. 2019, 21, 3937. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Shao, Z.; Li, G.; Lan, Y.; Liu, Q. Unmasking the ligand effect in manganese-catalyzed hydrogenation: Mechanistic insight and catalytic application. J. Am. Chem. Soc. 2019, 141, 17337. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Zubar, V.; Ganardi, R.-C.; Rueping, M. Chemoselective hydroboration of propargylic alcohols and amines using a manganese(II) catalyst. Org. Lett. 2020, 22, 3765. [Google Scholar] [CrossRef]
- Vasilenko, V.; Blasius, C.K.; Wadepohl, H.; Gade, L.H. Mechanism-based enantiodivergence in manganese reduction catalysis: A chiral pincer complex for the highly enantioselective hydroboration of ketones. Angew. Chem. Int. Ed. 2017, 56, 8393. [Google Scholar]
- Ma, X.; Zuo, Z.; Liu, G.; Huang, Z. Manganese-catalyzed asymmetric hydrosilylation of aryl ketones. ACS Omega 2017, 2, 4688. [Google Scholar]
- Zhang, G.; Zeng, H.; Wu, J.; Yin, Z.; Zheng, S.; Fettinger, J.C. Highly selective hydroboration of alkenes, ketones and aldehydes catalyzed by a well-defined manganese complex. Angew. Chem. Int. Ed. 2016, 55, 14369. [Google Scholar]
- Xu, Y.; Wang, T.; Li, Y.; Li, S.; Zhang, Y.; Wang, J.; Li, F.; Jiang, H. NNN pincer palladium(II) complexes with N-(2-(1H-pyrazol-1-yl)phenyl)-picolinamide ligands: Synthesis, characterization, and application to heck coupling reaction. Transition Met Chem. 2024, 49, 149. [Google Scholar] [CrossRef]
- Wang, T.; Hao, X.-Q.; Huang, J.-J.; Wang, K.; Gong, J.-F.; Song, M.-P. Chiral CNN pincer palladium(II) complexes with 2-aryl-6-(oxazolinyl)pyridine ligands: Synthesis, characterization, and application to enantioselective allylation of isatins and Suzuki–Miyaura coupling reaction. Organometallics 2014, 33, 194. [Google Scholar]
- Wang, T.; Hao, X.-Q.; Zhang, X.-X.; Gong, J.-F.; Song, M.-P. Synthesis, structure and catalytic properties of CNN pincer palladium(ii) and ruthenium(IIi) complexes with N-substituted-2-aminomethyl-6-phenylpyridines. Dalton Trans. 2011, 40, 8964. [Google Scholar]
- Wang, T.; Guo, J.; Wang, X.; Guo, H.; Jia, D.; Wang, H.; Liu, L. Cross coupling of benzylammonium salts with boronic acids using a well-defined N-heterocyclic carbene-palladium(II) precatalyst. RSC Adv. 2019, 9, 5738. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, K.; Meng, T.; Zhang, A.; Wang, H.; Shen, S.; Liu, L. N-Heterocyclic carbene-palladium(II) complexes with acridine ligand: Synthesis, characterization and catalytic applications. Chin. J. Org. Chem. 2017, 37, 1794. [Google Scholar]
- Wang, T.; Liu, L.; Xu, K.; Xie, H.; Shen, H.; Zhao, W.-X. Synthesis and characterization of trinuclear N-heterocyclic carbene-palladium(II) complexes and their applications in the Suzuki-Miyaura cross-coupling reaction. RSC Adv. 2016, 6, 100690. [Google Scholar]
- Wang, T.; Xie, H.; Liu, L.; Zhao, W.-X. N-heterocyclic carbene-palladium(II) complexes with benzoxazole or benzothiazole ligands: Synthesis, characterization, and application to Suzuki-Miyaura aross-coupling reaction. J. Organomet. Chem. 2016, 804, 73. [Google Scholar]
- Wang, T.; Wang, Y.; Xu, K.; Zhang, Y.; Guo, J.; Liu, L. Transition-metal-free DMAP-mediated aromatic esterification of amides with organoboronic acids. Eur. J. Org. Chem. 2021, 2021, 3274–3277. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Song, Y.; Huo, J.; Zhou, J.; Kang, Q.; Liu, L. Cu(OAc)2-Mediated C-H bond dithiolation of amide-oxazolines with aryl thiols. Chin. J. Org. Chem. 2021, 41, 1098. [Google Scholar]
- Daw, P.; Kumar, A.; Espinosa-Jalapa, N.A.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Synthesis of pyrazines and quinoxalines via acceptorless dehydrogenative coupling routes catalyzed by manganese pincer complexes. ACS Catal. 2018, 8, 7734. [Google Scholar]
- Singh, A.; Maji, A.; Joshi, M.; Choudhury, A.R.; Ghosh, K. Designed pincer ligand supported Co(II)-based catalysts for dehydrogenative activation of alcohols: Studies on N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines. Dalton Trans. 2021, 50, 8567. [Google Scholar]
- Singh, S.; Saini, R.; Chaudhary, V.K.; Ghosh, K. Organometallic Ru(III) catalysts for α-alkylation of carbonyl compounds using alcohols: Mechanistic insights via detection of key intermediates. Chem. Asian J. 2025, 20, e202400811. [Google Scholar]
- Deshmukh, G.; Murugavel, R. Bimetallic Ru(II) complex catalysed β-alkylation of secondary alcohols and α-alkylation of ketones: Selective formation of saturated ketones. Eur. J. Org. Chem. 2024, 27, e202400131. [Google Scholar]
- Samanta, A.; Behera, P.; Chaubey, A.; Mondal, A.; Pal, D.; Mohar, K.; Roy, L.; Srimani, D. Experimental and theoretical insights for designing Zn2+ complexes to trigger chemo-selective hetero-coupling of alcohols. Chem. Commun. 2024, 60, 4056. [Google Scholar]
- Xu, Q.; Chen, J.; Tian, H.; Yuan, X.; Li, S.; Zhou, C.; Liu, J. Catalyst-free dehydrative α-alkylation of ketones with alcohols: Green and selective autocatalyzed synthesis of alcohols and ketones. Angew. Chem. Int. Ed. 2014, 53, 225. [Google Scholar]
- Ouyang, K.; Xi, Z. Roles of bases in transition-metal catalyzed organic reactions. Acta Chim. Sinica. 2013, 71, 13. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]

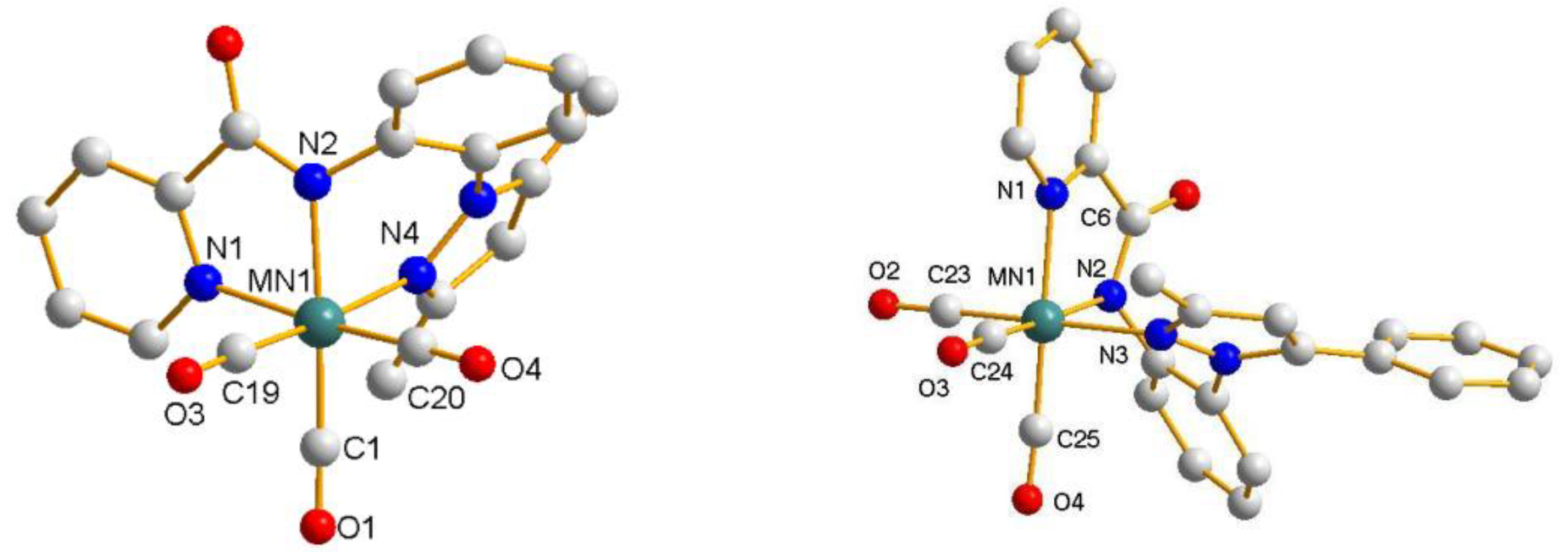
| 4a | 4c.H2O | |
|---|---|---|
| Mn1-N1 | 2.074(4) Å | 2.081(5) Å |
| Mn1-N2 | 2.003(3) Å | 2.025(5) Å |
| Mn1-N4/Mn1-N3 | 2.076(4) Å | 2.101(5) Å |
| Mn1-C1/Mn1-C24 | 1.806(5) Å | 1.805(7) Å |
| Mn1-C19/Mn1-C23 | 1.804(5) Å | 1.792(9) Å |
| Mn1-C20/Mn1-C25 | 1.808(5) Å | 1.801(7) Å |
| C1-Mn1-N2/C24-Mn1-N2 | 174.0° | 174.4° |
| N(1)-Mn1-N4/N(1)-Mn1-N3 | 92.83° | 91.3° |
| C19-Mn1-C1/C23-Mn1-C24 | 87.6(2)° | 89.8(3)° |
| C1-Mn1-C20/C24-Mn1-C25 | 88.6(2)° | 88.6(3)° |
| C19-Mn1-N2/C23-Mn1-N2 | 94.92(18)° | 94.1(3)° |
| C20-Mn1-N2/C25-Mn1-N2 | 96.82(19)° | 95.6(3)° |
| C1-Mn1-N1/C24-Mn1-N1 | 97.1(2)° | 98.1(3)° |
| N2-Mn1-N1 | 77.65(14)° | 78.0(2)° |
| C1-Mn1-N4/C24-Mn1-N3 | 97.6(2)° | 94.7(3)° |
| N2-Mn1-N4/N2-Mn1-N3 | 80.10(14)° | 81.5(2)° |
 | ||||
| Entry | Cat. | Base | Solvent | Yield (%) b |
| 1 | 4c | KOtBu | Toluene | 35 |
| 2 | 4c | KOH | Toluene | 37 |
| 3 | 4c | K2CO3 | Toluene | NR |
| 4 | 4c | Na2CO3 | Toluene | NR |
| 5 | 4c | NaOtBu | Toluene | 75 |
| 6 | 4c | NaOH | Toluene | 81 |
| 7 | 4c | Li2CO3 | Toluene | NR |
| 8 | 4c | LiOtBu | Toluene | 11 |
| 9 | 4c | Cs2CO3 | Toluene | 26 |
| 10 | 4c | Et3N | Toluene | NR |
| 11 | 4c | NaOH | 1,4-Dioxane | 37 |
| 12 | 4c | NaOH | m-xylene | 60 |
| 13 | 4c | NaOH | tert-amyl alcohol | 40 |
| 14 | 4c | NaOH | THF | 28 |
| 15 | 4c | NaOH | DMF | NR |
| 16 | 4a | NaOH | Toluene | 65 |
| 17 | 4b | NaOH | Toluene | 63 |
| 18 c | 4c | NaOH | Toluene | 57 |
| 19 d | 4c | NaOH | Toluene | 37 |
 |
 |
 |
 |
| 4a | 4c·H2O | |
|---|---|---|
| formula | C20H15MnN4O4 | C25H19MnN4O5 |
| Mr | 430.30 | 492.4 |
| temp (K) | 297 (2) | 296 (2) |
| crystsyst | Monoclinic | trigonal |
| space group | P2(1)/c | R3c:H |
| cryst size (mm) | 0.24 × 0.22 × 0.08 | 0.20 × 0.18 × 0.08 |
| a (Å) | 14.4012 (8) | 24.2887 (8) |
| b (Å) | 10.2731 (5) | 24.2887 (8) |
| c (Å) | 13.1141 (6) | 20.4273 (8) |
| α (deg) | 90.00 | 90 |
| β (deg) | 101.735 (2) | 90 |
| γ (deg) | 90 | 120 |
| V (Å3) | 1899.61 (17) | 10,436.4 (8) |
| Z | 4 | 18 |
| Dcalcd (g cm−3) | 1.505 | 1.462 |
| μ (mm−1) | 0.073 | 0.614 |
| θ range (deg) | 3.21–27.57 | 2.75–27.51 |
| index range | −18 ≤ h ≤ 18, −13 ≤ k ≤ 13, −17 ≤ l ≤ 17 | −31 ≤ h ≤ 31, −31 ≤ k ≤ 31, −25 ≤ l ≤ 26 |
| No. of data points collected | 48,737 | 93,102 |
| No. of unique data points | 4337 | 5282 |
| final R indices (I > 2σ(I)) R1 | 0.0736 | 0.0474 |
| wR2 | 0.1829 | 0.1187 |
| R indices (all data) R1 | 0.0795 | 0.0747 |
| wR2 | 0.1852 | 0.1535 |
| F(000) | 880 | 4716.0 |
| peak/hole (e·Å−3) | 0.859/−0.572 | 0.871/−0.462 |
| Goodness of fit on F2 | 1.390 | 1.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xu, Y.; Du, M.; Hu, Z.; Liu, L. Synthesis, Characterization and Application of NNN Pincer Manganese Complexes with Pyrazole Framework in α-Alkylation Reaction. Molecules 2025, 30, 1465. https://doi.org/10.3390/molecules30071465
Wang T, Xu Y, Du M, Hu Z, Liu L. Synthesis, Characterization and Application of NNN Pincer Manganese Complexes with Pyrazole Framework in α-Alkylation Reaction. Molecules. 2025; 30(7):1465. https://doi.org/10.3390/molecules30071465
Chicago/Turabian StyleWang, Tao, Yongli Xu, Mengxin Du, Zhiyuan Hu, and Lantao Liu. 2025. "Synthesis, Characterization and Application of NNN Pincer Manganese Complexes with Pyrazole Framework in α-Alkylation Reaction" Molecules 30, no. 7: 1465. https://doi.org/10.3390/molecules30071465
APA StyleWang, T., Xu, Y., Du, M., Hu, Z., & Liu, L. (2025). Synthesis, Characterization and Application of NNN Pincer Manganese Complexes with Pyrazole Framework in α-Alkylation Reaction. Molecules, 30(7), 1465. https://doi.org/10.3390/molecules30071465




