Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
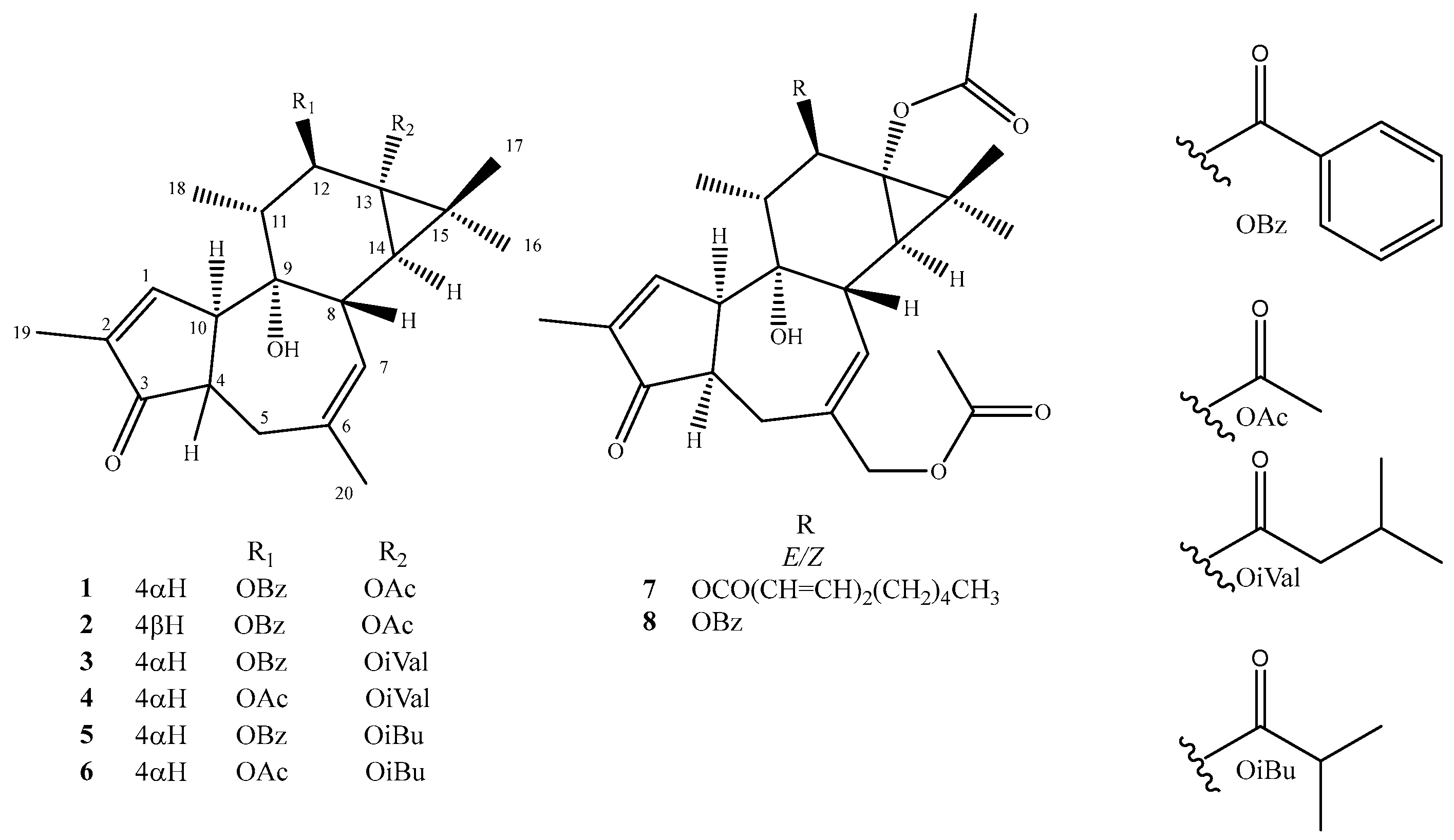
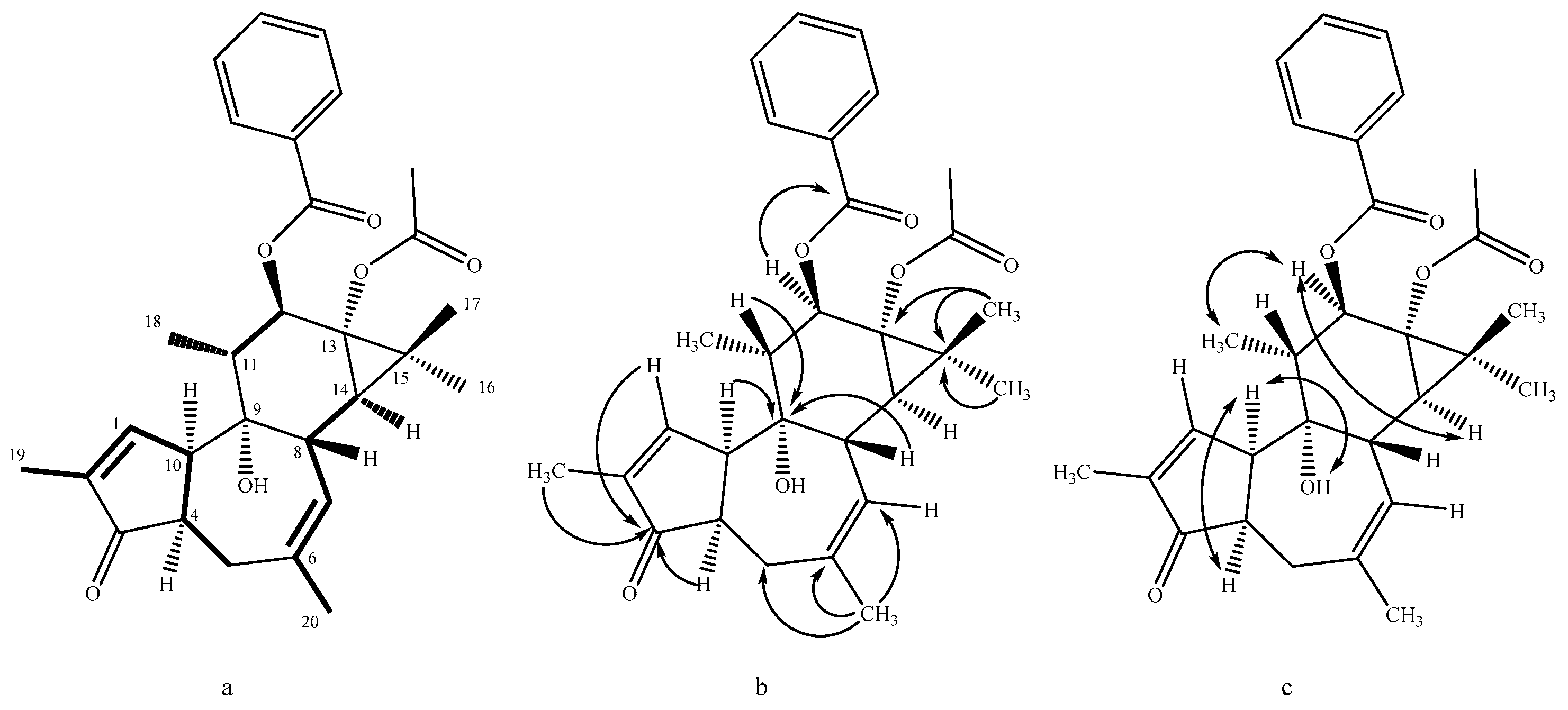
- Nicaeenin H (13α-Acetyloxy-12β-benzoyloxy-4-epi-4,20-dideoxyphorbol, 1): colorless, amorphous, solid substance; [α]D20 -14.0 (c 0.10, MeOH); UV (MeOH) λmax 195, 231 nm; IR (ATR) νmax 2970, 1729, 1244, 1120, 1015 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 501.2246 [M + Na]+ (calcd for C29H34NaO6+ 501.2248).
- Nicaeenin I (13α-Acetyloxy-12β-benzoyloxy-4,20-dideoxyphorbol, 2): colorless, amorphous, solid substance; [α]D20 +14.0 (c 0.10, MeOH); UV (MeOH) λmax 201, 231 nm; IR (ATR) νmax 2981, 1730, 1228, 1124, 1025 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 479.2416 [M + H]+ (calcd for C29H35O6+ 479.2428).
- Nicaeenin J (12β-Benzoyloxy-13α-isovaleryloxy-4-epi-4,20-dideoxyphorbol, 3): colorless, amorphous, solid substance; [α] D20 +9.9 (c 0.07, acetone); UV (MeOH) λmax 201, 232 nm; IR (ATR) νmax 2974, 1735, 1230, 1122, 1020 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 543.2714 [M + Na]+ (calcd for C32H40NaO6+ 543.2717).
- Nicaeenin K (12β-Acetyloxy-13α-isovaleryloxy-4-epi-4,20-dideoxyphorbol, 4): colorless, amorphous, solid substance; [α] D20 +7.8 (c 0.28, acetone); UV (MeOH) λmax 210, 236 nm; IR (ATR) νmax 2969, 1731, 1233, 1129, 1022 cm−1; 1H and 13C NMR data data in Table 1 and Table 2; HRESIMS m/z 481.2542 [M + Na]+ (calcd for C27H38NaO6+ 481.2561).
- Nicaeenin L (13α,20-Diacetyloxy-12β-(2′E,4′E-nonadienoyloxy)-4-epi-deoxyphorbol, 7): colorless, amorphous, solid; [α]D20 -1.3 (c 0.08, MeOH); UV (MeOH) λmax 199, 268 nm; IR (ATR) νmax 2974, 1733, 1235, 1126, 1024 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 583.3265 [M + H]+ (calcd for C34H47O8+ 583.3265).
3.4. Anti-HIV-Activity Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus. Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar]
- Zhao, H.; Sun, L.; Kong, C.H.; Mei, W.L.; Dai, H.F.; Xu, F.Q.; Huang, S.Z. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar]
- Mwine, J.T.; Van Damme, P. Why do Euphorbiaceae tick as medicinal plants?: A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Huang, X.; Tang, C.; Huang, X.; Yang, Y.; Li, Q.; Ma, M.; Zhao, L.; Yang, L.; Cui, Y.; Zhang, Z.; et al. Synthesis, and anti-HIV activities of phorbol derivatives. Chin. J. Nat. Med. 2024, 22, 1–16. [Google Scholar]
- Emerit, I.; Cerutti, P.A. Tumor promoter phorbol 12-myristate 13-acetate induces a clastogenic factor in human lymphocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 7509–7513. [Google Scholar]
- Otsuki, K.; Li, W. Tigliane and daphnane diterpenoids from Thymelaeaceae family: Chemistry, biological activity, and potential in drug discovery. J. Nat. Med. 2023, 77, 625–643. [Google Scholar]
- Cragg, G.M.; Newman, D.J.; Kingston, D.G.I. Comprehensive Natural Products II Chemistry and Biology; Mander, L., Lui, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 2, Chapter 2.02. [Google Scholar]
- Cashmore, A.R.; Seelye, R.N. The structure of prostratin: A toxic tetracyclic diterpene ester from Pimelea prostrata. Tetrahedron Lett. 1976, 20, 1737–1738. [Google Scholar]
- Wang, H.B.; Wang, X.Y.; Liu, L.P.; Qin, G.W.; Kang, T.G. Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 2000, 53, 457–464. [Google Scholar]
- Nothias-Scaglia, L.-F.; Pannecouque, C.; Renucci, F.; Delang, L.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. Antiviral activity of diterpene esters on chikungunya virus and HIV replication. J. Nat. Prod. 2015, 78, 1277–1283. [Google Scholar]
- Chen, H.; Zhang, R.; Luo, R.-H.; Yang, L.-M.; Wang, R.-R.; Ha, X.-J.; Zheng, Y.-T. Anti-HIV Activities and mechanism of 12-O-tricosanoylphorbol-20-acetate, a novel phorbol ester from Ostodes katharinae. Molecules 2017, 22, 1498. [Google Scholar] [CrossRef] [PubMed]
- Hemmers, H.; Gülz, P.-G. Epicuticular waxes from leaves of five Euphorbia species. Phytochemistry 1986, 25, 2103–2107. [Google Scholar]
- Öksüz, S.; Shieh, H.-L.; Pezzuto, J.M.; Özhatay, N.; Cordell, G.A. Biologically active compounds from the Euphorbiaceae; Part 1. Triterpenoids of Euphorbia nicaeensis subsp. glareosa. Planta Med. 1993, 59, 472–473. [Google Scholar]
- Krstić, G.B.; Novaković, M.M.; Jadranin, M.B.; Tešević, V.V. Tetracyclic triterpenoids from Euphorbia nicaeensis All. Adv. Technol. 2019, 8, 37–45. [Google Scholar]
- Cateni, F.; Zilic, J.; Falsone, G.; Hollan, F.; Frausin, F.; Scarcia, V. Preliminary biological assay on cerebroside mixture from Euphorbia nicaeensis All. Isolation and structure determination of five glucocerebrosides. Il Farm. 2003, 58, 809–817. [Google Scholar]
- Cateni, F.; Falsone, G.; Zilic, J.; Bonivento, P.; Zacchigna, M.; Žigon, D.; Sosa, S.; Altinier, G. Glyceroglycolipids from Euphorbia nicaeensis All. with antiinflamatory activity. ARKIVOC 2004, 5, 54–65. [Google Scholar] [CrossRef]
- Krstić, G.; Jadranin, M.; Todorović, N.; Pešić, M.; Stanković, T.; Aljančić, I.; Tešević, V. Jatrophane diterpenoids with multidrug-resistance modulating activity from the latex of Euphorbia nicaeensis. Phytochemistry 2018, 148, 104–112. [Google Scholar]
- Krstić, G.; Kostić, A.; Jadranin, M.; Pešić, M.; Novaković, M.; Aljančić, I.; Vajs, V. Two new jatrophane diterpenes from the roots of Euphorbia nicaeensis. J. Serb. Chem. Soc. 2021, 86, 1219–1228. [Google Scholar]
- Aichour, S.; Haba, H.; Benkhaled, M.; Harakat, D.; Lavaud, C. Terpenoids and other constituents from Euphorbia bupleuroides. Phytochem. Lett. 2014, 10, 198–203. [Google Scholar]
- Benmerache, A.; Alabdul Magid, A.; Labed, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Hubert, J.; Morjani, H.; Kabouche, Z. Isolation and characterisation of cytotoxic compounds from Euphorbia clementei Boiss. Nat. Prod. Res. 2017, 31, 2091–2098. [Google Scholar] [PubMed]
- Μiana, G.A.; Schmidt, R.; Hecker, E.; Shamma, M.; Moniot, J.L.; KlAmuddin, M. Notizen: 4α-Sapinine—A novel diterpene ester from Sapium indicum. Z. Naturforsch. B. 1977, 32, 727–728. [Google Scholar]
- Wang, L.-Y.; Wang, N.-L.; Yao, X.-S.; Miyata, S.; Kitanaka, S. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 2002, 65, 1246–1251. [Google Scholar] [PubMed]
- Lu, Y.; Huang, Y.S.; Chen, C.H.; Akiyama, T.; Morris-Natschke, S.L.; Cheng, Y.Y.; Chen, I.S.; Yang, S.Z.; Chen, D.F.; Lee, K.H. Anti-HIV tigliane diterpenoids from Reutealis trisperma. Phytochemistry 2020, 174, 112360. [Google Scholar]
- Asada, Y.; Sukemori, A.; Watanabe, T.; Malla, K.J.; Yoshikawa, T.; Li, W.; Koike, K.; Chen, C.-H.; Akiyama, T.; Qian, K.; et al. Stelleralides A–C, Novel Potent Anti-HIV Daphnane-Type Diterpenoids from Stellera chamaejasme L. Org. Lett. 2011, 13, 2904–2907. [Google Scholar]
- Otsuki, K.; Zhang, M.; Yamamoto, A.; Tsuji, M.; Tejima, M.; Bai, Z.S.; Zhou, D.; Huang, L.; Chen, C.H.; Lee, K.H.; et al. Anti-HIV Tigliane Diterpenoids from Wikstroemia scytophylla. J. Nat. Prod. 2020, 83, 3584–3590. [Google Scholar] [PubMed]
- Zhang, M.; Otsuki, K.; Kikuchi, T.; Bai, Z.-S.; Zhou, D.; Huang, L.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H.; Li, N.; et al. LC-MS identification, isolation, and structural elucidation of anti-HIV tigliane diterpenoids from Wikstroemia lamatsoensis. J. Nat. Prod. 2021, 84, 2366–2373. [Google Scholar]
- Ma, Q.-G.; Liu, W.-Z.; Wu, X.-Y.; Zhou, T.-X.; Qin, G.-W. Diterpenoids from Euphorbia fischeriana. Phytochemistry 1997, 44, 663–666. [Google Scholar]
- Shusterman, A.J.; McDougal, P.G.; Glasfeld, A. Dry-column flash chromatography. J. Chem. Educ. 1997, 10, 1222–1223. [Google Scholar]
| 1 | 2 | 3 | 4 | 7 | |
|---|---|---|---|---|---|
| 1 | 7.04, brs | 7.58, brs | 7.04, brs | 6.99, brs | 7.00, brs |
| 4 | 2.71, m | 2.49, m | 2.71, m | 2.68, m | 2.75, m |
| 5α | 3.41, d, 16 | 2.86, dd, 18, 10 | 3.47, m | 3.39, m | 3.38, brd, 16 |
| 5β | 2.38, dd, 16, 5 | 2.03, dd, 18, 10 | 2.38, dd, 15, 5 | 2.35, dd, 15, 5 | 2.47, dd, 16, 5 |
| 7 | 4.84, brs | 5.24, d, 5 | 4.85, brs | 4.81, brs | 5.18, brs |
| 8 | 1.97, brs | 2.41, m | 1.96, brs | 1.88, brs | 1.99, brs |
| 9(OH) | 5.13, s | 5.55, s | 5.24, s | 5.13, s | 5.17, brs |
| 10 | 3.46, m | 3.32, m | 3.48, m | 3.43, m | 3.47, m |
| 11 | 1.87, m | 1.72, m | 1.86, m | 1.68, m | 1.75, m |
| 12 | 5.74, d, 10 | 5.67, d, 10 | 5.73, d, 11 | 5.43, d, 10 | 5.55, d, 10 |
| 14 | 0.87, d, 5 | 1.07, d, 5 | 0.84, d, 5 | 0.77, d, 5 | 0.82, d, 5 |
| 16 | 1.18, s | 1.20, s | 1.18, s | 1.17, s | 1.25, s |
| 17 | 1.34, s | 1.32, s | 1.35, s | 1.19, s | 1.19, s |
| 18 | 1.12, d, 6 | 0.97, d, 7 | 1.11, d, 7 | 1.06, d, 7 | 1.08, d, 6 |
| 19 | 1.80, s | 1.73, brs | 1.80, s | 1.78, s | 1.77, s |
| 20 | 1.75, s | 1.75, brs | 1.76, s | 1.74, s | 4.47, d, 124.35, d, 12 |
| 12-OR | |||||
| 2′ | 2.11, s | 5.91, d, 15 | |||
| 3′ | 8.06, d, 7 | 8.02, d, 8 | 8.07, d, 8 | 7.66, dd, 15, 11 | |
| 4′ | 7.48, t, 7 | 7.46, t,8 | 7.48, t, 8 | 6.17, t, 11 | |
| 5′ | 7.60, t, 7 | 7.60, m | 7.60, t, 8 | - | 5.92, m |
| 6′ | 7.48, t, 7 | 7.46, t,8 | 7.48, t, 8 | - | 2.32, m |
| 7′ | 8.06, d, 7 | 8.02, d, 8 | 8.07, d, 8 | - | 1.44, m |
| 8′ | - | - | - | - | 1.33, m |
| 9′ | - | - | - | - | 1.32, m |
| 10′ | - | - | - | - | 0.91, t, 7 |
| 13-OR | |||||
| 2″ | 2.09, s | 2.13, s | 2.21, m | 2.18, m | 2.13, s |
| 3″ | - | - | 2.10, m | 2.10, m | - |
| 4″ | - | - | 0.97, d, 7 | 0.96, d, 7 | - |
| 5″ | - | - | 0.95, d, 7 | 0.94, d, 7 | - |
| 20-OR | |||||
| 2‴ | - | - | - | - | 2.09, s |
| 1 | 2 | 3 | 4 | 7 | |
|---|---|---|---|---|---|
| 1 | 155.5 | 160.2 | 155.7 | 155.7 | 155.4 |
| 2 | 143.2 | 136.7 | 143.4 | 143.3 | 143.6 |
| 3 | 211.7 | 210.2 | 212.0 | 211.9 | 211.2 |
| 4 | 49.2 | 44.8 | 49.5 | 49.4 | 49.1 |
| 5 | 30.0 | 34.3 | 30.2 | 30.1 | 26.6 |
| 6 | 134.9 | 139.3 | 135.1 | 134.9 | 133.0 |
| 7 | 124.2 | 125.9 | 124.5 | 124.4 | 129.0 |
| 8 | 40.9 | 42.5 | 41.2 | 41.0 | 41.2 |
| 9 | 78.1 | 78.2 | 78.3 | 78.1 | 78.0 |
| 10 | 47.1 | 54.6 | 47.3 | 47.2 | 47.1 |
| 11 | 43.3 | 42.8 | 43.8 | 43.4 | 43.4 |
| 12 | 76.7 | 78.1 | 77.0 | 76.3 | 75.7 |
| 13 | 65.4 | 65.7 | 65.3 | 65.2 | 65.5 |
| 14 | 37.7 | 36.2 | 38.1 | 37.8 | 37.1 |
| 15 | 25.2 | 25.6 | 25.7 | 25.6 | 25.4 |
| 16 | 24.2 | 24.0 | 24.5 | 24.4 | 16.6 |
| 17 | 16.6 | 17.2 | 16.9 | 16.5 | 24.3 |
| 18 | 11.9 | 15.4 | 12.2 | 12.1 | 12.1 |
| 19 | 10.5 | 10.4 | 10.7 | 10.6 | 10.7 |
| 20 | 29.7 | 26.0 | 29.2 | 29.0 | 70.4 |
| 12-OR | |||||
| 1′ | 166.2 | 166.5 | 166.3 | 170.8 | 167.2 |
| 2′ | 130.1 | 130.2 | 130.5 | 21.2 | 120.8 |
| 3′ | 129.7 | 128.7 | 130.0 | - | 140.5 |
| 4′ | 128.5 | 129.9 | 128.7 | - | 126.6 |
| 5′ | 133.1 | 133.4 | 133.3 | - | 142.6 |
| 6′ | 128.5 | 129.9 | 128.7 | - | 28.5 |
| 7′ | 129.7 | 128.7 | 130.0 | - | 29.2 |
| 8′ | - | - | - | - | 31.6 |
| 9′ | - | - | - | - | 22.7 |
| 10′ | - | - | - | - | 14.2 |
| 13-OR | |||||
| 1″ | 173.5 | 173.9 | 175.7 | 175.5 | 171.0 |
| 2″ | 21.1 | 21.4 | 43.7 | 43.6 | 21.3 |
| 3″ | - | - | 25.6 | 25.3 | - |
| 4″ | - | - | 22.7 | 22.5 | - |
| 5″ | - | - | 22.7 | 22.6 | - |
| 20-OR | |||||
| 1‴ | - | - | - | - | 173.7 |
| 2‴ | - | - | - | - | 21.3 |
| 1 | 2 | 3 | 4 | 5 | 6 | 8 | PMPA | AMD3100 | |
|---|---|---|---|---|---|---|---|---|---|
| HIV-1 | >42.0 ± 0.0 | 7.5 ± 0.2 | >18.0 ± 0.0 | >52.0 ± 0.0 | >34.0 ± 0.0 | >101.0 ± 0.0 | 3.3 ± 1.8 | 2.4 ± 0.7 | 0.008 ± 0.002 |
| HIV-2 | 9.4 ± 1.4 | 1.7 ± 1.3 | >18.0 ± 0.0 | >52.0 ± 0.0 | 4.6 ± 2.3 | >101.0 ± 0.0 | 1.1 ± 0.8 | 0.7 ± 0.5 | 0.0075 ± 0.0005 |
| Cellular toxicity | 42.0 ± 0.0 | 94.0 ± 0.0 | 17.6 ± 0.3 | 52.0 ± 0.0 | 33.5 ± 0.4 | 101.0 ± 0.0 | 20.8 ± 5.6 | >100.0 ± 0.0 | >10.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić, G.; Jadranin, M.; Schols, D.; Claes, S.; Tešević, V.; Mandić, B.; Milosavljević, S.; Wittine, K. Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules 2025, 30, 1452. https://doi.org/10.3390/molecules30071452
Krstić G, Jadranin M, Schols D, Claes S, Tešević V, Mandić B, Milosavljević S, Wittine K. Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules. 2025; 30(7):1452. https://doi.org/10.3390/molecules30071452
Chicago/Turabian StyleKrstić, Gordana, Milka Jadranin, Dominique Schols, Sandra Claes, Vele Tešević, Boris Mandić, Slobodan Milosavljević, and Karlo Wittine. 2025. "Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots" Molecules 30, no. 7: 1452. https://doi.org/10.3390/molecules30071452
APA StyleKrstić, G., Jadranin, M., Schols, D., Claes, S., Tešević, V., Mandić, B., Milosavljević, S., & Wittine, K. (2025). Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules, 30(7), 1452. https://doi.org/10.3390/molecules30071452






