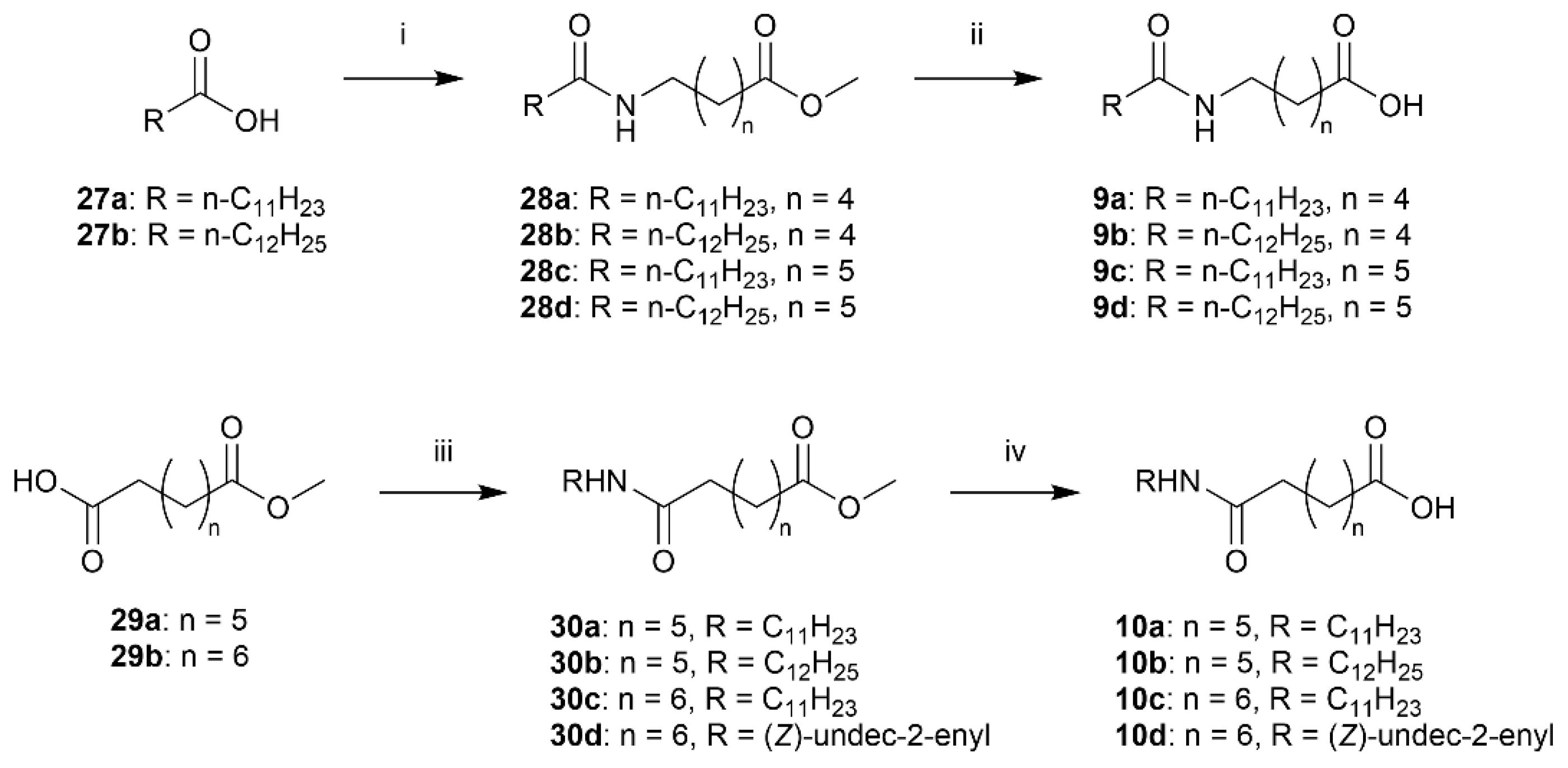
3.2.5. General Procedure E: Condensation Between Amine and Oxoester
The ethyl 2-alkylamino-2-oxoacetate is taken up in EtOH (abs.). Amine (1.00 equiv.) is added in one portion under an argon atmosphere and then stirred for 3 h at room temperature. CHCl3 is added to the suspension until all was dissolved before concentrating in vacuo. The product is purified by column chromatography (SiO2).
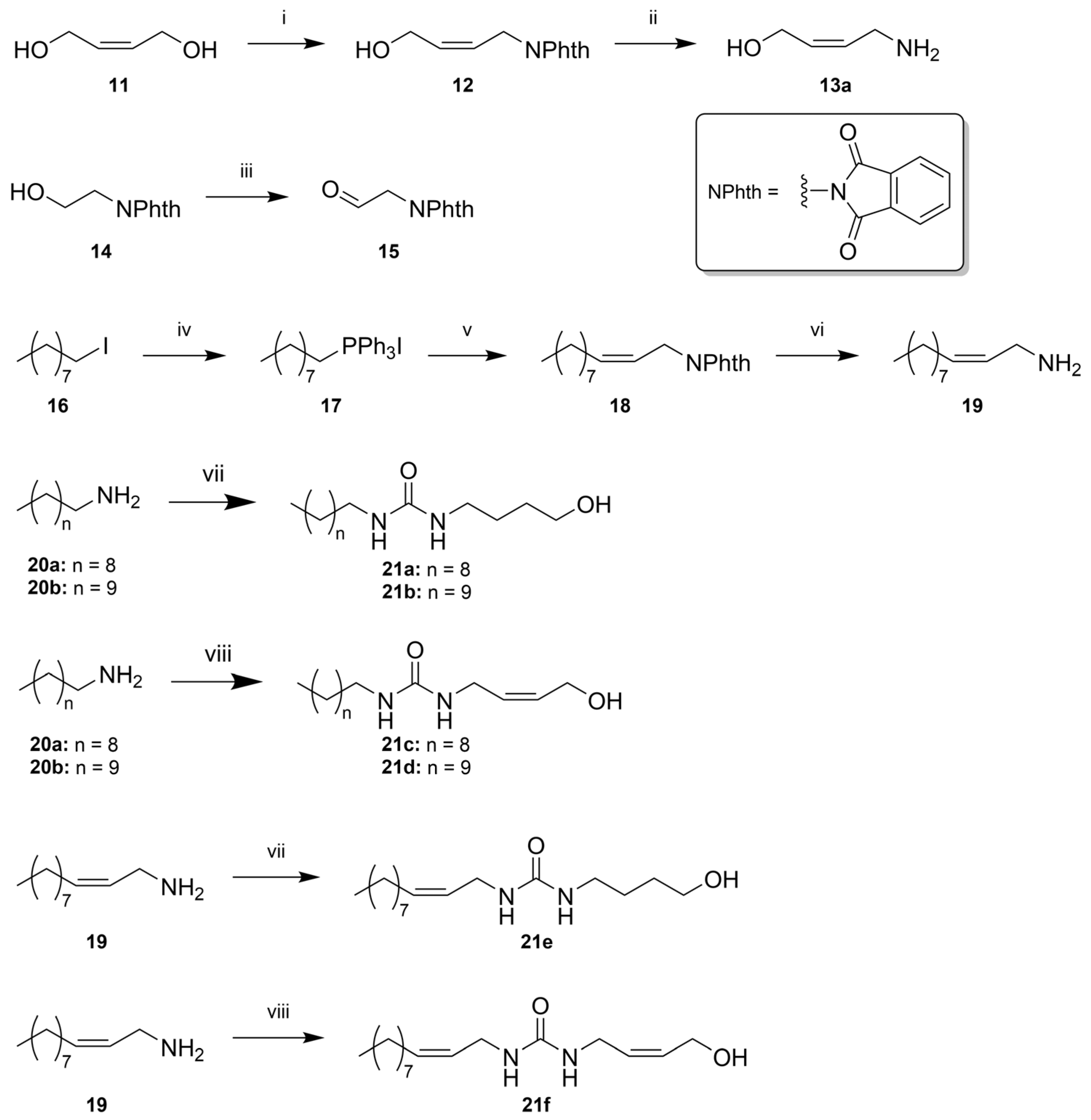
(Z)-1-Phthalimido-4-hydroxybut-2-ene (12)
To a solution of (
Z)-but-2-ene-1,4-diol (
11) (2.00 g, 22.7 mmol) in dry THF (91 mL), phthalimide (2.23 g, 15.1 mmol, 0.67 equiv.) and triphenylphosphine (3.97 g, 15.1 mmol, 0.667 equiv.) were added. The solution was cooled to 0 °C and diisopropyl azodicarboxylate (3.0 mL, 15 mmol, 0.67 equiv.) was added over 1 h, slowly warmed to room temperature, and stirred overnight. Concentration in vacuo and purification using column chromatography (SiO
2, 2:1
n-hexane:EtOAc) afforded
12 (2.26 g, 10.4 mmol, 46%) as colorless amorphous solids. R
f 0.50 (EtOAc, UV).
1H NMR (400 MHz, CDCl
3) δ 7.84 (dd,
J = 5.4, 3.1 Hz, 2H), 7.72 (dd,
J = 5.4, 3.1, 2H), 5.90 (dtt,
J = 10.8, 6.8, 1.2, 1H), 5.56 (dtt,
J = 10.38 7.7, 1.2, 1H), 4.39 (ddd,
J = 11.2, 7.3, 1.3 Hz, 4H);
13C NMR (101 MHz, CDCl
3) δ 168.3, 134.3, 133.4, 132.2, 125.1, 123.5, 58.2, 34.6. Spectroscopic data are consistent with that reported in the literature [
47].
(Z)-4-Aminobut-2-en-1-ol (13a)
Phthalimide
12 (1.81 g, 8.33 mmol) was dissolved in EtOH (42 mL), and hydrazine monohydrate (6.3 mL, 83 mmol, 10 equiv.) was added and refluxed for 2 h. The solution was cooled to room temperature, filtered, and the filtrate was concentrated in vacuo. Kugelrohr distillation (bp. 65 °C/0.8 mmHg) gave a colorless oil (532 mg, 6.11 mmol, 73%), which solidified at -18 °C.
1H NMR (400 MHz, CD
3OD) δ 5.63 (m, 2H), 4.14 (m, 2H), 3.31 (m, 2H);
13C NMR (101 MHz, CD
3OD) δ 131.3, 129.6, 57.0, 37.6. The
1H NMR spectrum is consistent with previously reported values [
48].
N-(2-oxoethyl)-phthalimide (15)
N-(2-Hydroxyethyl)phthalimide (
14) (0.961 g, 5.00 mmol, 1.00 equiv.) was dissolved in DCM (25 mL), and NaHCO
3 (1.27 g, 30.0 mmol, 3.00 equiv.) was added, followed by DMP (2.82 g, 6.5 mmol, 1.30 equiv.) at room temperature. The reaction mixture was stirred overnight (18 h) and quenched by addition of a saturated aqueous solution of Na
2S
2O
3 (50 mL). The layers were separated, and the aqueous layer was extracted with DCM (3 × 25 mL). The combined organic layers were washed with a saturated aq. NaHCO
3 (20 mL), brine (20 mL), dried (MgSO
4), filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel (EtOAc:hexane 1:1) afforded the desired compound
15 as a white solid (0.648 g, 3.42 mmol, 63%). mp. 111–114 °C; R
f: 0.28 (EtOAc:hexane 1:1);
1H NMR (400 MHz, CDCl
3) δ 9.65 (s, 1H), 7.88 (dd,
J = 5.5, 3.1 Hz, 2H), 7.75 (dd,
J = 5.5, 3.1 Hz, 2H), 4.55 (s, 2H);
13C NMR (101 MHz, CDCl
3) δ 194.0, 167.9 (2 × C), 134.8 (2 × C), 132.4 (2 × C), 124.1 (2 × C), 47.8. All spectroscopic and physical data were in agreement with those reported in the literature [
49].
Nonyltriphenylphosphonium Iodide (17)
1-Iodononane (
16) (1.27 g, 5.00 mmol, 1.00 equiv.) and PPh
3 (2.62 g, 10.0 mmol, 2.00 equiv.) were dissolved in acetonitrile (45 mL) and stirred at reflux overnight (18 h). The mixture was allowed to cool down. After evaporation of the solvent, the crude was purified by flash chromatography on silica gel, starting with pure DCM, then changing the eluent to DCM 95:5 MeOH. This afforded the desired Wittig salt
17 as a viscous yellow oil (2.50 g, 4.85 mmol, 97%). R
f 0.62 (DCM:MeOH 95:5);
1H NMR (400 MHz, CDCl
3) δ 7.87–7.62 (m, 15H), 3.68–3.49 (m, 2H), 1.63–1.55 (m, 4H), 1.31–1.05 (m, 10H), 0.81 (t,
J = 6.8 Hz, 3H);
13C NMR (101 MHz, CDCl
3) δ 135.2 (d,
J = 3.0 Hz, 3 × C), 133.7 (d,
J = 9.9 Hz, 6 × C), 130.6 (d,
J = 12.5 Hz, 6 × C), 118.2 (d,
J = 85.9 Hz, 3 × C), 31.8, 30.5 (d,
J = 15.5 Hz), 29.2 (3 × C), 23.2 (d,
J = 50.0 Hz), 22.7, 22.6, 14.1. All spectroscopic and physical data were in agreement with those reported in the literature [
50].
(Z)-N-(Undec-2-en-1-yl)phthalimide (18)
The Wittig salt 17 (1.83 g, 3.55 mmol, 1.00 equiv.) was dissolved in dry THF (72 mL) and HMPA (12.0 mL). The solution was degassed and purged three times with nitrogen. Subsequently, the mixture was cooled to −78 °C and then NaHMDS (6.20 mL, 0.6 M in toluene, 3.73 mmol, 1.05 equiv.) was added dropwise. The reaction mixture changed from colorless to an orange color. The mixture was brought up to 0 °C, stirred for five minutes, and then re-cooled to −78 °C. A solution of aldehyde 15 (0.671 g, 3.55 mmol, 1.00 equiv.) dissolved in dry THF (8.00 mL) was added in a dropwise manner. The mixture was allowed to warm up to room temperature slowly in a dry ice/acetone bath for 18 h before being quenched by the addition of saturated aq. NaH2PO4 (30 mL). The phases were separated, and the aqueous phase was extracted with Et2O (3 × 40 mL). The combined organic phases were washed with brine (2 × 25 mL), dried (MgSO4), filtered, and the solvent was removed in vacuo. The crude product was purified by flash chromatography on silica gel (hexane:EtOAc 9:1) to afford the desired Z-alkene 18 as an oil (0.613 g, 2.05 mmol, 57%). Rf 0.48 (hexane:EtOAc 9:1); 1H NMR (400 MHz, CDCl3) δ 7.83 (dd, J = 5.4, 3.0 Hz, 2H), 7.70 (dd, J = 5.5, 3.0 Hz, 2H), 5.64 – 5.54 (m, 1H), 5.50 – 5.37 (m, 1H), 4.31 (m, 2H), 2.45 – 2.07 (m, 2H), 1.46 – 1.15 (m, 12H), 0.91 – 0.83 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 168.5 (2 × C), 135.2 (2 × C), 134.3 (2 × C), 132.7 (2 × C), 123.6, 123.2, 35.3, 32.3, 29.9, 29.9, 29.8, 29.7, 27.8, 23.1, 14.6; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H25NO2Na 322.1777; found 322.1777.
(Z)-Undec-2-en-1-amine (19)
Phthalimide 18 (0.575 g, 1.93 mmol, 1.00 equiv.) was dissolved in EtOH (50 mL). Hydrazine monohydrate (1.54 mL, 19.3 mmol, 10.0 equiv.) was added and the mixture was refluxed until TLC revealed no starting material. After two hours, a white precipitate was formed, and the solution was cooled to 0 °C, filtrated, and the filtrate was concentrated in vacuo. Water (10 mL) was added, and the pH was adjusted to 12 with aq. NaOH (50%). Et2O (30 mL) was added, and the phases were separated. The aqueous phase was extracted with Et2O (3 × 30 mL), and the combined organic phases were washed with 1.0 M K2CO3 (20 mL) and brine (20 mL). The organic phases were dried over K2CO3, filtrated, and the solvent was removed in vacuo to afford the desired Z-amine 19 as a yellow oil (0.299 g, 1.77 mmol, 92%), which was used in the following step without further purification. 1H NMR (400 MHz, CDCl3) δ 5.67–5.18 (m, 2H), 3.30 (d, J = 6.2 Hz, 2H), 2.03 (q, J = 6.9 Hz, 2H), 1.51–1.39 (m, 2H), 1.37–1.19 (m, 12H), 0.90–0.83 (m, 3H); 13C NMR: (101 MHz, CDCl3) δ 131.4, 131.1, 39.3, 32.3, 30.1, 29.9, 29.7, 29.7, 27.7, 23.1, 14.5; IR: 3365 cm−1 and 3287 cm−1; HRMS (ESI/Q-TOF) m/z: [M+H]+ calcd. for C11H24N 170.1903; found 170.1903.
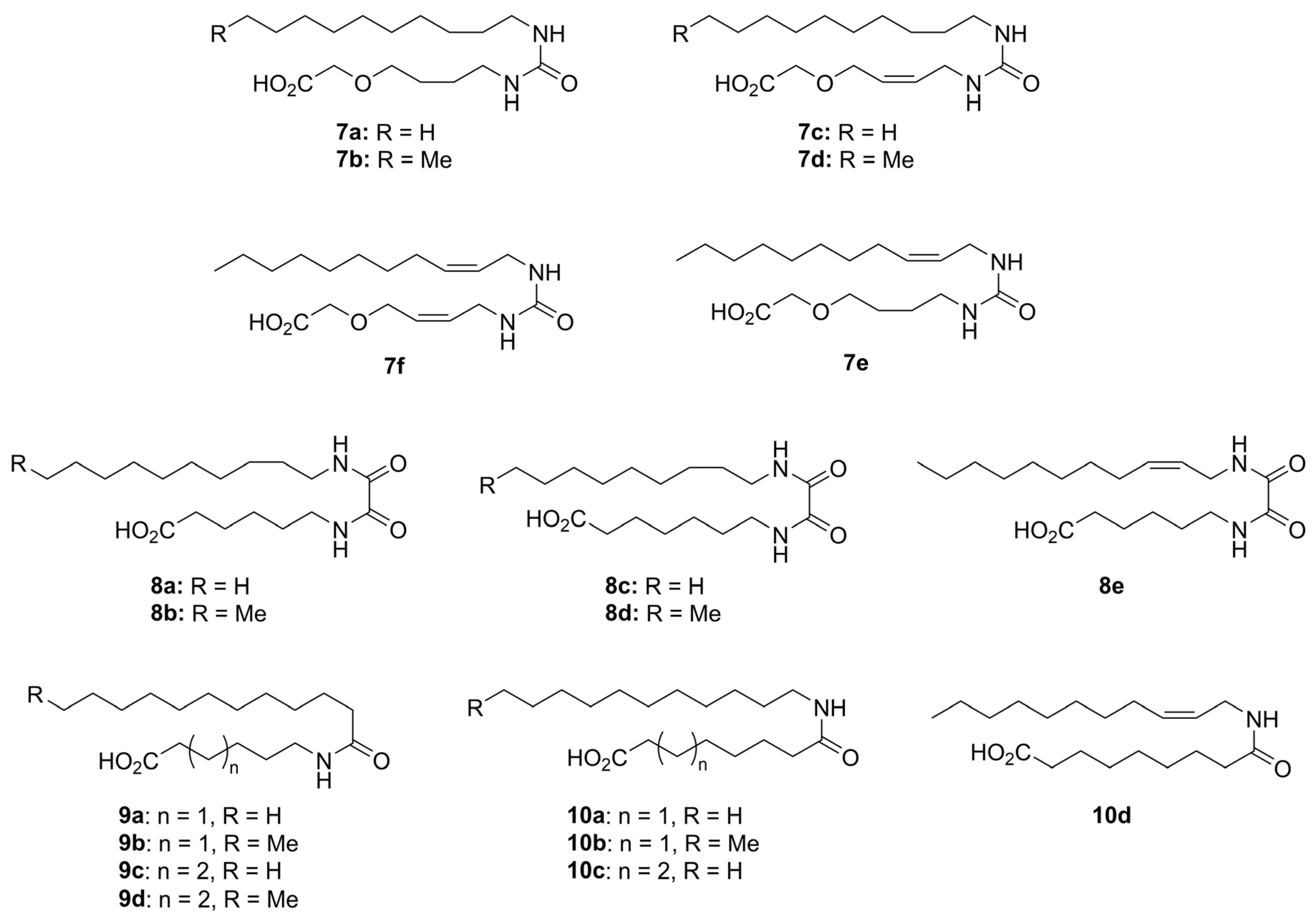
1-Decyl-3-(4-hydroxybutyl)urea (21a)
Prepared according to General Procedure A, from 4-aminobutanol (13b) and n-decylamine (20a). The solids formed during the reaction were recrystallized from MeCN to afford urea 21a (0.372 g, 1.37 mmol, 72%) as white needles. mp. 113–114; Rf 0.36 (9:1 DCM:MeOH); 1H NMR (400 MHz, CD3OD) δ 3.56 (m, 2H), 3.18–3.04 (m, 4H), 1.61–1.40 (m, 6H), 1.38–1.22 (m, 14H), 0.90 (m, 3H); 13C NMR (101 MHz, CD3OD) δ 160.7, 62.0, 40.4, 40.2, 32.4, 30.7, 30.2, 30.1, 30.1, 29.9, 29.8, 27.3, 27.2, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C15H32N2O2Na 295.2356; found 295.2355.
1-(4-Hydroxybutyl)-3-undecylurea (21b)
Prepared according to General Procedure A, using n-undecylamine (20b) and 4-aminobutanol (13b). Recrystallization from MeCN gave 21b (582 mg, 2.03 mmol, 91%) as white needles. mp. 116–117 °C; Rf 0.52 (9:1 DCM:MeOH). 1H NMR (400 MHz, CD3OD) δ 3.62–3.54 (m, 2H), 3.18–3.07 (m, 4H), 1.62–1.43 (m, 6H), 1.38–1.29 (m, 16H), 0.92 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 159.9, 61.2, 39.6, 39.4, 31.7, 30.0, 29.5, 29.3, 29.1, 29.1, 26.6, 26.4, 22.3, 13.0. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C16H34N2O2Na 309.2512; found 309.2512.
(Z)-1-Decyl-3-(4-hydroxybut-2-en-1-yl)urea (21c)
Prepared according to General Procedure A, from aminoalcohol 13a and n-decylamine (20a). The solids formed during the reaction were recrystallized from MeCN to give the urea 21c (223 mg, 72%) as white needles. mp 97–98 °C; Rf 0.41 (9:1 DCM:MeOH); 1H NMR (400 MHz, CD3OD) δ 5.65 (dtt, J = 11.1, 6.5, 1.6 Hz, 1H), 5.49 (dtt, J = 11.1, 6.8, 1.5 Hz, 1H), 4.16 (d, J = 6.6 Hz, 1H), 3.77 (d, J = 6.8 Hz, 2H), 3.09 (t, J = 7.0 Hz, 2H), 1.45 (q, J = 6.8 Hz, 2H), 1.38–1.23 (m, 14H), 0.90 (m, 3H); 13C NMR (101 MHz, CD3OD) δ 159.7, 130.3, 128.7, 57.0, 39.7, 36.6, 31.7, 29.9, 29.3, 29.3, 29.1, 29.1, 26.5, 22.3, 13.0; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C15H30N2O2Na 293.2199; found 293.2199.
(Z)-1-(4-Hydroxybut-2-en-1-yl)-3-undecylurea (21d)
Prepared according to General Procedure A, from n-undecylamine (20b) and aminoalcohol 13a. The product was recrystallized from first MeCN, then CHCl3, to give 21d (169 mg, 0.59 mmol, 52%), which was contaminated with diundecylurea (~20 mol%). mp. 100–102 °C; Rf 0.34 (9:1 DCM:MeOH); 1H NMR (400 MHz, CD3OD) δ 5.67 (ddd, J = 12.7, 8.1, 1.5 Hz, 1H), 5.51 (dtt, J = 11.0, 6.8, 1.5 Hz, 1H), 4.18 (d, J = 6.5 Hz, 2H), 3.80 (d, J = 7.2 Hz, 2H), 3.21–3.00 (m, 3H), 1.60–1.42 (m, 3H), 1.42–1.20 (m, 20H), 0.92 (t, J = 6.9 Hz, 4H); 13C NMR (101 MHz, CD3OD) δ 160.5, 131.1, 129.4, 57.8, 40.4, 37.3, 32.4, 30.7, 30.1, 29.9, 29.8, 27.3, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C16H32N2O2Na 307.2356; found 307.2355.
(Z)-1-(4-Hydroxybutyl)-3-(undec-2-en-1-yl)urea (21e)
Prepared according to General Procedure A, from 4-aminobutanol (13b) and allylic amine 19. Work-up: crystallization from MeCN and washing with cold MeCN afforded urea 21e (0.262 g, 0.92 mmol, 72%). mp. 90–92 °C; Rf 0.19 (DCM:MeOH 95:5); 1H NMR (400 MHz, CD3OD) δ 5.61–5.46 (m, 1H), 5.44–5.30 (m, 1H), 3.76 (dd, J = 6.7, 1.5 Hz, 2H), 3.63–3.50 (m, 2H), 3.18–3.09 (m, 2H), 2.10 (q, J = 6.8 Hz, 2H), 1.64–1.51 (m, 4H), 1.43–1.20 (m, 12H), 0.91 (t, J = 6.7, 3H); 13C NMR (101 MHz, CD3OD) δ 161.1, 133.3, 127.8, 62.6, 40.8, 38.0, 33.1, 30.9, 30.7, 30.6, 30.4, 30.4, 28.3, 27.8, 23.7, 14.4. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C16H32N2O2Na 307.2355; found 307.2356.
1-((Z)-4-Hydroxybut-2-en-1-yl)-3-((Z)-undec-2-en-1-yl)urea (21f)
Prepared from allylic amine 19 and aminoalcohol 13a according to General Procedure A. Column chromatography twice (SiO2, 6% MeOH in DCM) afforded 21f (28 mg, 99 µmol, 20%) as a waxy solid. Rf 0.22 (94:6 DCM:MeOH); 1H NMR (400 MHz, CD3OD) δ 5.65 (dtt, J = 11.0, 6.5, 1.6 Hz, 1H), 5.54–5.44 (m, 2H), 5.39 (dtt, J = 10.9, 6.7, 1.4 Hz, 1H), 4.16 (d, J = 6.5 Hz, 2H), 3.82–3.71 (m, 4H), 2.10 (app. q, J = 7.2, 6.6 Hz, 2H), 1.42–1.24 (m, 15H), 0.89 (app. t, 3H); 13C NMR (101 MHz, CD3OD) δ 160.3, 132.7, 131.2, 129.3, 127.1, 57.8, 49.0, 48.8, 48.6, 48.6, 48.4, 48.4, 48.1, 47.9, 47.7, 37.4, 37.3, 32.4, 30.1, 30.0, 29.8, 29.7, 27.6, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C16H30N2O2Na 305.2199; found 305.2198.
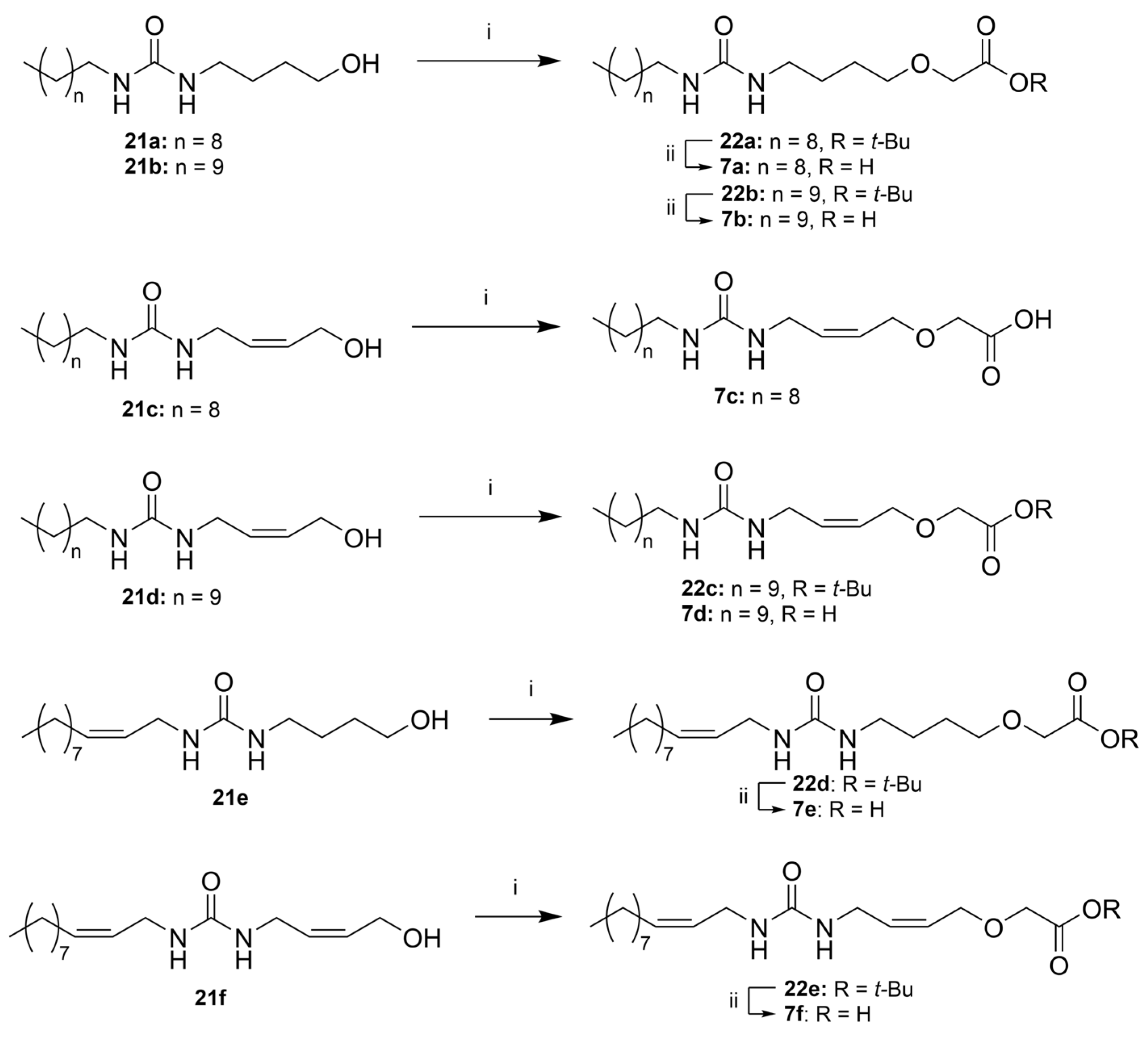
tert-Butyl 2-(4-(3-decylureido)butoxy)acetate (22a)
Prepared according to General Procedure B, starting from 21a. Reaction time: 5 days. Column chromatography (SiO2, 2:3 n-heptane:EtOAc) afforded 22a (226 mg, 0.58 mmol, 64%) as a white wax; Rf 0.45 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 5.20 (br, 2H), 3.96 (s, 2H), 3.52 (t, J = 5.5 Hz, 2H), 3.22 (t, J = 6.1 Hz, 2H), 3.15 (t, J = 7.1 Hz, 2H), 1.73–1.58 (m, 4H), 1.52–1.40 (m, 11H), 1.33–1.18 (m, 14H), 0.87 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 170.3, 158.9, 82.2, 71.7, 69.0, 40.6, 40.5, 32.0, 30.4, 29.7, 29.6, 29.5, 28.2, 27.8, 27.1, 27.1, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C21H42N2O4Na 409.3037; found 409.3037. Acidification of the combined aqueous phases with conc. HCl (37%) to pH 2, twice extraction with EtOAc and evaporation in vacuo yielded the carboxylic acid 7a (30 mg, 0.09 mmol, 10%) after recrystallization from MeOH. See the respective section for 7a below for characterization data.
tert-Butyl 2-(4-(3-undecylureido)butoxy)acetate (22b)
Prepared from 21b according to General Procedure B. Reaction time: 6 days. Purification by column chromatography (SiO2, EtOAc:n-heptane 3:1) gave 22b (211 mg, 0.52 mmol, 72%) as a white wax. Rf 0.24 (2:1 hexane:EtOAc); 1H NMR (400 MHz, CDCl3) δ 3.97 (s, 2H), 3.53 (t, J = 5.5 Hz, 2H), 3.24 (t, J = 6.0 Hz, 2H), 3.16 (t, J = 7.1 Hz, 2H), 1.72–1.60 (m, 4H), 1.48 (s, 10H), 1.34–1.22 (m, 20H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.3, 158.9, 82.2, 71.7, 69.0, 40.6, 40.6, 32.0, 30.4, 29.8, 29.7, 29.6, 29.5, 28.3, 27.8, 27.1, 22.8, 14.3; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C22H44N2O4Na 423.319; found 423.319.
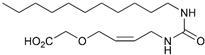
(Z)-2-((4-(3-Decylureido)but-2-en-1-yl)oxy)acetic Acid (7c)
Employing General Procedure B, starting from 21c. Reaction time: 2 days. The reaction resulted in primarily the carboxylic acid 7c. The aqueous phase was acidified with HCl (conc.) to pH 1–2 and extracted three times with EtOAc. Evaporation in vacuo and recrystallization from MeOH afforded 7c (115 mg, 0.35 mmol, 44%) as white solids. mp. 82–84 °C; 1H NMR (400 MHz, CD3OD) δ 5.70–5.57 (m, 2H), 4.23–4.14 (m, 2H), 4.08 (s, 2H), 3.83 – 3.75 (m, 2H), 3.09 (t, J = 7.0 Hz, 2H), 1.52–1.41 (m, 2H), 1.38–1.23 (m, 14H), 0.90 (app. t, 3H); 13C NMR (101 MHz, CD3OD) δ 173.4, 160.4, 131.8, 127.6, 67.3, 66.9, 40.4, 37.5, 32.4, 30.7, 30.1, 30.1, 29.9, 29.8, 27.3, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C17H32N2O4Na 351.2254; found 351.2253.
tert-Butyl (Z)-2-((4-(3-undecylureido)but-2-en-1-yl)oxy)acetate (22c)
Prepared according to General Procedure B, starting from 21d. Reaction time: 2 days. Column chromatography twice (SiO2, EtOAc:n-heptane 3:2 and CH2Cl2:EtOAc 1:1, respectively) gave 22c as a wax (111 mg, 0.28 mmol, 49%). The product was still contaminated with diundecylurea (~15 mol%). Rf 0.49 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.84–5.67 (m, 2H), 4.12 (d, J = 5.9 Hz, 2H), 3.98 (s, 2H), 3.87 (d, J = 6.5 Hz, 2H), 3.15 (t, J = 7.1 Hz, 2H), 1.48 (s, 10H), 1.35–1.22 (m, 17H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.1, 158.5, 132.4, 127.3, 82.3, 68.2, 66.8, 40.7, 37.6, 32.0, 30.4, 29.8, 29.7, 29.5, 29.5, 28.2, 28.2, 27.1, 22.8, 14.3; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C22H42N2O4Na 421.3037; found 421.3036.
tert-Butyl (Z)-2-(4-(3-(undec-2-en-1-yl)ureido)butoxy)acetate (22d) and (Z)-2-(4-(3-(undec-2-en-1-yl)ureido)butoxy)acetic Acid (7e)
Prepared according to General procedure B, starting from 21e. Reaction time: overnight. Work-up: purification of the product from the organic phase by column chromatography (SiO2, hexane:EtOAc 1:1) gave the tert-butyl ester 22d (69.0 mg, 0.17 mmol, 19%) as a clear oil. Rf 0.28 (hexane:EtOAc 4:1); 1H NMR (400 MHz, CDCl3) δ 5.51–5.33 (m, 2H), 3.94 (s, 2H), 3.78 (dd, J = 6.5, 1.3 Hz, 2H), 3.50 (t, J = 5.7 Hz, 2H), 3.20 (t, J = 6.2 Hz, 2H), 2.03 (q, J = 6.9 Hz, 2H), 1.78–1.58 (m, 4H), 1.45 (s, 9H), 1.34–1.23 (m, 12H), 0.85 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.2, 158.9, 132.7, 126.8, 82.1, 71.6, 68.9, 40.4, 37.6, 32.0, 29.7, 29.6, 29.4 (2 × C), 28.2 (3 × C), 27.7, 27.4, 27.0, 22.7, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C22H42N2O4Na 421.3037; found 421.3036.
The aqueous phase was acidified with HCl (conc.) to pH 2 and extracted with EtOAc (3 × 10 mL), and the combined organic phases were dried (MgSO4), filtrated, and concentrated in vacuo. The crude product was recrystallized using MeOH as a solvent. This afforded carboxylic acid 7e (29.1 mg, 0.08 mmol, 9%) as a crystalline solid. See the section for 7e below for characterization data.
tert-Butyl 2-(((Z)-4-(3-((Z)-undec-2-en-1-yl)ureido)but-2-en-1-yl)oxy)acetate (22e)
Prepared from 21f, according to General procedure B. Elution with EtOAc:n-heptane 1:1 gave 22e (12 mg, 29 µmol, 32%) as a clear oil. Rf 0.59 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 5.84–5.68 (m, 2H), 5.55–5.45 (m, 1H), 5.44–5.33 (m, 1H), 4.12 (d, J = 5.8 Hz, 2H), 3.97 (s, 2H), 3.88 (d, J = 6.2 Hz, 2H), 3.81 (d, J = 5.4 Hz, 2H), 2.05 (q, J = 7.3 Hz, 2H), 1.48 (s, 9H), 1.36–1.21 (m, 13H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.1, 158.4, 133.2, 132.2, 127.4, 126.3, 82.3, 68.2, 66.8, 37.8, 37.7, 32.0, 29.7, 29.6, 29.4, 28.3, 27.5, 22.8, 14.2. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C22H40N2O4Na 419.2880; found 419.2879.
2-(4-(3-Decylureido)butoxy)acetic Acid (7a)
Prepared according to General procedure C, from tert-butyl ester 22a. Afterwards, HCl (conc.) was added under cooling until pH 2, and the solution extracted with EtOAc (4 × 50 mL (the product is poorly soluble in EtOAc)). Evaporation in vacuo and recrystallization from MeOH afforded 7a (101 mg, 0.31 mmol, 64%) as a white solid, mp. 103–105 °C. 1H NMR (400 MHz, CD3OD) δ 4.05 (s, 2H), 3.55 (t, J = 6.1 Hz, 2H), 3.14 (t, J = 6.7 Hz, 2H), 3.09 (t, J = 7.0 Hz, 2H), 1.69–1.52 (m, 4H), 1.50–1.42 (m, 2H), 1.30 (m, 14H), 0.90 (m, 3H); 13C NMR (101 MHz, CD3OD) δ 173.7, 160.7, 71.7, 68.2, 40.4, 40.1, 32.4, 30.7, 30.1, 30.1, 29.9, 29.8, 27.3, 27.3, 23.1, 13.8. HRMS (ESI/Q-TOF) m/z: [M+Na] + calcd. for C17H34N2O4Na 353.2411; found 353.2410.
2-(4-(3-Undecylureido)butoxy)acetic Acid (7b)
Prepared according to General procedure C, starting from 21b. Evaporation of the organic phase and recrystallization from MeOH gave 7b (82 mg, 0.24 mmol, 52%) as white solids. mp. 109–110 °C. 1H NMR (400 MHz, CD3OD) δ 4.06 (s, 2H), 3.54 (t, J = 6.1 Hz, 2H), 3.14 (t, J = 6.9 Hz, 2H), 3.09 (t, J = 7.0 Hz, 2H), 1.69–1.50 (m, 4H), 1.52–1.41 (m, 2H), 1.37–1.22 (m, 16H), 0.89 (app. t, 3H). 13C NMR (101 MHz, CD3OD) δ 173.6, 160.7, 71.7, 68.1, 40.4, 40.1, 32.4, 30.7, 30.1, 29.9, 29.8, 27.3, 27.3, 27.3, 23.1, 13.8. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C18H36N2O4Na 367.2567; found 367.2567.
tert-Butyl (Z)-2-((4-(3-undecylureido)but-2-en-1-yl)oxy)acetate (7d)
Prepared according to General procedure C, starting from 21d. Column chromatography twice (SiO2, EtOAc:n-heptane 3:2 and CH2Cl2:EtOAc 1:1, respectively) gave 7d as a waxy solid (111 mg, 0.28 mmol, 49%). Rf 0.49 (EtOAc). The product was still contaminated with some diundecylurea (~15 mol%). 1H NMR (400 MHz, CDCl3) δ 5.84–5.67 (m, 2H), 4.12 (d, J = 5.9 Hz, 2H), 3.98 (s, 2H), 3.87 (d, J = 6.5 Hz, 2H), 3.15 (t, J = 7.1 Hz, 2H), 1.48 (s, 10H), 1.35–1.22 (m, 17H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.1, 158.5, 132.4, 127.3, 82.3, 68.2, 66.8, 40.7, 37.6, 32.0, 30.4, 29.8, 29.7, 29.5, 29.5, 28.2, 28.2, 27.1, 22.8, 14.3. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C22H42N2O4Na 421.3037; found 421.3036.
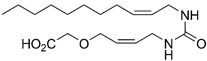
(Z)-2-(4-(3-(undec-2-en-1-yl)ureido)butoxy)acetic Acid (7e):
See the procedure for 22d above. The procedure gave 7e (29.1 mg, 0.08 mmol, 9%) as a crystalline solid. Rf 0.29 (DCM:MeOH 9:1); mp. 89–90 °C; 1H NMR (400 MHz, CD3OD) δ 5.54–5.45 (m, 1H), 5.44–5.35 (m, 1H), 4.05 (s, 2H), 3.75 (dd, J = 6.6, 1.4 Hz, 2H), 3.54 (t, J = 6.1 Hz, 2H), 3.14 (t, J = 6.6 Hz, 2H), 2.09 (q, J = 7.0 Hz, 2H), 1.75–1.51 (m, 4H), 1.40–1.13 (m, 12H), 0.90 (t, J = 6.6 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 174.3, 161.2, 133.3, 127.9, 72.3, 68.8, 40.8, 38.1, 33.1, 30.7, 30.7, 30.6, 30.4, 30.4, 28.3, 27.9, 23.7, 14.4; HRMS (ESI/Q-TOF) m/z: [M + 2Na]+ calcd. for C18H33NO4Na2 387.2230; found 387.2230.
2-(((Z)-4-(3-((Z)-undec-2-en-1-yl)ureido)but-2-en-1-yl)oxy)acetic Acid (7f)
Prepared from 22e according to General procedure C. The reaction mixture was diluted with water and acidified with HCl (conc.) until pH 2–3. The solution was extracted with EtOAc (2 × 30 mL), dried over MgSO4, and evaporated in vacuo. Column chromatography (SiO2, AcOH:EtOAc:heptane 1:5:5) gave 7f (5.5 mg, 16 µmol, 55%) as a waxy solid. 1H NMR (400 MHz, CD3OD) δ 5.71–5.57 (m, 1H), 5.55–5.33 (m, 1H), 4.18 (d, J = 5.0 Hz, 1H), 4.06 (s, 1H), 3.80 (d, J = 5.2 Hz, 1H), 3.75 (d, J = 6.7 Hz, 1H), 2.10 (app. q, J = 7.0 Hz, 1H), 1.42–1.27 (m, 6H), 0.94–0.86 (m, 1H). 13C NMR (101 MHz, CD3OD) δ 173.8, 160.3, 132.7, 131.7, 127.7, 127.1, 67.6, 66.8, 37.5, 37.5, 32.4, 30.1, 30.0, 29.8, 29.7, 27.6, 23.1, 13.8. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C18H32N2O4Na 363.2254; found 363.2254.
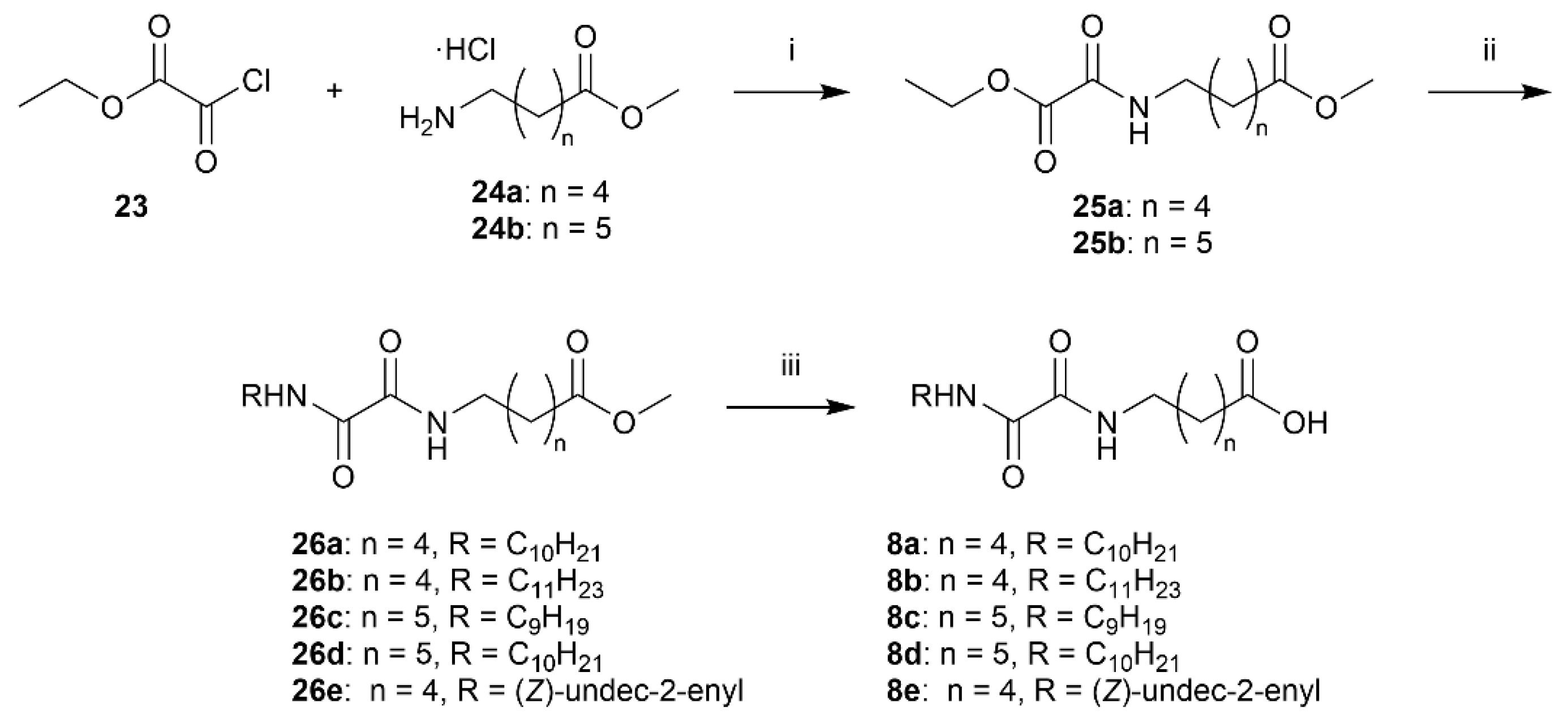
Methyl 6-(2-ethoxy-2-oxoacetamido)hexanoate (25a)
Methyl 6-aminohexanoate hydrochloride (24a) (99 mg, 0.56 mmol) is dissolved in DCM (6.9 mL) and cooled in an ice bath. TEA (169 µL, 1.22 mmol, 2.2 equiv.) and ethyl chlorooxoacetate (23) (67.7 µL, 0.61 mmol, 1.1 equiv.) is added. After 5 min, the ice bath is removed and the reaction is stirred for 2 h. The solution is transferred to a separatory funnel and washed with HCl (1 M). The organic phase is dried over Na2SO4 and evaporated in vacuo (Excess acid chloride is removed by evaporation) to afford 25a (127 mg, 0.53 mmol, 94%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.14 (s, 1H), 4.32 (q, J = 7.1 Hz, 2H), 3.65 (s, 3H), 3.32 (app. q, 2H), 2.30 (t, J = 7.4 Hz, 2H), 1.72–1.50 (m, 4H), 1.44–1.29 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 174.0, 160.9, 156.7, 63.3, 51.6, 39.7, 33.9, 28.9, 26.4, 24.5, 14.1. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C11H19NO5Na 268.1155; found 268.1155.
Methyl 7-(2-ethoxy-2-oxoacetamido)heptanoate (25b)
Methyl 6-aminoheptanoate hydrochloride (24b) (600 mg, 3.07 mmol) is dissolved in DCM (38 mL) and cooled in an ice bath. TEA (941 µL, 6.75 mmol, 2.2 equiv.) and ethyl chlorooxoacetate (23) (377 µL, 3.37 mmol, 1.1 equiv.) is added. After 5 min, the ice bath is removed and the reaction stirred for 2 h. The solution is transferred to a separatory funnel and washed with HCl (1 M). The organic phase is dried over Na2SO4 and evaporated in vacuo (excess acid chloride is removed by evaporation) to afford 25b as a yellow oil. Rf 0.51 (EtOAc) 1H NMR (400 MHz, CDCl3) δ 7.03 (s, 1H), 4.28 (q, J = 7.1 Hz, 2H), 3.60 (s, 3H), 3.27 (app. q, 2H), 2.24 (t, J = 7.5 Hz, 2H), 1.65–1.43 (m, 5H), 1.38–1.21 (m, 7H). 13C NMR (101 MHz, CDCl3) δ 174.1, 160.9, 156.6, 63.2, 51.5, 39.8, 33.9, 29.0, 28.7, 26.4, 24.7, 14.0. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C12H21NO5Na 282.1312; found 282.1314.
Methyl 6-(2-(decylamino)-2-oxoacetamido)hexanoate (26a)
General procedure E is employed using 25a and n-decylamine. Column chromatography (SiO2, 3:2 EtOAc:heptane) afforded 26a (158 mg, 0.44 mmol, 82%) as a white solid, mp. 100–101 °C. Rf 0.64 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.64–7.42 (m, 2H), 3.65 (s, 3H), 3.34–3.23 (m, 4H), 2.30 (t, J = 7.4 Hz, 2H), 1.70–1.47 (m, 6H), 1.40–1.18 (m, 16H), 0.86 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.0, 160.0, 159.9, 51.6, 39.8, 39.5, 33.9, 32.0, 29.6, 29.6, 29.4, 29.3, 29.0, 26.9, 26.4, 24.6, 22.8, 14.2. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H36N2O4Na 379.2567; found 379.2567.
Methyl 6-(2-oxo-2-(undecylamino)acetamido)hexanoate (26b)
Prepared from 25a and n-undecylamine according to General procedure E. Column chromatography (SiO2, 3:2 EtOAc:heptane) afforded 26b (162 mg, 0.44 mmol, 94%) as a white solid. mp. 104 °C. Rf 0.59 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.63–7.37 (m, 2H), 3.65 (s, 3H), 3.28 (app. p, 4H), 2.30 (t, J = 7.4 Hz, 2H), 1.70–1.48 (m, 6H), 1.42–1.17 (m, 18H), 0.86 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.0, 160.0, 159.9, 51.6, 39.8, 39.6, 33.9, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 29.0, 27.0, 26.4, 24.6, 22.8, 14.2. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H38N2O4Na 393.2724; found 393.2723.
Methyl 7-(2-(nonylamino)-2-oxoacetamido)heptanoate (26c)
Prepared from 25b and n-nonylamine according to General procedure E. Column chromatography (SiO2, 3:2 EtOAc:heptane) afforded 26c (419 mg, 1.18 mmol, 84%) as a white solid. mp. 114 °C. Rf 0.63 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.63–7.38 (m, 2H), 3.65 (s, 3H), 3.29 (q, J = 6.6 Hz, 4H), 2.29 (t, J = 7.5 Hz, 2H), 1.66–1.49 (m, 6H), 1.39–1.19 (m, 16H), 0.86 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.2, 160.0, 159.9, 51.6, 39.8, 39.7, 34.0, 32.0, 29.6, 29.3, 29.2, 28.8, 26.9, 26.6, 24.9, 22.8, 14.2. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H36N2O4Na 379.2567; found 379.2567.
Methyl 7-(2-(decylamino)-2-oxoacetamido)heptanoate (26d)
Prepared from 25b and n-decylamine according to General procedure E. Column chromatography (SiO2, 3:2 EtOAc:heptane) afforded 26d (411 mg, 1.11 mmol, 83%) as a white solid, mp. 111 °C. Rf 0.61 (EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.59–7.42 (m, 2H), 3.65 (s, 3H), 3.33–3.23 (m, 4H), 2.29 (t, J = 7.5 Hz, 2H), 1.66–1.48 (m, 6H), 1.39–1.18 (m, 18H), 0.86 (app. t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.2, 160.0, 159.9, 51.6, 39.8, 39.7, 34.0, 32.0, 29.6, 29.6, 29.4, 29.3, 29.2, 28.8, 27.0, 26.6, 24.9, 22.8, 14.2. HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H38N2O4Na 393.2724; found 393.2723.
Methyl (Z)-6-(2-oxo-2-(undec-2-en-1-ylamino)acetamido)hexanoate (26e)
The aminooxoacetate 25a (74.0 mg, 0.300 mmol, 1.00 equiv.) was dissolved in EtOH (0.5 mL) and added Z-amine 19 (51.0 mg, 0.300 mmol, 1.00 equiv.) in one portion at rt. The product precipitates quickly, and the reaction was stirred for 2.5 h. The solvent was evaporated, and the crude was purified by flash chromatography on silica gel (heptane:EtOAc 2:1) to afford the desired oxamide 26e (75.0 mg, 0.204 mmol, 68%) as a white crystalline solid. Rf 0.28 (heptane:EtOAc 2:1); mp. 85–86 °C; 1H NMR (400 MHz, CDCl3) δ 7.49–7.33 (m, 2H), 5.61 (dtt, J = 10.7, 7.4, 1.6 Hz, 1H), 5.39 (dtt, J = 10.4, 7.0, 1.6 Hz, 1H), 4.32–3.82 (m, 2H), 3.67 (s, 3H), 3.31 (q, J = 6.9 Hz, 2H), 2.31 (t, J = 7.4 Hz, 2H), 2.17–1.95 (m, 2H), 1.70–1.52 (m, 4H), 1.45–1.19 (m, 14H), 0.94–0.82 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 173.7, 159.6, 159.5, 134.7, 123.4, 51.4, 39.3, 36.6, 33.7, 31.7, 29.3, 29.2, 29.1 (2 × C), 28.8, 27.2, 26.1, 24.3, 22.5, 13.9; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H36N2O4Na 391.2567; found 391.2567.
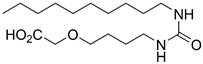
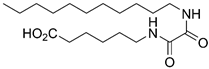
6-(2-(Decylamino)-2-oxoacetamido)hexanoic Acid (8a)
Ester 26a was hydrolyzed using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in diluted HCl, extracted with EtOAc (3 × 100 mL) and dried over MgSO4. Concentration in vacuo afforded the acid 8a (115 mg, 0.31 mmol, 82%) as a pale yellow solid. mp. 142–144 °C; 1H NMR (400 MHz, DMSO) δ 11.98 (s(br), 1H), 8.68 (app. q, 2H), 3.10 (q, J = 6.7 Hz, 4H), 2.18 (t, J = 7.4 Hz, 2H), 1.58–1.36 (m, 6H), 1.34–1.14 (m, 16H), 0.85 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 174.4, 160.0, 160.0, 38.8, 38.6, 33.6, 31.3, 29.0, 28.7, 28.7, 28.5, 26.3, 25.9, 24.2, 22.1, 14.0; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C18H34N2O4Na 365.2411; found 365.2410.
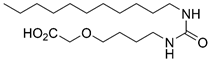
6-(2-Oxo-2-(undecylamino)acetamido)hexanoic Acid (8b)
Prepared by hydrolyzing 26b using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 100 mL), and dried over MgSO4. Concentration in vacuo afforded the acid 8b (104 mg, 0.29 mmol, 79%) as a pale yellow solid. mp. 142–145 °C. 1H NMR (400 MHz, DMSO) δ 11.97 (s, 1H), 8.78–8.59 (m, 2H), 3.11 (app. q, J = 6.8 Hz, 4H), 2.19 (t, J = 7.4 Hz, 2H), 1.46 (dq, J = 15.3, 7.6 Hz, 6H), 1.24 (s, 18H), 0.86 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 174.4, 160.0, 160.0, 38.8, 38.6, 33.6, 31.3, 29.0, 29.0, 28.7, 28.7, 28.5, 26.3, 25.9, 24.2, 22.1, 14.0; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H36N2O4Na 379.2567; found 379.2567.
7-(2-(Nonylamino)-2-oxoacetamido)heptanoic Acid (8c)
Prepared by hydrolyzing 26c using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 100 mL), and dried over MgSO4. Concentration in vacuo and recrystallization from first MeOH then CHCl3 afforded the acid 8c (130 mg, 0.38 mmol, 33%) as a white solid. mp. 148 °C; 1H NMR (400 MHz, DMSO) δ 11.96 (s, 1H), 8.76–8.57 (m, 2H), 3.10 (app. q, J = 6.8 Hz, 4H), 2.18 (t, J = 7.4 Hz, 2H), 1.52–1.37 (m, 6H), 1.32–1.16 (m, 16H), 0.85 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 174.5, 160.0, 160.0, 38.8, 38.7, 33.6, 31.3, 28.9, 28.7, 28.6, 28.6, 28.2, 26.3, 26.0, 24.4, 22.1, 13.9; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C18H34N2O4Na 365.2411; found 365.2410.
7-(2-(Decylamino)-2-oxoacetamido)heptanoic Acid (8d)
Prepared by hydrolyzing 27d using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 100 mL), and dried over MgSO4. Concentration in vacuo afforded the acid 8d (288 mg, 0.81 mmol, 74%) as a white solid. mp. 146–147 °C; 1H NMR (400 MHz, DMSO) δ 11.95 (s, 1H), 8.75–8.61 (m, 2H), 3.18–3.04 (m, 4H), 2.19 (t, J = 7.4 Hz, 2H), 1.54–1.39 (m, 6H), 1.32–1.15 (m, 19H), 0.91–0.80 (m, 3H); 13C NMR (101 MHz, DMSO) δ 174.9, 160.5, 39.2, 34.1, 31.8, 29.4, 29.2, 28.7, 26.8, 26.5, 24.9, 22.6, 14.4; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H36N2O4Na 379.2567; found 379.2567.
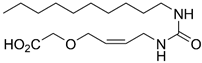
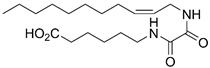
(Z)-6-(2-Oxo-2-(undec-2-en-1-ylamino)acetamido)hexanoic Acid (8e)
Prepared by hydrolyzing 26e using General procedure C. Work-up: the reaction mixture was acidified with 1.0 M HCl (7.0 mL) to pH 2 and extracted with EtOAc (3 × 10 mL). The combined organic phases were dried (Na2SO4), and the solvent was removed in vacuo. The crude mixture was purified by flash chromatography on silica gel (1:1 → 0:1 heptane:EtOAc) to afford the desired product 8e (20.1 mg, 0.057 mmol, 77%) as a white crystalline solid. Rf 0.49 (EtOAc); mp. 124–126 °C; 1H NMR (400 MHz, CDCl3) δ 7.74–7.58 (m, 1H), 7.57–7.46 (m, 1H), 5.75–5.48 (m, 1H), 5.46–5.33 (m, 1H), 4.11–3.82 (m, 2H), 3.32 (q, J = 6.9 Hz, 2H), 2.36 (t, J = 7.4 Hz, 2H), 2.15–2.01 (m, 2H), 1.73–1.50 (m, 4H), 1.47–1.13 (m, 14H), 0.88 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 178.2, 159.9, 159.9, 135.0, 123.7, 39.6, 36.9, 33.7, 32.0, 29.6, 29.6, 29.4 (2 × C), 29.0, 27.6, 26.3, 24.3, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H34N2O4Na 377.2411; found 377.2410.
Methyl 6-dodecanamidohexanoate (28a)
The acid chloride of dodecanoic acid (27a) (0.79 g, 4.0 mmol, 2.0 equiv.) was prepared according to General procedure D. Afterwards, methyl 6-aminohexanoate·HCl (24a) (363 mg, 2.00 mmol) was dissolved in MeCN (10 mL) and cooled to 0 °C. The acid chloride in DCM (3.0 mL) and DIPEA (0.70 mL, 4.0 mmol, 2.0 equiv.) was added and the ice bath removed. After stirring overnight, the suspension was concentrated in vacuo and taken up in DCM. The organic phase was washed twice with diluted HCl (1 M) and diluted K2CO3, before drying the organic phase over MgSO4 and evaporating in vacuo. Purification by column chromatography (SiO2, 1:1 EtOAc:n-heptane) afforded amide 28a (309 mg, 0.94 mmol, 47%) as a white solid. mp. 62–63 °C; Rf 0.50 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.58 (s, 1H), 3.66 (s, 3H), 3.30–3.18 (m, 2H), 2.31 (t, J = 7.4 Hz, 2H), 2.15 (app. t, 2H), 1.70–1.56 (m, 4H), 1.51 (p, J = 7.8 Hz, 2H), 1.40–1.18 (m, 18H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.1, 173.5, 51.5, 39.3, 36.7, 33.8, 31.9, 29.6, 29.6, 29.5, 29.4, 29.3, 29.3, 29.2, 26.3, 25.9, 24.4, 22.7, 14.1; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H37NO3Na 350.2666; found 350.2665.
Methyl 6-tridecanamidohexanoate (28b)
The acid chloride of tridecanoic acid (27b) (499 mg, 2.33 mmol, 1.6 equiv.) was prepared according to General procedure D. Afterwards, methyl 6-aminohexanoate·HCl (24a) (265 mg, 1.45 mmol) was dissolved in MeCN (7.3 mL) and cooled to 0 °C. The acid chloride in DCM (2.0 mL) and DIPEA (0.60 mL, 3.4 mmol, 2.4 equiv.) was added and the ice bath removed. After stirring overnight, the suspension was concentrated in vacuo. Purification by column chromatography (SiO2, 3:2 EtOAc:n-heptane) afforded amide 28b (439 mg, 1.29 mmol, 88%) as a white solid. mp. 68–69 °C; Rf 0.54 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.75 (s, 1H), 3.68 (s, 3H), 3.27 (app. q, 2H), 2.33 (t, J = 7.4 Hz, 2H), 2.18 (app. t, 2H), 1.72–1.58 (m, 4H), 1.58–1.48 (m, 2H), 1.43–1.21 (m, 20H), 0.89 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.1, 173.4, 51.5, 39.3, 36.8, 33.8, 31.9, 29.7, 29.6, 29.6, 29.5, 29.4, 29.4, 29.3, 29.2, 26.3, 25.9, 24.4, 22.7, 14.1; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2821.
Methyl 7-dodecanamidoheptanoate (28c)
The acid chloride of dodecanoic acid (27a) (300 mg, 1.50 mmol, 1.33 equiv.) was prepared according to General procedure D. Methyl 7-aminoheptanoate·HCl (24b) (220 mg, 1.12 mmol) was dissolved in MeCN (7.5 mL) and cooled to 0 °C. The acid chloride in DCM (1.0 mL) and TEA (0.37 mL, 2.6 mmol, 2.3 equiv.) were added and the ice bath removed. After stirring overnight, the suspension was concentrated in vacuo and taken up in DCM. The organic phase was washed with dilute HCl (1 M), dried over MgSO4, and evaporated in vacuo. Purification by column chromatography (SiO2, 3:2 EtOAc:n-heptane) afforded amide 28c (370 mg, 1.08 mmol, 96%) as a white solid. mp. 73 °C; Rf 0.55 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.66 (s, 1H), 3.65 (s, 3H), 3.22 (app. q, J = 6.7 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 2.14 (t, J = 7.7 Hz, 2H), 1.67–1.54 (m, 4H), 1.54–1.42 (m, 2H), 1.39–1.17 (m, 20H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.3, 173.5, 51.6, 39.5, 36.9, 34.0, 32.0, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 28.8, 26.6, 26.0, 24.9, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2822.
Methyl 7-tridecanamidoheptanoate (28d)
The acid chloride of tridecanoic acid (27b) (321 mg, 1.52 mmol, 1.33 equiv.) was prepared according to General procedure D. Afterwards, methyl 7-aminoheptanoate·HCl (24b) (221 mg, 1.12 mmol) was dissolved in MeCN (7.5 mL) and cooled to 0 °C. The acid chloride in DCM (1.0 mL) and TEA (0.37 mL, 2.6 mmol, 2.3 equiv.) were added, and the ice bath was removed. After stirring overnight, the suspension was concentrated in vacuo. Purification by column chromatography (SiO2, 1:1 EtOAc:n-heptane) afforded amide 28d (348 mg, 0.98 mmol, 91%) as a white solid. mp 72–73 °C; Rf 0.54 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.62 (s, 1H), 3.64 (s, 3H), 3.21 (app. q/m, J = 6.7 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 2.13 (t, J = 7.8 Hz, 2H), 1.66–1.54 (m, 4H), 1.53–1.42 (m, 2H), 1.37–1.17 (m, 22H), 0.86 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.3, 173.4, 51.6, 39.5, 36.9, 34.0, 32.0, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 28.8, 26.6, 26.0, 24.9, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C21H41NO3Na 378.2979; found 378.2978.
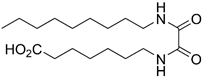
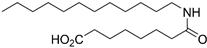
6-Dodecanamidohexanoic Acid (9a)
Ester 28a was hydrolyzed according to General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in diluted HCl, extracted thrice with EtOAc and dried over MgSO4. Concentration in vacuo afforded carboxylic acid 9a (104 mg, 0.33 mmol, 47%) as white solids. mp. 89–90 °C; 1H NMR (400 MHz, CD3OD) δ 3.16 (t, J = 7.0 Hz, 2H), 2.29 (t, J = 7.4 Hz, 2H), 2.16 (t, J = 7.5 Hz, 2H), 1.67–1.46 (m, 6H), 1.42–1.23 (m, 18H), 0.89 (app. t, 3H); 13C NMR (101 MHz, CD3OD) δ 176.9, 175.6, 39.5, 36.5, 34.2, 32.4, 30.1, 30.0, 29.8, 29.8, 29.6, 29.5, 26.9, 26.5, 25.1, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C18H35NO3Na 336.2509; found 336.2508.
6-Tridecanamidohexanoic Acid (9b)
Ester 28b was hydrolyzed using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in diluted HCl, extracted thrice with EtOAc and dried over MgSO4. Concentration in vacuo afforded the acid 9b (172 mg, 0.53 mmol, 45%) as a white solid. mp. 93–94 °C; 1H NMR (400 MHz, CD3OD) δ 3.16 (t, J = 7.0 Hz, 2H), 2.29 (t, J = 7.4 Hz, 2H), 2.16 (t, J = 7.5 Hz, 2H), 1.69–1.46 (m, 6H), 1.42–1.21 (m, 20H), 0.95–0.84 (m, 3H); 13C NMR (101 MHz, CD3OD) δ 176.8, 175.6, 39.5, 36.5, 34.2, 32.4, 30.1, 30.1, 30.1, 30.0, 29.8, 29.8, 29.6, 29.5, 26.9, 26.5, 25.1, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2821.
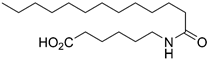
7-Dodecanamidoheptanoic Acid (9c)
Prepared by hydrolyzing 28c using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 30 mL) and dried over MgSO4. Concentration in vacuo afforded the acid 9c (280 mg, 0.85 mmol, 81%) as a white solid. mp. 104–105 °C; 1H NMR (400 MHz, CD3OD) δ 3.23–3.13 (m, 2H), 2.30 (t, J = 7.4 Hz, 2H), 2.18 (t, J = 7.4 Hz, 2H), 1.58 (dp, J = 43.5, 7.2 Hz, 6H), 1.42–1.25 (m, 20H), 0.92 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 176.9, 175.6, 39.6, 36.5, 34.2, 32.4, 30.1, 30.0, 29.8, 29.8, 29.6, 29.6, 29.2, 27.0, 26.5, 25.4, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H37NO3Na 350.2666; found 350.2665.
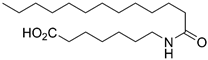
7-Tridecanamidoheptanoic Acid (9d)
Prepared by hydrolyzing 28d using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 30 mL) and dried over MgSO4. Concentration in vacuo afforded the acid 9d (278 mg, 0.81 mmol, 85%) as a white solid. mp. 105 °C; 1H NMR (400 MHz, CD3OD) δ 3.16 (t, J = 7.1 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 2.16 (t, J = 7.4 Hz, 2H), 1.68–1.43 (m, 6H), 1.43–1.17 (m, 22H), 0.97–0.83 (m, 3H); 13C NMR (101 MHz, CD3OD) δ 177.6, 176.2, 40.3, 37.2, 34.9, 33.1, 30.8, 30.7, 30.7, 30.5, 30.4, 30.3, 30.3, 29.9, 27.7, 27.1, 26.0, 23.7, 14.4; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2822.
Methyl 8-oxo-8-(undecylamino)octanoate (30a)
Methyl 7-carboxyheptanoate (187 µL, ~200 mg, 1.1 mmol) was taken up in dry DCM (5.3 mL). EDC hydrochloride (0.461 mg, 2.40 mmol, 2.2 equiv.) and N,N-dimethylaminopyridine (DMAP) (261 mg, 2.40 mmol, 2.0 equiv.) were added and the mixture stirred for 30 min. Then, n-undecylamine (229 µL, 1.06 mmol, 1.0 equiv.) was added in one portion and the reaction stirred overnight. The reaction was quenched with water (5 mL) and the phases separated. The organic phase was washed with dilute HCl, dried over MgSO4 and concentrated in vacuo. Column chromatography (SiO2, 1:1 EtOAc:heptane) gave 30a (202 mg, 0.59 mmol, 56%) as a white solid. mp. 71–72 °C; Rf 0.49 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.49 (s, 1H), 3.65 (s, 3H), 3.27–3.17 (m, 2H), 2.29 (t, J = 7.5 Hz, 2H), 2.14 (t, J = 7.6 Hz, 2H), 1.69–1.54 (m, 4H), 1.47 (p, J = 7.1 Hz, 2H), 1.39–1.15 (m, 20H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.3, 173.1, 51.6, 39.7, 36.8, 34.1, 32.0, 29.8, 29.7, 29.7, 29.5, 29.4, 29.0, 28.9, 27.1, 25.7, 24.9, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2822.
Methyl 8-(dodecylamino)-8-oxooctanoate (30b)
Methyl 7-carboxyheptanoate (195 mg, 1.04 mmol) was taken up in dry DCM (5.3 mL). EDC hydrochloride (415 mg, 2.16 mmol, 2.0 equiv.) and DMAP (260 mg, 2.13 mmol, 2.0 equiv.) were added and the mixture stirred for 30 min. Then, n-dodecylamine (190 mg, 1.03 mmol, 1.0 equiv.) was added in one portion and the reaction stirred overnight. The reaction was quenched with water (5 mL) and the phases separated. The organic phase was washed with dilute HCl, dried over MgSO4 and concentrated in vacuo. Column chromatography (SiO2, 1:1 EtOAc:heptane) gave 30b (250 mg, 0.70 mmol, 66%) as a white solid. mp. 73–74 °C; Rf 0.56 (EtOAc); 1H NMR (400 MHz, CDCl3) δ 5.53 (s, 1H), 3.65 (s, 3H), 3.21 (app. q, 2H), 2.28 (t, J = 7.5 Hz, 2H), 2.14 (t, J = 7.6 Hz, 2H), 1.68–1.55 (m, 4H), 1.53–1.41 (m, 2H), 1.39–1.19 (m, 22H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.3, 173.1, 51.6, 39.7, 36.8, 34.1, 32.0, 29.8, 29.8, 29.7, 29.7, 29.7, 29.5, 29.4, 29.0, 28.9, 27.0, 25.7, 24.9, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C21H41NO3Na 378.2979; found 378.2978.
Methyl 9-oxo-9-(undecylamino)nonanoate (30c)
n-Undecylamine (0.432 mL, 2.00 mmol 4.00 equiv.), DMAP (0.244 g, 2.00 mmol, 4.00 equiv.), and methyl 8-carboxyoctanoate (0.100 g, 0.49 mmol, 1.00 equiv.) were dissolved in dry DMF (4 mL) and EDCI hydrochloride (0.382 g, 2.00 mmol, 4.00 equiv.) was added portion-wise. After 18 h, the reaction mixture was diluted with water (10 mL) and extracted with Et2O (3 × 10 mL). The combined organic phases were washed with brine (10 mL), dried (Na2SO4), filtrated, and the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (heptane:EtOAc 1:1) to afford 30c (0.108 g, 0.30 mmol, 61%) as a crystalline solid. Rf 0.19 (heptane:EtOAc 1:1); mp.: 72–73 °C; 1H NMR (400 MHz, CDCl3) δ 5.52 (br s, 1H), 3.66 (s, 3H), 3.23 (td, J = 7.2, 5.5 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 2.15 (t, J = 7.5 Hz, 2H), 1.73–1.53 (m, 4H), 1.53–1.42 (m, 3H), 1.35–1.18 (m, 22H), 0.87 (t, J = 6.7 Hz, 3H); 13C NMR: (101 MHz, CDCl3) δ = 174.1, 173.1, 51.3, 39.5, 36.5, 33.8, 31.7, 29.4 (3 × C), 29.4, 29.1, 29.1, 28.8, 28.7 (2 × C), 26.7, 25.6, 24.7, 22.5, 13.9; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C21H41NO3Na 378.2978; found 378.2979.
Methyl (Z)-9-oxo-9-(undec-2-en-1-ylamino)nonanoate (30d)
EDCI hydrochloride (54.0 mg, 0.28 mmol, 1.20 equiv.) and DIPEA (76.0 mg, 0.591 mmol, 2.50 equiv.) were added to a solution of Z-amine 19 (40.0 mg, 0.243 mmol, 1.00 equiv.) and methyl 8-carboxyoctanoate (47.0 mg, 0.24 mmol, 1.00 equiv.) in dry DMF (2.00 mL). The solution was stirred overnight at rt. The solvent was removed in vacuo and the residue was purified by flash chromatography on silica gel (heptane:EtOAc 1:1) to afford 30d (31.0 mg, 0.09 mmol, 36%) as a crystalline solid. Rf 0.33 (heptane:EtOAc 1:1); mp.: 54–55 °C; 1H NMR (400 MHz, CDCl3) δ 5.61–5.42 (m, 2H), 5.43–5.29 (m, 1H), 3.91–3.83 (m, 2H), 3.64 (s, 3H), 2.28 (t, J = 7.5 Hz, 2H), 2.14 (t, J = 7.6 Hz, 2H), 2.05 (q, J = 7.3 Hz, 2H), 1.70–1.49 (m, 4H), 1.36–1.13 (m, 19H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 174.4, 173.0, 134.1, 125.1, 51.6, 36.8, 36.8, 34.1, 32.0, 29.6, 29.6, 29.4, 29.4, 29.2, 29.0, 29.0, 27.5, 25.8, 25.0, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C21H39NO3Na 376.2822; found 376.2822.
8-Oxo-8-(undecylamino)octanoic Acid (10a)
Prepared by hydrolyzing 30a using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 30 mL) and dried over MgSO4. Concentration in vacuo afforded 10a (117 mg, 0.36 mmol, 68%) as a white solid. mp. 104–105 °C; 1H NMR (400 MHz, CD3OD) δ 3.15 (t, J = 7.0 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 2.17 (t, J = 7.5 Hz, 2H), 1.67–1.55 (m, 4H), 1.55–1.43 (m, 2H), 1.40–1.22 (m, 20H), 0.90 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 176.9, 175.5, 48.1, 47.9, 47.7, 39.7, 36.4, 34.2, 32.4, 30.1, 30.1, 30.1, 29.8, 29.8, 29.3, 29.2, 27.4, 26.3, 25.3, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C19H37NO3Na 350.2666; found 350.2665.
8-(Dodecylamino)-8-oxooctanoic Acid (10b)
Prepared by hydrolyzing 30b using General procedure C. After cooling to 0 °C, the solution was filtered and washed with cold water. The solids were taken up in dilute HCl, extracted with EtOAc (3 × 30 mL) and dried over MgSO4. Concentration in vacuo afforded 10b (201 mg, 0.59 mmol, 86%) as a white solid. mp. 104–105 °C; 1H NMR (400 MHz, CD3OD) δ 7.92 (s, 1H), 3.20–3.11 (m, 2H), 2.28 (t, J = 7.4 Hz, 2H), 2.17 (t, J = 7.5 Hz, 2H), 1.67–1.55 (m, 4H), 1.55–1.43 (m, 2H), 1.39–1.23 (m, 22H), 0.90 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 176.9, 175.5, 39.7, 36.4, 34.2, 32.4, 30.1, 30.1, 30.1, 29.8, 29.8, 29.3, 29.2, 27.4, 26.3, 25.3, 23.1, 13.8; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2822.
9-Oxo-9-(undecylamino)nonanoic Acid (10c)
Prepared by hydrolyzing 30c using General procedure C. The aqueous solution was acidified with HCl (conc.) to pH 2 and extracted with EtOAc (3 × 10 mL). The combined organic phases were washed with brine (25 mL), dried (Na2SO4), and the solvent was removed in vacuo. The crude product was recrystallized using MeOH as a solvent, and the crystals were washed with cold MeOH to afford the desired product 10c (41.0 mg, 0.120 mmol, 85%) as a white crystalline solid. Rf 0.21 (DCM:MeOH 96:4; mp.: 96–98 °C; 1H NMR (400 MHz, CD3OD) δ 3.16 (t, J = 7.0 Hz, 2H), 2.29 (t, J = 7.4 Hz, 2H), 2.18 (t, J = 7.4 Hz, 2H), 1.72–1.55 (m, 4H), 1.55–1.44 (m, 2H), 1.41–1.23 (m, 22H), 0.91 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 177.7, 176.2, 40.3, 37.1, 35.0, 33.1, 30.7 (3 × C), 30.5, 30.4 (2 × C), 30.1 (3 × C), 28.0, 27.1, 26.1, 23.7, 14.4; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H39NO3Na 364.2822; found 364.2821.
(Z)-9-Oxo-9-(undec-2-en-1-ylamino)nonanoic Acid (10d)
Prepared by hydrolyzing 30d using General procedure C. The reaction mixture was acidified with 1.0 M HCl (6.0 mL) to pH 2 and extracted with EtOAc (3 × 10 mL). The combined organic phases were dried (Na2SO4), and the solvent was removed in vacuo. The crude mixture was purified by flash chromatography on silica gel (DCM, 1:0 → 0:1 heptane:EtOAc) to afford the desired product 10d (21.0 mg, 0.062 mmol, 84%) as a white crystalline solid. Rf 0.41 (EtOAc); mp. 73–74 °C; 1H NMR: (400 MHz, CDCl3) δ 5.62–5.51 (m, 1H), 5.47 (br. s, 1H), 5.43–5.31 (m, 1H), 3.88 (t, J = 6.3 Hz, 2H), 2.33 (t, J = 7.5 Hz, 2H), 2.24–2.12 (m, 2H), 2.07 (q, J = 7.2 Hz, 2H), 1.69–1.55 (m, 4H), 1.38–1.17 (m, 18H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 179.0, 173.4, 134.2, 125.0, 36.9, 36.8, 34.1, 32.0, 29.6, 29.6, 29.4, 29.4, 29.1, 29.0, 29.0, 27.5, 25.8, 24.8, 22.8, 14.2; HRMS (ESI/Q-TOF) m/z: [M+Na]+ calcd. for C20H37NO3Na 362.2666; found 362.2665.
Measurement of Human sEH and mEH Inhibition
The inhibition potency of the compounds against the recombinant purified human and mouse sEH and human mEH were measured using sensitive fluorescent assays [
51,
52]. The enzymes were diluted to the proper buffer and aliquoted in black 96-well plates. The enzymes were incubated with the inhibitors (0.4 < [I] < 50,000 nM) for 5 min at 37 °C before the introduction of the reporting substrate. For the sEH, nonfluorescent cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate MNPC was used as the reporting substrate at a final concentration of 5 μM [
51]. For the mEH, cyano(6-methoxy-naphthalen-2-yl)methyl glycidyl carbonate (MNGC) was used at a final concentration of 5 µM. The formation of the product (6-methoxynaphthaldehyde) was measured (λ
em = 330 nm, λ
ex = 465 nm) every 30 s for 10 min by a Molecular Device M-2 plate reader. All measurements were performed in triplicate. The inhibitory potency (IC
50) was calculated by regression of at least four data points on both sides of the 50% mark.