Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy
Abstract
1. Introduction
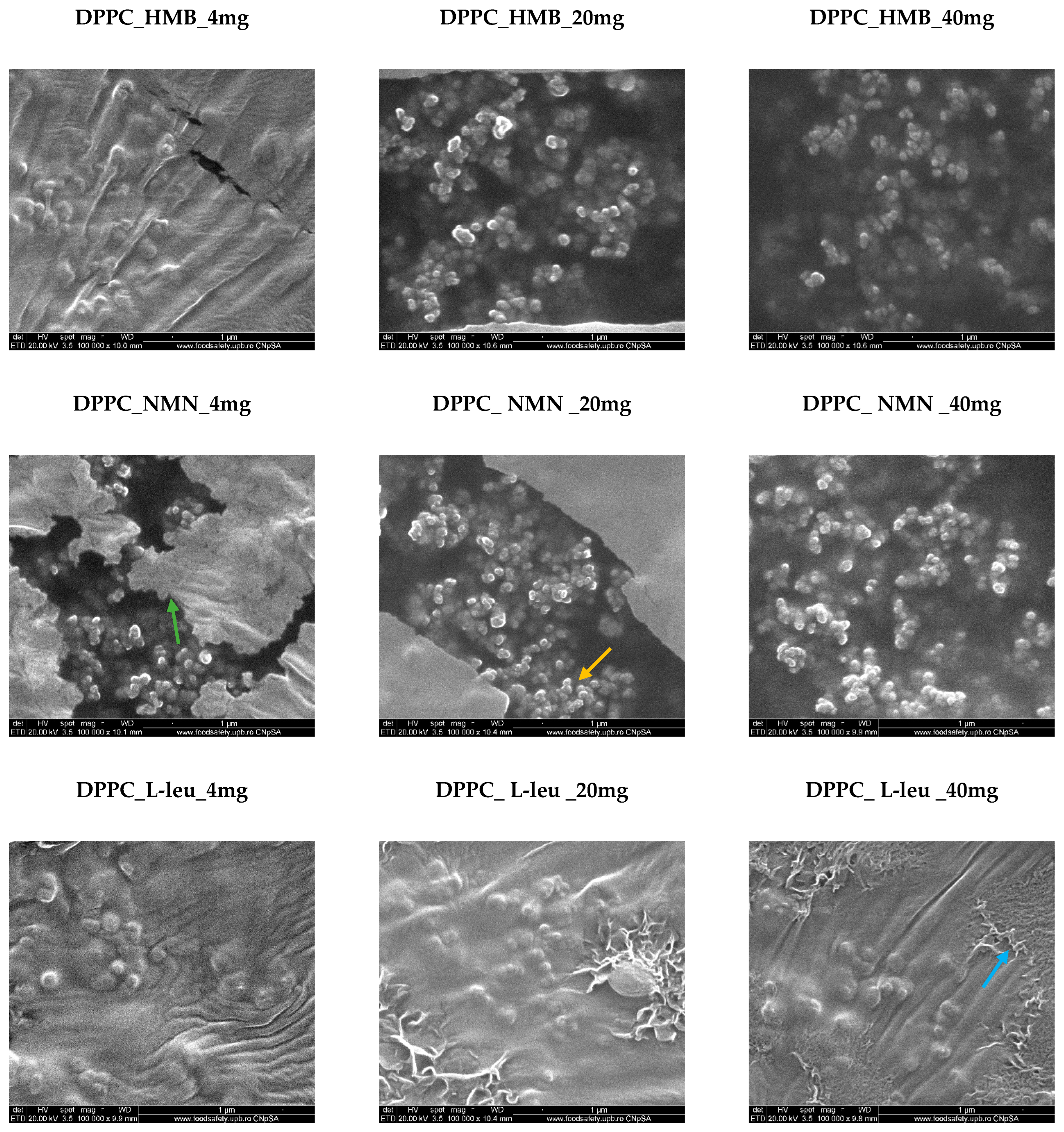
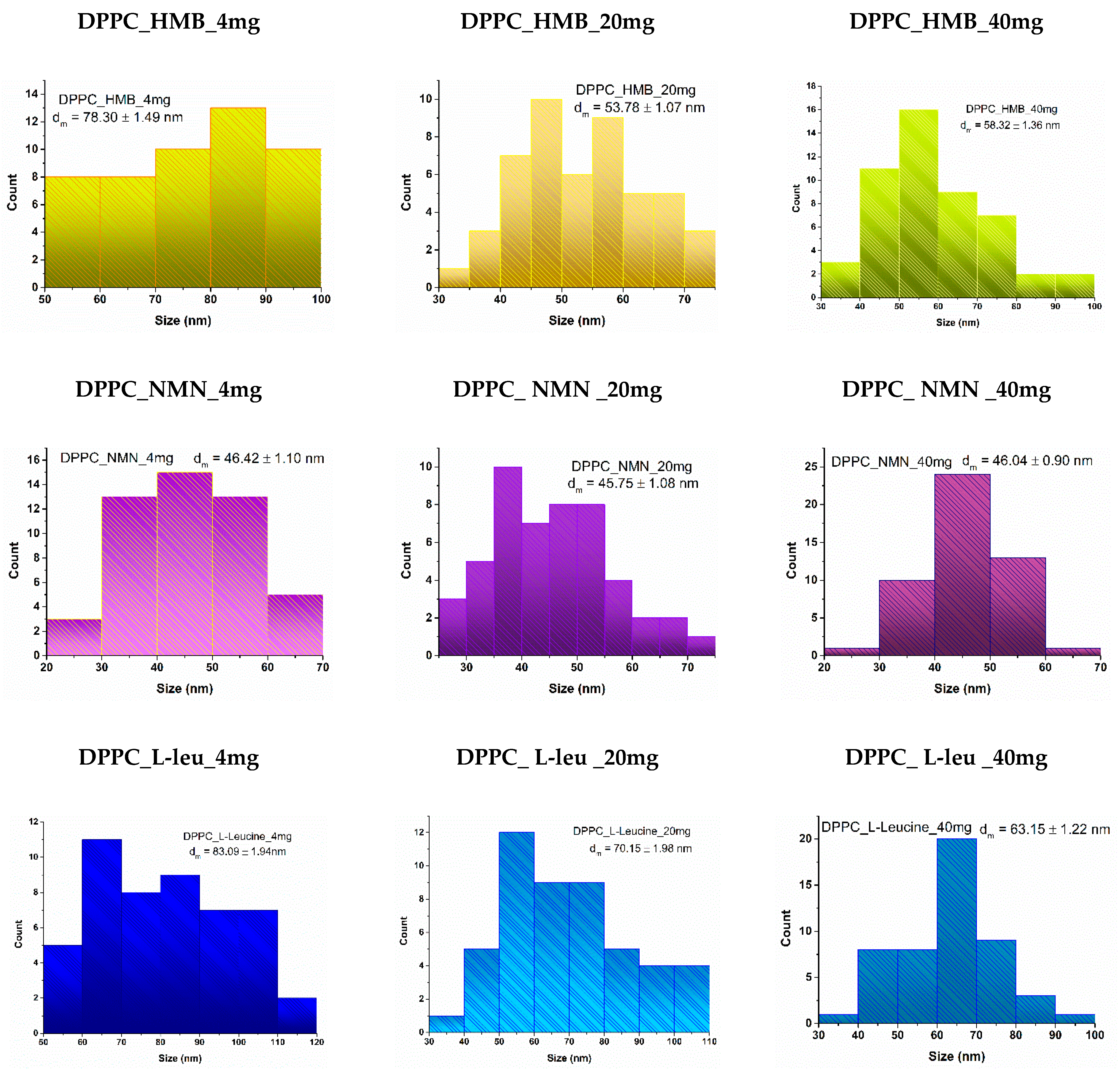
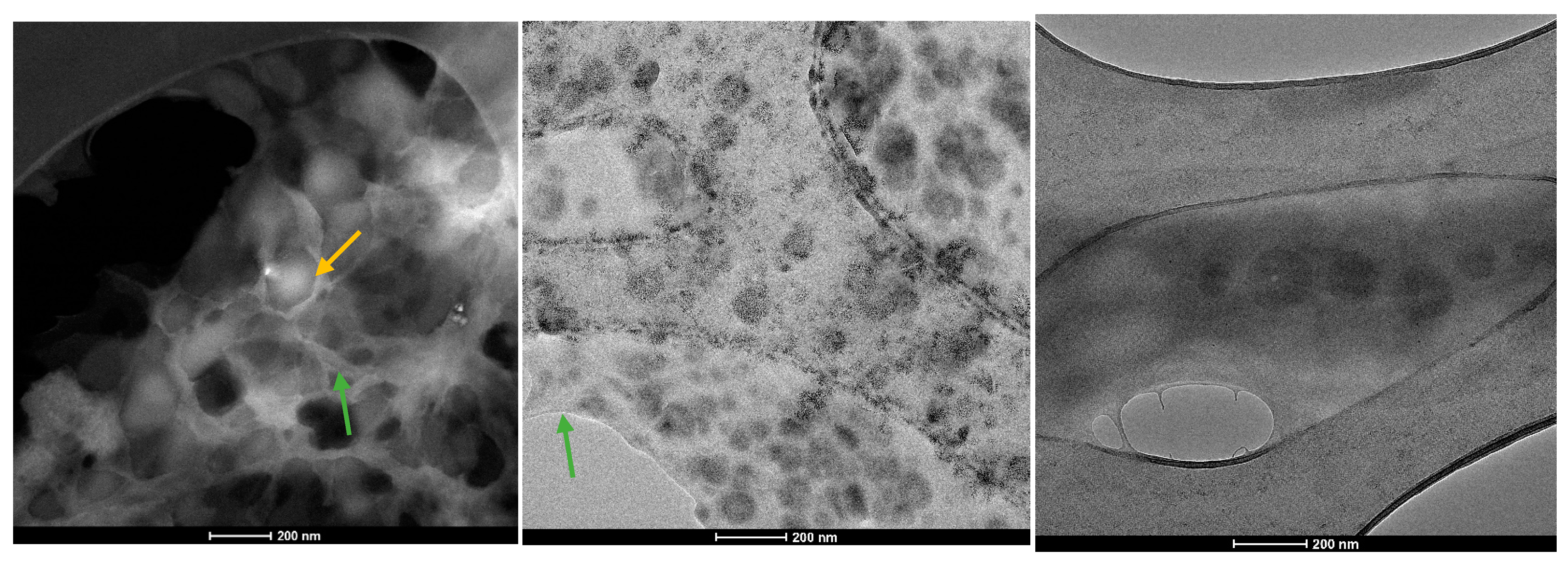
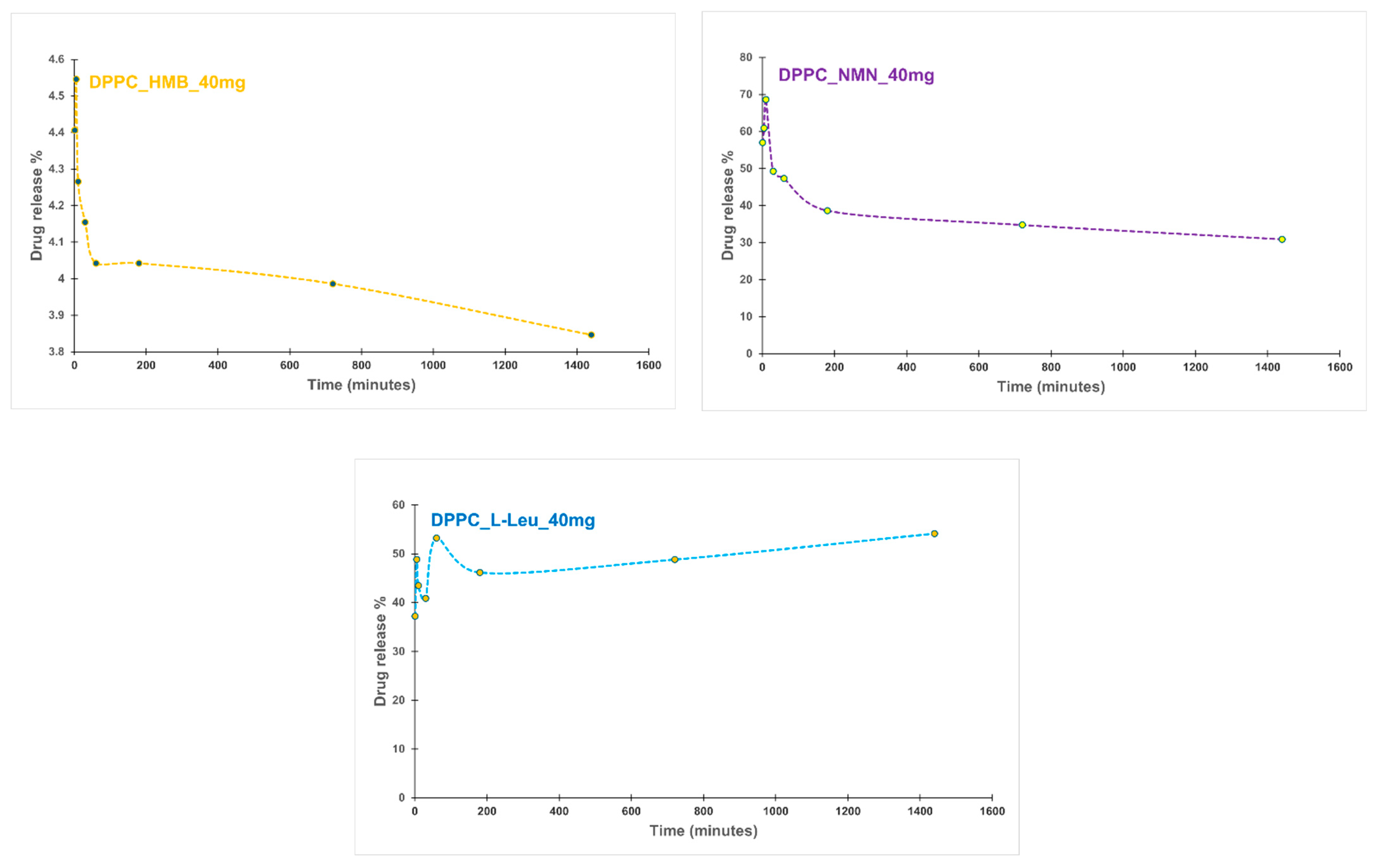
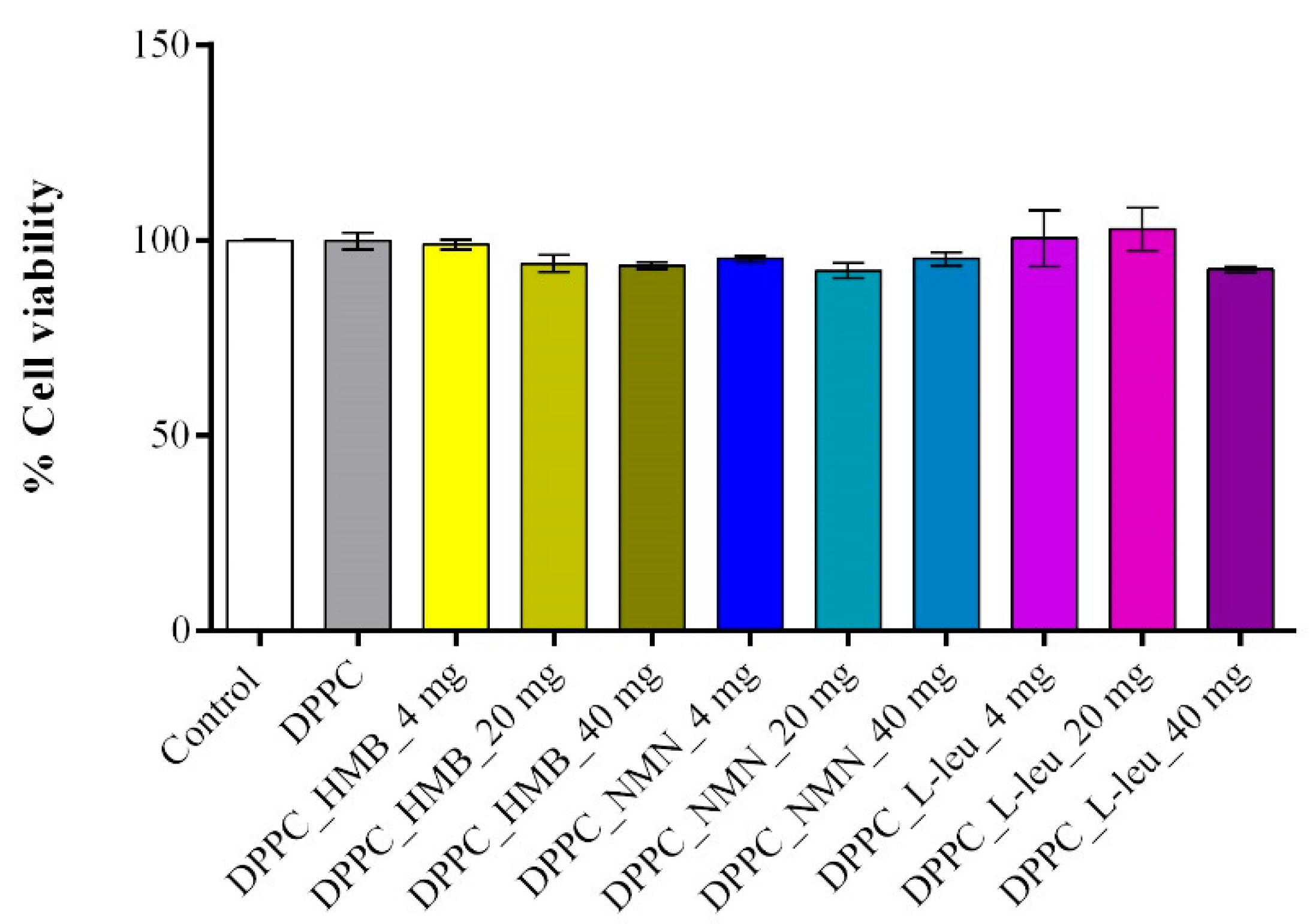
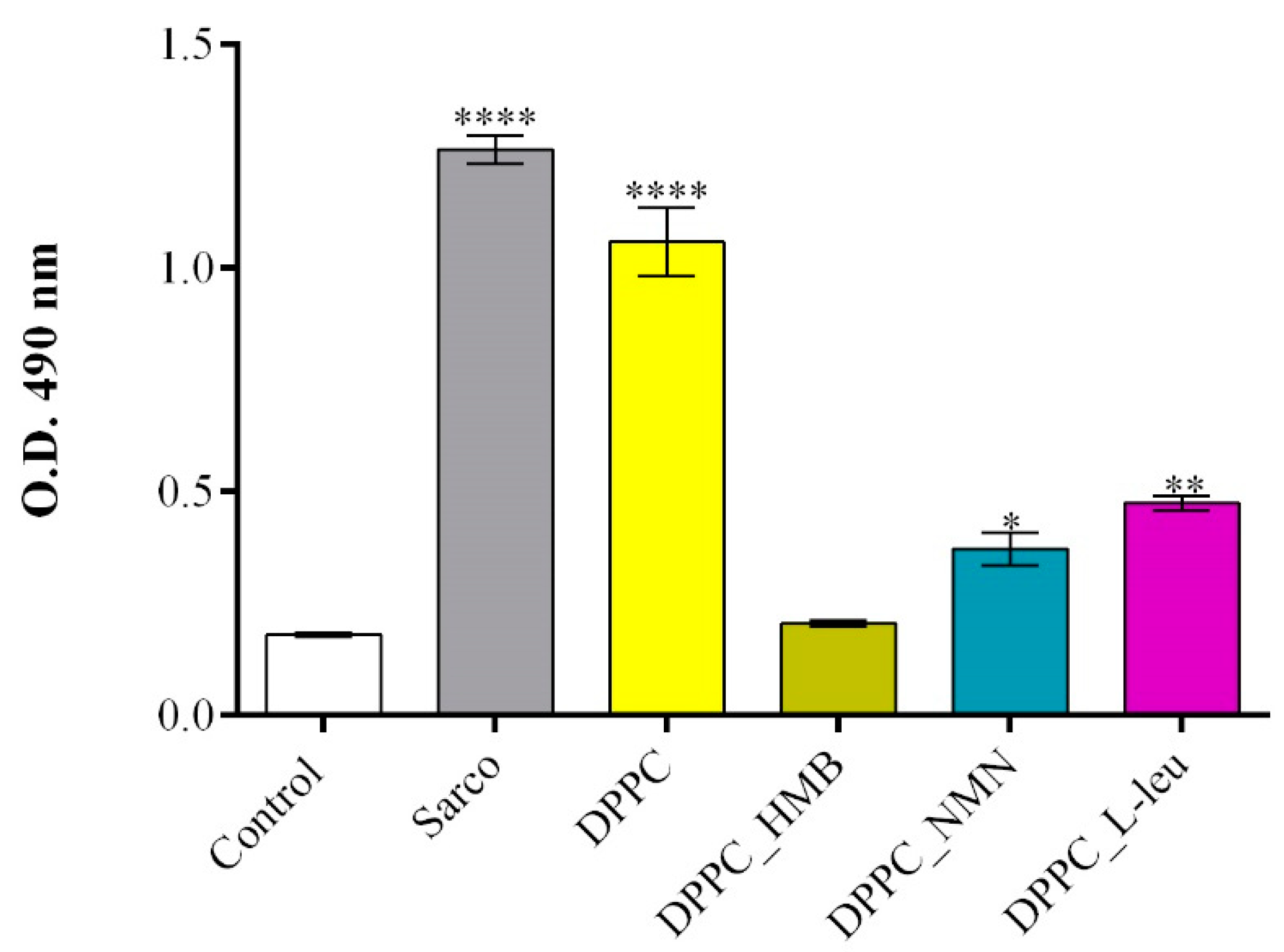
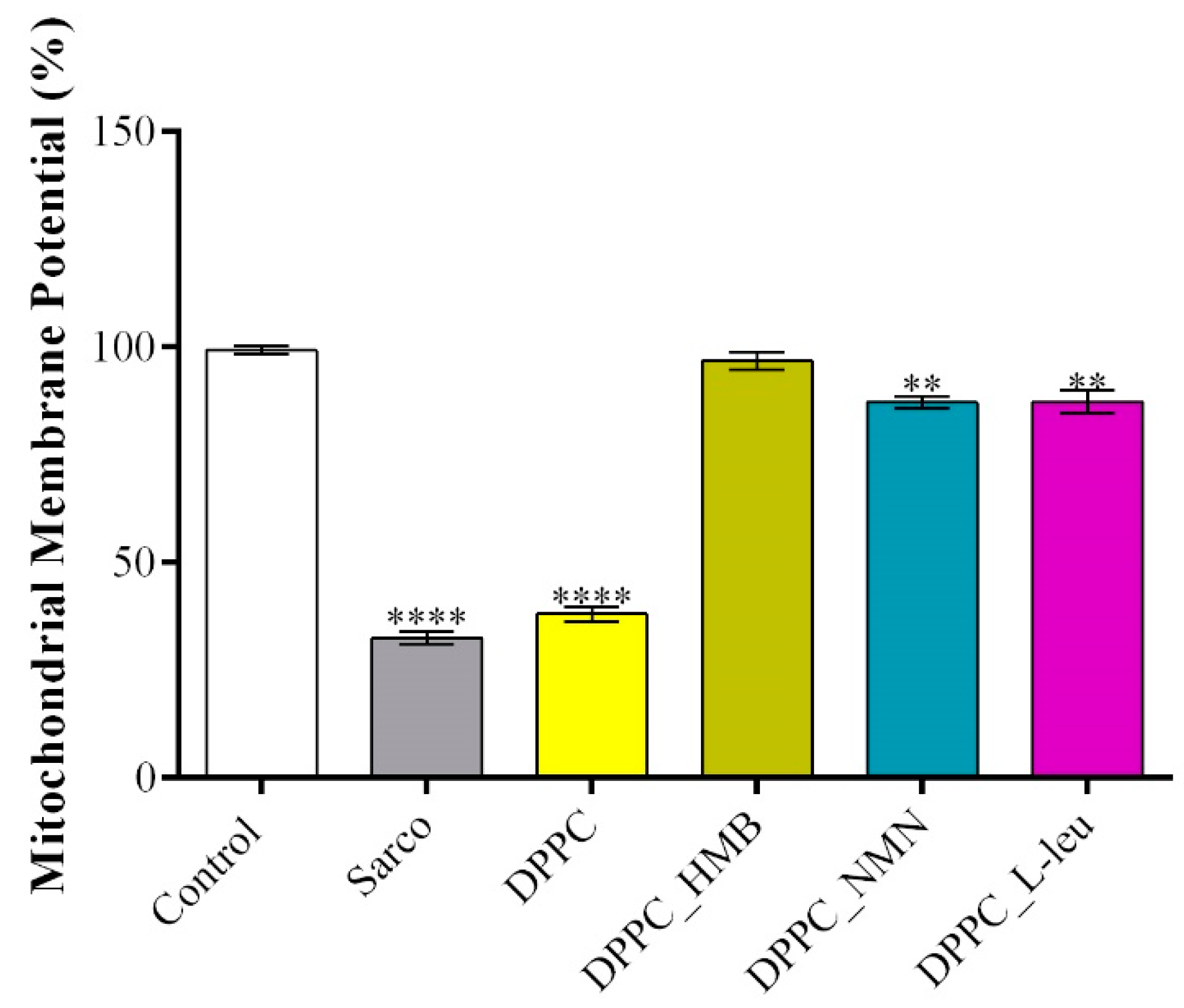
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of DPPC-Based Liposomal Vesicles and HMB, NMN, and L-Leucine Drug Encapsulation
4.2. Analysis Methods
4.2.1. Dynamic Light Scattering (DLS)
4.2.2. Scanning Electron Microscopy (SEM)
4.2.3. Transmission Electron Microscopy (TEM)
4.2.4. Encapsulation Efficiency (EE%)
4.2.5. Drug Release%
4.3. In Vitro Biological Evaluation of HMB, NMN and L-Leucine Drug-Loaded DPPC Lipid Vesicles
4.3.1. Cell Culture and Treatments
- Control group—Differentiation medium refreshed without additional treatment.
- Model group—Myotubes exposed to 100 μM H2O2 to induce a sarcopenia-like phenotype.
- DPPC group—Myotubes treated with 100 μM H2O2 + DPPC lipid vesicles.
- DPPC + HMB group—Myotubes treated with 100 μM H2O2 + DPPC lipid vesicles loaded with HMB.
- DPPC + NMN group—Myotubes treated with 100 μM H2O2 + DPPC lipid vesicles loaded with NMN.
- DPPC + L-Leucine group—Myotubes treated with 100 μM H2O2 + DPPC lipid vesicles loaded with L-Leucine.
4.3.2. Cell Viability Assessment
4.3.3. Cytotoxicity Assessment
4.3.4. Reactive Oxygen Species (ROS) Measurement
4.3.5. Nitric Oxide (NO) Assay
4.3.6. Mitochondrial Membrane Potential (MMP) Assessment
4.3.7. Cytoskeleton Investigation
4.3.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Haehling, S.; Morley, J.E.; Anker, S.D. From muscle wasting to sarcopenia and myopenia: Update 2012. J. Cachexia Sarcopenia Muscle 2012, 3, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Keller, K. Sarcopenia. Wien. Med. Wochenschr. 2019, 169, 157–172. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. La Radiol. Medica 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metab. -Clin. Exp. 2023, 144, 155533. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.-J.; Bae, G.-U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef]
- Hadjispyrou, S.; Giannopoulos, A.; Philippou, A.; Theos, A. Mitochondrial Dysfunction and Sarcopenic Obesity: The Role of Exercise. J. Clin. Med. 2023, 12, 5628. [Google Scholar] [CrossRef]
- Kim, M.-J.; Sinam, I.S.; Siddique, Z.; Jeon, J.-H.; Lee, I.-K. The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4. Diabetes Metab. J. 2023, 47, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Mitochondrial Impairment in Sarcopenia. Biology 2021, 10, 31. [Google Scholar] [CrossRef]
- Alway, S.E.; Mohamed, J.S.; Myers, M.J. Mitochondria Initiate and Regulate Sarcopenia. Exerc. Sport. Sci. Rev. 2017, 45, 58–69. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, W. NAD+: An old but promising therapeutic agent for skeletal muscle ageing. Ageing Res. Rev. 2023, 92, 102106. [Google Scholar] [CrossRef]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD +Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090.e5. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Y.-b.; Tao, X.-h.; Pan, W.-l.; Wu, Y.-x.; Wang, X.-h.; He, Y.-q.; Xiao, W.-f.; Li, Y.-s. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2022, 12, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Nakagawa, T. The therapeutic perspective of NAD+ precursors in age-related diseases. Biochem. Biophys. Res. Commun. 2024, 702, 149590. [Google Scholar] [CrossRef]
- Melouane, A.; Yoshioka, M.; St-Amand, J. Extracellular matrix/mitochondria pathway: A novel potential target for sarcopenia. Mitochondrion 2020, 50, 63–70. [Google Scholar] [CrossRef]
- Liu, D.; Sun, H.; Xiao, Y.; Chen, S.; Cornel, E.J.; Zhu, Y.; Du, J. Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes. J. Control. Release 2020, 326, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Ichikawa, S. Lipid Vesicles and Other Polymolecular Aggregates—From Basic Studies of Polar Lipids to Innovative Applications. Appl. Sci. 2021, 11, 10345. [Google Scholar] [CrossRef]
- Apolinário, A.C.; Hauschke, L.; Nunes, J.R.; Lopes, L.B. Lipid nanovesicles for biomedical applications: ‘What is in a name’? Prog. Lipid Res. 2021, 82, 101096. [Google Scholar] [CrossRef]
- de Oliveira, D.C.S.; de Freitas, C.F.; Calori, I.R.; Goncalves, R.S.; Cardinali, C.A.E.F.; Malacarne, L.C.; Ravanelli, M.I.; de Oliveira, H.P.M.; Tedesco, A.C.; Caetano, W.; et al. Theranostic verteporfin- loaded lipid-polymer liposome for photodynamic applications. J. Photochem. Photobiol. B Biol. 2020, 212, 112039. [Google Scholar] [CrossRef]
- Zumbuehl, A. Artificial Phospholipids and Their Vesicles. Langmuir 2019, 35, 10223–10232. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, Y.; Matalon, S.; Franses, E.I. Effect of buffer composition and preparation protocol on the dispersion stability and interfacial behavior of aqueous DPPC dispersions. Colloids Surf. B Biointerfaces 2008, 67, 253–260. [Google Scholar] [CrossRef]
- Pereira-Leite, C.; Lopes-de-Campos, D.; Fontaine, P.; Cuccovia, I.M.; Nunes, C.; Reis, S. Licofelone-DPPC Interactions: Putting Membrane Lipids on the Radar of Drug Development. Molecules 2019, 24, 516. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-hydroxy-β-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; D’Introno, A.; Rubele, S.; Caliari, C.; Gattazzo, S.; Zoico, E.; Mazzali, G.; Fantin, F.; Zamboni, M. The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging 2017, 34, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, Y.; Li, T.; Chen, X.; Zhou, J.; Pan, Q.; Jiang, W.; Wang, M.; Jia, H. Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Older Adults with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Nutr. Health Aging 2023, 27, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Cruz-Jentoft, A.J.; Stout, J.R. β-hydroxy-β-methylbutyrate supplementation in older persons—An update. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Igarashi, M.; Yamauchi, T. Insight into the application of nicotinamide mononucleotide (NMN) to age-related disorders. J. Cell. Mol. Immunol. 2023, 2, 9–13. [Google Scholar] [CrossRef]
- Liang, R.; Xiang, Q.; Dai, M.; Lin, T.; Xie, D.; Song, Q.; Liu, Y.; Yue, J. Identification of nicotinamide N-methyltransferase as a promising therapeutic target for sarcopenia. Aging Cell 2024, 23, e14236. [Google Scholar] [CrossRef]
- Dimet-Wiley, A.L.; Latham, C.M.; Brightwell, C.R.; Neelakantan, H.; Keeble, A.R.; Thomas, N.T.; Noehren, H.; Fry, C.S.; Watowich, S.J. Nicotinamide N-methyltransferase inhibition mimics and boosts exercise-mediated improvements in muscle function in aged mice. Sci. Rep. 2024, 14, 15554. [Google Scholar] [CrossRef]
- Ito, N.; Takatsu, A.; Ito, H.; Koike, Y.; Yoshioka, K.; Kamei, Y.; Imai, S.I. Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging. Cell Rep. 2022, 40, 111131. [Google Scholar] [CrossRef]
- Wu, W.; Yuan, S.; Tang, Y.; Meng, X.; Peng, M.; Hu, Z.; Liu, W. Effect of Exercise and Oral Niacinamide Mononucleotide on Improving Mitochondrial Autophagy in Alzheimer’s Disease. Nutrients 2023, 15, 2851. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Manickam, R.; Brotto, M.; Tipparaju, S.M. NAD(+) centric mechanisms and molecular determinants of skeletal muscle disease and aging. Mol. Cell. Biochem. 2022, 477, 1829–1848. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef] [PubMed]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef]
- Kim, M.; Isoda, H.; Okura, T. Effect of Citrulline and Leucine Intake with Exercises on Body Composition, Physical Activity, and Amino Acid Concentration in Older Women: A Randomized Double-Blind Placebo-Controlled Study. Foods 2021, 10, 3117. [Google Scholar] [CrossRef]
- Viana, L.R.; Chiocchetti, G.d.M.e.; Oroy, L.; Vieira, W.F.; Busanello, E.N.B.; Marques, A.C.; Salgado, C.d.M.; de Oliveira, A.L.R.; Vieira, A.S.; Suarez, P.S.; et al. Leucine-Rich Diet Improved Muscle Function in Cachectic Walker 256 Tumour-Bearing Wistar Rats. Cells 2021, 10, 3272. [Google Scholar] [CrossRef]
- Pandur, Ž.; Dogsa, I.; Dular, M.; Stopar, D. Liposome destruction by hydrodynamic cavitation in comparison to chemical, physical and mechanical treatments. Ultrason. Sonochemistry 2020, 61, 104826. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Garcia, A.; Zou, H.; Hossain, K.R.; Xu, Q.H.; Buda, A.; Clarke, R.J. Polar Interactions Play an Important Role in the Energetics of the Main Phase Transition of Phosphatidylcholine Membranes. ACS Omega 2019, 4, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Solid-State NMR Study of Drug Interaction with Phospholipid Bilayers Chlorpromazine, Olanzapine and Articaine Interacting with Saturated and Unsaturated Phosphatidylserines and Saturated Phosphatidylcholines; The University of Bergen: Bergen, Norway, 2007. [Google Scholar]
- Kumarage, T.; Morris, N.B.; Ashkar, R. The effects of molecular and nanoscopic additives on phospholipid membranes. Front. Phys. 2023, 11, 1251146. [Google Scholar] [CrossRef]
- Marinescu, G.C.; Popescu, R.G.; Dinischiotu, A. Size Exclusion Chromatography Method for Purification of Nicotinamide Mononucleotide (NMN) from Bacterial Cells. Sci. Rep. 2018, 8, 4433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; King, E.; Black, W.B.; Heckmann, C.M.; Wolder, A.; Cui, Y.; Nicklen, F.; Siegel, J.B.; Luo, R.; Paul, C.E.; et al. Directed evolution of phosphite dehydrogenase to cycle noncanonical redox cofactors via universal growth selection platform. Nat. Commun. 2022, 13, 5021. [Google Scholar] [CrossRef] [PubMed]
- Dua, J.; Rana, A.; Bhandari, A. Liposome: Methods of preparation and applications. Int. J. Pharm. Stud. Res. 2012, 3, 14–20. [Google Scholar]
- Ong, S.G.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Ong, S.G.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef]
- Buczek, A.; Kupka, T.; Broda, M.A.; Maślanka, S.; Pentak, D. Liposomes as nonspecific nanocarriers for 5-Fluorouracil in the presence of cyclodextrins. J. Mol. Liq. 2021, 343, 117623. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.; Kostarelos, K. Chemical Components for the Design of Temperature-Responsive Vesicles as Cancer Therapeutics. Chem. Rev. 2016, 116, 3883–3918. [Google Scholar] [CrossRef]
- McGorm, H.; Roberts, L.A.; Coombes, J.S.; Peake, J.M. Turning Up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports Med. 2018, 48, 1311–1328. [Google Scholar] [CrossRef]
- Zanoli, G.; Albarova-Corral, I.; Ancona, M.; Grattagliano, I.; Hotfiel, T.; Iolascon, G.; Krüger, K.; Rodríguez Maruri, G. Current Indications and Future Direction in Heat Therapy for Musculoskeletal Pain: A Narrative Review. Muscles 2024, 3, 212–223. [Google Scholar] [CrossRef]
- Behera, D.; Shetty, A. Effectiveness of heat and cold therapy in muscle spasm: A review. Int. J. Phys. Educ. Sports Health 2023, 10, 196–200. [Google Scholar]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer—Lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef]
- Paun, V.-A.; Popa, M.; Desbrieres, J.; Peptu, C.A.; Dragan, S.V.; Zegan, G.; Cioca, G. Liposome loaded chitosan hydrogels, a promising way to reduce the burst effect in drug releasea comparativ analysis. Mater. Plast. 2016, 53, 590–593. [Google Scholar]
- Guillot, A.J.; Jornet-Mollá, E.; Landsberg, N.; Milián-Guimerá, C.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Cyanocobalamin Ultraflexible Lipid Vesicles: Characterization and In Vitro Evaluation of Drug-Skin Depth Profiles. Pharmaceutics 2021, 13, 418. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, N.; He, L.; Xu, M.; Li, Y. 5′-Cytimidine Monophosphate Ameliorates H(2)O(2)-Induced Muscular Atrophy in C2C12 Myotubes by Activating IRS-1/Akt/S6K Pathway. Antioxidants 2024, 13, 249. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
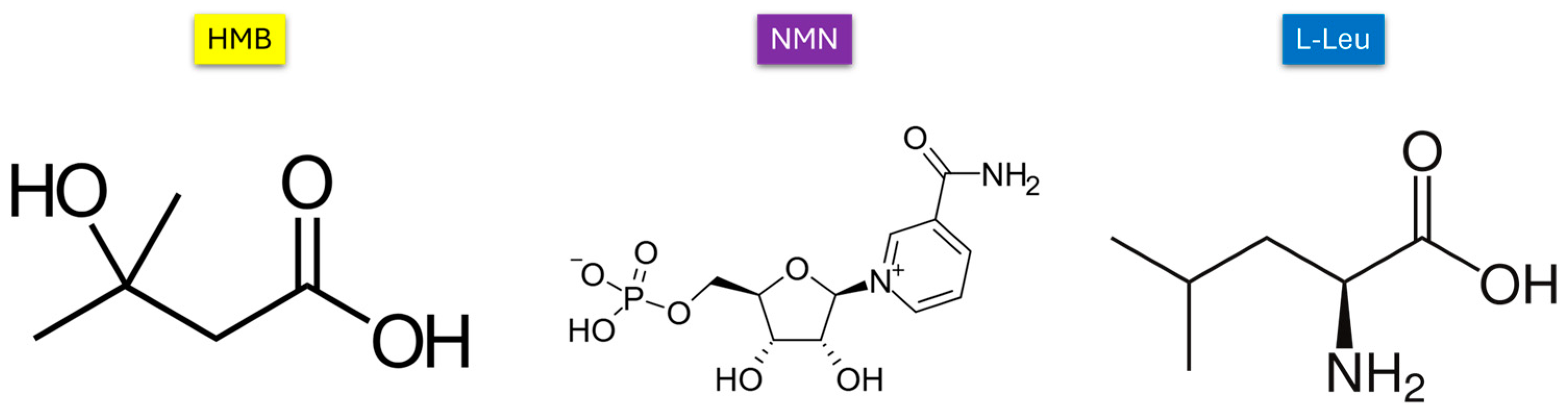
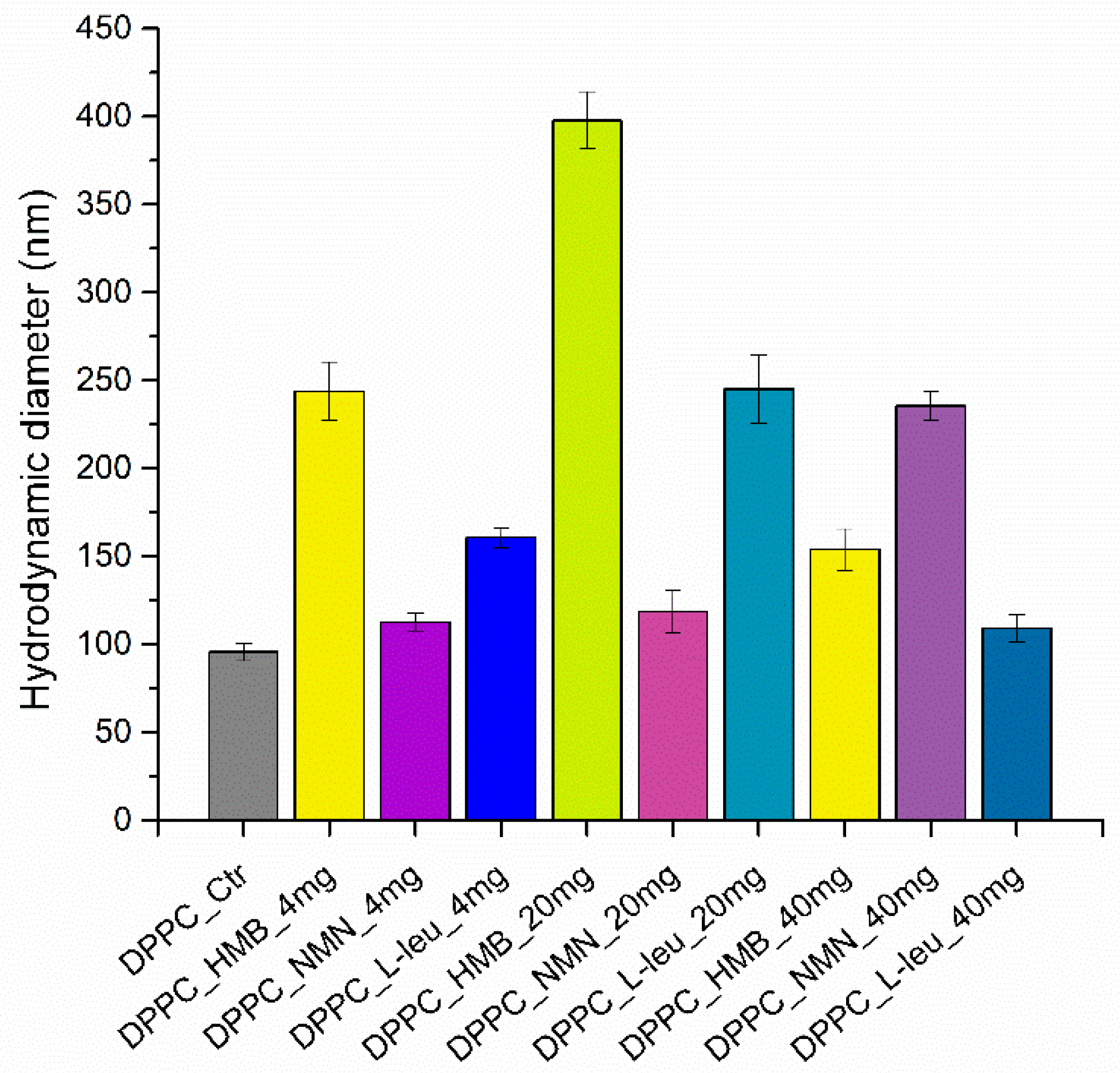
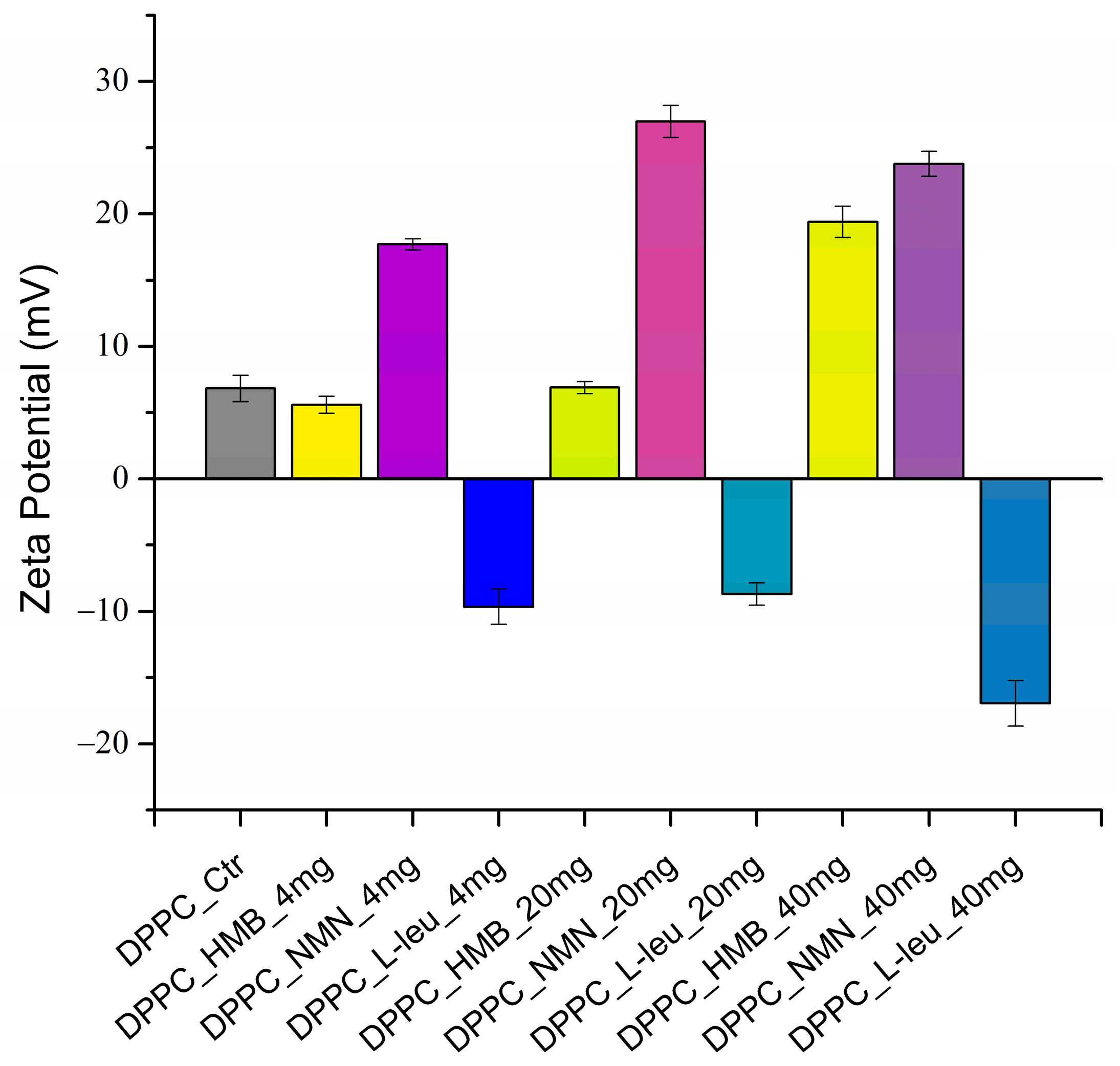
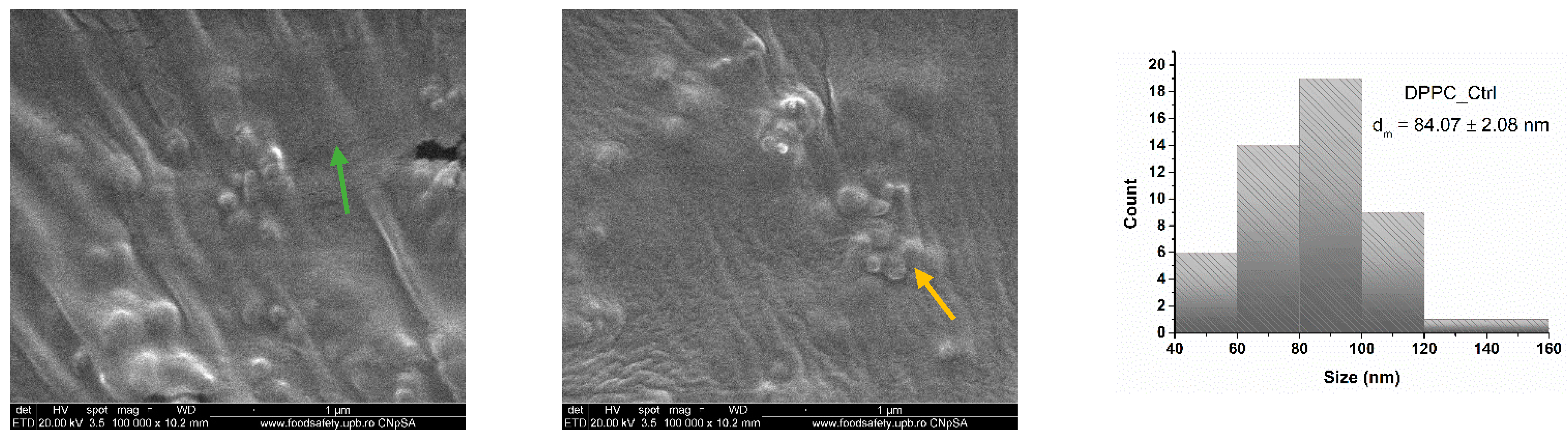





| Hydrodynamic Diameter (nm) | |||
|---|---|---|---|
| 4 mg | 20 mg | 40 mg | |
| DPPC_HMB | 243.76 ± 16.65 | 397.80 ± 16.30 | 153.80 ± 11.72 |
| DPPC_NMN | 112.46 ± 4.92 | 118.56 ± 12.20 | 235.60 ± 8.22 |
| DPPC_L-Leucine | 160.60 ± 5.6 | 245.16 ± 19.15 | 109.13 ± 7.74 |
| Zeta Potential (mV) | |||
|---|---|---|---|
| 4 mg | 20 mg | 40 mg | |
| DPPC_HMB | 5.59 ± 0.63 | 6.90 ± 0.45 | 19.39 ± 1.17 |
| DPPC_NMN | 17.70 ± 0.43 | 26.99 ± 1.21 | 23.78 ± 0.94 |
| DPPC_L-Leucine | −9.66 ± 1.33 | −8.69 ± 0.84 | −16.94 ± 1.71 |
| PDI | ||||||
|---|---|---|---|---|---|---|
| 4 mg | PDI Range | 20 mg | PDI Range | 40 mg | PDI Range | |
| DPPC_HMB | 0.081 | <0.1 (Monodisperse) | 0.011 | <0.1 (Monodisperse) | 0.081 | <0.1 (Monodisperse) |
| DPPC_NMN | 0.137 | 0.1–0.3 (Moderate) | 0.102 | 0.1–0.3 (Moderate) | 0.066 | <0.1 (Monodisperse) |
| DPPC_L-Leucine | 0.082 | <0.1 (Monodisperse) | 0.081 | <0.1 (Monodisperse) | 0.097 | <0.1 (Monodisperse) |
| Synthesis Series | Follow the Process | ||||
|---|---|---|---|---|---|
| DPPC stock solution | 40 mL of H2O for hydrating the DPPC dried lipid film |  | |||
| Drug aqueous solution | 20 mL of H2O | 4 mg/20 mg/40 mg of HMB in 20 mL of H2O | 4 mg/20 mg/40 mg of NMN in 20 mL of H2O | 4 mg/20 mg/40 mg of L-Leu in 20 mL of H2O |  |
| Mix of DPPC with drugs | 10 mL DPPC solution + 20 mL of H2O | 10 mL DPPC solution + HMB solution | 10 mL DPPC solution + NMN solution | 10 mL DPPC solution + L-Leucine solution |  |
| Sample code | DPPC_Ctrl | DPPC_HMB_4mg/20mg/40mg | DPPC_NMN_4mg/20mg/40mg | DPPC_L-Leucine_4mg/20mg/40mg | Finish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najm, A.; Bîrcă, A.C.; Niculescu, A.-G.; Alberts, A.; Grumezescu, A.M.; Gălățeanu, B.; Vasile, B.Ș.; Beuran, M.; Gaspar, B.S.; Hudiță, A. Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy. Molecules 2025, 30, 1437. https://doi.org/10.3390/molecules30071437
Najm A, Bîrcă AC, Niculescu A-G, Alberts A, Grumezescu AM, Gălățeanu B, Vasile BȘ, Beuran M, Gaspar BS, Hudiță A. Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy. Molecules. 2025; 30(7):1437. https://doi.org/10.3390/molecules30071437
Chicago/Turabian StyleNajm, Alfred, Alexandra Cătălina Bîrcă, Adelina-Gabriela Niculescu, Adina Alberts, Alexandru Mihai Grumezescu, Bianca Gălățeanu, Bogdan Ștefan Vasile, Mircea Beuran, Bogdan Severus Gaspar, and Ariana Hudiță. 2025. "Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy" Molecules 30, no. 7: 1437. https://doi.org/10.3390/molecules30071437
APA StyleNajm, A., Bîrcă, A. C., Niculescu, A.-G., Alberts, A., Grumezescu, A. M., Gălățeanu, B., Vasile, B. Ș., Beuran, M., Gaspar, B. S., & Hudiță, A. (2025). Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy. Molecules, 30(7), 1437. https://doi.org/10.3390/molecules30071437











