Structural Rearrangement in Cyclic Cu(II) Pyridyltriazole Complexes: Oxidation of Dabco to Oxalate and CO2 Conversion to Carbonate
Abstract
1. Introduction
2. Results and Discussion
2.1. Interactions of Supramolecular Complexes with Dabco in Air
2.2. Spectroscopic Monitoring of the Interaction Between Supramolecules and Dabco
2.3. Role of Water in Carbonate/Oxalate Formation
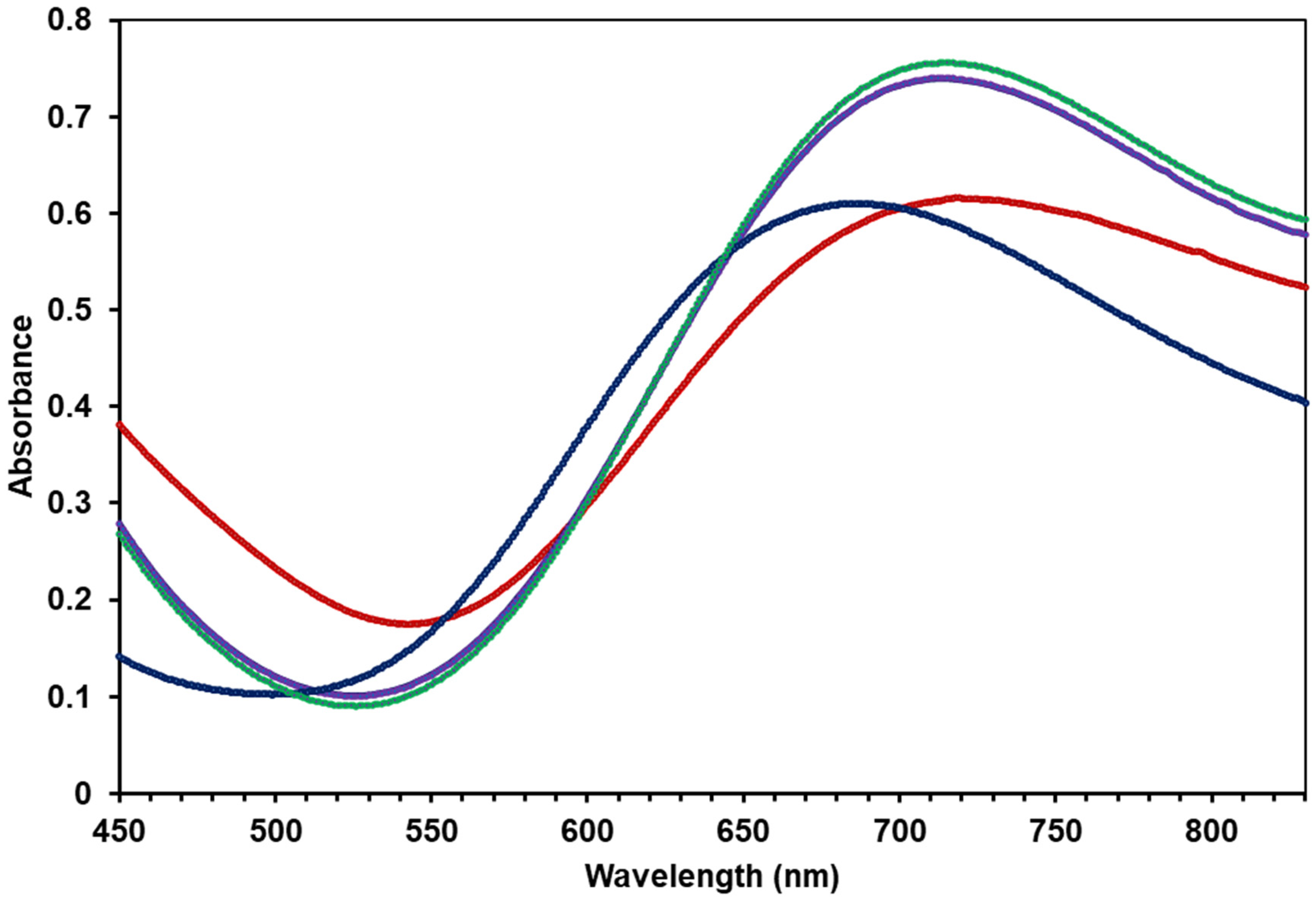
2.4. Spectroscopic Characterization
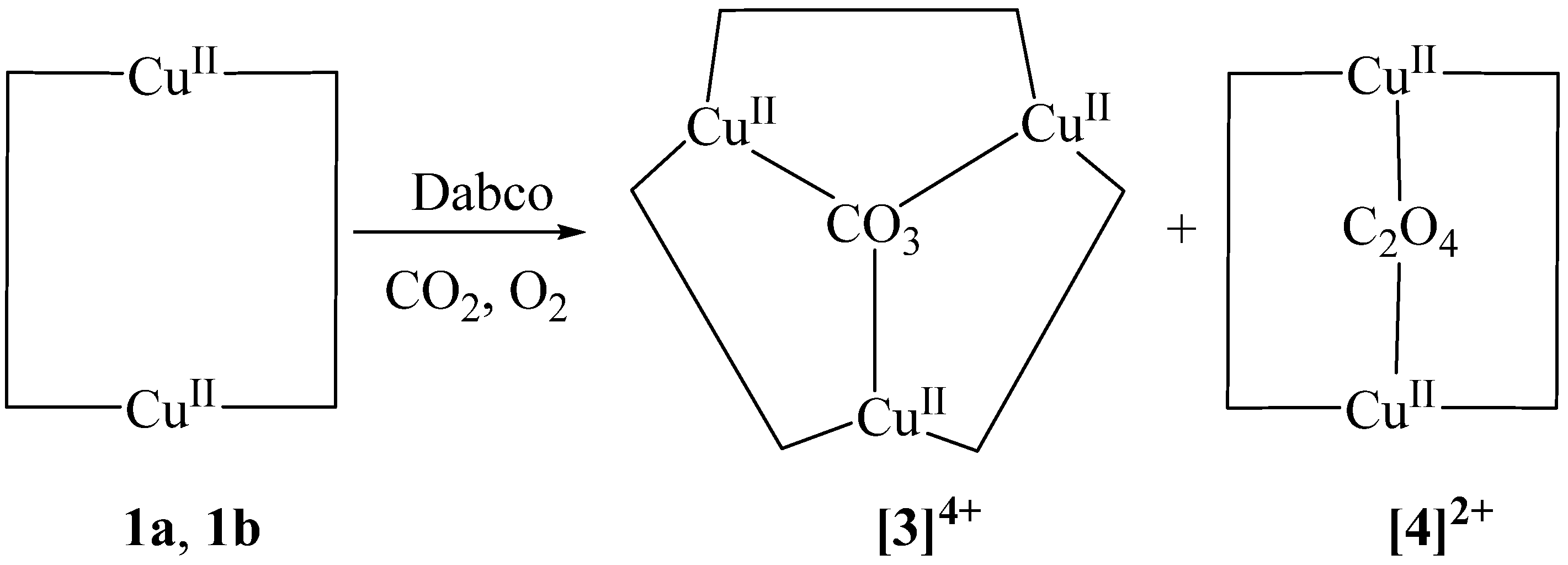
2.5. Sources of Carbonate and Oxalate
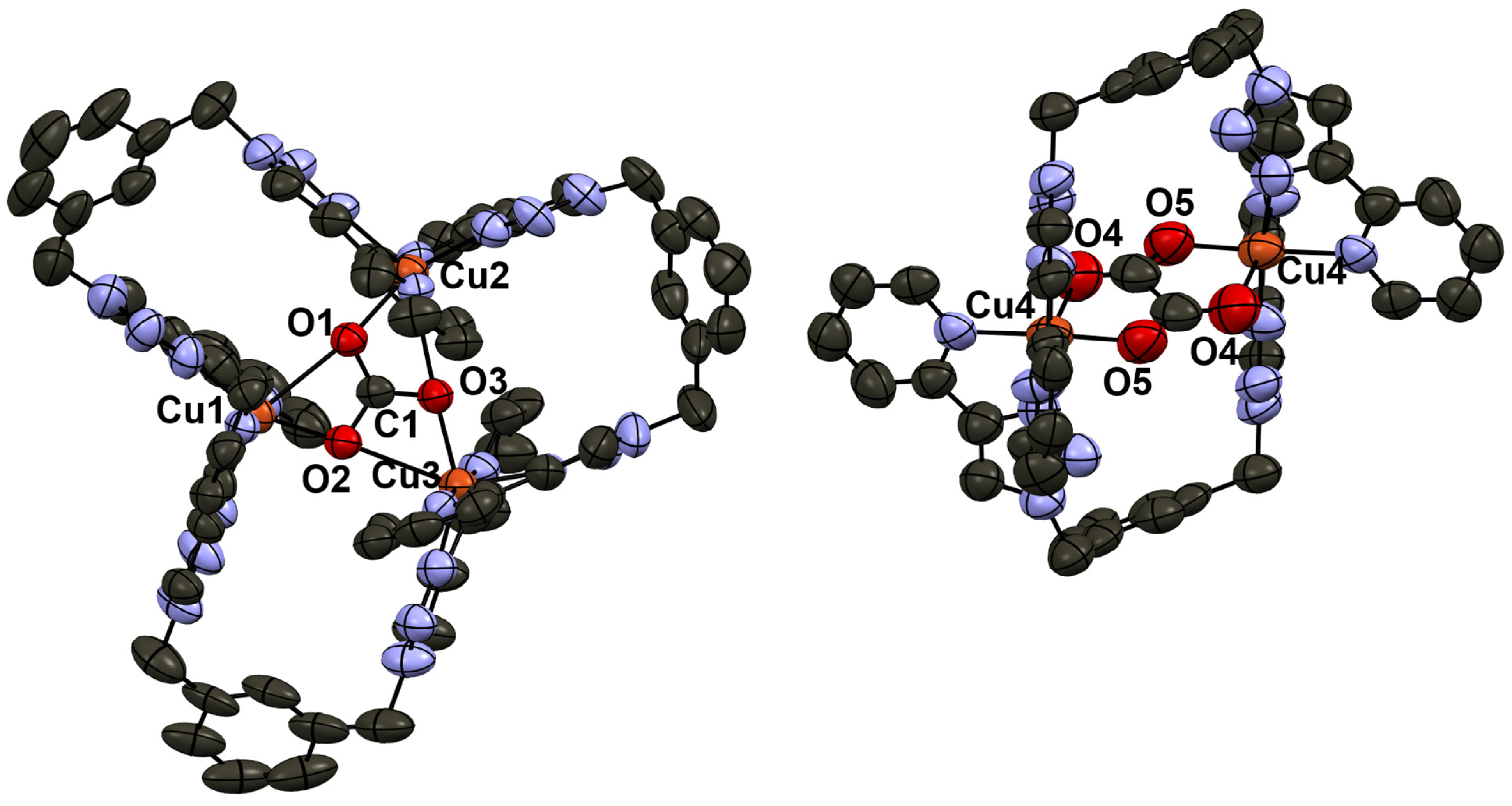
2.6. Release of Carbonate Ion from Complex [3]4+
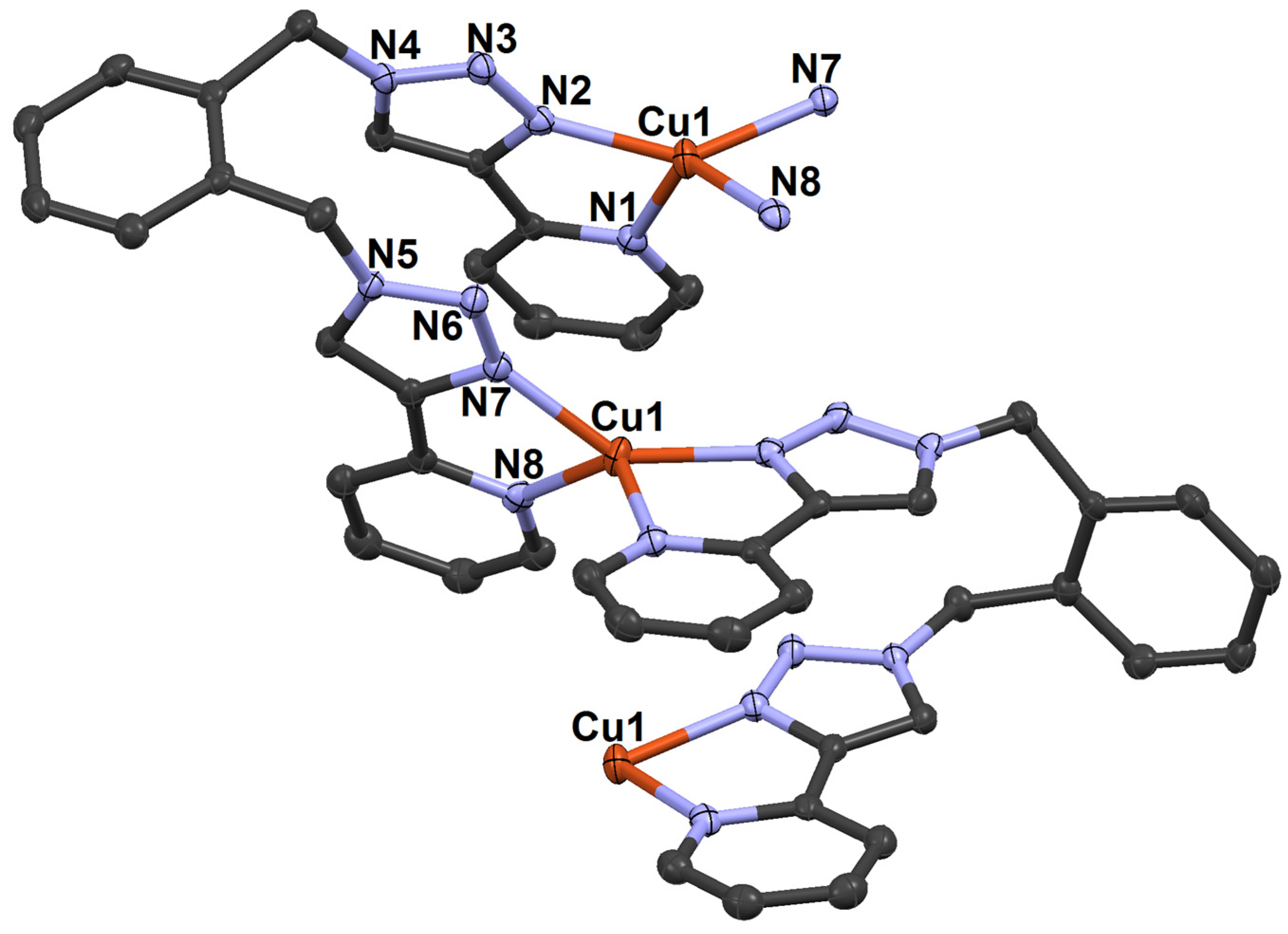
2.7. Synthesis of Cu(I) Complex of ortho-Xylylenebis(Pyridyltriazole) (o-xpt) and Its Reactivity with CO2-Enriched Air
3. Materials and Methods
3.1. General Procedures
3.2. Experimental Procedures
3.3. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 611–661. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Zhang, L.; Dong, L.-Z.; Wang, X.-H.; Li, R.-H.; Guo, C.; Li, X.; Yan, Y.; Li, S.-L.; Lan, Y.-Q. Bridging the Homogeneous and Heterogeneous Catalysis by Supramolecular Metal-Organic Cages with Varied Packing Modes. Adv. Mater. 2024, 36, 2310061. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Clever, G.H. Catalysis in confined space: Relationship between metal-organic frameworks and discrete coordination cages. Monogr. Supramol. Chem. 2021, 31, 247–281. [Google Scholar] [CrossRef]
- Tong, H.-Y.; Liang, J.; Wu, Q.-J.; Zou, Y.-H.; Huang, Y.-B.; Cao, R. Soluble imidazolium-functionalized coordination cages for efficient homogeneous catalysis of CO2 cycloaddition reactions. Chem. Commun. 2021, 57, 2140–2143. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220. [Google Scholar] [CrossRef]
- Ngai, C.; da Camara, B.; Woods, C.Z.; Hooley, R.J. Size- and Shape-Selective Catalysis with a Functionalized Self-Assembled Cage Host. J. Org. Chem. 2021, 86, 12862–12871. [Google Scholar] [CrossRef]
- Chang, X.; Lin, S.; Wang, G.; Shang, C.; Wang, Z.; Liu, K.; Fang, Y.; Stang, P.J. Self-Assembled Perylene Bisimide-Cored Trigonal Prism as an Electron-Deficient Host for C60 and C70 Driven by “Like Dissolves Like”. J. Am. Chem. Soc. 2020, 142, 15950–15960. [Google Scholar] [CrossRef]
- Fan, W.; Peh, S.B.; Zhang, Z.; Yuan, H.; Yang, Z.; Wang, Y.; Chai, K.; Sun, D.; Zhao, D. Tetrazole-Functionalized Zirconium Metal-Organic Cages for Efficient C2H2/C2H4 and C2H2/CO2 Separations. Angew. Chem. Int. Ed. 2021, 60, 17338–17343. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Su, J.; Wu, L.-X.; Luo, D.; Wang, X.-Z.; Zhou, X.-C.; Zhou, C.-W.; Zhou, X.-P.; Li, D. Selective separation of pyrene from mixed polycyclic aromatic hydrocarbons by a hexahedral metal-organic cage. Chin. Chem. Lett. 2024, 35, 108326. [Google Scholar] [CrossRef]
- Nguyen, B.-N.T.; Thoburn, J.D.; Grommet, A.B.; Howe, D.J.; Ronson, T.K.; Ryan, H.P.; Bolliger, J.L.; Nitschke, J.R. Coordination cages selectively transport molecular cargoes across liquid membranes. J. Am. Chem. Soc. 2021, 143, 12175–12180. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Zou, Y.-Q.; Nitschke, J.R. Metal-organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Imran Anwar, M.; Abbas, A.; Younas, A.; Hussain, S.; Gao, R.; Li, L.-K.; Shahid, M.; Khan, S. AIE based luminescent porous materials as cutting-edge tool for environmental monitoring: State of the art advances and perspectives. Coord. Chem. Rev. 2022, 463, 214539. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J.E. Supramolecular Coordination Complexes as Optical Biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yang, F.; Wang, X.; Shan, W.-L.; Liu, D.; Zhang, L.; Yuan, G. Trefoil-Shaped Metal-Organic Cages as Fluorescent Chemosensors for Multiple Detection of Fe3+, Cr2O72−, and Antibiotics. Inorg. Chem. 2023, 62, 1297–1305. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Pan, M.; Su, C.-Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2019, 59, 209–219. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Cyclic pyridyltriazole–Cu (II) dimers as supramolecular hosts. Dalton Trans. 2013, 42, 14064–14067. [Google Scholar] [CrossRef]
- Wood, D.M.; Meng, W.; Ronson, T.K.; Stefankiewicz, A.R.; Sanders, J.K.M.; Nitschke, J.R. Guest-Induced Transformation of a Porphyrin-Edged FeII4L6 Capsule into a CuIFeII2L4 Fullerene Receptor. Angew. Chem. Int. Ed. 2015, 54, 3988–3992. [Google Scholar] [CrossRef]
- Cherutoi, J.K.; Sandifer, J.D.; Pokharel, U.R.; Fronczek, F.R.; Pakhomova, S.; Maverick, A.W. Externally and Internally Functionalized Copper(II) β-Diketonate Molecular Squares. Inorg. Chem. 2015, 54, 7791–7802. [Google Scholar] [CrossRef] [PubMed]
- Turega, S.; Whitehead, M.; Hall, B.R.; Meijer, A.J.H.M.; Hunter, C.A.; Ward, M.D. Shape-, Size-, and Functional Group-Selective Binding of Small Organic Guests in a Paramagnetic Coordination Cage. Inorg. Chem. 2013, 52, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Regeni, I.; Holstein, J.J.; Clever, G.H. Guest-Induced Reversible Transformation between an Azulene-Based Pd2L4 Lantern-Shaped Cage and a Pd4L8 Tetrahedron. J. Am. Chem. Soc. 2023, 145, 25365–25371. [Google Scholar] [CrossRef]
- Percástegui, E.G. Guest-Induced Transformations in Metal-Organic Cages. Eur. J. Inorg. Chem. 2021, 2021, 4425–4438. [Google Scholar] [CrossRef]
- Bravin, C.; Badetti, E.; Scaramuzzo, F.A.; Licini, G.; Zonta, C. Triggering Assembly and Disassembly of a Supramolecular Cage. J. Am. Chem. Soc. 2017, 139, 6456–6460. [Google Scholar] [CrossRef]
- Begato, F.; Licini, G.; Zonta, C. Programmed guest confinement via hierarchical cage to cage transformations. Chem. Sci. 2023, 14, 8147–8151. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mitra, A.K. Versatility of DABCO as a Reagent in Organic Synthesis: A Review. Org. Biomol. Chem. 2023, 21, 6830–6880. [Google Scholar] [CrossRef]
- Kupietz, K.; Trouvé, J.; Roisnel, T.; Kahlal, S.; Gramage-Doria, R. A Highly Sterically Congested Bis-Zinc-Porphyrin Containing a Single Buta-1,3-diyne Linkage: From a Serendipitous Finding to Supramolecular Encapsulation. Eur. J. Org. Chem. 2023, 26, e202300621. [Google Scholar] [CrossRef]
- Samanta, S.K.; Samanta, D.; Bats, J.W.; Schmittel, M. DABCO as a Dynamic Hinge between Cofacial Porphyrin Panels and Its Tumbling inside a Supramolecular Cavity. J. Org. Chem. 2011, 76, 7466–7473. [Google Scholar] [CrossRef]
- Maverick, A.W.; Buckingham, S.C.; Yao, Q.; Bradbury, J.R.; Stanley, G.G. Intramolecular coordination of bidentate Lewis bases to a cofacial binuclear copper(II) complex. J. Am. Chem. Soc. 1986, 108, 7430–7431. [Google Scholar] [CrossRef]
- Fasano, F.; Bolgar, P.; Iadevaia, G.; Hunter, C.A. Supramolecular template-directed synthesis of triazole oligomers. Chem. Sci. 2022, 13, 13085–13093. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W.; Kuil, M.; Lutz, M.; Tooke, D.M.; Spek, A.L.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Supramolecular zinc(II) salphen motifs: Reversible dimerization and templated dimeric structures. Inorg. Chim. Acta 2006, 359, 1807–1814. [Google Scholar] [CrossRef]
- Knope, K.E.; Cahill, C.L. Hydrothermal Synthesis of a Novel Uranium Oxalate/Glycolate via In-Situ Ligand Formation. Inorg. Chem. 2007, 46, 6607–6612. [Google Scholar] [CrossRef]
- Pokharel, U.R.; Maverick, A.W.; Fronczek, F.R. Fronczek CCDC 987918: Experimental Crystal Structure Determination. 2014. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/cc1250bl&sid=DataCite (accessed on 17 March 2025). [CrossRef]
- Mao, Z.-W.; Liehr, G.; van Eldik, R. Structural and mechanistic information on the reaction of bicarbonate with Cu(II) and Zn(II) complexes of tris(2-aminoethyl)amine. Identification of intermediate and product species. J. Chem. Soc. Dalton Trans. 2001, 1593–1600. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Cowie, M. Unusual mixed-metal carbonate-bridged complexes via oxidation of a carbonyl ligand in [RhM(CO)4(Ph2PCH2PPh2)2] (M = Mn, Re) and [IrRe(CO)5(Ph2PCH2PPh2)2]. Organometallics 1991, 10, 2173–2177. [Google Scholar] [CrossRef]
- Sadique, A.R.; Brennessel, W.W.; Holland, P.L. Reduction of CO2 to CO using low-coordinate iron: Formation of a four-coordinate iron dicarbonyl complex and a bridging carbonate complex. Inorg. Chem. 2008, 47, 784–786. [Google Scholar] [CrossRef]
- Lozano, A.A.; Sáez, M.; Pérez, J.; García, L.; Lezama, L.; Rojo, T.; López, G.; García, G.; Santana, M.D. Structure and magnetic properties of carbonate-bridged five-coordinate nickel(II) complexes controlled by solvent effect. Dalton Trans. 2006, 3906–3911. [Google Scholar] [CrossRef]
- Sakamoto, S.; Yamauchi, S.; Hagiwara, H.; Matsumoto, N.; Sunatsuki, Y.; Re, N. Carbonate-bridged tetranuclear NiII2GdIII2 complex generated by atmospheric CO2 fixation. Inorg. Chem. Commun. 2012, 26, 20–23. [Google Scholar] [CrossRef]
- Sołtys-Brzostek, K.; Terlecki, M.; Sokołowski, K.; Lewiński, J. Chemical fixation and conversion of CO2 into cyclic and cage-type metal carbonates. Coord. Chem. Rev. 2017, 334, 199–231. [Google Scholar] [CrossRef]
- Dussart, Y.; Harding, C.; Dalgaard, P.; McKenzie, C.; Kadirvelraj, R.; McKee, V.; Nelson, J. Cascade chemistry in azacryptand cages: Bridging carbonates and methylcarbonates. J. Chem. Soc. Dalton Trans. 2002, 1704–1713. [Google Scholar] [CrossRef]
- Fondo, M.; García-Deibe, A.M.; Bermejo, M.R.; Sanmartín, J.; Llamas-Saiz, A.L. Spontaneous carbon dioxide fixation: A µ4-carbonate bridged tetranuclear zinc(II) complex of a heptadentate Schiff base. J. Chem. Soc. Dalton Trans. 2002, 4746–4750. [Google Scholar] [CrossRef]
- Wikstrom, J.P.; Filatov, A.S.; Mikhalyova, E.A.; Shatruk, M.; Foxman, B.M.; Rybak-Akimova, E.V. Carbonate formation within a nickel dimer: Synthesis of a coordinatively unsaturated bis(μ-hydroxo)dinickel complex and its reactivity toward carbon dioxide. Dalton Trans. 2010, 39, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Drew, M.G.; Estrader, M.; Ghosh, A. Coordination-driven self-assembly of a novel carbonato-bridged heteromolecular neutral nickel (II) triangle by atmospheric CO2 fixation. Inorg. Chem. 2008, 47, 7784–7791. [Google Scholar] [CrossRef]
- Escuer, A.; Vicente, R.; Kumar, S.B.; Solans, X.; Font-Bardia, M.; Caneschi, A. A Novel Pentadentate Coordination Mode for the Carbonato Bridge: Synthesis, Crystal Structure, and Magnetic Behavior of (μ3-CO3)[Ni3(Medpt)3(NCS)4], a New Trinuclear Nickel(II) Carbonato-Bridged Complex with Strong Antiferromagnetic Coupling. Inorg. Chem. 1996, 35, 3094. [Google Scholar] [CrossRef]
- Evans, W.J.; Seibel, C.A.; Ziller, J.W. Organosamarium-mediated transformations of CO2 and COS: Monoinsertion and disproportionation reactions and the reductive coupling of CO2 to [O2CCO2]2−. Inorg. Chem. 1998, 37, 770–776. [Google Scholar] [CrossRef]
- Evans, W.J.; Lorenz, S.E.; Ziller, J.W. Investigating Metal Size Effects in the Ln2(μ-η2: η2-N2) Reduction System: Reductive Reactivity with Complexes of the Largest and Smallest Trivalent Lanthanide Ions, La3+ and Lu3+. Inorg. Chem. 2009, 48, 2001–2009. [Google Scholar] [CrossRef]
- Tanaka, K.; Kushi, Y.; Tsuge, K.; Toyohara, K.; Nishioka, T.; Isobe, K. Catalytic generation of oxalate through a coupling reaction of two CO2 molecules activated on [(Ir(η5-C5Me5)2)(Ir(η4-C5Me5)CH2CN)(μ3-S)2]. Inorg. Chem. 1998, 37, 120–126. [Google Scholar] [CrossRef]
- Stibrany, R.T.; Schugar, H.J.; Potenza, J.A. A copper(II)-oxalate compound resulting from the fixation of carbon dioxide: μ-oxalato-bis[bis(1-benzyl-1H-pyrazole)(trifluoromethanesulfonato)copper(II)]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2005, 61, M1904–M1906. [Google Scholar] [CrossRef]
- Farrugia, L.J.; Lopinski, S.; Lovatt, P.A.; Peacock, R.D. Fixing carbon dioxide with copper: Crystal structure of [LCu(μ-C2O4)CuL][Ph4B]2 (L = N,N′,N′′-triallyl-1,4,7-triazacyclononane). Inorg. Chem. 2001, 40, 558–559. [Google Scholar] [CrossRef]
- Wong, W.K.; Zhang, L.L.; Xue, F.; Mak, C.W. Synthesis and X-ray crystal structure of an unexpected neutral oxalate-bridged ytterbium(III) porphyrinate dimer. J. Chem. Soc. Dalton Trans. 2000, 2245–2246. [Google Scholar] [CrossRef]
- Khamespanah, F.; Marx, M.; Crochet, D.B.; Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W.; Beller, M. Oxalate production via oxidation of ascorbate rather than reduction of carbon dioxide. Nat. Commun. 2021, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; Bandeen, P.H. A multicomponent CuAAC “click” approach to a library of hybrid polydentate 2-pyridyl-1,2,3-triazole ligands: New building blocks for the generation of metallosupramolecular architectures. Dalton Trans. 2010, 39, 612–623. [Google Scholar] [CrossRef] [PubMed]
- McCarney, E.P.; Hawes, C.S.; Blasco, S.; Gunnlaugsson, T. Synthesis and structural studies of 1,4-di(2-pyridyl)-1,2,3-triazole dpt and its transition metal complexes; a versatile and subtly unsymmetric ligand. Dalton Trans. 2016, 45, 10209–10221. [Google Scholar] [CrossRef] [PubMed]
- Fleischel, O.; Wu, N.; Petitjean, A. Click-triazole: Coordination of 2-(1,2,3-triazol-4-yl)-pyridine to cations of traditional tetrahedral geometry (Cu(I), Ag(I)). Chem. Commun. 2010, 46, 8454–8456. [Google Scholar] [CrossRef]
- Findlay, J.A.; McAdam, C.J.; Sutton, J.J.; Preston, D.; Gordon, K.C.; Crowley, J.D. Metallosupramolecular Architectures Formed with Ferrocene-Linked Bis-Bidentate Ligands: Synthesis, Structures, and Electrochemical Studies. Inorg. Chem. 2018, 57, 3602–3614. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A Found. Adv. 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]




| Compound | [3](PF6)4 | [3]2[4](PF6)10 |
|---|---|---|
| Chemical formula | C67H54Cu3N24O3(PF6)4·8CH3CN | 2[C67H54Cu3N24O3(PF6)4]·C46H36Cu2N16O4(PF6)2·13.2C3H7NO |
| Mr | 2342.27 | 6286.48 |
| Deposition number | 2422201 | 2422202 |
| Crystal system, space group | Monoclinic, C2/c | Triclinic, P |
| Temperature (K) | 100.0 (5) | 90.0 (5) |
| a, b, c (Å) | 24.980 (2), 16.581 (2), 25.075 (2) | 14.0459 (12), 14.8950 (15), 31.686 (3) |
| α (deg) | 90 | 93.007 (7) |
| β (deg) | 108.560 (8) | 101.480 (6) |
| γ (deg) | 90 | 102.415 (6) |
| V (Å3) | 9845.9 (17) | 6313.1 (10) |
| Z | 4 | 1 |
| Radiation type | Mo Kα | Cu Kα |
| µ (mm−1) | 0.82 | 2.42 |
| Crystal size (mm) | 0.13 × 0.11 × 0.09 | 0.19 × 0.08 × 0.04 |
| Tmin, Tmax | 0.901, 0.930 | 0.656, 0.909 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 34,413, 10,126, 6908 | 46,744, 21,356, 7056 |
| Rint | 0.074 | 0.109 |
| (sin θ/λ)max (Å−1) | 0.628 | 0.588 |
| R[I > 2σ(I)], wR(F2), S | 0.047, 0.124, 0.98 | 0.085, 0.238, 0.79 |
| No. of parameters | 579 | 1495 |
| No. of restraints | 0 | 526 |
| ∆ρmax, ∆ρmin (e Å−3) | 0.42, −0.42 | 0.55, −0.42 |
| Compound | 5 | 6 |
|---|---|---|
| Chemical formula | C22H18CuN8(PF6)·2(C3H7NO) | C69H58Cu4N24O6(PF6)4·0.5C3H7NO·0.5C4H10O·CH3CN·0.48H2O |
| Mr | 749.15 | 2276.74 |
| Deposition number | 2422203 | 2422204 |
| Crystal system, space group | Monoclinic, P21/c | Triclinic, P |
| Temperature (K) | 100.0 (5) | 90.0 (5) |
| a, b, c (Å) | 17.7197 (17), 7.9426 (8), 24.116 (3) | 19.4804 (10), 20.2673 (10), 22.6368 (11) |
| α (deg) | 90 | 87.796 (2) |
| β (deg) | 108.252 (5) | 88.026 (2) |
| γ (deg) | 90 | 88.919 (2) |
| V (Å3) | 3223.4 (6) | 8924.1 (8) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Cu Kα |
| µ (mm−1) | 0.81 | 2.81 |
| Crystal size (mm) | 0.42 × 0.16 × 0.07 | 0.18 × 0.15 × 0.07 |
| Tmin, Tmax | 0.728, 0.946 | 0.622, 0.827 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 44,222, 6547, 5476 | 134,009, 25,452, 16,636 |
| Rint | 0.038 | 0.206 |
| (sin θ/λ)max (Å−1) | 0.626 | 0.556 |
| R[I > 2σ(I)], wR(F2), S | 0.033, 0.085, 1.03 | 0.089, 0.252, 1.02 |
| No. of parameters | 474 | 2524 |
| No. of restraints | 45 | 27 |
| ∆ρmax, ∆ρmin (e Å−3) | 0.34, −0.33 | 1.31, −0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Structural Rearrangement in Cyclic Cu(II) Pyridyltriazole Complexes: Oxidation of Dabco to Oxalate and CO2 Conversion to Carbonate. Molecules 2025, 30, 1430. https://doi.org/10.3390/molecules30071430
Pokharel UR, Fronczek FR, Maverick AW. Structural Rearrangement in Cyclic Cu(II) Pyridyltriazole Complexes: Oxidation of Dabco to Oxalate and CO2 Conversion to Carbonate. Molecules. 2025; 30(7):1430. https://doi.org/10.3390/molecules30071430
Chicago/Turabian StylePokharel, Uttam R., Frank R. Fronczek, and Andrew W. Maverick. 2025. "Structural Rearrangement in Cyclic Cu(II) Pyridyltriazole Complexes: Oxidation of Dabco to Oxalate and CO2 Conversion to Carbonate" Molecules 30, no. 7: 1430. https://doi.org/10.3390/molecules30071430
APA StylePokharel, U. R., Fronczek, F. R., & Maverick, A. W. (2025). Structural Rearrangement in Cyclic Cu(II) Pyridyltriazole Complexes: Oxidation of Dabco to Oxalate and CO2 Conversion to Carbonate. Molecules, 30(7), 1430. https://doi.org/10.3390/molecules30071430







