Abstract
Structural rearrangements in metal–organic supramolecules constructed from the coordination of Cu(II) with m-xpt (m-xylylenebis(pyridyltriazole)) are investigated upon their interaction with 1,4-diazabicyclo[2.2.2]octane (dabco) and carbon dioxide-enriched air. The binuclear [Cu2(m-xpt)2]4+ complexes react with dabco to produce a carbonate-bridged trinuclear complex, [Cu3(m-xpt)3(µ-CO3)]4+, and an oxalate-bridged binuclear complex, [Cu2(m-xpt)2(µ-C2O4)]2+, where carbonate and oxalate likely originate from CO2 and dabco, respectively. The trinuclear complex reassembles the original dimer upon the removal of the carbonate ion. Similarly, polymeric [Cu(o-xpt)(PF6)]n, formed from Cu(I) and o-xpt (o-xylylenebis(pyridyltriazole)) coordination, undergoes oxidation in CO2-enriched air to yield a tetranuclear Cu(II) complex, Cu4(o-xpt)3(μ4-CO3)(μ2-OH)(μ2-OCOCH3)4+. The reaction progress is monitored by UV-Vis spectroscopy, and the major products are characterized by single-crystal X-ray diffraction.
1. Introduction
The design of coordination-driven metal–organic cages has emerged as an important subfield of supramolecular chemistry recently due to their diverse potential applications in areas such as catalysis [1,2,3,4,5,6], separation [7,8,9,10,11], and sensing [12,13,14,15]. The rational design of these supramolecular cages relies on the careful consideration of the stereochemical preference of the transition metal ions, the coordination sites, and the geometrical preference of multidentate ligands, as well as the solubility profile of counter-anions [16,17,18]. These cages possess well-defined cavities capable of encapsulating guest molecules through secondary interactions, including Lewis acid–base interactions [19], π-π stacking [20,21], and hydrogen bonding [22].
Typically, the interactions of guest species preserve the structural topology of the host molecules in supramolecular host–guest interactions. However, in some cases, guest species can induce the structural rearrangement of the host [23,24]. Zonta and co-workers demonstrated that diacids as guests can trigger the disassembly of macrocyclic cages formed from tris(2-pyridylmethyl)amine and a zinc(II) complex via imine condensation [25,26]. Despite these findings, the role of guest species in simultaneously inducing disassembly and mediating subsequent transformations or reassembly remains largely underexplored.
Dabco (1,4-diazabicyclo[2.2.2]octane) is a bicyclic compound containing two nitrogen atoms in a rigid, cage-like structure. It is widely used as a base or nucleophile in organic synthesis, catalysis, and materials science [27]. In coordination chemistry, dabco often serves as a guest molecule in metal–organic self-assemblies due to its bidentate coordination capability and basicity [19,28,29,30]. While it has been employed as a structural template in the assembly of metal–organic complexes [31,32], there are limited reports of its chemical transformation during the metal–organic self-assembly process. Notably, Knope and Cahill observed that dabco undergoes oxidation to oxalate when heated with uranium oxynitrate hexahydrate and tetraethylammonium hydroxide under hydrothermal conditions [33]. This suggests that dabco can undergo chemical transformation in specific reaction environments.
Previously, we synthesized Cu(II) complexes of tetradentate pyridyltriazole-based ligands separated by a 2,7-dimethylnaphthalene spacer [19]. In this macrocyclic complex, the Cu···Cu separation was 7.5385(9) Å when the dabco occupied its cavity as a guest. Similarly, the treatment of meta-xylylenebis(pyridyltriazole) (m-xpt) with CuCl2 in the presence of dabco yielded a binuclear complex [Cu2(m-xpt)(dabco)]4+, which exhibited a Cu···Cu separation of 6.634(1) Å (Figure 1i). These findings prompted us to investigate the possibility of incorporating dabco as an internal guest of dimeric macrocycles constructed from m-xpt and Cu2+. Herein, we present the structural rearrangements caused by dabco in these macrocycles (Figure 1ii) and the concurrent formation of oxalate and carbonate species during the reassembly process. As a complement to this study, we report the reactivity of Cu(I) complexes of ortho-xylylenebis(pyridyltriazole) (o-xpt) for the activation of small guest molecules.
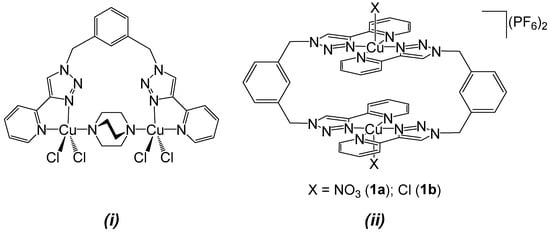
Figure 1.
Examples of copper(II) complexes assembled from the m-xpt ligand. (i) Dabco-bridged binuclear complex with one m-xpt ligand; and (ii) metallacyclic complexes used as starting materials in this study: [Cu2(m-xpt)2(NO3)2](PF6)2 (1a) and [Cu2(m-xpt)2Cl2](PF6)2 (1b).
2. Results and Discussion
2.1. Interactions of Supramolecular Complexes with Dabco in Air
Figure 1 shows the two Cu(II) complexes employed as starting materials in our experiments: [Cu2(m-xpt)2(NO3)2](PF6)2 (1a) and [Cu2(m-xpt)2Cl2](PF6)2 (1b). These [Cu2(m-xpt)2]4+ complexes display of range of Cu···Cu distances in the solid state, some of which are 7.6 Å or greater [19]. This is more than enough to accommodate dabco as an internal guest molecule. Our initial investigation of host–guest interactions between these macrocycles with dabco in air showed a subtle color change in complex 1a in acetonitrile and a pronounced color change in complex 1b in DMF upon the addition of five equivalents of dabco to each complex (Figure S1). The slow vapor diffusion of diethyl ether facilitated crystallization, yielding blue plate-shaped crystals at the upper part of the test tube. X-ray crystallographic analysis confirmed these as a carbonate-bridged trinuclear complex, [Cu3(m-xpt)3(µ-CO3)](PF6)4, [3](PF6)4 (Figure 2). Meanwhile, the needle-shaped green crystals that formed at the lower part of the test tube were identified as the carbonate-bridged trinuclear complex ([3]4+) co-crystallized with an oxalate-bridged binuclear complex, [Cu2(m-xpt)2(µ-C2O4)]2+ ([4]2+), with a stoichiometry of [3]2[4](PF6)10 (Figure 3).
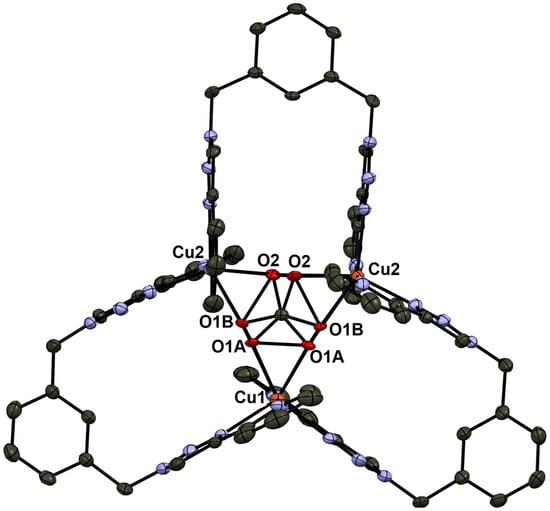
Figure 2.
Ellipsoid plot of [Cu3(m-xpt)3(μ-CO3)](PF6)4, ([3](PF6)4). The complex has crystallographically imposed two-fold rotation symmetry, with the axis approximately vertical in this drawing. This results in the disorder of the carbonate anion. In this and other crystal structure illustrations, ellipsoids are at the 50% probability level, and hydrogen atoms, solvents, and counterions are omitted for clarity.
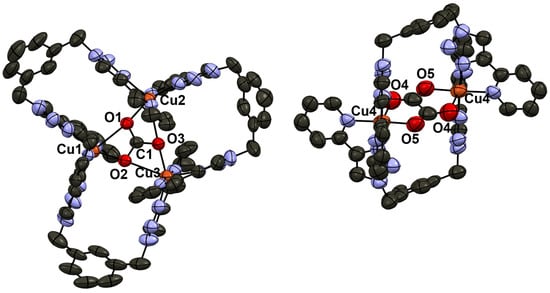
Figure 3.
Ellipsoid plot of the two cations in [Cu3(m-xpt)3(μ-CO3)]2[Cu2(m-xpt)2(μ-C2O4)](PF6)10, [3]2[4](PF6)10.
In the structure of [3](PF6)4 (Figure 2), the cation lies on a two-fold axis (passing through one m-xylylene group, the central carbonate C, and Cu1) in the crystal. The carbonate anion is bound asymmetrically; Cu1···Cu2 4.6058(8) and Cu2···Cu2′ 4.6126(8) Å. The structure of [3]2[4](PF6)10 (Figure 3) contains two different cations. The carbonate-bridged trimer [3]4+ lies in a general position, with Cu···Cu distances of 4.534(2), 4.705(1), and 4.583(1) Å. The oxalate-bridged dimer [4]2+ lies on an inversion center; Cu4···Cu4′ 5.501(2) Å.
2.2. Spectroscopic Monitoring of the Interaction Between Supramolecules and Dabco
These observations prompted us to investigate the reactions under controlled conditions and monitor the progress by UV-Vis spectrometry. Upon adding one equivalent of dabco under a nitrogen atmosphere, the primary d-d absorption band of compound 1a in CH3CN showed a red shift from 686 nm to 718 nm. No further shift in absorbance was observed with additional dabco, suggesting the formation of a 1:1 host–guest complex. Microanalysis of the green microcrystalline product (2) supported the formation of the 1:1 adduct with dabco (see experimental section). The compound may have contained dabco internally bound within the complex or externally coordinated, potentially forming a chain-like arrangement: –Cu2–dabco–Cu2–dabco–. Unfortunately, we were unable to obtain suitable crystals for X-ray analysis. The UV-vis absorption spectrum of compound 2 showed a minimal shift (ca. 5 nm) in the presence of CO2, but its intensity was increased (Figure 4). The spectrum of this solution was very similar to that of [3](PF6)4 in acetonitrile (λmax 715 nm), suggesting that 2 was converted to the carbonate-bridged trinuclear complex.
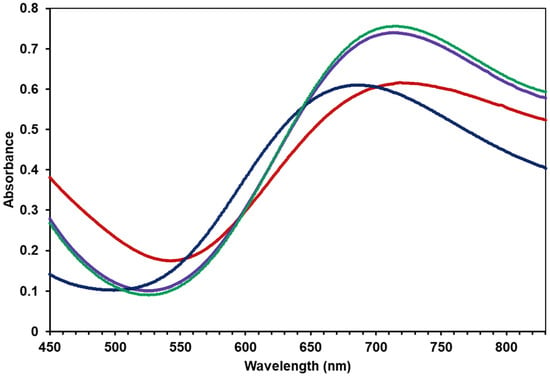
Figure 4.
A portion of the electronic spectrum of a 3.75 mM solution of [Cu2(m-xpt)2(NO3)2](PF6)2 (1a) in acetonitrile (▬▬), with one equivalent of dabco (▬▬) under N2. The solution was then kept under CO2 for 10 min (▬▬) and for 24 h (▬▬).
The above changes primarily reflected the host–guest binding of 1a with dabco. In contrast, the treatment of 1b with dabco led to reduction to Cu(I). This difference in behavior is shown in Figure S1. Attempts to monitor the reaction of complex 1b with dabco in acetonitrile were unsuccessful due to its low solubility. However, UV-Vis monitoring of 1b in DMF under nitrogen showed a gradual increase in signal intensity at ~440 nm (Figure S2), which we attribute to a metal-to-ligand charge transfer (MLCT) band, characteristic of Cu(I) complexes.
2.3. Role of Water in Carbonate/Oxalate Formation
Repeated experiments revealed that complex 1a produces more carbonate-bridged complex [3]4+ in bulk solvents than in dry solvents. This suggests that the carbonate ions in [3]4+ are generated in situ by the hydration of carbon dioxide in the presence of dabco as a base. Dabco reacts with residual water in the solvent and carbon dioxide to form bicarbonate. The bicarbonate coordinates with Cu(II) and then loses a proton to produce carbonate. In fact, one of the byproducts of the reaction is protonated dabco molecules linked by intermolecular hydrogen bonding [34] (Figure S3). Complex 1b is more effective for forming the co-crystallized oxalate/carbonate complex [3]2[4](PF6)10 than 1a. Based on the yields of [3](PF6)4 and [3]2[4](PF6)10 from 1b, the overall amount of oxalate complex [4]2+ formed is approx. 10% that of the carbonate complex [3]4+.
2.4. Spectroscopic Characterization
The crystals of [3](PF6)4 and [3]2[4](PF6)10 were manually separated for further analysis. The ESI-MS analysis of the trinuclear carbonate complex [3](PF6)4 showed a monoisotopic peak at 1663.2112 corresponding to a fully reduced trimer [Cu3(m-xpt)3(PF6)2]+. However, we were not able to detect any peak associated with CO32−, suggesting that the coordination of carbonate ions to the metal centers is weak. The IR of the product showed a prominent peak at 1459 cm−1, corresponding to the C=O stretching (Figure S4) of the carbonate group, as reported in carbonate-bridged Cu complexes [35]. The co-crystallized compound [3]2[4](PF6)10 exhibited signals at 1659 cm−1 and 1457 cm−1 in IR (Figure S5), corresponding to C=O stretching in the oxalate and carbonate. ESI-MS analysis showed a monoisotopic peak at 1147.1636, corresponding to [Cu2(m-xpt)2(C2O4)(PF6)]+.
2.5. Sources of Carbonate and Oxalate
Carbon dioxide, oxygen, and water are frequently present in metal–organic self-assembly reactions. The role of carbon dioxide in carbonate formation is well documented [36,37,38,39,40,41,42,43,44,45]. There have been reports of oxalate formation during self-assembly [46,47,48,49,50,51], though its sources are not always clearly identified. In our previous study, we demonstrated the high affinity of both complexes 1a and 1b to host oxalate. Upon the reduction of the Cu(II) centers to Cu(I) using ascorbic acid, followed by the exposure of the mixture to air, oxalate was observed as an oxidation product of ascorbic acid [52].
Although the chloro group is normally a better electron donor than nitrate for stabilizing Cu(II) centers, the redox potentials of the two complexes are nearly identical (−0.27 V for complex 1a and −0.28 V for complex 1b versus Fc/Fc+). Interestingly, the dihedral angle between the two pyridyltriazole moieties around the Cu(II) center is greater for 1b than for 1a. This greater twisting in the coordination environment of 1b might hinder the efficient coordination of dabco, causing dabco to act as a reducing agent rather than as a guest in this complex. We propose that the oxidation of dabco is responsible for the formation of oxalate, and that carbon dioxide is the source of carbonate. The carbonate and oxalate ions serve as templates for the self-assembly of trinuclear complex [3]4+ and binuclear complex [4]2+ (Scheme 1).
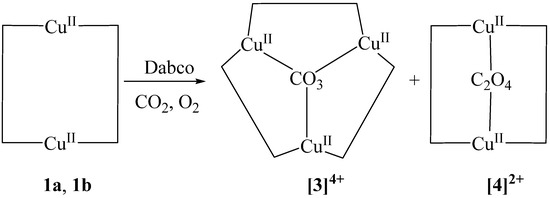
Scheme 1.
Rearrangement of macrocyclic Cu-m-xpt complexes.
2.6. Release of Carbonate Ion from Complex [3]4+
The release of carbonate ions from trinuclear complex [3]4+ was observed upon treatment with BaBr2 in DMF. Upon the addition of BaBr2 to the solution, the color changed from blue to green, accompanied by the formation of a white precipitate. When treated with dilute HCl, the precipitate dissolved with the effervescence of CO2, confirming the formation of BaCO3. The slow diffusion of diethyl ether into the green solution yielded the “empty” macrocycle [Cu2(m-xpt)2Br2](PF6)2, as evidenced by preliminary crystallographic data (Figure S6). The empty macrocycle contained bromo groups occupying the axial positions with a Cu···Cu separation of 7.592 Å.
In the presence of acid, the trinuclear complex [3]4+ also rearranged into a binuclear species. The addition of dilute HCl to an acetonitrile solution of complex [3](PF6)4 produced a green–blue precipitate, which dissolved in DMF. The slow vapor diffusion of diethyl ether into the DMF solution gave a dark-green crystalline product. X-ray crystallographic analysis revealed the formation of [Cu2(m-xpt)2Cl2]Cl2, as reported previously [19].
2.7. Synthesis of Cu(I) Complex of ortho-Xylylenebis(Pyridyltriazole) (o-xpt) and Its Reactivity with CO2-Enriched Air
In our previous work, we reported the formation of a carbonate-bridged trinuclear complex, [3]4+, when the Cu(I) complex of m-xpt was oxidized in CO2-enriched air [52]. However, we were unable to determine the structure of the Cu(I) complex by X-ray crystallography. To investigate the impact of Cu···Cu separation on CO2 fixation to CO32−, we synthesized a Cu(I) complex of ortho-xylylenebis(pyridyltriazole) (o-xpt) and crystallized this under a nitrogen atmosphere. The product was a bright yellow microcrystalline solid (Figure S7), and single-crystal X-ray analysis revealed the formation of a polymeric network of [Cu(o-xpt)(PF6)]n (5) (Scheme 2; Figure 5). Although the Cu···Cu separation in this complex was similar to the Ag···Ag separation in [Ag2(o-xpt)2]2+, as reported by Crowley et al. [53], the dimerization in this case was possibly less favorable due to the preferred tetrahedral geometry of Cu(I) coordination.
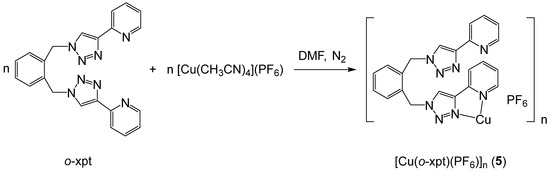
Scheme 2.
Synthesis of [Cu(o-xpt)(PF6)]n (5).
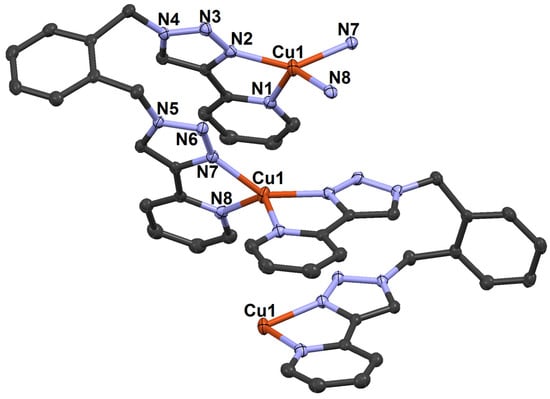
Figure 5.
Ellipsoid plot of [Cu(o-xpt)(PF6)]n (5). The Cu···Cu separation is 4.0304(5) Å.
In the structure of 5, the two Cu(pyridyltriazole) planes made an angle of 65.34°, representing some distortion compared to the ideal angle of 90°. This may be compared to the values found in three other [CuI(pyridyltriazole)2]+ structures: 63.85° [54], 85.18° [55], and 89.75° and 81.46° [56]. It was also well within the range of the interligand angles for [Cu(2,2′-bpy)2]+ structures (37.6–85.7°, 95 examples in 55 structures) [57].
When a DMF solution of complex 5 was exposed to CO2-enriched air, it gradually changed to blue. Attempts to grow crystals from DMF resulted in the formation of a non-crystalline blue precipitate. Therefore, the precipitate was redissolved in acetonitrile, and diethyl ether vapor was diffused into the solution. X-ray crystallographic analysis of the resulting product revealed the formation of a tetrameric complex, [Cu4(o-xpt)3(μ4-CO3)(μ2-OH)(μ2-OCOCH3)4](PF6)4 (6) (Figure 6). Unlike complex [3]4+, the carbonate ion in this complex bridged four Cu(II) centers, two of which were further linked by hydroxy (OH−) and acetate (CH3COO−) ligands. The carbonyl stretch of the acetate bridge appeared at 1668 cm−1, while the carbonate stretch was observed at 1458 cm−1 (Figure S8). The acetate was likely formed via the hydrolysis of acetonitrile, while the hydroxy bridge (μ-OH) between the Cu(II) centers may have arisen from water coordination followed by proton loss. In the crystal structure of 6, there were two crystallographically independent [Cu4(o-xpt)3(μ4-CO3)(μ2-OH)(μ2-OAc)]4+ cations with very similar conformations. One of them is shown in Figure 6.
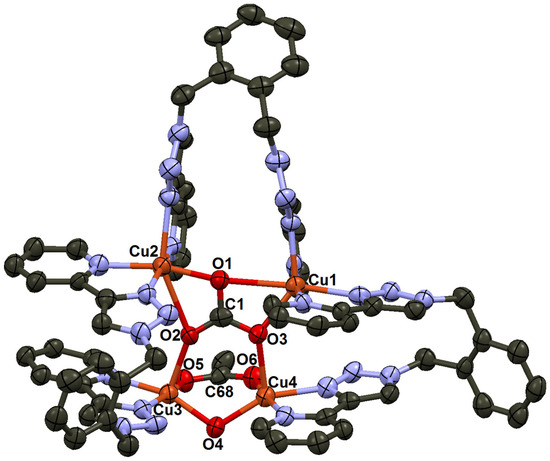
Figure 6.
Ellipsoid plot of [Cu4(o-xpt)3(μ4-CO3)(μ2-OH)(μ2-OCOCH3](PF6)4 (6). One of the two independent cations in the asymmetric unit is shown. Cu1···Cu2, 4.412(2) Å; and Cu3···Cu4 3.171(2) Å.
3. Materials and Methods
3.1. General Procedures
All commercially available reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Alfa Aesar (Ward Hill, MA, USA) and were used as received. NMR spectra were recorded on a Bruker 400 MHz (Avance) spectrometer (Bruker, Billerica, MA, USA). ESI mass spectra were measured on an Agilent 6230 instrument (Agilent Technologies, Santa Clara, CA, USA). FTIR spectra were recorded on a Bruker Tensor 27 spectrometer (Bruker, Billerica, MA, USA) equipped with a diamond crystal ATR sample holder in a range between 400 and 4000 cm−1. Elemental analyses were performed by M-H-W Laboratories, Phoenix, AZ, USA. UV-visible spectra were recorded in a custom-designed reaction flask connected to the cuvette using an Aviv 14DS spectrometer (Aviv Biomedical, Inc., Lakewood, NJ, USA). The ligands o-xpt [53] and m-xpt [19] were synthesized following the literature procedures. For the preparation, structures, and electrochemical properties of the complexes [Cu2(m-xpt)2X2](PF6)2 [where X = NO3 (1a), Cl (1b)] (Figure 1ii), see [52] and references therein.
3.2. Experimental Procedures
[Cu2(m-xpt)2](PF6)4·dabco (2): In a nitrogen-purged Schlenk flask, complex 1a (100 mg, 0.08 mmol) was dissolved in dry acetonitrile (20 mL), and dabco (25 mg, 0.23 mmol) was added. The complex changed its color from blue to blue–green as soon as dabco was added. N2 purged diethyl ether was slowly diffused into the reaction mixture via a cannula over a period of 4–5 days; by then, the green solid was deposited as a microcrystalline product. The mother liquor was decanted, and the product was washed with N2-purged diethyl ether. Then, it was dried under vacuum to give 2 (87 mg) as a green solid. ESI-MS: m/z 1349.0783, [Cu2(m-xpt)2(PF6)3]+ (calcd 1349.0821). Anal. Calcd [Cu2(m-xpt)2](PF6)4·Dabco: C 37.35, H 3.01, and N 15.68, and the following were found: C 36.88, H 2.92, and N 15.40.
[Cu3(m-xpt)3(µ-CO3)](PF6)4 ([3](PF6)4) and [Cu2(m-xpt)2(µ-C2O4)](PF6)2 ([4](PF6)2): In a test tube, complex 1a (100 mg, 0.08 mmol) was dissolved in acetonitrile or DMF (5 mL) and dabco (45 mg, 0.4 mmol) was added. The blue solution turned slightly lighter in the presence of dabco. To the reaction mixture, a small chunk of dry ice was added, and the test tube was enclosed in a CO2-purged jar containing diethyl ether for one week. We observed the formation of two distinct types of crystals in the test tube, which were separately analyzed using single-crystal X-ray crystallography. Blue plate-shaped crystals that formed towards the top of the test tube were identified as a carbonate-bridged trinuclear complex, [Cu3(m-xpt)3(µ-CO3)](PF6)4 ([3](PF6)4 (Figure 2). Green needle-like crystals formed towards the bottom of the test tube, which were identified as a co-crystallized complex comprising the carbonate-bridged trinuclear complex [Cu3(m-xpt)3(µ-CO3)]4+ ([3]4+) and the oxalate-bridged binuclear complex [Cu2(m-xpt)2(µ-C2O4)]2+ ([4]2+), with the stoichiometry of [3]2[4](PF6)10 (Figure 3).
Complex 1b was also treated in the same mole ratio with dabco, which led to the formation of the same two types of crystals: [3](PF6)4 and [3]2[4](PF6)10. The proportion of [3]2[4](PF6)10 in the mixture of products appeared to be higher when 1b was the reactant (as compared to 1a). Since 1b had poor solubility in acetonitrile, we performed further experiments with it in DMF only.
In these experiments, we sometimes observed crystals of the unreacted starting material forming along with [3](PF6)4 and [3]2[4](PF6)10. The same carbonate and carbonate-oxalate products were obtained in ordinary air (i.e., containing only natural amounts of CO2), but in lower yields.
[3](PF6)4: FT-IR (cm−1): 1458.8 (µ3-CO3), 823.8, 779.0, and 741.8 (PF6). Anal. Calcd for [Cu3(m-xpt)3(µ-CO3)](PF6)4: C 39.96, H 2.70, and N 16.69, and the following were found: C 40.30, H 3.00, and N 16.49. [3]2[4](PF6)10: FT-IR (cm−1): 1659.8 (C=O, oxalate), 1458.8 (C=O, carbonate), 820.0, 777.8, and 743.7 (PF6). Anal. Calcd for [Cu3(m-xpt)3(µ-CO3)]2[Cu2(m-xpt)2(µ-C2O4)](PF6)10: C 40.63, H 2.73, and N 16.85, and the following were found: C 39.39, H 3.39, and N 16.94. The discrepancy in microanalysis may have been due to the poorly defined solvents in the crystals. For example, formulae with water of crystallization gave a better agreement with the observed results; however, the solvents in the crystal could not be identified in the X-ray analysis.
[Cu(o-xpt)(PF6)]n (5): To nitrogen-purged dry DMF (5 mL) in a Schlenk flask, o-xpt (25 mg, 0.06 mmol) and [Cu(CH3CN)4]PF6 (24 mg, 0.06 mmol) were simultaneously added. The mixture produced an orange solution, which was stirred for 12 h. Diethyl ether vapor was then slowly diffused into the solution under nitrogen to give 5 (38 mg, 99%) as a bright yellow microcrystalline solid. 1H NMR (DMSO-d6): 8.71 (br, 4H), 8.30 (br, 4H), 7.87 (br, 8H), 7.62 (s, 8H), 7.31 (br, 4H), and 5.97 (s, 8H). 13C NMR (DMSO-d6): 149.2, 146.8, 145.5, 138.7, 133.6, 132.4, 130.5, 125.3, 124.4, 121.8, and 52.4. NMR showed the presence of DMF as an impurity in the crystalline product. ESI-MS: m/z 1059.1542 [Cu2(o-xpt)2(PF6)]+ (calcd 1059.1537). The product was characterized by single-crystal X-ray analysis as its DMF solvate.
[Cu4(o-xpt)3(µ4-CO3)(µ2-OH)(µ2-CH3COO)](PF6)4 (6): To DMF (4 mL) in a Schlenk flask, complex 5 (25 mg, 0.02 mmol) was added and stirred under a mixture of CO2 and air. The yellow color progressively turned to green. Diethyl ether vapor was then slowly diffused into the reaction mixture, which led to the formation of a precipitate. The solid was separated, redissolved in acetonitrile, and diffused further with diethyl ether vapor to give 6 (18 mg, 85%). IR: 1668 (C=O, acetate) and 1457 (C=O, carbonate). The product was analyzed using single-crystal X-ray crystallography.
[Cu2(m-xpt)2Br2](PF6)2 (7): To a stirred solution of 3 (100 mg, 0.05 mmol) in DMF (4 mL), a few drops of 1.0 M BaBr2 in DMF were added. The blue solution immediately turned to green, accompanied by the formation of a white precipitate. The precipitate was confirmed to be BaCO3: it was separated by centrifugation and dissolved in 1.0 M HCl (2-3 drops), giving the effervescence of carbon dioxide. The green DMF solution was collected and subjected to vapor diffusion with diethyl ether. The solution gave the green blocks of 7 (97 mg, 95%), which was characterized by single-crystal X-ray crystallography. Although the complete refinement was not successful, the analysis was sufficient for the identity of the product (Figure S6). ESI-MS: 1283.033 [Cu2(m-xpt)2Br(PF6)2]+ (calcd 1283.036). Anal. Calcd for [Cu2(m-xpt)2Br2](PF6)2·6H2O: C 35.86, H 3.28, N 15.21, and Br 10.84, and the following were found: C 35.65, H 3.47, N 14.70, and Br 9.62.
3.3. X-Ray Crystallography
X-ray diffraction data were measured at a low temperature on a Bruker Kappa Apex-II DUO CCD diffractometer (Bruker, Madison, WI, USA) with either MoKα (0.71073 Å) or CuKα (1.54184 Å) radiation. Absorption corrections were performed by a multi-scan technique. The structures were solved using SHELXS [58] and refined using SHELXL [59]. Hydrogen atoms were mostly visible in difference maps and were placed in idealized positions during refinement and treated as riding. Disordered solvent contributions in [3](PF6)2 and [3]2[4](PF6)10 were removed using the SQUEEZE [60] procedure. The trimeric cation in [3](PF6)2 lay on a two-fold axis, so the carbonate ligand was disordered across that axis. The PF6− anion in 5 was rotationally disordered into two orientations. The crystal of 6 was a two-component twin and hydrogen atoms of partially occupied water molecules were not located. The crystal data and refinement parameters of the structures are reported in Table 1 and Table 2; details are available in CIF format as CCDC 2422201–2422204 from https://www.ccdc.cam.ac.uk/structures/.

Table 1.
Crystal data and structure refinement parameters for compounds [3](PF6)4 and [3]2[4](PF6)10.

Table 2.
Crystal data and structure refinement parameters for compounds 5 and 6.
Two of the structures were of less than ideal quality, as judged by R and wR factors, and by the esd values for the C–C bond distances. The crystals of [3]2[4](PF6)10 were small, diffracting relatively weakly, and the asymmetric unit contained a large number of atoms. The crystals of 6 were twinned, with disordered solvent molecules; this limited the quality of the structure that could be obtained. In both cases, repeated crystallization experiments did not yield higher quality crystals.
4. Conclusions
This study elucidates the dabco-induced rearrangements in metal–organic supramolecules, emphasizing the distinct roles of dabco and air in facilitating the formation of oxalate and carbonate ions, respectively. The behavior of dabco differs between the macrocycles 1a and 1b, despite their subtle differences in ligand environments and coordination geometries. The complex 1a may accommodate dabco either externally or internally under a nitrogen atmosphere. However, it disassembles and reassembles in the presence of CO2 using carbonate ions as a template for the reassembly process. In contrast, complex 1b participates in a redox reaction with dabco, ultimately oxidizing dabco to oxalate. These reactions demonstrate the influence of the solvent environment: bulk solvents favor carbonate formation possibly due to the presence of water in the solvent, while anhydrous conditions promote oxalate generation. The removal of the carbonate ion from the trinuclear macrocycle [3]4+ by chemical methods induces a structural rearrangement from a trimer to a dimer.
Further, we investigate the effect of the distance between copper centers by employing ortho-xylylenebis(pyridyltriazole), o-xpt, as a ligand to coordinate with Cu(I). This modification leads to the formation of a polymeric network rather than a dimeric structure. The resulting polymer exhibits the ability to activate CO2 into carbonate while simultaneously facilitating the hydrolysis of acetonitrile to acetate in the presence of air.
These conversions are of interest as facets of metal-based host–guest and redox chemistry. However, they are not directly applicable to large-scale CO2 conversion, because they are stoichiometric, not catalytic. Also, the oxalate produced is likely from the oxidation of dabco rather than the reduction of CO2.
We are currently exploring the reactivity of structurally similar mononuclear copper complexes of pyridyltriazole or closely related bipyridine derivatives with dabco in the presence of O2 and CO2.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30071430/s1: illustrations of colors of complexes, IR and UV-Vis spectra, and crystal structure drawings.
Author Contributions
Conceptualization, U.R.P. and A.W.M.; Data curation, F.R.F.; Formal analysis, F.R.F.; Funding acquisition, A.W.M.; Investigation, U.R.P. and F.R.F.; Methodology, U.R.P. and F.R.F.; Software, F.R.F.; Validation, F.R.F. and A.W.M.; Visualization, U.R.P. and F.R.F.; Writing—original draft, U.R.P.; Writing—review and editing, U.R.P. and A.W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Albemarle Corporation and the Louisiana Board of Regents, and by the LSU West Professorship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available from CCDC at https://www.ccdc.cam.ac.uk/structures/, reference numbers 2422201-2422204.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 611–661. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Zhang, L.; Dong, L.-Z.; Wang, X.-H.; Li, R.-H.; Guo, C.; Li, X.; Yan, Y.; Li, S.-L.; Lan, Y.-Q. Bridging the Homogeneous and Heterogeneous Catalysis by Supramolecular Metal-Organic Cages with Varied Packing Modes. Adv. Mater. 2024, 36, 2310061. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Clever, G.H. Catalysis in confined space: Relationship between metal-organic frameworks and discrete coordination cages. Monogr. Supramol. Chem. 2021, 31, 247–281. [Google Scholar] [CrossRef]
- Tong, H.-Y.; Liang, J.; Wu, Q.-J.; Zou, Y.-H.; Huang, Y.-B.; Cao, R. Soluble imidazolium-functionalized coordination cages for efficient homogeneous catalysis of CO2 cycloaddition reactions. Chem. Commun. 2021, 57, 2140–2143. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220. [Google Scholar] [CrossRef]
- Ngai, C.; da Camara, B.; Woods, C.Z.; Hooley, R.J. Size- and Shape-Selective Catalysis with a Functionalized Self-Assembled Cage Host. J. Org. Chem. 2021, 86, 12862–12871. [Google Scholar] [CrossRef]
- Chang, X.; Lin, S.; Wang, G.; Shang, C.; Wang, Z.; Liu, K.; Fang, Y.; Stang, P.J. Self-Assembled Perylene Bisimide-Cored Trigonal Prism as an Electron-Deficient Host for C60 and C70 Driven by “Like Dissolves Like”. J. Am. Chem. Soc. 2020, 142, 15950–15960. [Google Scholar] [CrossRef]
- Fan, W.; Peh, S.B.; Zhang, Z.; Yuan, H.; Yang, Z.; Wang, Y.; Chai, K.; Sun, D.; Zhao, D. Tetrazole-Functionalized Zirconium Metal-Organic Cages for Efficient C2H2/C2H4 and C2H2/CO2 Separations. Angew. Chem. Int. Ed. 2021, 60, 17338–17343. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Su, J.; Wu, L.-X.; Luo, D.; Wang, X.-Z.; Zhou, X.-C.; Zhou, C.-W.; Zhou, X.-P.; Li, D. Selective separation of pyrene from mixed polycyclic aromatic hydrocarbons by a hexahedral metal-organic cage. Chin. Chem. Lett. 2024, 35, 108326. [Google Scholar] [CrossRef]
- Nguyen, B.-N.T.; Thoburn, J.D.; Grommet, A.B.; Howe, D.J.; Ronson, T.K.; Ryan, H.P.; Bolliger, J.L.; Nitschke, J.R. Coordination cages selectively transport molecular cargoes across liquid membranes. J. Am. Chem. Soc. 2021, 143, 12175–12180. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Zou, Y.-Q.; Nitschke, J.R. Metal-organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Imran Anwar, M.; Abbas, A.; Younas, A.; Hussain, S.; Gao, R.; Li, L.-K.; Shahid, M.; Khan, S. AIE based luminescent porous materials as cutting-edge tool for environmental monitoring: State of the art advances and perspectives. Coord. Chem. Rev. 2022, 463, 214539. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J.E. Supramolecular Coordination Complexes as Optical Biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yang, F.; Wang, X.; Shan, W.-L.; Liu, D.; Zhang, L.; Yuan, G. Trefoil-Shaped Metal-Organic Cages as Fluorescent Chemosensors for Multiple Detection of Fe3+, Cr2O72−, and Antibiotics. Inorg. Chem. 2023, 62, 1297–1305. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Pan, M.; Su, C.-Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2019, 59, 209–219. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Cyclic pyridyltriazole–Cu (II) dimers as supramolecular hosts. Dalton Trans. 2013, 42, 14064–14067. [Google Scholar] [CrossRef]
- Wood, D.M.; Meng, W.; Ronson, T.K.; Stefankiewicz, A.R.; Sanders, J.K.M.; Nitschke, J.R. Guest-Induced Transformation of a Porphyrin-Edged FeII4L6 Capsule into a CuIFeII2L4 Fullerene Receptor. Angew. Chem. Int. Ed. 2015, 54, 3988–3992. [Google Scholar] [CrossRef]
- Cherutoi, J.K.; Sandifer, J.D.; Pokharel, U.R.; Fronczek, F.R.; Pakhomova, S.; Maverick, A.W. Externally and Internally Functionalized Copper(II) β-Diketonate Molecular Squares. Inorg. Chem. 2015, 54, 7791–7802. [Google Scholar] [CrossRef] [PubMed]
- Turega, S.; Whitehead, M.; Hall, B.R.; Meijer, A.J.H.M.; Hunter, C.A.; Ward, M.D. Shape-, Size-, and Functional Group-Selective Binding of Small Organic Guests in a Paramagnetic Coordination Cage. Inorg. Chem. 2013, 52, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Regeni, I.; Holstein, J.J.; Clever, G.H. Guest-Induced Reversible Transformation between an Azulene-Based Pd2L4 Lantern-Shaped Cage and a Pd4L8 Tetrahedron. J. Am. Chem. Soc. 2023, 145, 25365–25371. [Google Scholar] [CrossRef]
- Percástegui, E.G. Guest-Induced Transformations in Metal-Organic Cages. Eur. J. Inorg. Chem. 2021, 2021, 4425–4438. [Google Scholar] [CrossRef]
- Bravin, C.; Badetti, E.; Scaramuzzo, F.A.; Licini, G.; Zonta, C. Triggering Assembly and Disassembly of a Supramolecular Cage. J. Am. Chem. Soc. 2017, 139, 6456–6460. [Google Scholar] [CrossRef]
- Begato, F.; Licini, G.; Zonta, C. Programmed guest confinement via hierarchical cage to cage transformations. Chem. Sci. 2023, 14, 8147–8151. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mitra, A.K. Versatility of DABCO as a Reagent in Organic Synthesis: A Review. Org. Biomol. Chem. 2023, 21, 6830–6880. [Google Scholar] [CrossRef]
- Kupietz, K.; Trouvé, J.; Roisnel, T.; Kahlal, S.; Gramage-Doria, R. A Highly Sterically Congested Bis-Zinc-Porphyrin Containing a Single Buta-1,3-diyne Linkage: From a Serendipitous Finding to Supramolecular Encapsulation. Eur. J. Org. Chem. 2023, 26, e202300621. [Google Scholar] [CrossRef]
- Samanta, S.K.; Samanta, D.; Bats, J.W.; Schmittel, M. DABCO as a Dynamic Hinge between Cofacial Porphyrin Panels and Its Tumbling inside a Supramolecular Cavity. J. Org. Chem. 2011, 76, 7466–7473. [Google Scholar] [CrossRef]
- Maverick, A.W.; Buckingham, S.C.; Yao, Q.; Bradbury, J.R.; Stanley, G.G. Intramolecular coordination of bidentate Lewis bases to a cofacial binuclear copper(II) complex. J. Am. Chem. Soc. 1986, 108, 7430–7431. [Google Scholar] [CrossRef]
- Fasano, F.; Bolgar, P.; Iadevaia, G.; Hunter, C.A. Supramolecular template-directed synthesis of triazole oligomers. Chem. Sci. 2022, 13, 13085–13093. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W.; Kuil, M.; Lutz, M.; Tooke, D.M.; Spek, A.L.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Supramolecular zinc(II) salphen motifs: Reversible dimerization and templated dimeric structures. Inorg. Chim. Acta 2006, 359, 1807–1814. [Google Scholar] [CrossRef]
- Knope, K.E.; Cahill, C.L. Hydrothermal Synthesis of a Novel Uranium Oxalate/Glycolate via In-Situ Ligand Formation. Inorg. Chem. 2007, 46, 6607–6612. [Google Scholar] [CrossRef]
- Pokharel, U.R.; Maverick, A.W.; Fronczek, F.R. Fronczek CCDC 987918: Experimental Crystal Structure Determination. 2014. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/cc1250bl&sid=DataCite (accessed on 17 March 2025). [CrossRef]
- Mao, Z.-W.; Liehr, G.; van Eldik, R. Structural and mechanistic information on the reaction of bicarbonate with Cu(II) and Zn(II) complexes of tris(2-aminoethyl)amine. Identification of intermediate and product species. J. Chem. Soc. Dalton Trans. 2001, 1593–1600. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Cowie, M. Unusual mixed-metal carbonate-bridged complexes via oxidation of a carbonyl ligand in [RhM(CO)4(Ph2PCH2PPh2)2] (M = Mn, Re) and [IrRe(CO)5(Ph2PCH2PPh2)2]. Organometallics 1991, 10, 2173–2177. [Google Scholar] [CrossRef]
- Sadique, A.R.; Brennessel, W.W.; Holland, P.L. Reduction of CO2 to CO using low-coordinate iron: Formation of a four-coordinate iron dicarbonyl complex and a bridging carbonate complex. Inorg. Chem. 2008, 47, 784–786. [Google Scholar] [CrossRef]
- Lozano, A.A.; Sáez, M.; Pérez, J.; García, L.; Lezama, L.; Rojo, T.; López, G.; García, G.; Santana, M.D. Structure and magnetic properties of carbonate-bridged five-coordinate nickel(II) complexes controlled by solvent effect. Dalton Trans. 2006, 3906–3911. [Google Scholar] [CrossRef]
- Sakamoto, S.; Yamauchi, S.; Hagiwara, H.; Matsumoto, N.; Sunatsuki, Y.; Re, N. Carbonate-bridged tetranuclear NiII2GdIII2 complex generated by atmospheric CO2 fixation. Inorg. Chem. Commun. 2012, 26, 20–23. [Google Scholar] [CrossRef]
- Sołtys-Brzostek, K.; Terlecki, M.; Sokołowski, K.; Lewiński, J. Chemical fixation and conversion of CO2 into cyclic and cage-type metal carbonates. Coord. Chem. Rev. 2017, 334, 199–231. [Google Scholar] [CrossRef]
- Dussart, Y.; Harding, C.; Dalgaard, P.; McKenzie, C.; Kadirvelraj, R.; McKee, V.; Nelson, J. Cascade chemistry in azacryptand cages: Bridging carbonates and methylcarbonates. J. Chem. Soc. Dalton Trans. 2002, 1704–1713. [Google Scholar] [CrossRef]
- Fondo, M.; García-Deibe, A.M.; Bermejo, M.R.; Sanmartín, J.; Llamas-Saiz, A.L. Spontaneous carbon dioxide fixation: A µ4-carbonate bridged tetranuclear zinc(II) complex of a heptadentate Schiff base. J. Chem. Soc. Dalton Trans. 2002, 4746–4750. [Google Scholar] [CrossRef]
- Wikstrom, J.P.; Filatov, A.S.; Mikhalyova, E.A.; Shatruk, M.; Foxman, B.M.; Rybak-Akimova, E.V. Carbonate formation within a nickel dimer: Synthesis of a coordinatively unsaturated bis(μ-hydroxo)dinickel complex and its reactivity toward carbon dioxide. Dalton Trans. 2010, 39, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Drew, M.G.; Estrader, M.; Ghosh, A. Coordination-driven self-assembly of a novel carbonato-bridged heteromolecular neutral nickel (II) triangle by atmospheric CO2 fixation. Inorg. Chem. 2008, 47, 7784–7791. [Google Scholar] [CrossRef]
- Escuer, A.; Vicente, R.; Kumar, S.B.; Solans, X.; Font-Bardia, M.; Caneschi, A. A Novel Pentadentate Coordination Mode for the Carbonato Bridge: Synthesis, Crystal Structure, and Magnetic Behavior of (μ3-CO3)[Ni3(Medpt)3(NCS)4], a New Trinuclear Nickel(II) Carbonato-Bridged Complex with Strong Antiferromagnetic Coupling. Inorg. Chem. 1996, 35, 3094. [Google Scholar] [CrossRef]
- Evans, W.J.; Seibel, C.A.; Ziller, J.W. Organosamarium-mediated transformations of CO2 and COS: Monoinsertion and disproportionation reactions and the reductive coupling of CO2 to [O2CCO2]2−. Inorg. Chem. 1998, 37, 770–776. [Google Scholar] [CrossRef]
- Evans, W.J.; Lorenz, S.E.; Ziller, J.W. Investigating Metal Size Effects in the Ln2(μ-η2: η2-N2) Reduction System: Reductive Reactivity with Complexes of the Largest and Smallest Trivalent Lanthanide Ions, La3+ and Lu3+. Inorg. Chem. 2009, 48, 2001–2009. [Google Scholar] [CrossRef]
- Tanaka, K.; Kushi, Y.; Tsuge, K.; Toyohara, K.; Nishioka, T.; Isobe, K. Catalytic generation of oxalate through a coupling reaction of two CO2 molecules activated on [(Ir(η5-C5Me5)2)(Ir(η4-C5Me5)CH2CN)(μ3-S)2]. Inorg. Chem. 1998, 37, 120–126. [Google Scholar] [CrossRef]
- Stibrany, R.T.; Schugar, H.J.; Potenza, J.A. A copper(II)-oxalate compound resulting from the fixation of carbon dioxide: μ-oxalato-bis[bis(1-benzyl-1H-pyrazole)(trifluoromethanesulfonato)copper(II)]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2005, 61, M1904–M1906. [Google Scholar] [CrossRef]
- Farrugia, L.J.; Lopinski, S.; Lovatt, P.A.; Peacock, R.D. Fixing carbon dioxide with copper: Crystal structure of [LCu(μ-C2O4)CuL][Ph4B]2 (L = N,N′,N′′-triallyl-1,4,7-triazacyclononane). Inorg. Chem. 2001, 40, 558–559. [Google Scholar] [CrossRef]
- Wong, W.K.; Zhang, L.L.; Xue, F.; Mak, C.W. Synthesis and X-ray crystal structure of an unexpected neutral oxalate-bridged ytterbium(III) porphyrinate dimer. J. Chem. Soc. Dalton Trans. 2000, 2245–2246. [Google Scholar] [CrossRef]
- Khamespanah, F.; Marx, M.; Crochet, D.B.; Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W.; Beller, M. Oxalate production via oxidation of ascorbate rather than reduction of carbon dioxide. Nat. Commun. 2021, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; Bandeen, P.H. A multicomponent CuAAC “click” approach to a library of hybrid polydentate 2-pyridyl-1,2,3-triazole ligands: New building blocks for the generation of metallosupramolecular architectures. Dalton Trans. 2010, 39, 612–623. [Google Scholar] [CrossRef] [PubMed]
- McCarney, E.P.; Hawes, C.S.; Blasco, S.; Gunnlaugsson, T. Synthesis and structural studies of 1,4-di(2-pyridyl)-1,2,3-triazole dpt and its transition metal complexes; a versatile and subtly unsymmetric ligand. Dalton Trans. 2016, 45, 10209–10221. [Google Scholar] [CrossRef] [PubMed]
- Fleischel, O.; Wu, N.; Petitjean, A. Click-triazole: Coordination of 2-(1,2,3-triazol-4-yl)-pyridine to cations of traditional tetrahedral geometry (Cu(I), Ag(I)). Chem. Commun. 2010, 46, 8454–8456. [Google Scholar] [CrossRef]
- Findlay, J.A.; McAdam, C.J.; Sutton, J.J.; Preston, D.; Gordon, K.C.; Crowley, J.D. Metallosupramolecular Architectures Formed with Ferrocene-Linked Bis-Bidentate Ligands: Synthesis, Structures, and Electrochemical Studies. Inorg. Chem. 2018, 57, 3602–3614. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A Found. Adv. 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).