Phytochemical Profile, Antioxidant Capacity and Anticancer Potential of Water Extracts from In Vitro Cultivated Salvia aethiopis
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analyses
2.1.1. Total Polyphenol and Flavonoid Content and Antioxidant Activity
2.1.2. LC-HRMS Analysis
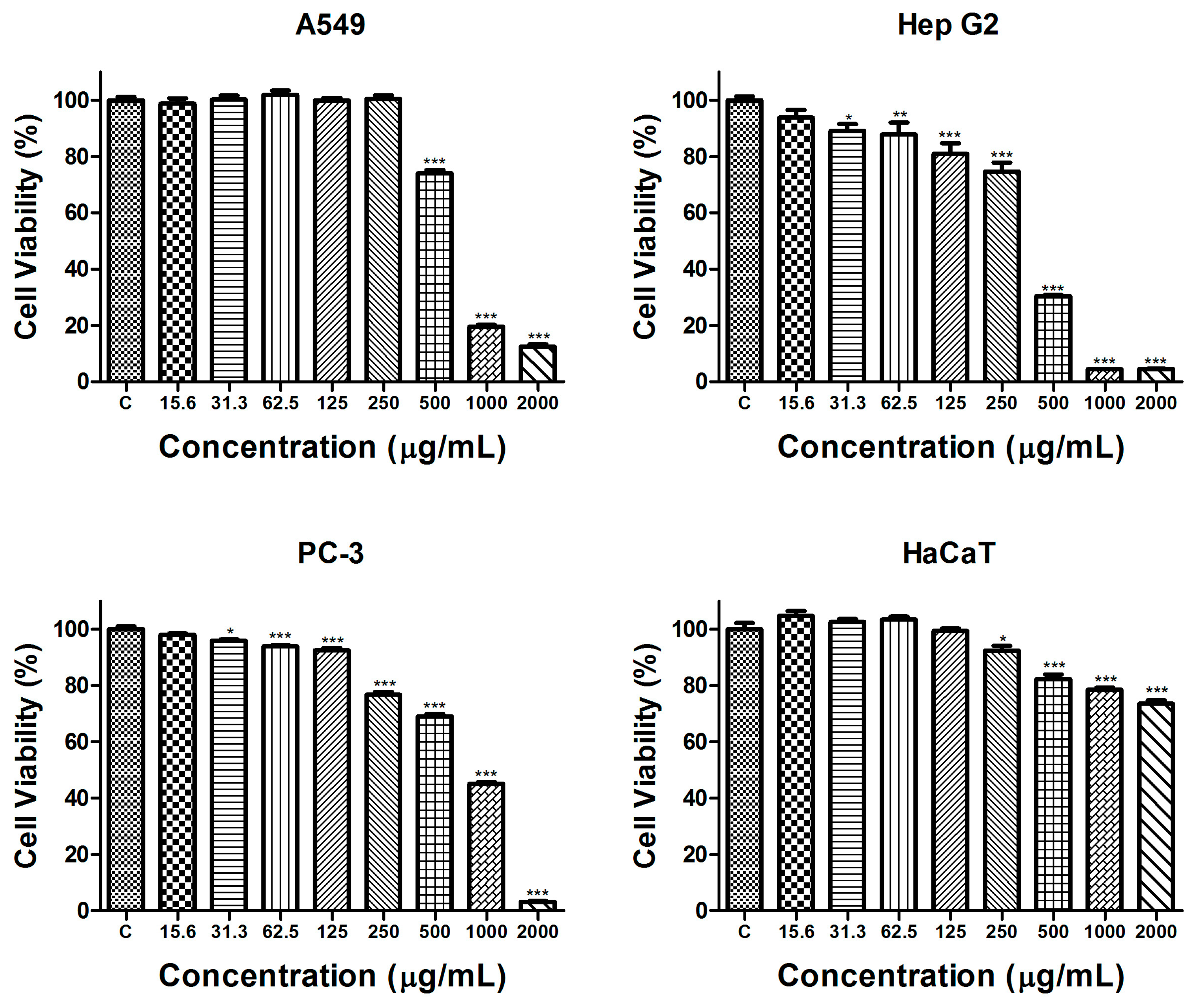
2.2. Assessment of Cytotoxicity and Antiproliferative Activity of S. aetiopis Extract
2.2.1. Cytotoxicity Evaluation
2.2.2. Antiproliferative Activity
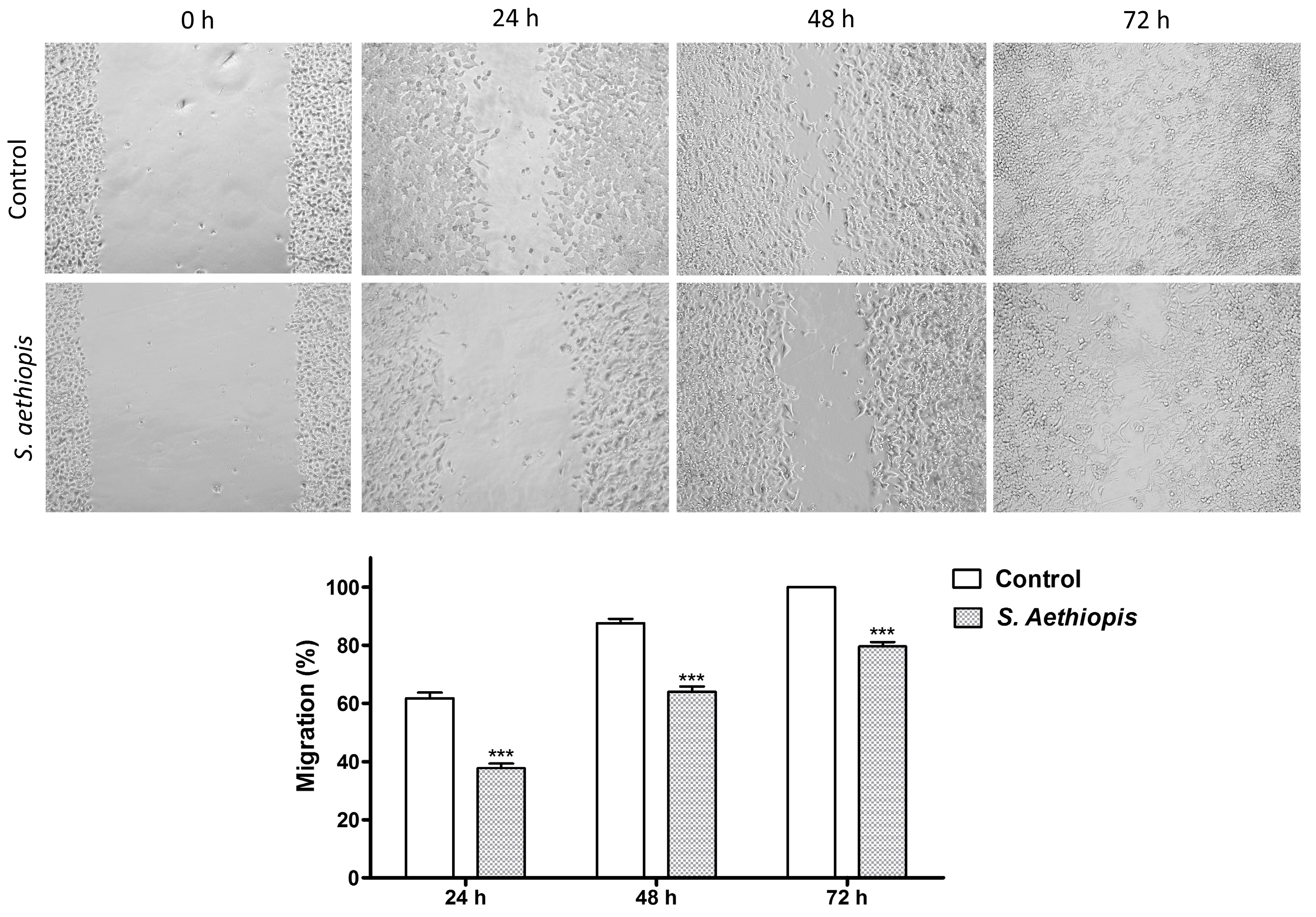
2.2.3. Migration Assay
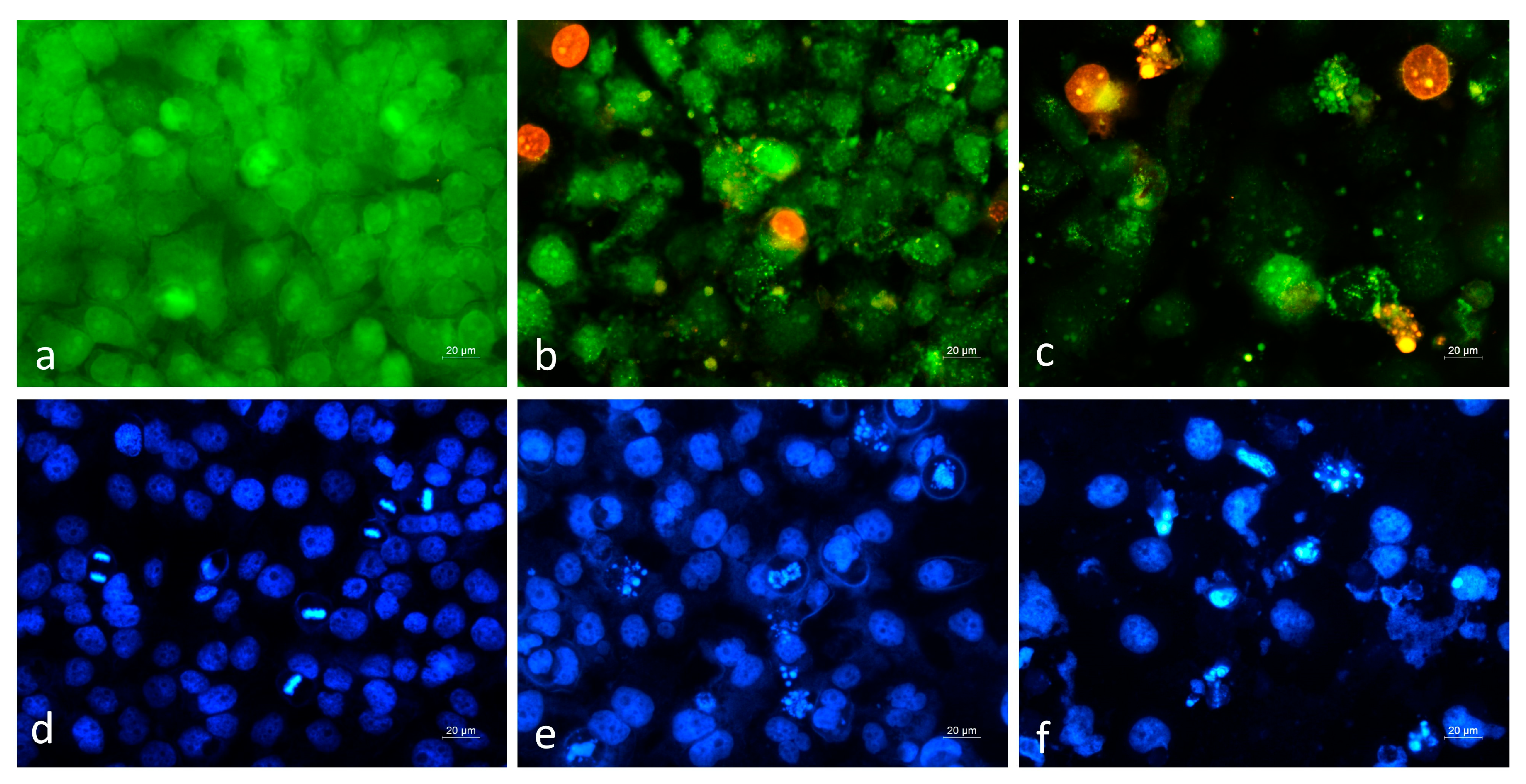
2.2.4. Cytopathological Analysis
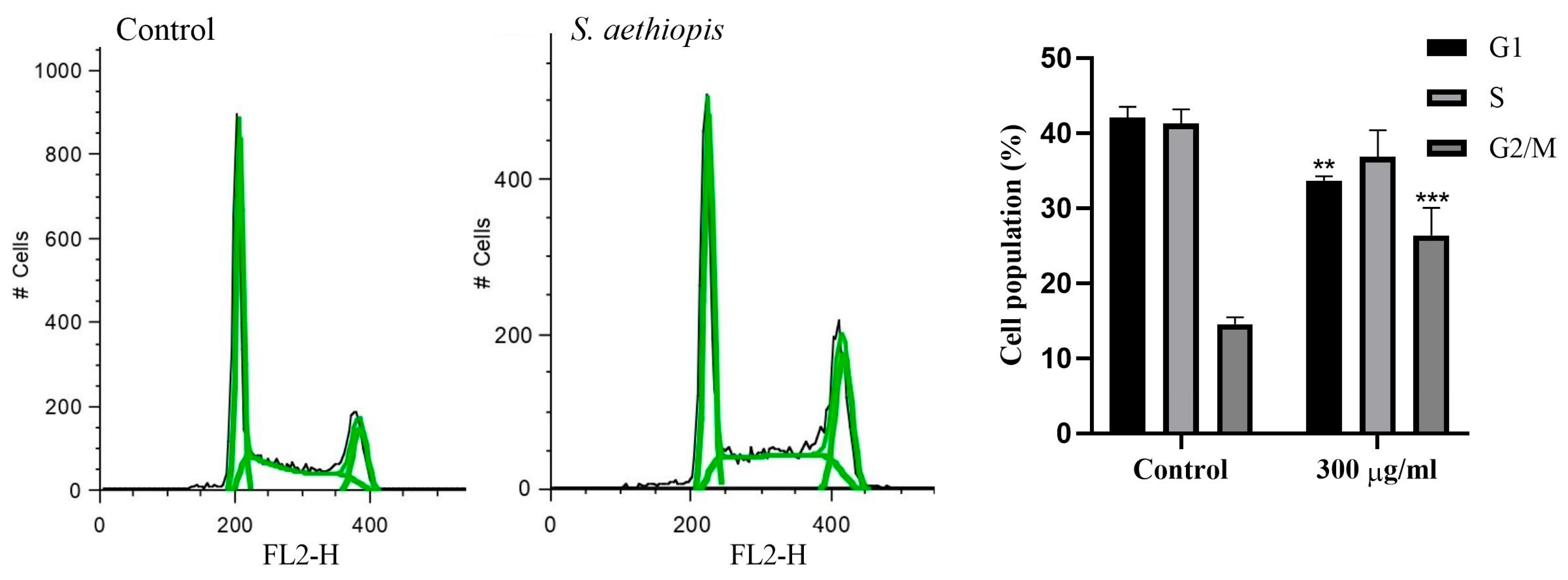
2.2.5. Cell Cycle Analysis
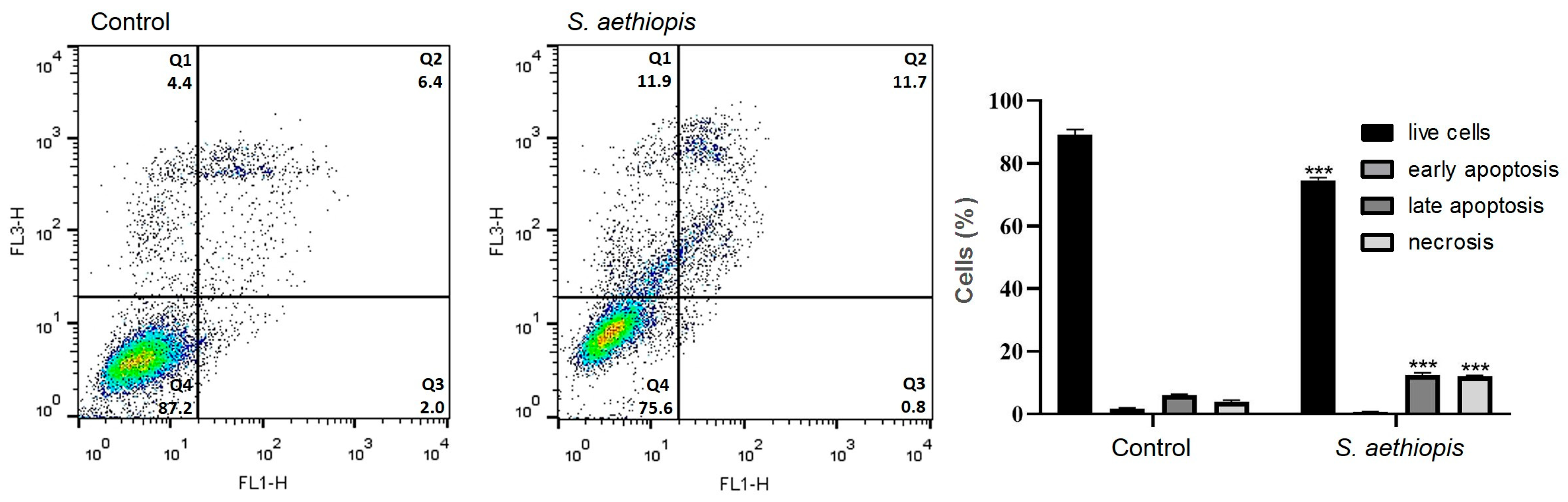
2.2.6. Apoptosis Assay
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phytochemical Analysis
4.2.1. Extraction and Freeze-Dried Procedure
4.2.2. Quantification of Total Polyphenols and Flavonoid Content
4.2.3. Evaluation of Antioxidant Activity
4.2.4. Liquid Chromatography–High Resolution Mass Spectrometry Analysis (LC-HRMS)
Sample Preparation
LC-HRMS Analysis
4.3. Antitumor Activity
4.3.1. Cell Lines
4.3.2. BALB/3T3 Neutral Red Uptake Assay
4.3.3. Assessment of Antiproliferative Activity
4.3.4. Cytopathological Studies—Live/Dead Staining with Acridine Orange and Ethidium Bromide
4.3.5. Nuclear Morphology Analysis by DAPI Staining
4.3.6. Flow Cytometric Analysis
4.3.7. Cell Cycle Assay
4.3.8. Apoptosis Detection Assay
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar]
- Poyraz, İ.E.; Çiftçi, G.A.; Öztürk, N. Phenolic contents, in vitro antioxidant and cytotoxicity activities of Salvia aethiopis L. and S. ceratophylla L. (Lamiaceae). Rec. Nat. Prod. 2017, 11, 345–355. [Google Scholar]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [PubMed]
- Moshari Nasirkandi, A.; Iaccarino, N.; Romano, F.; Graziani, G.; Alirezalu, A.; Alipour, H.; Amato, J. Chemometrics based analysis of the phytochemical profile and antioxidant activity of Salvia species from Iran. Sci. Rep. 2024, 14, 17317. [Google Scholar]
- Boya, M.T.; Valverde, S. An orthoquinone isolated from Salvia aethiopis. Phytochemistry 1981, 20, 1367–1368. [Google Scholar]
- Rodríguez, B.; Fernandez-Gadea, F.; Savona, G. A rearranged abietane diterpenoid from the root of Salvia aethiopis. Phytochemistry 1984, 23, 1805–1806. [Google Scholar]
- Zhumaliyeva, G.; Zhussupova, A.; Zhusupova, G.E.; Błońska-Sikora, E.; Cerreto, A.; Omirbekova, N.; Zhunusbayeva, Z.; Gemejiyeva, N.; Ramazanova, M.; Wrzosek, M.; et al. Natural compounds of Salvia L. genus and molecular mechanism of their biological activity. Biomedicines 2023, 11, 3151. [Google Scholar] [CrossRef]
- Kostić, M.; Miladinović, B.; Milutinović, M.; Branković, S.; Živanović, S.; Zlatković, B.; Kitić, D. Rosmarinic and caffeic acid content and antioxidant potential of the Salvia aethiopis L. extracts. Acta Medica Median. 2017, 56, 121–128. [Google Scholar]
- Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.-T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical profile and bioactivity evaluation of Salvia species from Eastern Europe. Antioxidants 2023, 12, 1514. [Google Scholar] [CrossRef]
- Veličković, D.T.; Ranđelović, N.V.; Ristić, M.S.; Šmelcerović, A.A.; Veličković, A.S. Chemical composition and antimicrobial action of the ethanol extracts of Salvia pratensis L. Salvia glutinosa L. and Salvia aethiopis L. J. Serb. Chem. Soc. 2002, 67, 639–646. [Google Scholar]
- Tosun, F.; Kizilay, C.A.; Sener, B.; Vural, M.; Palittapongarnpim, P. Antimycobacterial screening of some Turkish plants. J. Ethnopharmacol. 2004, 95, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, M.; Rabanal, R.M.; de la Torre, M.C.; Rodríguez, B. Analgesic, anti-inflammatory, antipyretic and haematological effects of aethiopinone, an o-naphthoquinone diterpenoid from Salvia aethiopis roots and two hemisynthetic derivatives. Planta Med. 1995, 61, 505–509. [Google Scholar] [CrossRef]
- Hernandez-Perez, M.; Rabanal, R.M.; Arias, A.; De La Torre, M.C.; Rodriguez, B. Aethiopinone, an antibacterial and cytotoxic agent from Salvia aethiopis roots. Pharm. Biol. 1999, 37, 17–21. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosaddegh, M.; Mohammadi Motamed, M.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2010, 4, 63–79. [Google Scholar]
- Şenol, F.S.; Orhan, I.; Celep, F.; Kahraman, A.; Doğan, M.; Yilmaz, G.; Şener, B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010, 120, 34–43. [Google Scholar] [CrossRef]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.C.; Alfieri, M.; Malafronte, N.; De Tommasi, N.; Leone, A. Increasing the synthesis of bioactive abietane diterpenes in Salvia sclarea hairy roots by elicited transcriptional reprogramming. Plant Cell Rep. 2017, 36, 375–386. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran. J. Pharm. Res. 2013, 12, 801–810. [Google Scholar]
- Nwosu, N. Cancer: A Disease of Modern Times? Cureus 2024, 16, e74666. [Google Scholar] [CrossRef]
- Njoya, E.M. Chapter 31—Medicinal plants, antioxidant potential, and cancer. In Cancer, Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 349–357. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sorriento, D. Oxidative stress and inflammation in cancer. Antioxidants 2024, 13, 1403. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Nilofar; Sinan, K.I.; Darendelioglu, E.; Bahsi, M.; Polat, R.; Cakilcioglu, U.; Orlando, G.; Ferrante, C.; Zengin, G. Incorporating the HPLC-ESI-Q-TOF-MS profiles with the biochemical properties of eight Salvia species. eFood 2025, 6, e70021. [Google Scholar] [CrossRef]
- MassBank of North America (MoNA037822). Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/MoNA037822 (accessed on 14 February 2025).
- Zengin, G.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops. Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Z.; Zhu, F.; Xu, Y.; Zhou, F. Development of a high-resolution mass-spectrometry-based method and software for human leukocyte antigen typing. Front. Immunol. 2023, 14, 1188381. [Google Scholar] [CrossRef]
- El-Gazar, A.A.; Emad, A.M.; Ragab, G.M.; Rasheed, D.M. Mentha pulegium L.(Pennyroyal, Lamiaceae) extracts impose abortion or fetal-mediated toxicity in pregnant rats; evidenced by the modulation of pregnancy hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 protein expressions, inflammatory state, certain related signaling pathways, and metabolite profiling via UPLC-ESI-TOF-MS. Toxins 2022, 14, 347. [Google Scholar] [CrossRef]
- MassBank of North America (MoNA035378). Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/MoNA035378 (accessed on 14 February 2025).
- Stochmal, A.; Simonet, A.M.; Macias, F.A.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 2. Tricin and chrysoeriol glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- Shirota, O.; Nagamatsu, K.; Sekita, S. Neo-clerodane diterpenes from the hallucinogenic sage Salvia divinorum. J. Nat. Prod. 2006, 69, 1782–1786. [Google Scholar] [CrossRef]
- Ezema, C.A.; Ezeorba, T.P.C.; Aguchem, R.N.; Okagu, I.U. Therapeutic benefits of Salvia species: A focus on cancer and viral infection. Heliyon 2022, 8, e08763. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Jaglanian, A.; Termini, D.; Tsiani, E. Rosemary (Rosmarinus officinalis L.) extract inhibits prostate cancer cell proliferation and survival by targeting Akt and mTOR. Biomed. Pharmacother. 2020, 131, 110717. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Delavari, S.; Ebrahimi, S.N.; Asili, J.; Emami, S.A.; Tayarani-Najaran, Z. A new tricyclic abietane diterpenoid from Salvia chloroleuca and evaluation of cytotoxic and apoptotic activities. Rev. Bras. Farmacogn. 2019, 29, 30–35. [Google Scholar] [CrossRef]
- Shakeri, A.; Farahmand, S.S.; Tayarani-Najaran, Z.; Emami, S.A.; Kúsz, N.; Hohmann, J.; Boozari, M.; Tavallaie, F.Z.; Asili, J. 4,5-Seco-5,10-friedo-abietane-type diterpenoids with anticancer activity from Salvia atropatana Bunge. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 241–248. [Google Scholar] [CrossRef]
- Telang, N. Antiproliferative and pro apoptotic effects of rosemary and constituent terpenoids in a model for the HER 2 enriched molecular subtype of clinical breast cancer. Oncol. Lett. 2018, 16, 5489–5497. [Google Scholar]
- Roszkowski, S. Application of polyphenols and flavonoids in oncological therapy. Molecules 2023, 28, 4080. [Google Scholar] [CrossRef] [PubMed]
- de Luna, F.C.F.; Ferreira, W.A.S.; Casseb, S.M.M.; de Oliveira, E.H.C. Anticancer potential of flavonoids: An overview with an emphasis on tangeretin. Pharmaceuticals 2023, 16, 1229. [Google Scholar] [CrossRef]
- Hanganu, D.; Olah, N.K.; Pop, C.E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Pu¸sca¸s, C.M.; Silaghi-Dumitrescu, R.; Benedec, D. Evaluation of polyphenolic profile and antioxidant activity for some Salvia species. Farmacia 2019, 67, 801–805. [Google Scholar] [CrossRef]
- Dogan, H. Minerals and bioactive content of some Salvia species in cultivated condition. C. R. Acad. Bulg. Sci. 2020, 73, 1398–1408. [Google Scholar]
- Caylak, E. Determination of antioxidant and anti-inflammatory properties of Salvia aethiopis/sclarea and synthesized silver nanoparticles. Ann. Med. Res. 2024, 31, 169–176. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, S.; Qian, L.; Tian, Y.; Chen, Y.; Bi, L.; Chen, W. OCF can repress tumor metastasis by inhibiting epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway in lung cancer cells. PLoS ONE 2017, 12, e0174021. [Google Scholar] [PubMed]
- Qin, T.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J.; et al. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256–2264. [Google Scholar]
- Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Yılmaz, M.A.; Gülçin, İ.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS Analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef]
- Hossan, S.; Rahman, S.; Jahan, R.; Al-Nahain, A.; Rahmatullah, M. Rosmarinic acid: A review of its anticancer action. WJPPS 2014, 3, 57–70. [Google Scholar]
- El Kantar, S.; Yassin, A.; Nehmeh, B.; Labaki, L.; Mitri, S.; Naser Aldine, F.; Hirko, A.; Caballero, S.; Monck, E.; Garcia-Maruniak, A.; et al. Deciphering the therapeutical potentials of rosmarinic acid. Sci. Rep. 2022, 12, 15489. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother. 2023, 162, 114687. [Google Scholar] [PubMed]
- Tao, L.; Wang, S.; Zhao, Y.; Sheng, X.; Wang, A.; Zheng, S.; Lu, Y. Phenolcarboxylic acids from medicinal herbs exert anticancer effects through disruption of COX-2 activity. Phytomedicine 2014, 21, 1473–1482. [Google Scholar]
- Jin, B.; Liu, J.; Gao, D.; Xu, Y.; He, L.; Zang, Y.; Li, N.; Lin, D. Detailed studies on the anticancer action of rosmarinic acid in human Hep-G2 liver carcinoma cells: Evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. J. BUON 2020, 25, 1383–1389. [Google Scholar]
- An, Y.; Zhao, J.; Zhang, Y.; Wu, W.; Hu, J.; Hao, H.; Qiao, Y.; Tao, Y.; An, L. Rosmarinic acid induces proliferation suppression of hepatoma cells associated with NF-κB signaling pathway. Asian Pac. J. Cancer Prev. 2021, 22, 1623–1632. [Google Scholar]
- Villegas, C.; Cortez, N.; Ogundele, A.V.; Burgos, V.; Pardi, P.C.; Cabrera-Pardo, J.R.; Paz, C. Therapeutic applications of rosmarinic acid in cancer-chemotherapy-associated Resistance and Toxicity. Biomolecules 2024, 14, 867. [Google Scholar] [CrossRef]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic acids: Potential source of natural drugs for the treatment of fibrosis disease and cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Salvianolic-acid-K (accessed on 18 February 2025).
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an endemic Portuguese plant: Phytochemical profiling, antioxidant, anti-proliferative and anti-inflammatory activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.-Y.; Jiang, Q.; Li, K.-R.; Zhao, Y.-X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [PubMed]
- Wang, X.; Wang, C.; Zhang, L.; Li, Y.; Wang, S.; Wang, J.; Yuan, C.; Niu, J.; Wang, C.; Lu, G. Salvianolic acid A shows selective cytotoxicity against multidrug-resistant MCF-7 breast cancer cells. Anticancer Drugs 2015, 26, 210–223. [Google Scholar] [PubMed]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar]
- Tang, C.; Jiang, S.T.; Li, C.X.; Jia, X.F.; Yang, W.L. The effect of salvianolic acid A on tumor-associated macrophage polarization and its mechanisms in the tumor microenvironment of triple-negative breast cancer. Molecules 2024, 29, 1469. [Google Scholar] [CrossRef]
- Chen, F.Y.; Bi, L.; Qian, L.; Gao, J.; Jiang, Y.C.; Chen, W.P. Identification of multidrug resistance gene MDR1 associated microRNA of salvianolic acid A reversal in lung cancer. China J. Chin. Mater. Med. 2016, 41, 3279–3284. [Google Scholar]
- Cai, J.; Chen, S.; Zhang, W.; Zheng, X.; Hu, S.; Pang, C.; Lu, J.; Xing, J.; Dong, Y. Salvianolic acid A reverses paclitaxel resistance in human breast cancer MCF-7 cells via targeting the expression of transgelin 2 and attenuating PI3 K/Akt pathway. Phytomedicine 2014, 21, 1725–1732. [Google Scholar]
- Casas, E.; Kim, J.; Bendesky, A.; Ohno-Machado, L.; Wolfe, C.J.; Yang, J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer 2011, 71, 245–254. [Google Scholar]
- Cao, Y.; Lu, K.; Xia, Y.; Wang, Y.; Wang, A.; Zhao, Y. Danshensu attenuated epithelial-mesenchymal transformation and chemoresistance of colon cancer cells induced by platelets. Front. Biosci. 2022, 27, 160. [Google Scholar] [CrossRef]
- Cao, H.Y.; Ding, R.L.; Li, M.; Yang, M.N.; Yang, L.L.; Wu, J.B.; Yang, B.; Wang, J.; Luo, C.L.; Wen, Q.L. Danshensu, a major water-soluble component of Salvia miltiorrhiza, enhances the radioresponse for Lewis Lung Carcinoma xenografts in mice. Oncol. Lett. 2017, 13, 605–612. [Google Scholar] [PubMed]
- Duan, S.P.; Zhu, L.H.; Li, P.; Song, X.W.; Wang, H.W.; Shen, B.S. Effect and mechanism of danshensu on hepatitis B virus reverse transcriptase and antigen expression. Zhongguo Zhong Yao Za Zhi 2016, 41, 1297–1301. (In Chinese) [Google Scholar]
- Lan, Y.; Yan, R.; Shan, W.; Chu, J.; Sun, R.; Wang, R.; Zhao, Y.; Wang, Z.; Zhang, N.; Yao, J. Salvianic acid A alleviates chronic alcoholic liver disease by inhibiting HMGB1 translocation via down-regulating BRD4. J. Cell Mol. Med. 2020, 24, 8518–8531. [Google Scholar]
- Cao, G.; Zhu, R.; Jiang, T.; Tang, D.; Kwan, H.Y.; Su, T. Danshensu, a novel indoleamine 2,3-dioxygenase1 inhibitor, exerts anti-hepatic fibrosis effects via inhibition of JAK2-STAT3 signaling. Phytomedicine 2019, 63, 153055. [Google Scholar] [PubMed]
- Arslan, M.E. Anticarcinogenic properties of malic acid on glioblastoma cell line through necrotic cell death mechanism. MANAS J. Eng. 2021, 9, 22–29. [Google Scholar]
- Chen, X.; Lv, Q.; Liu, Y.; Deng, W. Effect of Food Additive Citric Acid on The Growth of Human Esophageal Carcinoma Cell Line EC109. Cell J. 2017, 18, 493–502. [Google Scholar]
- Mycielska, M.E.; Mohr, M.T.J.; Schmidt, K.; Drexler, K.; Rümmele, P.; Haferkamp, S.; Schlitt, H.J.; Gaumann, A.; Adamski, J.; Geissler, E.K. Potential use of gluconate in cancer therapy. Front. Oncol. 2019, 9, 522. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolibdiphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel Fluorometric Assay for Hydroxyl Radical Prevention Capacity Using Fluorescein as the Probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [PubMed]


| Sample | Total Polyphenols (mg GAE/g DW) | Total Flavonoids (mg QE/g DW) | ORAC (µmol TE/g DW) | HORAC (µmol GAE/g DW) |
|---|---|---|---|---|
| S. aethiopis (cultivated) | 110.03 ± 0.7 | 7.88 ± 0.25 | 3677.9 ± 24.8 | 889.6 ± 14.3 |
| Peak | RT (min) | Experimental m/z Error (ppm) | Molecular Formula | MS2 Fragments m/z, (R.I., %) | Proposed Compound |
|---|---|---|---|---|---|
| 1 | 0.48 | 195.0483 (−13.7) | C6H12O7 | 71.0125 (8), 75.0073 (51), 85.0280 (7), 87.0071 (19), 99.0070 (14), 105.0176 (21), 129.0173 (32), 147.0275 (7), 159.0273 (6), 177.0378 (11) | Gluconic acid [25,26] |
| 2 | 0.54 | 341.1038 (−14.9) | C12H22O11 | 59.0127 (76), 71.0125 (62), 89.0229 (100), 95.0122 (9), 101.0227 (70),113.0226 (59), 119.0331 (39), 131.0329 (9), 143.0328 (17), 161.0430 (15), 179.0534 (44) | Disaccharide [25,27] |
| 3 | 0.56 | 133.0122 (−14.9) | C4H6O5 | 71.0124 (38), 72.9916 (15), 89.0227 (7), 115.0017 (100) | Malic acid [10,25] |
| 4 | 1.20 | 191.0172 (−13.8) | C6H8O7 | 85.0279 (29), 99.0070 (6), 111.0069 (100), 117.0173 (21), 129.0172 (14), 154.9962 (16), 173.0065 (27) | Citric acid [25] |
| 5 | 4.55 | 197.0429 (−13.5) | C9H10O5 | 72.9917 (55), 123.0433 (78), 134.0353 (13), 135.0432 (100), 179.0326 (58) | Danshensu (3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoic acid) [28] |
| 6 | 5.94 | 315.0679 (−13.5) | C13H16O9 | 153.0170 (34), 152.0092 (98), 109.0278 (36), 108.0200 (90) | Protocatechuic acid hexoside [25,29] |
| 7 | 6.91 | 175.0586 (−14.9) | C7H12O5 | 85.0643 (32), 113.0589 (36), 115.0381 (87), 131.0693 (8), 157.0482 (8), 175.0585 (90) | Isopropylmalic acid [25,30] |
| 8 | 8.29 | 475.1754 (−14.0) | C21H32O12 | 71.0122 (12), 85.0277 (12), 101.0225 (9), 103.0380 (8) 113.0223 (72), 149.0583 (6), 161.0427 (8), 329.1194 (8) | 2-(3-Hydroxy-4-methoxyphenyl)ethyl-O-(rhamnosyl)glucopyranoside |
| 9 | 9.43 | 377.0832 (−13.4) | C18H18O9 | 133.0274 (5), 135.0432 (17), 137.02220 (7), 161.0220 (100), 179.0323 (17), 197.0427 (31), 359.0721 (22) | Salvianic acid C |
| 10 | 9.92 | 569.1068 (−14.4) | C24H26O16 | 111.0069 (23), 121.0275 (9), 129.0173 (100.00), 138.0303 (5), 147.0276 (28), 153.0532 (28), 173.0063 (26), 182.0193(5), 191.0169 (16), 197.0424 (96), 265.0676 (23), 327.0671 (33), 371.0561 (52), 389.0666 (21) | Ester of salvianic acid C and 2,3,4,5-tetrahydroxyhexanedioic acid |
| 11 | 12.02 | 651.1111 (−13.9) | C28H28O18 | 113.0226 (35), 175.0221 (9), 193.0323 (23), 284.0286 (6), 285.0367 (6), 289.0525 (4), 299.0520 (15), 351.0520 (100) | O-(O-Glucuronyl-O-glucuronide)methoxylated flavonoid |
| 12 | 13.09 | 359.0722 (−14.5) | C18H16O8 | 123.0434 (5), 132.0202 (7), 135.0432 (13), 161.0220 (100), 179.0324 (18), 197.0427 (30) | Rosmarinic acid [10] |
| 13 | 13.76 | 475.0817 (−17.3) | C22H20O12 | 113.0225 (26), 175.0220 (7), 284.0284 (32), 299.0516 (100) | Methoxylated flavonoid-O-glucoronide [25] |
| 14 | 13.96 | 207.0634 (−14.2) | C11H12O4 | 135.0429 (100), 136.0510 (5), 162.0299 (6), 163.0375 (11), 164.0456 (8), 174.0295 (9), 177.0529, 192.0399 (15) | Unknown |
| 15 | 14.39 | 629.2346 (−16.2) | C29H42O15 | 153.0533 (5), 165.1258 (5), 196.0346 (5), 197.0423 (47), 207.1357 (11), 209.1150 (30), 211.0580 (15), 224.0293 (6), 225.1462 (8), 239.0523 (81), 251.1250 (8), 269.1352 (100), 285.0577 (6), 299.0731 (27), 359.0930 (5) | Unknown |
| 16 | 14.87 | 813.1404 (−14.0) | C37H34O21 | 113.0225 (35), 135.0430 (45), 161.0218 (39), 175.0219 (15), 179.0322 (80), 193.0322 (29), 203.0318 (6), 245.0420 (18), 284.0287 (14), 285.0366 (17), 289.0526 (5), 299.0521 (45), 333.0417 (21), 337.0517 (14), 351.0517 (55), 355.0616 (5), 513.0811 (100), 633.0996 (6) | O-(O-Caffeoyl-O-glucuronyl-O-glucuronide)methoxylated flavonoid |
| 17 | 15.04 | 555.1065 (−14.2) | C27H24O13 | 133.0274 (5), 135.0431 (80), 161.0220 (100), 179.0322 (25), 197.0426 (33), 295.0573 (10), 359.0718 (97), 401.0821 (7), 493.1060 (19) | Salvianolic acid K [27] |
| 18 | 15.65 | 857.1658 (−13.8) | C39H38O22 | 113.0225 (26), 149.0220 (16), 164.0452 (31), 175.0218 (10), 179.0685 (5), 223.0579 (79), 284.0287 (16), 285.0362 (6), 299.0521 (41), 333.0417 (25), 351.0516 (7), 399.0880 (5), 557.1068 (100), 633.1000 (6) | O-(O-Sinapoyl-O-glucuronyl-O-glucuronide)methoxylated flavonoid |
| 19 | 16.03 | 827.1561 (−13.2) | C38H36O21 | 113.0225 (27), 134.0351 (37), 149.0584 (7), 175.0217 (10), 193.0473 (87), 284.0284 (16), 285.0365 (7), 299.0518 (44), 333.0413 (27), 351.0527 (7), 369.0772 (5), 527.0952 (100), 633.0990 (6) | O-(O-Feruloyl-O-glucuronyl-O-glucuronide)methoxylated flavonoid |
| 20 | 17.49 | 327.2132 (−12.0) | C18H32O5 | 137.0952 (13), 171.1001 (100), 201.1100 (8), 211.1310 (18), 221.1160 (5), 229.1415 (11) | Dihydroxy-oxo-octadecenoic acid [10,25] |
| 21 | 17.58 | 313.0675 (−11.7) | C17H14O6 | 133.0273 (14), 151.0376 (7), 161.0219 (100) | 2-(3,4-dihydroxyphenyl)ethenyl 3-(3,4-dihydroxyphenyl)prop-2-enoate |
| Cell Lines | IC50 (μg/mL) | Selectivity Index (SI) |
|---|---|---|
| HaCaT (non-tumorigenic) | >2000 | - |
| A549 (lung carcinoma) | 683.4 ± 7.5 | >2.9 |
| Hep G2 (hepatocellular carcinoma) | 353.8 ± 21.8 | >5.7 |
| PC-3 (prostate carcinoma) | 720.1 ± 15.2 | >2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasheva, K.; Sulikovska, I.; Georgieva, A.; Djeliova, V.; Lozanova, V.; Vasileva, A.; Ivanov, I.; Denev, P.; Lazarova, M.; Vassileva, V.; et al. Phytochemical Profile, Antioxidant Capacity and Anticancer Potential of Water Extracts from In Vitro Cultivated Salvia aethiopis. Molecules 2025, 30, 1427. https://doi.org/10.3390/molecules30071427
Tasheva K, Sulikovska I, Georgieva A, Djeliova V, Lozanova V, Vasileva A, Ivanov I, Denev P, Lazarova M, Vassileva V, et al. Phytochemical Profile, Antioxidant Capacity and Anticancer Potential of Water Extracts from In Vitro Cultivated Salvia aethiopis. Molecules. 2025; 30(7):1427. https://doi.org/10.3390/molecules30071427
Chicago/Turabian StyleTasheva, Krasimira, Inna Sulikovska, Ani Georgieva, Vera Djeliova, Vesela Lozanova, Anelia Vasileva, Ivaylo Ivanov, Petko Denev, Maria Lazarova, Valya Vassileva, and et al. 2025. "Phytochemical Profile, Antioxidant Capacity and Anticancer Potential of Water Extracts from In Vitro Cultivated Salvia aethiopis" Molecules 30, no. 7: 1427. https://doi.org/10.3390/molecules30071427
APA StyleTasheva, K., Sulikovska, I., Georgieva, A., Djeliova, V., Lozanova, V., Vasileva, A., Ivanov, I., Denev, P., Lazarova, M., Vassileva, V., & Petkova-Kirova, P. (2025). Phytochemical Profile, Antioxidant Capacity and Anticancer Potential of Water Extracts from In Vitro Cultivated Salvia aethiopis. Molecules, 30(7), 1427. https://doi.org/10.3390/molecules30071427













