All chemicals used in this study were of reagent grade and were purchased from commercial suppliers and used as received without further purification. Solvents, reagents and isatins were purchased from Energy Chemical (Shanghai, China), Macklin (Shanghai, China), Beijing Chemical Plant (Beijing, China), Quanrui Reagent Co., Ltd., (Jinzhou, China) and Tongguang Fine Chemicals Company (Beijing, China). Thin-layer chromatography (TLC) analysis was performed using TLC silica gel 60 F254 25 aluminum sheets 20 × 20 cm (supelco, Darmstadt, Germany), and column chromatography analysis was performed using 200–300 mesh silica gel (Qingdao Marine Chemical Co., Ltd., Qingdao, China). The 1H NMR and 13C NMR spectra were obtained on a Bruker AVANCE III-400 MHz instrument (Karlsruhe, Germany). The solvents were CDCl3 and deuterated DMSO, and the internal standard was TMS; all deuterated reagents were purchased from Energy Chemical (Shanghai, China). The high-resolution mass spectrometry was obtained on a Thermo Q Exactive HF-X (Thermo Fisher Scientific, MA, USA). An X4 microscopic melting point apparatus was used as the melting point instrument. It was purchased from Shanghai Precision Instrument Science Co., Ltd. (Shanghai, China). The thermometer was not corrected.
4.1.3. Method for the Synthesis of Intermediate 3b–s
To a solution of ethyl 2-(2,3-dioxoindolin-1-yl)acetate (500 mg, 2.14 mmol) in EtOH (1 mL), Et2NH (265 μL, 1.2 eq) and the corresponding ketones (1.2 eq) were added. The reaction mixture was stirred at room temperature until the complete disappearance of compound 2 as evidenced by TLC. The solid crude products 3b–s were filtered, washed with EtOH and dried, and these crude products were used directly in the next step.
Ethyl (E)-2-(3-hydroxy-2-oxo-3-(2-oxo-4-phenylbut-3-en-1-yl)indolin-1-yl)acetate (3b)
White powder, 55% yield, m.p. 90.7–91.2 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 16.3 Hz, 1H, Ph-CH=), 7.53–7.48 (m, 2H, Ar-H2′, Ar-H6′), 7.44 (d, J = 7.4 Hz, 1H, Ar-H4), 7.42–7.35 (m, 3H, Ar-H3′, Ar-H4′, Ar-H5′), 7.29 (t, J = 7.8 Hz, 1H, Ar-H6), 7.07 (t, J = 7.5 Hz, 1H, Ar-H5), δ 6.73 (d, J = 7.9 Hz, 1H, Ar-H7), 6.70 (d, J = 16.2 Hz, 1H, CO-CH=CH), 4.93 (s, 1H, OH), 4.57 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.35 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.22 (q, J = 7.1 Hz, 2H, OCH2), 3.45 (d, J = 16.7 Hz, 1H, CO-CH2-C), 3.18 (d, J = 16.7 Hz, 1H, CO-CH2-C), 1.27 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.9, 176.1, 167.6, 144.8, 142.0, 134.0, 131.1, 129.9, 129.0, 128.6, 126.1, 124.4, 123.5, 108.6, 74.8, 61.9, 45.7, 41.4, 14.2. HRMS m/z: 380.1477 [M + H]+, calcd for C22H22NO5: 380.1492.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-phenylethyl)indolin-1-yl)acetate (3c)
White powder, 83% yield, m.p. 162.8–163.4 °C. 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 7.1 Hz, 2H, Ar-H3′, Ar-H5′), 7.58 (t, J = 7.4 Hz, 1H, Ar-H4), 7.49–7.41 (m, 3H, Ar-H2′, Ar-H4′, Ar-H6′), 7.31 (t, J = 7.8 Hz, 1H, Ar-H6), 7.06 (t, J = 7.3 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 4.59–4.42 (m, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.85 (d, J = 17.4 Hz, 1H, CO-CH2-C), 3.57 (d, J = 17.4 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.5, 176.2, 167.7, 142.3, 136.4, 133.9, 130.0, 129.9, 128.7, 128.3, 124.3, 123.4, 108.6, 74.5, 61.9, 44.4, 41.4, 14.1. HRMS m/z: 354.1322 [M + H]+, calcd for C20H20NO5: 354.1336.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-(p-tolyl)ethyl)indolin-1-yl)acetate (3d)
White powder, 63% yield, m.p. 143.6–144.4 °C. 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.2 Hz, 2H, Ar-H3′, Ar-H5′), 7.45 (d, J = 7.4 Hz, 1H, Ar-H4), 7.30 (t, J = 7.6 Hz, 1H, Ar-H6), 7.25 (d, J = 8.1 Hz, 2H, Ar-H2′, Ar-H6′), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 5.05 (s, 1H, OH), 4.56 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.44 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 1H, OCH2), 3.80 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.52 (d, J = 17.3 Hz, 1H, CO-CH2-C), 2.41 (s, 3H, CH3), 1.30 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, CDCl3) δ 198.4, 176.2, 167.7, 144.9, 142.2, 134.0, 130.1, 129.8, 129.4, 128.4, 124.3, 123.4, 108.5, 74.7, 61.9, 44.0, 41.4, 21.7, 14.2. HRMS m/z: 368.1479 [M + H]+, calcd for C21H22NO5: 368.1492.
Ethyl 2-(3-(2-(4-ethylphenyl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3e)
White powder, 63% yield, m.p. 139.3–140.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 2H, Ar-H3′, Ar-H5′), 7.45 (d, J = 7.3 Hz, 1H, Ar-H4), 7.36–7.21 (m, 3H, Ar-H2′, Ar-H6′, Ar-H6), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 5.01 (s, 1H, OH), 4.57 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.42 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.0 Hz, 2H, OCH2), 3.80 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.50 (d, J = 17.3 Hz, 1H, CO-CH2-C), 2.70 (q, J = 7.5 Hz, 2H, CH2), 1.35–1.19 (m, 6H, (CH3)2). 13C NMR (101 MHz, CDCl3) δ 198.5, 176.1, 167.6, 151.1, 142.2, 134.2, 130.1, 129.9, 128.5, 128.3, 124.4, 123.4, 108.6, 74.7, 61.9, 44.0, 41.4, 29.0, 15.1, 14.2. HRMS m/z: 382.1635 [M + H]+, calcd for C22H24NO5: 382.1649.
Ethyl 2-(3-hydroxy-3-(2-(4-isopropylphenyl)-2-oxoethyl)-2-oxoindolin-1-yl)acetate (3f)
White powder, 26% yield, m.p. 128.7–129.5 °C. 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.3 Hz, 2H, Ar-H3′, Ar-H5′), 7.45 (d, J = 7.3 Hz, 1H, Ar-H4), 7.39–7.25 (m, 3H, Ar-H2′, Ar-H6′, Ar-H6), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 5.01 (s, 1H, OH), 4.58 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.42 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.80 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.50 (d, J = 17.3 Hz, 1H, CO-CH2-C), 2.97 (hept, J = 6.9 Hz, 1H, CH), 1.29 (m, J = 9H, (CH3)3). 13C NMR (101 MHz, CDCl3) δ 198.6, 176.1, 167.6, 155.6, 142.2, 134.3, 130.1, 129.9, 128.6, 126.8, 124.4, 123.4, 108.6, 74.7, 61.9, 43.9, 41.4, 34.3, 23.6, 14.2. HRMS m/z: 396.1789 [M + H]+, calcd for C23H26NO5: 396.1805.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-(4-pentylphenyl)ethyl)indolin-1-yl)acetate (3g)
White powder, 16% yield, m.p. 75.2–76.8 °C. 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 2H, Ar-H3′, Ar-H5′), 7.46 (d, J = 7.4 Hz, 1H, Ar-H4), 7.29 (dd, J = 14.9, 6.9 Hz, 3H, Ar-H6, Ar-H2′, Ar-H6′), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 4.98 (s, 1H, OH), 4.58 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.41 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.79 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.49 (d, J = 17.3 Hz, 1H, CO-CH2-C), 2.66 (t, J = 7.7 Hz, 2H, Ar-CH2), 1.63 (p, J = 7.1 Hz, 2H, Ar-CH2CH2), 1.31 (q, J = 7.0 Hz, 7H, CH2CH2CH3, CH2CH2CH3, OCH2CH3), 0.90 (t, J = 6.8 Hz, 3H, CH2CH2CH3). 13C NMR (101 MHz, CDCl3) δ 198.7, 176.1, 167.6, 149.9, 142.2, 134.2, 130.1, 129.9, 128.8, 128.5, 124.4, 123.4, 108.6, 74.8, 61.9, 43.9, 41.4, 36.0, 31.4, 30.7, 22.5, 14.1, 14.0. HRMS m/z: 424.2103 [M + H]+, calcd for C25H30NO5: 424.2118.
Ethyl 2-(3-hydroxy-3-(2-(4-methoxyphenyl)-2-oxoethyl)-2-oxoindolin-1-yl)acetate (3h)
White powder, 31% yield, m.p. 113.6–114.3 °C. 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 8.8 Hz, 2H, Ar-H2′, Ar-H6′), 7.46 (d, J = 7.3 Hz, 1H, Ar-H4), 7.30 (t, J = 7.6 Hz, 1H, Ar-H6), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.92 (d, J = 8.8 Hz, 2H, Ar-H3′, Ar-H5′), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 5.14 (s, 1H, OH), 4.59 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.39 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.87 (s, 3H, OCH3), 3.72 (t, J = 17.1 Hz, 1H, CO-CH2-C), 3.45 (d, J = 17.1 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.5, 176.1, 167.6, 164.2, 142.1, 130.7, 130.2, 129.8, 129.5, 124.4, 123.4, 113.9, 108.5, 74.8, 61.9, 55.6, 43.5, 41.4, 14.2. HRMS m/z: 384.1427 [M + H]+, calcd for C21H22NO6: 384.1442.
Ethyl 2-(3-(2-(4-ethoxyphenyl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3i)
White powder, 84% yield, m.p. 135.7–136.7 °C. 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 2H, Ar-H2′, Ar-H6′), 7.46 (d, J = 7.3 Hz, 1H, Ar-H4), 7.30 (d, J = 7.8 Hz, 1H, Ar-H6), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.90 (d, J = 8.9 Hz, 2H, Ar-H3′, Ar-H5′), 6.75 (d, J = 7.8 Hz, 1H, Ar-H7), 5.16 (s, 1H, OH), 4.59 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.39 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 4.10 (q, J = 7.0 Hz, 2H, OCH2), 3.73 (d, J = 17.1 Hz, 1H, CO-CH2-C), 3.44 (d, J = 17.1 Hz, 1H, CO-CH2-C), 1.44 (t, J = 7.0 Hz, 3H, CH3), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.5, 176.1, 167.6, 163.6, 142.1, 130.7, 130.2, 129.8, 129.3, 124.4, 123.4, 114.3, 108.5, 74.8, 63.9, 61.9, 43.4, 41.4, 14.6, 14.2. HRMS m/z: 398.1584 [M + H]+, calcd for C22H24NO6: 398.1598.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (3j)
White powder, 65% yield, m.p. 121.7–122.4 °C. 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.1 Hz, 1H, Ar-H4), 7.30 (td, J = 7.8, 0.9 Hz, 1H, Ar-H6), 7.15 (s, 2H, Ar-H2′, Ar-H6′), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.75 (d, J = 7.8 Hz, 1H, Ar-H7), 4.93 (s, 1H, OH), 4.55 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.40 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.21 (q, J = 7.1 Hz, 2H, OCH2), 3.88 (d, 9H, 3′, 4′, 5′-(OCH3)3), 3.79 (d, J = 17.1 Hz, 1H, C-CH2-CO), 3.50 (d, J = 7.1 Hz, 1H, C-CH2-CO), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.5, 176.3, 167.6, 153.1, 143.2, 142.2, 131.5, 130.0, 129.9, 124.3, 123.5, 108.6, 105.7, 74.7, 61.9, 61.0, 56.3, 43.9, 41.4, 14.1. HRMS m/z: 444.1637 [M + H]+, calcd for C23H26NO8: 444.1653.
Ethyl 2-(3-(2-(4-fluorophenyl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3k)
White powder, 96% yield, m.p. 154.6–155.4 °C. 1H NMR (400 MHz, CDCl3) δ 7.94 (dd, J = 8.7, 5.4 Hz, 2H, Ar-H2′, Ar-H6), 7.43 (d, J = 7.3 Hz, 1H, Ar-H4), 7.31 (t, J = 6.2 Hz, 1H, Ar-H6), 7.12 (t, J = 8.6 Hz, 2H, Ar-H3′, Ar-H5′), 7.07 (t, J = 7.6 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.47 (d, J = 17.7 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.82 (d, J = 17.3 Hz, 1H, N-CH2-CO), 3.54 (d, J = 17.3 Hz, 1H, N-CH2-CO), 1.31 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 196.8, 176.3, 167.8, 166.2 (J = 256.0 Hz), 142.3, 132.8 (J = 3.0 Hz), 131.0 (J = 9.5 Hz), 129.9, 124.2, 123.5, 115.9 (J = 22.0 Hz), 108.6, 74.4, 60.0, 44.4, 41.4, 14.1. HRMS m/z: 372.1228 [M + H]+, calcd for C20H19FNO5: 372.1242.
Ethyl 2-(3-(2-(4-chlorophenyl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3l)
White powder, 86% yield, m.p. 152.7–153.5 °C. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.5 Hz, 2H, Ar-H2′, Ar-H6′), 7.46–7.39 (m, 3H, Ar-H3′, Ar-H5′, Ar-H4), 7.32 (d, J = 7.7 Hz, 1H, Ar-H6), 7.07 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 4.52 (d, J = 17.8 Hz, 1H, N-CH2-CO), 4.47 (d, J = 17.9 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.82 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.54 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.1, 176.3, 167.9, 142.3, 140.4, 134.7, 130.0, 129.9, 129.7, 129.0, 124.2, 123.5, 108.6, 74.4, 62.0, 44.5, 41.4, 14.2. HRMS m/z: 388.0933 [M + H]+, calcd for C20H19ClNO5: 388.0946.
Ethyl 2-(3-(2-(4-bromophenyl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3m)
White powder, 75% yield, m.p. 145.4–146.8 °C. 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.5 Hz, 2H, Ar-H3′, Ar-H5′), 7.59 (d, J = 8.5 Hz, 2H, Ar-H2′, Ar-H6′), 7.43 (d, J = 7.4 Hz, 1H, Ar-H4), 7.31 (t, J = 7.8 Hz, 1H, Ar-H6), 7.07 (t, J = 7.5 Hz, 1H, Ar-H5), 6.77 (d, J = 7.8 Hz, 1H, Ar-H7), 4.55 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.43 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.79 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.50 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.5, 176.1, 167.6, 142.3, 135.1, 132.1, 130.0, 129.8, 129.7, 129.2, 124.3, 123.5, 108.6, 74.5, 62.0, 44.3, 41.4, 14.2. HRMS m/z: 432.0428 [M + H]+, calcd for C20H19BrNO5: 432.0441.
Ethyl 2-(3-hydroxy-3-(2-(4-nitrophenyl)-2-oxoethyl)-2-oxoindolin-1-yl)acetate (3n)
White powder, 90% yield, m.p. 153.9–154.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 8.6 Hz, 2H, Ar-H3′, Ar-H5′), 8.07 (d, J = 8.6 Hz, 2H, Ar-H2′, Ar-H6′), 7.43 (d, J = 7.3 Hz, 1H, Ar-H4), 7.33 (t, J = 7.7 Hz, 1H, Ar-H6), 7.08 (t, J = 7.5 Hz, 1H, Ar-H5), 6.79 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.45 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.90 (d, J = 17.2 Hz, 1H, CO-CH2-C), 3.61 (d, J = 17.2 Hz, 1H, CO-CH2-C), 1.31 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 196.6, 176.2, 167.6, 150.6, 142.4, 140.7, 130.2, 129.4, 129.3, 124.2, 123.9, 123.6, 108.8, 74.4, 62.1, 45.3, 41.4, 14.2. HRMS m/z: 399.1172 [M + H]+, calcd for C20H19N2O7: 399.1187.
Ethyl 2-(3-(2-([1,1′-biphenyl]-4-yl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3o)
White powder, 20% yield, m.p. 127.9–128.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 6.9 Hz, 2H, Diphenyl-H2, H6), 7.65 (dd, J = 21.9, 7.7 Hz, 4H, Diphenyl-H3, H5, H2′, H6′), 7.52–7.38 (m, 4H, Ar-H4, Diphenyl-H3′, H4′, H5′), 7.32 (t, J = 7.2 Hz, 1H, Ar-H6), 7.09 (t, J = 7.5 Hz, 1H, Ar-H5), 6.79 (d, J = 7.8 Hz, 1H, Ar-H7), 4.59 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.44 (d, J = 17.5 Hz, 1H, N-CH2-CO), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 3.87 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.56 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.31 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.4, 176.2, 167.6, 146.6, 142.2, 139.6, 135.1, 130.0, 129.9, 129.0, 128.9, 128.4, 127.4, 127.3, 124.4, 123.5, 108.6, 74.7, 61.9, 44.2, 41.5, 14.2. HRMS m/z: 430.1634 [M + H]+, calcd for C26H24NO5: 430.1649.
Ethyl 2-(3-hydroxy-3-(2-(naphthalen-2-yl)-2-oxoethyl)-2-oxoindolin-1-yl)acetate (3p)
White powder, 84% yield, m.p. 138.6–139.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.45 (s, 1H, Napht-H1), 7.97 (dd, J = 8.7, 1.4 Hz, 1H, Napht-H4), 7.94 (d, J = 8.1 Hz, 1H, Napht-H8), 7.87 (d, J = 8.9 Hz, 1H, Napht-H3), 7.86 (d, J = 8.0 Hz, 1H, Napht-H5), 7.62 (t, J = 7.1 Hz, 1H, Napht-H7), 7.55 (t, J = 7.2 Hz, 1H, Napht-H6), 7.50 (d, J = 7.3 Hz, 1H, Ar-H4), 7.32 (t, J = 7.7 Hz, 1H, Ar-H6), 7.07 (t, J = 7.5 Hz, 1H, Ar-H5), 6.78 (d, J = 7.8 Hz, 1H, Ar-H7), 4.57 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.48 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 4.00 (d, J = 17.2 Hz, 1H, CO-CH2-C), 3.69 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.31 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.6, 176.3, 167.9, 142.3, 135.9, 133.7, 132.4, 130.6, 130.1, 129.9, 129.9, 128.9, 128.6, 127.8, 127.0, 124.3, 123.5, 108.6, 74.7, 62.0, 44.4, 41.4, 14.2. HRMS m/z: 404.1479 [M + H]+, calcd for C24H22NO5: 404.1492.
Ethyl 2-(3-(2-(furan-2-yl)-2-oxoethyl)-3-hydroxy-2-oxoindolin-1-yl)acetate (3q)
White powder, 44% yield, m.p. 136.2–137.7 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 1H, furan-H5), 7.44 (d, J = 7.4 Hz, 1H, Ar-H4), 7.30 (t, J = 7.6 Hz, 1H, Ar-H6), 7.23 (d, J = 3.5 Hz, 1H, furan-H4), 7.06 (t, J = 7.5 Hz, 1H, Ar-H5), 6.74 (d, J = 7.8 Hz, 1H, Ar-H7), 6.54 (d, J = 2.0 Hz, 1H, furan-H3), 4.88 (s, 1H, OH), 4.55 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.39 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.23 (q, J = 7.1 Hz, 2H, OCH2), 3.64 (d, J = 16.8 Hz, 1H, CO-CH2-C), 3.34 (d, J = 16.8 Hz, 1H, CO-CH2-C), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 187.0, 175.98, 167.6, 152.1, 147.4, 142.1, 130.0, 129.6, 124.4, 123.5, 118.8, 112.7, 108.6, 74.7, 61.9, 43.8, 41.4, 14.1. HRMS m/z: 344.1118 [M + H]+, calcd for C18H18NO6: 344.1129.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-(1H-pyrrol-2-yl)ethyl)indolin-1-yl)acetate (3r)
White powder, 76% yield, m.p. 149.9–151.1 °C. 1H NMR (400 MHz, CDCl3) δ 9.92 (s, 1H, NH), 7.42 (d, J = 7.3 Hz, 1H, Ar-H4), 7.30 (t, J = 7.7 Hz, 1H, Ar-H6), 7.07 (m, 2H, Ar-H5, pyrrol-H5), 6.89 (m, 1H, pyrrol-H3), 6.74 (d, J = 7.8 Hz, 1H, Ar-H7), 6.25 (d, J = 2.4 Hz, 1H, pyrrol-H4), 4.56 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.37 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.23 (q, J = 7.1 Hz, 2H, OCH2), 3.52 (d, J = 16.2 Hz, 1H, CO-CH2-C), 3.29 (d, J = 16.2 Hz, 1H, CO-CH2-C), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 188.0, 176.4, 167.7, 142.0, 131.6, 129.9, 129.8, 126.5, 124.3, 123.5, 118.5, 111.2, 108.5, 75.0, 61.9, 42.9, 41.4, 14.1. HRMS m/z: 343.1277 [M + H]+, calcd for C18H19N2O5: 343.1288.
Ethyl 2-(3-hydroxy-2-oxo-3-(2-oxo-2-(pyridin-2-yl)ethyl)indolin-1-yl)acetate (3s)
White powder, 93% yield, m.p. 160.4–161.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.70 (d, J = 4.4 Hz, 1H, Pyr-H6), 8.11 (d, J = 7.8 Hz, 1H, Pyr-H3), 7.93 (t, J = 7.3 Hz, 1H, Pyr-H4), 7.59–7.53 (m, 1H, Pyr-H5), 7.34 (t, J = 7.4 Hz, 1H, Ar-H4), 7.30 (t, J = 7.7 Hz, 1H, Ar-H6), 7.07 (t, J = 7.5 Hz, 1H, Ar-H5), 6.76 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (d, J = 17.5 Hz, 1H, N-CH2-CO), 4.38 (d, J = 17.5 Hz, 1H, N-CH2-CO), 4.23 (q, J = 7.1 Hz, 2H, OCH2), 3.90 (d, J = 15.3 Hz, 1H, CO-CH2-C), 3.59 (d, J = 15.3 Hz, 1H, CO-CH2-C), 1.27 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.3, 176.8, 167.5, 152.7, 148.4, 141.9, 137.9, 130.3, 129.8, 127.7, 124.1, 123.4, 122.6, 108.6, 74.5, 61.9, 46.9, 41.5, 14.1. HRMS m/z: 355.1275 [M + H]+, calcd for C19H19N2O5: 355.1288.
4.1.4. Method for the Synthesis of Target Compounds 4a–q
A mixture of the corresponding intermediate of 3a–q (200 mg) and HCl (aq, conc, 400 μL) in EtOH (2 mL) was stirred at reflux until the disappearance of the intermediate was evidenced by TLC. After the reaction mixture was cooled to room temperature, the resulting solid was filtered. The solid products were purified by silica gel column chromatography (silica gel, petroleum ether/ethyl acetate = 3:1).
Ethyl (E)-2-(2-oxo-3-(2-oxopropylidene)indolin-1-yl)acetate (4a)
Orange powder, 31% yield, m.p. 111.3–112.4 °C. 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 7.7 Hz, 1H, Ar-H4), 7.36 (td, J = 7.8, 1.1 Hz, 1H, Ar-H6), 7.22 (s, 1H, -CO-CH=C), 7.07 (td, J = 7.7, 0.8 Hz, 1H, Ar-H5), 6.68 (d, J = 7.8 Hz, 1H, Ar-H7), 4.48 (s, 2H, N-CH2-CO), 4.22 (q, J = 7.1 Hz, 2H, OCH2), 2.49 (s, 3H, COCH3), 1.26 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 198.4, 168.3, 167.3, 144.8, 134.7, 132.9, 128.4, 128.2, 123.3, 120.2, 108.2, 61.9, 41.5, 32.3, 14.1. HRMS m/z: 274.1067 [M + H]+, calcd for C15H16NO4: 274.1074.
Ethyl 2-((E)-2-oxo-3-((E)-2-oxo-4-phenylbut-3-en-1-ylidene)indolin-1-yl)acetate (4b)
Orange powder, 73% yield, m.p. 131.4–132.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.57 (d, J = 7.7 Hz, 1H, Ar-H4), 7.80 (d, J = 16.2 Hz, 1H, Ph-CH=), 7.69–7.61 (m, 2H, Ar-H2′, Ar-H6′), 7.58 (s, 1H, CO-CH=), 7.50–7.42 (m, 3H, Ar-H3′, Ar-H4′, Ar-H5′), 7.38 (t, J = 7.7 Hz, 1H, Ar-H6), 7.10 (t, J = 7.8 Hz, 1H, Ar-H5), 7.08 (d, J = 16.2 Hz, 1H, CO-CH=CH), 6.72 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.9, 168.3, 167.4, 145.4, 144.7, 135.5, 134.3, 132.7, 131.2, 129.1, 128.7, 128.4, 128.3, 127.5, 123.3, 120.4, 108.3, 61.9, 41.5, 14.2. HRMS m/z: 362.1377 [M + H]+, calcd for C22H20NO4: 362.1387.
Ethyl (E)-2-(2-oxo-3-(2-oxo-2-phenylethylidene)indolin-1-yl)acetate (4c)
Orange powder, 60% yield, m.p. 99.1–100.3 °C. 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 7.7 Hz, 1H, Ar-H4), 8.21–8.05 (m, 2H, Ar-H2′, Ar-H6′), 7.92 (s, 1H, Ar-CO-CH=C), 7.64 (t, J = 7.4 Hz, 1H, Ar-H4′), 7.54 (t, J = 7.6 Hz, 2H, Ar-H3′, Ar-H5′), 7.36 (t, J = 7.7 Hz, 1H, Ar-H6), 7.05 (t, J = 7.7 Hz, 1H, Ar-H5), 6.71 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 191.1, 168.0, 167.4, 144.7, 137.5, 135.9, 133.9, 132.6, 129.0, 128.9, 127.9, 126.9, 123.2, 120.2, 108.3, 61.9, 41.5, 14.2. HRMS m/z: 336.1222 [M + H]+, calcd for C20H18NO4: 336.1230.
Ethyl (E)-2-(2-oxo-3-(2-oxo-2-(p-tolyl)ethylidene)indolin-1-yl)acetate (4d)
Orange powder, 64% yield, m.p. 150.8–151.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 7.7 Hz, 1H, Ar-H4), 8.01 (d, J = 8.2 Hz, 2H, Ar-H2′, Ar-H6′), 7.90 (s, 1H, Ar-CO-CH=C), 7.34 (m, 3H, Ar-H6, Ar-H3′, Ar-H5′), 7.04 (t, J = 7.8 Hz, 1H, Ar-H5), 6.70 (d, J = 7.8 Hz, 1H, Ar-H7), 4.52 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 2.44 (s, 3H, CH3-Ar), 1.27 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 190.7, 168.0, 167.4, 145.0, 144.6, 135.5, 135.1, 132.4, 129.7, 129.0, 127.8, 127.3, 123.2, 120.2, 108.3, 61.9, 41.5, 21.8, 14.2. HRMS m/z: 350.1379 [M + H]+, calcd for C21H20NO4: 350.1387.
Ethyl (E)-2-(3-(2-(4-ethylphenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4e)
Orange powder, 66% yield, m.p. 109.4–110.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 7.7 Hz, 1H, Ar-H4), 8.06 (d, J = 8.3 Hz, 2H, Ar-H2′, Ar-H6′), 7.93 (s, 1H, Ar-CO-CH=C), 7.36 (m, 3H, Ar-H6, Ar-H3′, Ar-H5′), 7.06 (td, J = 7.7, 0.9 Hz, 1H, Ar-H5), 6.73 (d, J = 7.8 Hz, 1H, Ar-H7), 4.55 (s, 2H, N-CH2-CO), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 2.76 (q, J = 7.6 Hz, 2H, CH2-Ar), 1.37–1.23 (m, 6H, (CH3)2). 13C NMR (101 MHz, CDCl3) δ 190.8, 168.0, 167.4, 151.2, 144.6, 135.5, 135.3, 132.4, 129.1, 128.5, 127.8, 127.4, 123.2, 120.2, 108.3, 61.9, 41.5, 29.1, 15.2, 14.2. HRMS m/z: 364.1535 [M + H]+, calcd for C22H22NO4: 364.1543.
Ethyl (E)-2-(3-(2-(4-isopropylphenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4f)
Orange powder, 55% yield, m.p. 97.3–98.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 7.7 Hz, 1H, Ar-H4), 8.05 (d, J = 8.3 Hz, 2H, Ar-H2′, Ar-H6′), 7.91 (s, 1H, Ar-CO-CH=C), 7.38 (d, J = 8.3 Hz, 2H, Ar-H3′, Ar-H5′), 7.34 (td, J = 7.8, 1.0 Hz, 1H, Ar-H6), 7.04 (td, J = 7.7, 0.7 Hz, 1H, Ar-H5), 6.71 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.00 (m, 1H, CH), 1.45–1.18 (m, 9H, (CH3)3). 13C NMR (101 MHz, CDCl3) δ 190.7, 168.0, 167.4, 155.7, 144.6, 135.5, 135.4, 132.4, 129.2, 127.8, 127.4, 127.1, 123.2, 120.2, 108.3, 61.9, 41.5, 34.4, 23.7, 14.2. HRMS m/z: 378.1692 [M + H]+, calcd for C23H24NO4: 378.1700.
Ethyl (E)-2-(2-oxo-3-(2-oxo-2-(4-pentylphenyl)ethylidene)indolin-1-yl)acetate (4g)
Orange powder, 89% yield, m.p. 75.3–76.5 °C.1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 7.9 Hz, 1H, Ar-H4), 8.05 (d, J = 8.3 Hz, 2H, Ar-H2′, Ar-H6′), 7.93 (s, 1H, Ar-CO-CH=C), 7.38 (s, 1H, Ar-H6), 7.35 (d, J = 8.0 Hz, 2H, Ar-H3′, Ar-H5′), 7.06 (t, J = 7.2 Hz, 1H, Ar-H5), 6.73 (d, J = 7.8 Hz, 1H, Ar-H7), 4.55 (s, 2H, N-CH2-CO), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 2.71 (t, J = 7.8 Hz, 2H, Ar-CH2), 1.67 (m, 2H, Ar-CH2CH2), 1.38–1.31 (m, 7H, CH2CH2CH3, CH2CH2CH3, OCH2CH3), 0.92 (t, J = 6.6 Hz, 3H, CH2CH2CH3). 13C NMR (101 MHz, CDCl3) δ 190.8, 168.0, 167.4, 150.0, 144.6, 135.5, 135.3, 132.4, 129.0, 129.0, 127.8, 127.5, 123.2, 120.2, 108.3, 61.9, 41.5, 36.1, 31.4, 30.7, 22.5, 14.2, 14.0. HRMS m/z: 406.2004 [M + H]+, calcd for C25H28NO4: 406.2013.
Ethyl (E)-2-(3-(2-(4-methoxyphenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4h)
Orange powder, 42% yield, m.p. 105.1–106.8 °C. 1H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 7.7 Hz, 1H, Ar-H4), 8.10 (d, J = 8.8 Hz, 2H, Ar-H2′, Ar-H6′), 7.88 (s, 1H, Ar-CO-CH=C), 7.33 (t, J = 7.7 Hz, 1H, Ar-H6), 7.03 (t, J = 7.8 Hz, 1H, Ar-H5), 6.99 (d, J = 8.8 Hz, 2H, Ar-H3′, Ar-H5′), 6.70 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.89 (s, 3H, OCH3), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.6, 168.0, 167.5, 164.3, 144.4, 135.1, 132.3, 131.3, 130.6, 127.7, 127.6, 123.1, 120.2, 114.2, 108.3, 61.9, 55.6, 41.5, 14.2. HRMS m/z: 366.1330 [M + H]+, calcd for C21H20NO5: 366.1336.
Ethyl (E)-2-(3-(2-(4-ethoxyphenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4i)
Orange powder, 88% yield, m.p. 123.1–124.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 7.6 Hz, 1H, Ar-H4), 8.10 (d, J = 8.8 Hz, 2H, Ar-H2′, Ar-H6′), 7.90 (s, 1H, Ar-CO-CH=C), 7.34 (t, J = 7.5 Hz, 1H, Ar-H6), 7.04 (t, J = 7.7 Hz, 1H, Ar-H5), 6.99 (d, J = 8.8 Hz, 2H, Ar-H3′, Ar-H5′), 6.72 (d, J = 7.8 Hz, 1H, Ar-H7), 4.54 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 4.14 (q, J = 7.0 Hz, 2H, OCH2), 1.47 (t, J = 7.0 Hz, 3H, CH3), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.6, 168.1, 167.5, 163.8, 144.4, 135.0, 132.2, 131.4, 130.4, 127.8, 127.7, 123.2, 120.2, 114.6, 108.3, 64.0, 61.9, 41.5, 14.7, 14.2. HRMS m/z: 380.1485 [M + H]+, calcd for C22H22NO5: 380.1492.
Ethyl (E)-2-(2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (4j)
Orange powder, 84% yield, m.p. 127.1–128.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 7.7 Hz, 1H, Ar-H4), 7.87 (s, 1H, Ar-CO-CH=C), 7.51–7.30 (m, 3H, Ar-H6, Ar-H2′, Ar-H6′), 7.05 (t, J = 7.7 Hz, 1H, Ar-H5), 6.71 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.0 Hz, 2H, OCH2), 3.95 (s, 9H, 3′, 4′, 5′-(OCH3)3), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.9, 168.0, 167.4, 153.3, 144.6, 143.4, 135.9, 132.7, 132.6, 127.8, 127.0, 123.2, 120.1, 108.3, 106.2, 62.0, 61.1, 56.4, 41.5, 14.2. HRMS m/z: 426.1536 [M + H]+, calcd for C23H24NO7: 426.1547.
Ethyl (E)-2-(3-(2-(4-fluorophenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4k)
Orange powder, 64% yield, m.p. 123.4–124.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 7.6 Hz, 1H, Ar-H4), 8.16 (dd, J = 7.5, 5.8 Hz, 2H, Ar-H2′, Ar-H6′), 7.88 (s, 1H, Ar-CO-CH=C), 7.37 (t, J = 7.6 Hz, 1H, Ar-H6), 7.22 (t, J = 8.3 Hz, 2H, Ar-H3′, Ar-H5′), 7.07 (t, J = 7.6 Hz, 1H, Ar-H5), 6.73 (d, J = 7.8 Hz, 1H, Ar-H7), 4.54 (s, 2H, N-CH2-CO), 4.26 (q, J = 7.0 Hz, 2H, OCH2), 1.30 (t, J = 7.0 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.4, 167.9, 167.4, 166.2 (J = 257.6 Hz), 144.7, 136.2, 134.0 (J = 3.0 Hz), 132.8, 131.6 (J = 10.1 Hz), 127.9, 126.3, 123.2, 120.1, 116.2 (J = 22.2 Hz), 108.4, 62.0, 41.5, 14.2. HRMS m/z: 354.1129 [M + H]+, calcd for C20H17FNO4: 354.1136.
Ethyl (E)-2-(3-(2-(4-chlorophenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4l)
Orange powder, 64% yield, m.p. 130.3–131.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 7.7 Hz, 1H, Ar-H4), 8.06 (d, J = 8.5 Hz, 2H, Ar-H2′, Ar-H6′), 7.86 (s, 1H, Ar-CO-CH=C), 7.51 (d, J = 8.4 Hz, 2H, Ar-H3′, Ar-H5′), 7.37 (t, J = 7.7 Hz, 1H, Ar-H6), 7.07 (t, J = 7.7 Hz, 1H, Ar-H5), 6.72 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.67, 167.88, 167.36, 144.82, 140.42, 136.49, 135.91, 132.91, 130.22, 129.28, 128.06, 125.87, 123.26, 120.04, 108.38, 61.96, 41.50, 14.18. HRMS m/z: 370.0834 [M + H]+, calcd for C20H17ClNO4: 370.0841.
Ethyl (E)-2-(3-(2-(4-bromophenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4m)
Orange powder, 66% yield, m.p. 137.2–138.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 7.7 Hz, 1H, Ar-H4), 7.97 (d, J = 8.4 Hz, 2H, Ar-H2′, Ar-H6′), 7.84 (s, 1H, Ar-CO-CH=C), 7.67 (d, J = 8.4 Hz, 2H Ar-H3′, Ar-H5′), 7.36 (t, J = 7.7 Hz, 1H, Ar-H6), 7.06 (t, J =7.7 Hz, 1H, Ar-H5), 6.71 (d, J = 7.9 Hz, 1H, Ar-H7), 4.52 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.9, 167.9, 167.4, 144.8, 136.6, 136.3, 132.9, 132.3, 130.3, 129.3, 128.1, 125.8, 123.3, 120.1, 108.4, 62.0, 41.5, 14.2. HRMS m/z: 414.0327 [M + H]+, calcd for C20H17BrNO4: 414.0335.
Ethyl (E)-2-(3-(2-(4-nitrophenyl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4n)
Orange powder, 83% yield, m.p. 200.4–201.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.41 (d, J = 9.0 Hz, 2H, Ar-H3′, Ar-H5′), 8.32 (d, J = 9.0 Hz, 2H, Ar-H2′, Ar-H6′), 8.18 (d, J = 7.5 Hz, 1H, Ar-H4), 7.85 (s, 1H, Ar-CO-CH=C), 7.46 (td, J = 7.8, 1.1 Hz, 1H, Ar-H6), 7.09 (m, 2H, Ar-H5, Ar-H7), 4.68 (s, 2H, N-CH2-CO), 4.17 (q, J = 7.1 Hz, 2H, OCH2), 1.22 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 190.5, 168.2, 167.5, 150.7, 145.6, 142.0, 136.2, 133.9, 130.6, 127.3, 126.6, 124.6, 123.2, 119.6, 110.1, 61.8, 41.7, 14.5. HRMS m/z: 381.1074 [M + H]+, calcd for C20H17N2O6: 381.1081.
Ethyl (E)-2-(3-(2-([1,1′-biphenyl]-4-yl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4o)
Orange powder, 50% yield, m.p. 132.4–133.4 °C. 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 7.7 Hz, 1H, Ar-H4), 8.18 (d, J = 8.3 Hz, 2H, Diphenyl-H2, H6), 7.95 (s, 1H, Ar-CO-CH=C), 7.74 (d, J = 8.3 Hz, 2H, Diphenyl-H3, H5), 7.65 (d, J = 7.5 Hz, 2H, Diphenyl-H2′, H6′), 7.48 (t, J = 7.5 Hz, 2H, Diphenyl-H3′, H5′), 7.41 (t, J = 7.2 Hz, 1H, Diphenyl-H4′), 7.35 (t, J = 7.7 Hz, 1H, Ar-H6), 7.05 (t, J = 7.7 Hz, 1H, Ar-H5), 6.71 (d, J = 7.8 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 190.5, 168.0, 167.4, 146.5, 144.7, 139.6, 136.3, 135.9, 132.7, 129.5, 129.1, 128.5, 128.0, 127.6, 127.4, 126.9, 123.2, 120.2, 108.3, 62.0, 41.5, 14.2. HRMS m/z: 412.1535 [M + H]+, calcd for C26H22NO4: 412.1543.
Ethyl (E)-2-(3-(2-(naphthalen-2-yl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4p)
Orange powder, 85% yield, m.p. 134.5–135.4 °C. 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H, Napht-H1), 8.41 (d, J = 7.7 Hz, 1H, Ar-H4), 8.19 (dd, J = 8.6, 1.5 Hz, 1H, Napht-H4), 8.09 (s, 1H, Ar-CO-CH=C), 8.02 (d, J = 8.0 Hz, 1H, Napht-H8), 7.97 (d, J = 8.7 Hz, 1H, Napht-H5), 7.91 (d, J = 8.1 Hz, 1H, Napht-H3), 7.65 (t, J = 7.1 Hz, 1H, Napht-H7), 7.60 (t, J = 7.4 Hz, 1H, Napht-H6), 7.37 (t, J = 7.7 Hz, 1H, Ar-H6), 7.08 (t, J = 7.7 Hz, 1H, Ar-H5), 6.74 (d, J = 7.8 Hz, 1H, Ar-H7), 4.57 (s, 2H, N-CH2-CO), 4.28 (q, J = 7.1 Hz, 2H, OCH2), 1.31 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 190.9, 168.1, 167.5, 144.7, 135.9, 134.9, 132.6, 132.5, 131.3, 129.9, 129.1, 129.0, 127.9, 127.9, 127.1, 127.0, 123.9, 123.3, 120.2, 108.3, 62.0, 41.5, 14.2. HRMS m/z: 386.1379 [M + H]+, calcd for C24H20NO4: 386.1387.
Ethyl (E)-2-(3-(2-(furan-2-yl)-2-oxoethylidene)-2-oxoindolin-1-yl)acetate (4q)
Orange powder, 34% yield, m.p. 145.7–146.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 7.7 Hz, 1H, Ar-H4), 7.81 (s, 1H, Ar-CO-CH=C), 7.71 (d, J = 0.7 Hz, 1H, furan-H5), 7.44 (d, J = 3.5 Hz, 1H, furan-H3), 7.38 (t, J = 7.5 Hz, 1H, Ar-H6), 7.10 (t, J = 7.6 Hz, 1H, Ar-H5), 6.70 (d, J = 7.8 Hz, 1H, Ar-H7), 6.64 (dd, J = 3.5, 1.5 Hz, 1H, furan-H4), 4.52 (s, 2H, N-CH2-CO), 4.23 (q, J = 7.1 Hz, 2H, OCH2), 1.27 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 177.7, 168.1, 167.4, 154.0, 147.7, 145.0, 137.0, 133.1, 129.2, 124.9, 123.3, 120.3, 119.2, 113.0, 108.2, 61.9, 41.5, 14.2. HRMS m/z: 326.1017 [M + H]+, calcd for C18H16NO5: 326.1023.
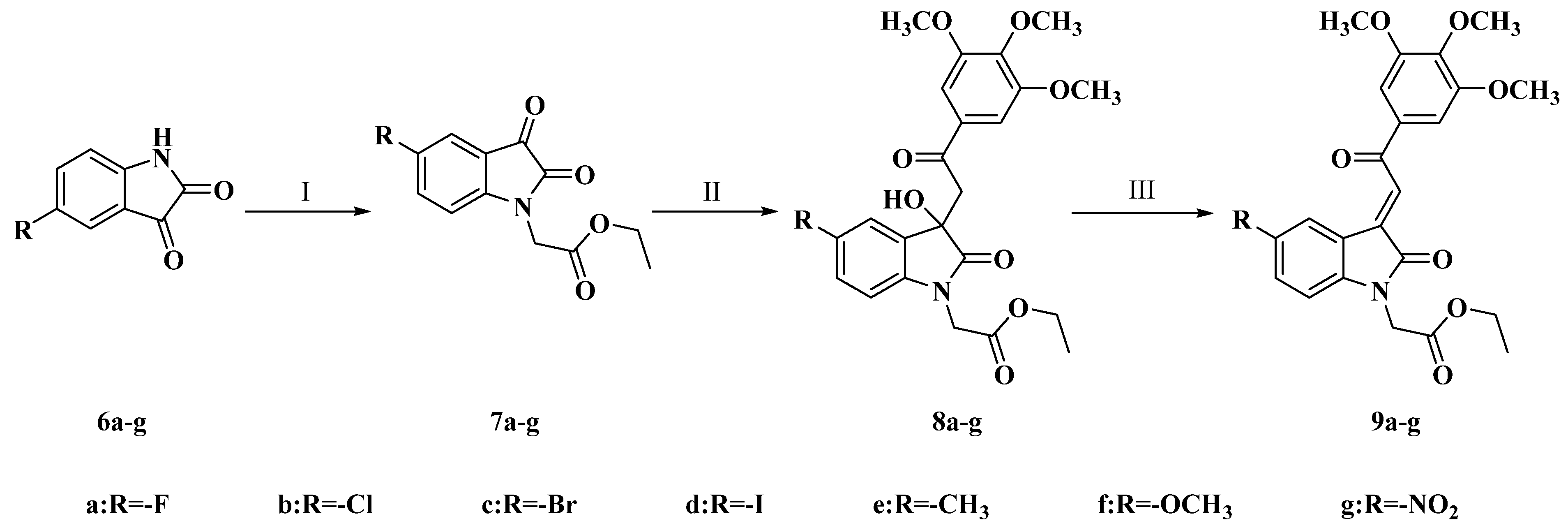
4.1.17. Method for the Synthesis of Intermediate 8a–g
To a solution of compounds 7a–g (300 mg) in EtOH (400 μL), Et2NH (1.2 eq) and 3,4,5-Trimethoxyphenylethanone (1.2 eq) were added. The reaction mixture was stirred at room temperature until the complete disappearance of 7a–g was evidenced by TLC. The solvent was removed under vacuum, 5 mL of water was added to the residue, and then the mixture was extracted with ethyl acetate (3 × 10 mL). The organic fractions were combined, dried over Na2SO4, filtered and concentrated. The crude product was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 1:1).
Ethyl 2-(5-fluoro-3-hydroxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8a)
White powder, 61% yield, m.p. 138.6–139.3 °C. 1H NMR (400 MHz, CDCl3) δ 7.23 (dd, J = 7.7, 2.6 Hz, 1H, Ar-H4), 7.18 (s, 2H, Ar-H2′, Ar-H6′), 7.01 (td, J = 8.8, 2.6 Hz, 1H, Ar-H6), 6.70 (dd, J = 8.6, 4.0 Hz, 1H, Ar-H7), 4.56 (d, J = 17.7 Hz, 1H, N-CH2-CO), 4.41 (d, J = 17.7 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.91 (d, J = 9.3 Hz, 9H, (OCH3)3), 3.81 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.50 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.2, 175.9, 167.5, 159.6 (d, J = 242.5 Hz), 153.1, 143.5, 138.1 (d, J = 2.2 Hz), 131.5 (d, J = 7.7 Hz), 131.3, 116.1 (d, J = 23.8 Hz), 112.7 (d, J = 24.9 Hz), 109.3 (d, J = 8.1 Hz), 105.8, 74.8, 62.0, 61.0, 56.3, 43.9, 41.5, 14.1. HRMS m/z: 462.1540 [M + H]+, calcd for C23H25FNO8: 462.1559.
Ethyl 2-(5-chloro-3-hydroxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8b)
White powder, 71% yield, m.p. 102.5–103.2 °C. 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 2.1 Hz, 1H, Ar-H4), 7.29 (d, J = 3.3 Hz, 1H, Ar-H6), 7.17 (s, 2H, Ar-H2′, Ar-H6′), 6.70 (d, J = 8.4 Hz, 1H, Ar-H7), 4.54 (d, J = 17.7 Hz, 1H, N-CH2-CO), 4.42 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.91 (d, J = 9.6 Hz, 9H, (OCH3)3), 3.82 (d, J = 17.4 Hz, 1H, CO-CH2-C), 3.53 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.0, 175.8, 167.5, 153.1, 143.4, 140.9, 131.6, 131.2, 129.8, 128.8, 125.0, 109.7, 105.8, 74.6, 62.1, 61.0, 56.3, 44.0, 41.5, 14.1. HRMS m/z: 478.1247 [M + H]+, calcd for C23H25ClNO8: 478.1263.
Ethyl 2-(5-bromo-3-hydroxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8c)
White powder, 69% yield, m.p. 96.9–98.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 1H, Ar-H4), 7.44 (d, J = 8.4 Hz, 1H, Ar-H6), 7.18 (s, 2H, Ar-H2′, Ar-H6′), 6.66 (d, J = 8.3 Hz, 1H, Ar-H7), 4.88 (s, 1H, OH), 4.54 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.42 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.24 (q, J = 7.1 Hz, 2H, OCH2), 3.92 (d, J = 8.9 Hz, 9H, (OCH3)3), 3.81 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.52 (d, J = 17.3 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.0, 175.7, 167.4, 153.1, 143.5, 141.4, 132.7, 131.9, 131.2, 127.7, 116.1, 110.2, 105.8, 74.5, 62.1, 61.0, 56.3, 44.0, 41.4, 14.1. HRMS m/z: 522.0741 [M + H]+, calcd for C23H25BrNO8: 522.0758.
Ethyl 2-(3-hydroxy-5-iodo-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8d)
White powder, 31% yield, m.p. 111.4–112.7 °C. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 1.7 Hz, 1H, Ar-H4), 7.62 (dd, J = 8.2, 1.7 Hz, 1H, Ar-H6), 7.17 (s, 2H, Ar-H2′, Ar-H6′), 6.56 (d, J = 8.2 Hz, 1H, Ar-H7), 4.87 (s, 1H, OH), 4.52 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.41 (d, J = 17.7 Hz, 1H, N-CH2-CO), 4.23 (q, J = 7.2 Hz, 2H, OCH2), 3.91 (d, J = 9.7 Hz, 9H, (OCH3)3), 3.80 (d, J = 17.3 Hz, 1H, CO-CH2-C), 3.53 (d, J = 17.4 Hz, 1H, CO-CH2-C), 1.30 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.0, 175.5, 167.4, 153.1, 143.4, 142.1, 138.7, 133.2, 132.2, 131.3, 110.7, 105.8, 86.0, 74.4, 62.1, 61.0, 56.3, 44.0, 41.4, 14.1. HRMS m/z: 570.0600 [M + H]+, calcd for C23H25INO8: 570.0619.
Ethyl 2-(3-hydroxy-5-methyl-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8e)
White powder, 29% yield, m.p. 131.7–132.2 °C. 1H NMR (400 MHz, CDCl3) δ 7.27 (d, J = 4.8 Hz, 1H, Ar-H4), 7.18 (s, 2H, Ar-H2′, Ar-H6′), 7.11 (d, J = 8.7 Hz, 1H, Ar-H6), 6.66 (d, J = 7.9 Hz, 1H, Ar-H7), 4.55 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.38 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.22 (q, J = 7.2 Hz, 2H, OCH2), 3.90 (d, J = 10.4 Hz, 9H, (OCH3)3), 3.79 (d, J = 17.1 Hz, 1H, CO-CH2-C), 3.49 (d, J = 17.1 Hz, 1H, CO-CH2-C), 2.31 (s, 3H, Ar-CH3), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.6, 176.2, 167.7, 153.1, 143.2, 139.8, 133.1, 131.6, 130.2, 129.9, 125.1, 108.3, 105.8, 74.9, 61.8, 61.0, 56.3, 43.8, 41.4, 21.1, 14.1. HRMS m/z: 458.1796 [M + H]+, calcd for C24H28NO8: 458.1809.
Ethyl 2-(3-hydroxy-5-methoxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8f)
White powder, 34% yield, m.p. 131.7–132.9 °C. 1H NMR (400 MHz, CDCl3) δ 7.18 (s, 2H, Ar-H2′, Ar-H6′), 7.08 (d, J = 2.6 Hz, 1H, Ar-H4), 6.83 (dd, J = 8.5, 2.6 Hz, 1H, Ar-H6), 6.68 (d, J = 8.5 Hz, 1H, Ar-H7), 4.55 (d, J = 17.6 Hz, 1H, N-CH2-CO), 4.37 (d, J = 17.5 Hz, 1H, N-CH2-CO), 4.23 (q, J = 7.1 Hz, 2H, OCH2), 3.90 (d, J = 10.8 Hz, 9H, (OCH3)3), 3.76 (d, J = 17.1 Hz, 4H, CO-CH2-C, Ar-OCH3), 3.48 (d, J = 17.1 Hz, 1H, CO-CH2-C), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 197.5, 176.1, 167.7, 156.5, 153.1, 143.3, 135.5, 131.5, 131.1, 114.3, 111.7, 109.1, 105.8, 75.1, 61.9, 61.0, 56.3, 55.8, 43.8, 41.5, 14.1. HRMS m/z: 474.1741 [M + H]+, calcd for C24H28NO9: 474.1759.
Ethyl 2-(3-hydroxy-5-nitro-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethyl)indolin-1-yl)acetate (8g)
White powder, 73% yield, m.p. 156.4–157.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 2.2 Hz, 1H, Ar-H4), 8.26 (d, J = 8.6 Hz, 1H, Ar-H6), 7.14 (s, 2H, Ar-H2′, Ar-H6′), 6.86 (d, J = 8.6 Hz, 1H, Ar-H7), 4.60 (d, J = 17.8 Hz, 2H, N-CH2-CO), 4.27 (q, J = 7.1 Hz, 2H, OCH2), 3.95 (d, J = 17.6 Hz, 1H, CO-CH2-C), 3.90 (d, J = 9.0 Hz, 9H, (OCH3)3), 3.70 (d, J = 17.6 Hz, 1H, CO-CH2-C), 1.32 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 196.2, 176.3, 167.2, 153.1, 148.2, 143.9, 143.5, 130.9, 130.8, 126.9, 120.1, 108.5, 105.8, 73.8, 62.4, 61.0, 56.3, 44.7, 41.6, 14.1. HRMS m/z: 489.1487 [M + H]+, calcd for C23H25N2O10: 489.1504.
4.1.18. Method for the Synthesis of Target Compounds 9a–g
A mixture of the corresponding intermediate of 8a–g (200 mg) and HCl (aq, conc, 400 μL) in EtOH (2 mL) was stirred at reflux until its disappearance was evidenced by TLC. After the reaction mixture was cooled to room temperature, the resulting solid product was filtered. The solid products were purified by silica gel column chromatography (silica gel, petroleum ether/ethyl acetate = 3:1).
Ethyl (E)-2-(5-fluoro-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9a)
Orange powder, 75% yield, m.p. 147.8–148.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.15 (dd, J = 9.0, 2.7 Hz, 1H, Ar-H4), 7.95 (s, 1H, Ar-CO-CH=C), 7.36 (s, 2H, Ar-H2′, Ar-H6′), 7.09 (td, J = 8.6, 2.7 Hz, 1H, Ar-H6), 6.66 (dd, J = 8.6, 4.1 Hz, 1H, Ar-H7), 4.53 (s, 2H, N-CH2-CO), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 3.97 (d, J = 2.1 Hz, 9H, 3′, 4′, 5′-(OCH3)3), 1.30 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.4, 167.8, 167.3, 159.1 (d, J = 240.3 Hz), 153.4, 143.7, 140.8, 135.6 (d, J = 3.3 Hz), 132.6, 128.0, 121.1 (d, J = 9.9 Hz), 118.9 (d, J = 24.2 Hz), 115.5 (d, J = 26.8 Hz), 108.8 (d, J = 8.1 Hz), 106.4, 62.0, 61.1, 56.5, 41.6, 14.1. HRMS m/z: 444.1446 [M + H]+, calcd for C23H23FNO7: 444.1453.
Ethyl (E)-2-(5-chloro-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9b)
Orange powder, 57% yield, m.p. 166.5–167.1 °C. 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H, Ar-H4), 7.93 (s, 1H, Ar-CO-CH=C), 7.36 (m, 3H, Ar-H2′, Ar-H6′, Ar-H6), 6.66 (d, J = 8.3 Hz, 1H, Ar-H7), 4.52 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.2 Hz, 2H, OCH2), 3.97 (s, 9H, 3′, 4′, 5′-(OCH3)3), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.3, 167.6, 167.1, 153.4, 143.7, 143.1, 135.1, 132.6, 132.2, 128.6, 128.1, 128.0, 121.3, 109.3, 106.4, 77.4, 77.1, 76.7, 62.0, 61.1, 56.5, 41.5, 14.1. HRMS m/z: 460.1149 [M + H]+, calcd for C23H23ClNO7: 460.1158.
Ethyl (E)-2-(5-bromo-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9c)
Orange powder, 75% yield, m.p. 171.6–172.4 °C. 1H NMR (400 MHz, CDCl3) δ 8.52 (s, 1H, Ar-H4), 7.93 (s, 1H, Ar-CO-CH=C), 7.49 (dd, J = 8.3, 2.0 Hz, 1H, Ar-H6), 7.36 (s, 2H, Ar-H2′, Ar-H6′), 6.62 (d, J = 8.3 Hz, 1H, Ar-H7), 4.52 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.97 (s, 9H, 3′, 4′, 5′-(OCH3)3), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.2, 167.4, 167.1, 153.4, 143.7, 143.6, 135.0, 134.9, 132.6, 130.7, 128.1, 121.7, 115.9, 109.7, 106.4, 62.0, 61.1, 56.5, 41.5, 14.1. HRMS m/z: 504.0643 [M + H]+, calcd for C23H23BrNO7: 504.0652.
Ethyl (E)-2-(5-iodo-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9d)
Orange powder, 60% yield, m.p. 172.0–173.3 °C. 1H NMR (400 MHz, CDCl3) δ 8.68 (s, 1H, Ar-H4), 7.91 (s, 1H, Ar-CO-CH=C), 7.69 (d, J = 8.3 Hz, 1H, Ar-H6), 7.36 (s, 2H, Ar-H2′, Ar-H6′), 6.52 (d, J = 8.2 Hz, 1H, Ar-H7), 4.52 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.0 Hz, 2H, OCH2), 3.97 (s, 9H, 3′, 4′, 5′-(OCH3)3), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.3, 167.3, 167.1, 153.4, 144.2, 143.7, 141.0, 136.3, 134.7, 132.6, 128.0, 122.1, 110.3, 106.4, 85.8, 62.1, 61.1, 56.5, 41.5, 14.2. HRMS m/z: 552.0504 [M + H]+, calcd for C23H23INO7: 552.0514.
Ethyl (E)-2-(5-methyl-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9e)
Orange powder, 62% yield, m.p. 157.4–158.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.14 (s, 1H, Ar-H4), 7.86 (s, 1H, Ar-CO-CH=C), 7.36 (s, 2H, Ar-H2′, Ar-H6′), 7.17 (d, J = 7.9 Hz, 1H, Ar-H6), 6.61 (d, J = 8.0 Hz, 1H, Ar-H7), 4.51 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.96 (s, 9H, 3′, 4′, 5′-(OCH3)3), 2.34 (s, 3H, Ar-CH3), 1.29 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.9, 168.0, 167.5, 153.3, 143.4, 142.5, 136.2, 133.0, 132.9, 132.7, 128.4, 126.5, 120.1, 108.0, 106.3, 61.9, 61.0, 56.4, 41.5, 21.1, 14.1. HRMS m/z: 440.1695 [M + H]+, calcd for C24H26NO7: 440.1704.
Ethyl (E)-2-(5-methoxy-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9f)
Brown powder, 69% yield, m.p. 157.9–158.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 2.6 Hz, 1H, Ar-H4), 7.88 (s, 1H, Ar-CO-CH=C), 7.36 (s, 2H, Ar-H2′, Ar-H6′), 6.92 (dd, J = 8.6, 2.6 Hz, 1H, Ar-H6), 6.63 (d, J = 8.5 Hz, 1H, Ar-H7), 4.50 (s, 2H, N-CH2-CO), 4.25 (q, J = 7.1 Hz, 2H, OCH2), 3.97 (s, 9H, 3′, 4′, 5′-(OCH3)3), 3.82 (s, 3H, 5-OCH3), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 189.7, 167.9, 167.5, 156.0, 153.3, 143.4, 138.6, 136.4, 132.9, 126.9, 120.8, 118.5, 113.7, 108.8, 106.3, 61.9, 61.0, 56.5, 55.9, 41.6, 14.1. HRMS m/z: 456.1642 [M + H]+, calcd for C24H26NO8: 456.1653.
Ethyl (E)-2-(5-nitro-2-oxo-3-(2-oxo-2-(3,4,5-trimethoxyphenyl)ethylidene)indolin-1-yl)acetate (9g)
Orange powder, 72% yield, m.p. 179.5–180.8 °C. 1H NMR (400 MHz, CDCl3) δ 9.29 (d, J = 2.3 Hz, 1H, Ar-H4), 8.33 (dd, J = 8.7, 2.3 Hz, 1H, Ar-H6), 8.04 (s, 1H, Ar-CO-CH=C), 7.38 (s, 2H, Ar-H2′, Ar-H6′), 6.85 (d, J = 8.7 Hz, 1H, Ar-H7), 4.61 (s, 2H, N-CH2-CO), 4.28 (q, J = 7.1 Hz, 2H, OCH2), 3.98 (s, 9H, 3′, 4′, 5′-(OCH3)3), 1.32 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 188.7, 167.9, 166.7, 153.4, 149.2, 144.0, 143.8, 133.9, 132.3, 129.8, 128.5, 123.7, 120.4, 108.2, 106.6, 62.3, 61.1, 56.5, 41.7, 14.1. HRMS m/z: 471.1391 [M + H]+, calcd for C23H23N2O9: 471.1398.