Abstract
Iodosulfonylation of an ethynyl group at the C2 position of 2′-deoxyadenosine or adenosine with TsI provides (E)-2-(β-iodovinyl)sulfones. The latter undergo nucleophilic substitution with amines via an addition–elimination to give β-sulfonylvinylamines (enamines). Acid-catalyzed hydrolysis of the β-sulfonylvinylamines provides 2-(β-keto)sulfones, mechanistically different probes that react with alkyl halides, resulting in α-alkylation. Adenine nucleosides with a β-ketosulfone group at C2, during conversion to their 5′-triphosphate form, undergo an unexpected conversion to 2-carboxylic acid nucleotides. The 5′-triphosphate of 2′-deoxyadenosine-2-carboxylic acid was incorporated by a human DNA polymerase into a one-nucleotide gap DNA substrate.
1. Introduction
Modification of purine nucleobases provides a strategy for the development of anticancer and antiviral nucleoside therapeutics [1,2]. Methods for the synthesis of C2-substituted purine nucleosides, although, in general, more challenging than those for the C8-substituted counterparts, can now be achieved quite efficiently using several strategies which include (i) nucleophilic aromatic substitutions [1], (ii) diazoniation/dediazoniation sequences [3], (iii) lithiation followed by captures of electrophiles [4], and most effectively by (iv) transition metal-catalyzed cross-coupling reactions [5,6,7], which include methods for direct C-H activation [8], and recently reported (v) regioselective homolytic C2-H borylation through photocatalysis or thermal activation [9].
Purine nucleosides with C2 substitution are a frequently occurring motif in approved drugs and bioactive adenine derivatives [9,10,11,12,13,14], including adenosine receptor ligands [15,16]. Matsuda and Jacobsen groups described C2-substituted adenine nucleosides as adenosine A2 receptor agonists [17], and A2A adenosine receptor antagonists [18], respectively. dATP modified with a reactive formyl group at C2 is used for crosslinking with peptides and proteins, as well as for fluorescent labelling [19]. 2-methyladenosine (m2A) promotes protein translation by facilitating the decoding of tandem m2A-tRNA-dependent codons [20], and the introduction of hydrophilic PEG substituents at the C2 position of adenine improves water solubility without affecting binding properties [21].
The Hocek group has reported C2-substituted dATP derivatives bearing Cl, NH2, CH3, vinyl, ethynyl, and phenyl substituents to study the effect of substituents on their polymerase-catalyzed DNA incorporation [22]. They found that all of dATP derivatives were good substrates in primer-extension experiments, except for the bulky 2-phenyl-dATP. Among them, the vinyl-modified DNA reacted with the thiol 4-methylmercaptocoumarin, and ethynyl-modified DNA underwent reaction with Cy3 azide to enzymatically label the minor grove via nucleobase modification. Previously, 2-chloroadenine [23] and 2,6-diaminopurine [24,25] dNTPs were the only reported minor-groove base-modified nucleotides as substrates for DNA polymerases. The Hocek group also reported 2-alkylamino-2′-deoxyadenosine triphosphates, which incorporated only one modified nucleotide into the primer by Terminator DNA polymerase [26]. The allylamino-substituted DNA was utilized for the thiol–ene addition, while the propargylamino-DNA reacted with an azide to label the DNA with a fluorescent dye in the minor groove. Also, 2-substituted 2′-deoxyinosine triphosphates containing a CH3 or vinyl group were shown to be good substrates for DNA polymerase and for the enzymatic synthesis of minor-groove base-modified DNA [27].
We reported 5-(1-halo-2-tosylvinyl) pyrimidine nucleosides, their reactions with nucleophiles, and their crosslinking with amino acids via an addition–elimination reaction [28,29,30], as well as the reactivity of the corresponding 5-(2-tosylacetyl) analogues with electrophiles and their polymerase-catalyzed incorporation into DNA [30]. Recently, we developed purine nucleosides modified at C8 with (β-halovinyl)sulfone, and (β-keto)sulfone probes and determined their DNA incorporation by human or Escherichia coli DNA–polymerase-catalyzed reactions [31]. Herein, we describe adenine nucleosides modified at C2 with reactive (β-halovinyl)sulfone or β-ketosulfone group, as well as the DNA incorporation of 2′-deoxyadenosine-2-carboxylic acid triphosphate by a DNA–polymerase-catalyzed reaction.
2. Results and Discussions
The substrates 2-iodo-2′-deoxyadenosine 1 [32] and 2-iodoadenosine 2 [33] (Scheme 1) were synthesized as reported. Standard tert-butyldimethylsilyl (TBS) protection of 1 and 2, followed by the Sonogashira coupling of the resulting 3 [34] and 4 [5], and selective removal of trimethylsilyl (TMS) protection from the 2-ethynyl group, gave the precursors 3′,5′-di-O-TBS-2-ethynyl-2′-deoxyadenosine 5 and 2′,3′,5′-tri-O-TBS-2-ethynyladenosine 6.
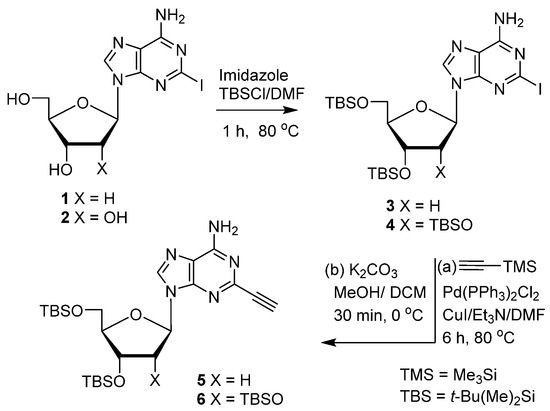
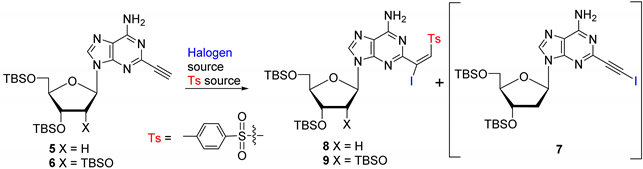
Scheme 1.
Synthesis of 2-ethynyladenine nucleoside substrates.
We initially attempted iodosulfonylation [35,36] of 5 with the I2/TsNa/NaOAc [37] (MeCN/80 °C) protocol, which was successful for the iodovinylsulfonylation of 8-alkynyladenine nucleosides [31]. However, the isolated product was identified as 2-(iodoethynyl)-2′-deoxyadenosine 7 (80%; Table 1, entry 1). An analogous reaction in the absence of NaOAc in H2O or H2O/MeCN mixture also provided 7 but with lower yields (entries 2 and 3). It is noteworthy that such iodination of the terminal alkyne was also observed with 8-ethynyl-2′-deoxyguanosine but not with the adenine counterpart [31]. Also, iodosulfonylation of 5 with TsNHNH2/KI/benzoyl peroxide failed to give the expected product 8, resulting only in partial removal of the TBS-protecting groups (entry 4), although these conditions were successful with 8-alkynylguanine nucleosides [31]. The use of CuI as a source of iodide (entry 5) and application of other methods [38,39,40,41] were also not successful (entries 6 and 7). Furthermore, treatment of 5 with freshly prepared TsI [42] in THF, although it effected iodosulfonylation, resulted mainly in the cleavage of the glycosidic bond (entry 8). However, a similar iodosulfonylation of 5 with TsI (2 equiv.) in the presence of NaOAc (3 equiv.) afforded the expected iodovinylsulfone 8 as a single E-isomer (81%; entry 9). Analogous treatment of the adenosine substrate 6 gave 9 in even higher yield (E; 92%, entry 10). This condition has a general character and was also successful for the iodosulfonylation of 8-alkynyl nucleosides. For example, treatment of 3′,5′-tri-O-(tert-butyldimethylsilyl)-8-ethynyl-2′-deoxyadenosine with TsI in the presence of NaOAc gave (E)-3′,5′-tri-O-(tert-butyldimethylsilyl)-8-(1-iodo-2-tosylvinyl)-2′-deoxyadenosine [31] in 78% yield.

Table 1.
Synthesis of C2-modified β-iodovinylsulfones of 2′-deoxyadenosine and adenosine.
Treatment of 8 or 9 with NH4F/AcOH in MeOH at 60 °C for 6 h gave unprotected 2-(β-iodovinyl)sulfone 10 (78%) or 11 (80%) as single E-isomers (Scheme 2). Subsequent treatment of 10 or 11 with NH3/MeOH (rt, 1 h) provided the corresponding (Z)-β-aminovinylsulfones 14 (70%) or 15 (70%). The Z stereochemistry is believed to be the result of hydrogen bonding between the hydrogen from the NH2 group and the oxygen from a sulfonyl group [43,44]. The protected (Z)-β-aminovinylsulfones 12 and 13 were analogously prepared from 8 or 9. Reaction of 14 or 15 with AcOH (aq) in MeOH (rt, 24 h) effected efficient conversion to the β-ketosulfones [37,45] 18 (84%) or 19 (82%). Similarly, TBS-protected β-ketosulfones 16 (86%) and 17 (85%) were prepared from 12 or 13. Subsequent removal of the silyl protection group with tetrabutylammonium fluoride (TBAF) gave unprotected β-ketosulfones 18 (87%) and 19 (88%).
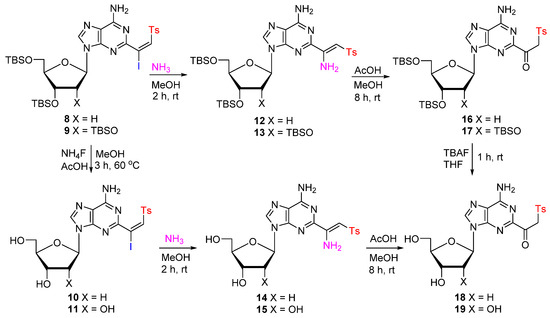
Scheme 2.
Synthesis of C2-modified β-ketosulfone from (β-iodo)vinylsulfone.
As expected [1,2,9], the C2-modified (at electron-deficient pyrimidine ring) adenine nucleosides have different physical, chemical, and spectroscopical properties as compared to C8-modified (at electron-excessive imidazole ring) counterparts [31]. For example, C2 aminovinylsulfones 12–15 partially convert into corresponding β-ketosulfones 16–19 during silica column purification, while C8 aminovinylsulfones [31] are stable on the silica column. Polarity and Rf value for TLC (Thin-Layer Chromatography) are also quite different, probably due to different internal hydrogen bonding. Especially, the β-ketosulfones at the C2 position of purine rings 16–19 are much polar than the corresponding C8 analogues.
(β-halovinyl)sulfones react efficiently with nucleophiles [28,30,31,36]. Thus, treatment of (β-iodovinyl)sulfone 11 with propanethiol (1.0 equiv) in the presence of Et3N (1.2 equiv) at rt for 24 h afforded (β-thiovinyl)sulfones 20 (60%) as a single E-isomer (Scheme 3). Reaction of 11 with diisopropylamine in MeOH (rt, 20 min) gave β-(dialkylamino)vinyl sulfone 21 (E/Z, ~46:54; 74%).
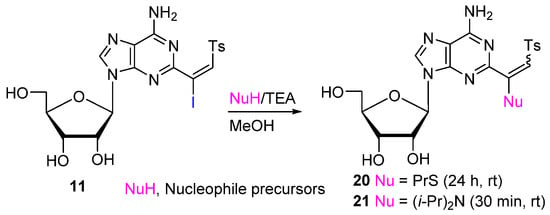
Scheme 3.
Reactions of 2-(β-iodovinyl)sulfone of adenosine with nucleophiles.
Reactivity of β-ketosulfone probes was tested in reactions with electrophiles. Thus, treatment of 19 with benzyl bromide (BnBr) in the presence of NaOH (aq) at rt afforded a diastereotopic mixture of the α-monobenzylated product 22 (50/50; 75%) with no α,α-dialkylated byproduct observed (Scheme 4). Alkylation of 19 with allyl bromide gave a diastereotopic mixture of α-allylated ketone 23 (50/50; 68%). These results demonstrate that β-ketosulfone and (β-iodovinyl)sulfone groups at the C2 position of the adenine ring can act as mechanistically different probes for further modification and potential covalent inhibition of proteins after incorporation into oligonucleotide fragments.
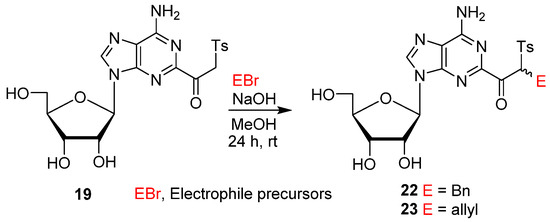
Scheme 4.
Alkylation of the 2-(β-ketosulfone) of adenosine.
5′-Phosphorylation of 18 with POCl3 in the presence of proton sponge followed by treatment with tributylammonium pyrophosphate (TBAPP, 5.0 equiv.) and tributylamine (TBA, 4.0 equiv.) at 0 °C did not provide the expected 5′-triphosphate of β-ketosulfone 26 (Scheme 5). Surprisingly, purification of the crude phosphorylation mixture on a Sephadex column with triethylammonium bicarbonate buffer (TEAB) provided the 2-carboxylic acid of 2′-deoxyadenosine-5′-triphosphate 24 (2-CdATP; 25%). Analogous phosphorylation of 19 led to the formation of the 2-carboxylic acid derivative of adenosine-5′-triphosphate 25 (2-CATP; 22%), instead of the expected 27.
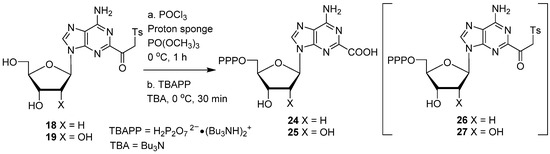
Scheme 5.
Conversion of 2-(β-ketosulfone) group of adenine nucleosides to adenine-2-carboxylate derivatives.
To confirm the instability of the β-ketosulfone moiety at the adenine C2 position during phosphorylation and/or purification steps, we treated 18 and 19 with the Sephadex resin in 0.5 M TEAB solution at rt overnight. TLC and 1H NMR analysis showed stability of 18 and 19 under these conditions, which might indicate that the reagents used for the phosphorylation and/or the presence of the triphosphate group at C5′ might be involved in/facilitate the conversion β-ketosulfones to carboxylic acids 24 or 25. The tentative pathway for this unexpected conversion of the β-ketosulfone group to carboxylic acid with the concomitant loss of (chloromethyl)sulfonyl fragment E is presented in Scheme 6. Thus, the initial reaction of POCl3 with the enol form of β-ketosulfones A yielded the dichlorophosphate ester B. Subsequent intramolecular electrophilic chlorination [46] produced the oxo carbocation C. The ensuing SN2 substitution at the trivalent phosphorus atom [47] D with concomitant elimination of the (chloromethyl)sulfone fragment [48,49] E led to the formation of a carboxylic acid. It is noteworthy that adenine nucleosides substituted with β-ketosulfone moiety at C8 were stable under the same phosphorylation conditions [31].
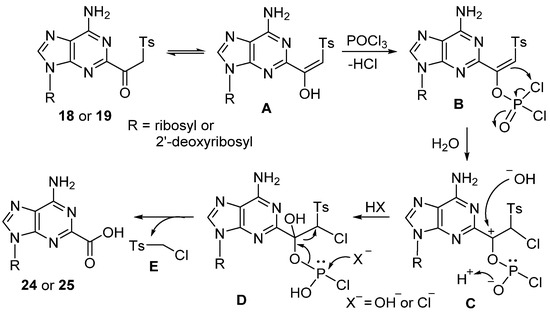
Scheme 6.
Tentative mechanism for the formation of 2-carboxylic acid of adenine nucleosides from C2 β-ketosulfones via electrophilic chlorination. SN2 substitution at trivalent phosphorus atom and cleavage of carbon–carbon bond with elimination of (chloromethyl)sulfone fragment.
To verify the structure of 2-CATP 25, an independent synthesis of 25 was undertaken. Our initial effort to oxidize 2-methyladenosine [6] with basic KMnO4 at reflux failed to produce adenosine-2-carboxylic acid. Also, hydrolysis of 2-cyanoadenosine 28 [50] with NaOH did not succeed in producing adenosine-2-carboxylic acid. However, treatment of 28 with NaOMe, followed by hydrolysis with dilute HCl (aq), afforded the corresponding methyl ester 29 (80%; Scheme 7). 5′-Phosphorylation of 29 and hydrolysis of the carboxylic ester with aqueous NaOH afforded 25 in a 25% yield after Sephadex purification. Triphosphate 25 prepared using both methods (Scheme 5 and Scheme 7) has identical NMR spectra, and its structure was also confirmed by HRMS (High-Resolution Mass Spectrometry). Analogues 24 and 25 can be considered as displaced isoguanosine analogues [51] in which the 2 carboxylate group can be involved in hydrogen bonding, instead of the C2 keto group present in isoguanosine [51,52]. The reactive carboxylic group can be explored for further bioconjugation [53,54,55] with reactive fluorescent amines and DNA–protein crosslink.
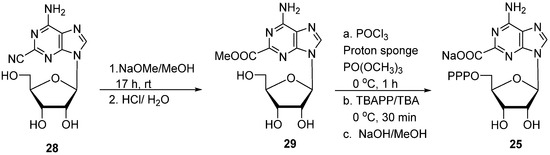
Scheme 7.
Alternative synthesis of 2-carboxylic acid of adenosine-5′-triphosphate 25.
The incorporation of triphosphate 24 into a duplex DNA by Pol β was examined by incubating a DNA (50 nM) substrate containing a 1 nucleotide (nt) gap and 5′-phosphate with increasing concentrations of Pol β (1 nM–100 nM) in the presence of 24 (50 µM) (Figure 1). The results showed that Pol β readily incorporated 24 to fill in the 1 nt gap at a low concentration of 5 nM (Figure 1, lane 2). Increasing concentrations of Pol β from 5 nM to 25 nM led to significantly elevated incorporation of nucleotide 24 (lanes 2–5). However, Pol β at 50 nM and 100 nM exhibited a similar percentage of nucleotide insertion products as 25 nM (compare lanes 6–7 with lane 5). The results indicate that nucleotide 24 can be readily incorporated into the DNA substrate by a repair DNA polymerase.
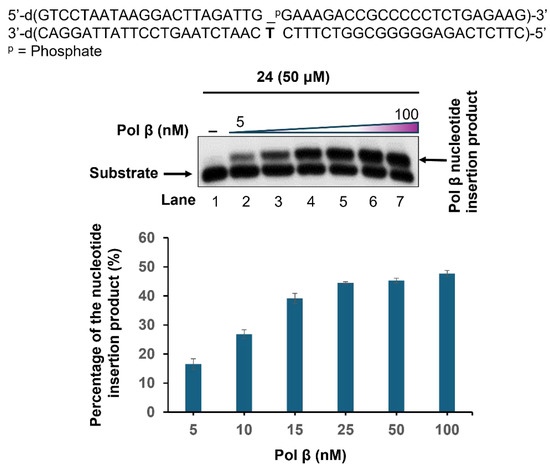
Figure 1.
Incorporation of triphosphate 24 by Pol β. The incorporation of 24 at 1 nt gap by Pol β was examined in the presence of 50 nM of 1 nt gap substrate and increasing concentrations of Pol β (5 nM–100 nM). Lane 1 indicates substrate only. Lanes 2–7 show 24 insertion products by Pol β at 5 nM–100 nM. The percentage of 24 insertion products by Pol β was quantified and illustrated in the bar chart below the gel. The substrate was 32P-labeled at the 5′-end of the upstream primer. Substrates and products were separated by 15% 7M urea-denaturing polyacrylamide gel and detected by a PhosphorImager. The experiments were repeated at least three times independently. A representative gel image is illustrated.
It should be noted that the incorporation of 24 by Pol β was less efficient than with analogs containing the β-keto)sulfone group [30,31]. The results may suggest that the carboxylic group in 24 could be more reactive and can react with the amino acid residues in the catalytic center of Pol β and partially inactivate the enzyme, leading to less efficient nucleotide incorporation. This indicates that under cellular physiological conditions, the carboxylate group can be further developed into anticancer and crosslink agents for biomedical applications.
A previous study has shown the incorporation of C2-modified purine nucleotides and the potential application of their fluorescence conjugates in detecting DNA and RNA in cells [22,25]. It is possible that 24 can also be conjugated with a fluorescent tag to be developed as a new probe for DNA staining in cells. Additionally, the modification at C2 can be used to investigate the effect of the functional groups at the position on their insertion by DNA polymerases [22].
3. Experimental Section
General Information. 1H NMR spectra at 400 MHz and 13C NMR at 101 MHz were recorded in DMSO-d6 or CDCl3 unless otherwise noted. All chemical shift values are reported in parts per million (ppm) and referenced to the residual solvent peaks of CDCl3 (7.26 ppm), DMSO-d6 (2.50 ppm), MeOD-d4 (3.31 ppm) and D2O (4.79 ppm) for 1H NMR, and referenced to the CDCl3 (77.16 ppm), DMSO-d6 (39.52 ppm) and MeOD-d4 (49.00 ppm) peaks for the 13C NMR spectra, with coupling constant (J) values reported in Hz. HRMS data were recorded in TOF mode, negative or positive, unless otherwise noted. Reaction progress was monitored by TLC on Merck Kieselgel 60-F254 sheets, with product detection by 254 nm light. Products were purified by column chromatography using Merck (Boston, MA, USA) Kiselgel 60 (230–400 mesh). Reagent-grade chemicals were used, and solvents were purchased from commercial suppliers and used without further purification unless otherwise specified. DNA oligonucleotides were synthesized by Eurofins Genomics (Louisville, KY, USA). Radionucleotide, 32P-ATP (6000 µCi/mmol), was purchased from PerkinElmer Inc. (Boston, MA, USA). Micro Bio-Spin 6 chromatography columns were from Bio-Rad (Hercules, CA, USA). DNA polymerase β (Pol β) was purified as previously described [56]. All other standard chemical reagents were from Sigma–Aldrich (St. Louis, MO, USA) and Thermo Fisher Scientific (Pittsburgh, PA, USA).
- 3′,5′-Di-O-(tert-butyldimethylsilyl)-2-ethynyl-2′-deoxyadenosine (5). Step a. Pd(PPh3)2Cl2 (70.0 mg, 0.10 mmol) and Cu(I)I (38.1 mg, 0.2 mmol) were added to dry DMF (50 mL) and Et3N (5.0 mL, 3.63 g, 35.9 mmol) in a flame-dried flask equipped with a stirring bar under N2 at rt. Then, 3 [34] (3.0 g, 4.95 mmol) was added followed by TMS–acetylene (1.41 mL, 973 mg, 9.90 mmol). The resulting mixture was stirred at 80 °C for 6 h. Volatiles were evaporated, and the residue was purified by column chromatography (20 → 50% EtOAc/hexane) to give TMS-protected alkyne 5 as a brown solid. Step b. The solid was dissolved in anhydrous MeOH (50 mL) and stirred at 0 °C. Anhydrous K2CO3 (3.2 g, 23.1 mmol) was added portion-wise at room temperature, and the mixture was stirred at rt. After 30 min, the reaction was concentrated under reduced pressure, and the residue was suspended in a mixture of H2O (30 mL) and EtOAc (50 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered, and concentrated to dryness under vacuum to give a light-yellow crude solid. The solid was recrystallized with hexane to give 5 (2.25 g, 90%, overall) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.26 (s, 1H), 6.47 (t, J = 6.3 Hz, 1H), 6.07 (s, 2H), 4.61 (dt, J = 5.9, 3.9 Hz, 1H), 3.99 (q, J = 3.5 Hz, 1H), 3.90 (dd, J = 11.2, 3.9 Hz, 1H), 3.77 (dd, J = 11.2, 3.0 Hz, 1H), 3.00 (s, 1H), 2.58 (dt, J = 12.5, 6.1 Hz, 1H), 2.45 (ddd, J = 9.1, 6.3, 3.2 Hz, 1H), 0.92 (s, 10H), 0.91 (s, 9H), 0.10 (s, 6H), 0.09 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 155.49, 149.43, 145.25, 140.19, 119.86, 88.02, 84.54, 82.68, 73.22, 71.84, 62.81, 41.69, 26.09, 25.90, 18.55, 18.14, −4.52, −4.70, −4.98, −5.26, −5.37; HRMS (TOF, ESI) m/z calcd for C24H42N5O3Si2 504.2821 [M + H]+, found 504.2832.
- 2′,3′,5′-Tri-O-(tert-butyldimethylsilyl)-2-ethynyladenosine (6). Step a. Treatment of 4 [5] (5.0 g, 6.79 mmol) with TMS–acetylene (1.93 mL, 1.33 g, 13.6 mmol) in the presence of Pd(PPh3)2Cl2 (71.5 mg, 0.102 mmol), Cu(I)I (38.8 mg, 0.204 mmol) and Et3N (5.0 mL, 3.63 g, 35.9 mmol) followed by treatment with K2CO3 (3.2 g, 23.1 mmol; Step b), as described for 5, gave 6 (3.79 g, 88%, overall) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 1H), 6.00 (d, J = 5.2 Hz, 1H), 4.70 (t, J = 4.8 Hz, 1H), 4.31 (t, J = 3.8 Hz, 1H), 4.10–4.14 (m, 1H), 4.06 (dd, J = 11.2, 4.8 Hz, 1H), 3.78 (dd, J = 11.2, 2.8 Hz, 1H), 2.93 (s, 1H), 0.95 (s, 9H), 0.93 (s, 9H), 0.81 (s, 9H), 0.14 (s, 3H), 0.13 (s, 3H), 0.103 (s, 3H), 0.097 (s, 3H), −0.04 (s, 3H), −0.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 155.39, 149.79, 145.27, 140.84, 120.07, 88.84, 85.69, 82.66, 75.79, 72.93, 72.08, 62.60, 26.22, 25.99, 25.84, 18.64, 18.24, 18.02, −4.24, −4.58, −4.62, −4.98, −5.241, −5.238; HRMS (TOF, ESI) m/z calcd for C30H56N5O4Si3 634.3635 [M + H]+, found 634.3641.
- 3′,5′-Di-O-(tert-butyldimethylsilyl)-2-(2-iodoethynyl)-2′-deoxyadenosine (7). Sodium acetate (7.4 mg, 0.09 mmol), sodium p-toluenesulfinate (32.1 mg, 0.18 mmol), and I2 (22.8 mg, 0.09 mmol) were added to a stirring solution of 5 (31 mg, 0.06 mmol) in MeCN at rt. The resulting mixture was then heated at 80 °C for 1.5 h. The reaction mixture was quenched by saturated aqueous Na2S2O3. The volatiles were removed under reduced pressure, and the residue was suspended in a mixture of H2O (30 mL) and EtOAc (50 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered, and concentrated to dryness under vacuum to give a light-yellow crude solid. The residue was purified by column chromatography (30 → 50% EtOAc/hexane) to give 7 (30 mg, 80%) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 6.45 (t, J = 6.4 Hz, 1H), 5.92 (s, 2H), 4.61 (dt, J = 6.0, 3.6 Hz, 1H), 3.99 (q, J = 3.4 Hz, 1H), 3.90 (dd, J = 11.2, 4.0 Hz, 1H), 3.77 (dd, J = 11.6, 3.2 Hz, 1H), 2.65–2.56 (m, 1H), 2.43 (ddd, J = 13.2, 6.0, 4.4 Hz, 1H), 0.92 (s, 9H), 0.91 (s, 9H), 0.10 (s, 12H); 13C NMR (101 MHz, CDCl3) δ 154.92, 149.31, 145.40, 140.46, 119.76, 93.73, 88.09, 84.69, 71.85, 62.78, 41.62, 26.14, 26.09, 25.96, 25.90, 24.84, 18.58, 18.18, −4.47, −4.54, −4.65, −4.71, −5.20, −5.26, −5.31, −5.37; HRMS (TOF, ESI) m/z calcd for C24H41IN5O3Si2 630.1787 [M + H]+, found 630.1778.
- (E)-3′,5′-Di-O-(tert-butyldimethylsilyl)-2-(1-iodo-2-tosylvinyl)-2′-deoxyadenosine (8). NaOAc (342 mg, 4.17 mmol) and freshly prepared TsI (784 mg, 2.78 mmol) were added to a stirring solution of 5 (700 mg, 1.39 mmol) in THF (15 mL) at rt. After stirring for 40 h, the reaction mixture was quenched with saturated aqueous Na2S2O3. The volatiles were removed under reduced pressure, and the residue was suspended in a mixture of H2O (30 mL) and EtOAc (50 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered, and concentrated to dryness under vacuum. The residue was purified by column chromatography (30 → 50% EtOAc/hexane) to give 8 (885 mg, 81%) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 1H), 7.75, (d, J = 8.4 Hz, 2H), 7.22 (d, J = 7.8 Hz, 2H), 7.23 (s, 1H), 6.41 (t, J = 6.4 Hz, 1H), 6.22 (s, 2H), 4.63 (dt, J = 6.0, 3.6 Hz, 1H), 4.03 (q, J = 3.6 Hz, 1H), 3.92 (dd, J = 11.2, 4.4 Hz, 1H), 3.79 (dd, J = 10.8, 3.2 Hz, 1H), 2.76–2.68 (m, 1H), 2.44 (ddd, J = 13.2, 6.0, 4.0 Hz, 1H), 2.31 (s, 3H), 0.93 (s, 9H), 0.92 (s, 9H), 0.12–0.11 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 158.29, 155.16, 149.08, 144.94, 140.63, 140.54, 137.22, 129.86, 128.56, 119.33, 110.84, 88.29, 84.89, 72.12, 63.12, 41.46, 26.24, 26.04, 21.18, 18.68, 18.27, −4.36, −4.53, −5.08, −5.17; HRMS (TOF, ESI) m/z calcd for C31H49IN5O5SSi2 786.2032 [M + H]+, found 786.2021.
- (E)-2′,3′,5′-Tri-O-(tert-butyldimethylsilyl)-2-(1-iodo-2-tosylvinyl)adenosine (9). Treatment of 6 (1.5 g, 2.36 mmol) with NaOAc (582 mg, 7.09 mmol) and freshly prepared TsI (1.33 g, 4.72 mmol), as described for 8, gave 9 (1.99 g, 92%) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.26 (s, 1H); 7.73 (d, J = 8.4 Hz, 2H), 7.23–7.18 (m, 3H), 6.28 (s, 2H), 5.93 (d, J = 4.0 Hz, 1H), 4.60 (t, J = 4.0 Hz, 1H), 4.34–4.32 (m, 1H), 4.16 (td, J = 4.8, 2.8 Hz, 1H), 4.10 (dd, J = 11.2, 4.4 Hz, 1H), 3.83 (dd, J = 11.2, 2.6 Hz, 1H), 2.30 (s, 3H), 0.97 (s, 9H), 0.93 (s, 9H), 0.84 (s, 9H), 0.17 (s, 3H), 0.16 (s, 3H), 0.11 (s, 3H), 0.09 (s, 3H), 0.02 (s, 3H), −0.07 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 158.34, 155.04, 149.13, 144.83, 140.68, 140.40, 137.02, 129.74, 128.53, 119.24, 110.06, 89.29, 84.93, 75.83, 71.31, 62.25, 26.29, 26.01, 25.97, 21.70, 18.72, 18.23, 18.07, −4.14, −4.51, −4.60, −4.67, −5.09, −5.17; HRMS (TOF, ESI) m/z calcd for C37H63IN5O6SSi3 916.2846 [M + H]+, found 916.2867.
- (E)-2-(1-Iodo-2-tosylvinyl)-2′-deoxyadenosine (10). CH3CO2H (1.7 mL, 1.78 g, 29.6 mmol) and NH4F (944 mg, 25.5 mmol) were added to a stirring solution of 8 (400 mg, 0.51 mmol) in MeOH (15 mL) at rt. The resulting mixture was stirred at 60 °C for 3.0 h. The volatiles were evaporated at reduced pressure and co-evaporated with acetonitrile (3 × 5 mL) yielding an off-white solid, which was suspended in 20% MeOH in CH2Cl2. The white precipitate was removed by vacuum filtration, and the mother liquor evaporated at reduced pressure. The residue was purified by column chromatography (5 → 10% MeOH/CH2Cl2) to give 10 (222 mg, 78%) as a white solid. 1H NMR (400 MHz, MeOD-d4) δ 8.38 (s, 1H), 7.71 (d, J = 8.0 Hz, 2H), 7.45 (s, 1H), 7.32 (d, J = 8.0 Hz, 2H), 6.42 (t, J = 6.8 Hz, 1H), 4.60 (quint, J = 2.8 Hz, 1H), 4.09 (q, J = 3.2 Hz, 1H), 3.87 (dd, J = 12.4, 3.2 Hz, 1H), 3.75 (dd, J = 12.0, 3.6 Hz, 1H), 2.86–2.77 (m, 1H), 2.43 (ddd, J = 13.6, 6.4, 3.2 Hz, 1H), 2.37 (s, 3H); 13C NMR (101 MHz, MeOD-d4) δ 160.11, 156.90, 149.69, 146.53, 142.38, 141.36, 138.06, 130.82, 129.43, 119.80, 110.85, 89.85, 86.78, 73.04, 63.64, 41.57, 21.64; HRMS (TOF, ESI) m/z calcd for C19H21IN5O5S 558.0303 [M + H]+, found 558.0330.
- (E)-2-(1-Iodo-2-tosylvinyl)adenosine (11). Treatment of 9 (800 mg, 0.87 mmol) with CH3CO2H (3.1 mL, 3.25 g, 54.2 mmol) and NH4F (1.61 g, 43.5 mmol) in MeOH (20 mL), as described for 10, gave 11 (499 mg, 80%) as a white solid. 1H NMR (400 MHz, MeOD-d4) δ 8.38 (s, 1H), 7.70 (d, J = 8.4 Hz, 2H), 7.46 (s, 1H), 7.31 (d, J = 8.0 Hz, 2H), 5.98 (d, J = 6.4 Hz, 1H), 4.75 (dd, J = 6.2, 5.0 Hz, 1H), 4.36 (dd, J = 5.0, 3.0 Hz, 1H), 4.20 (q, J = 2.8 Hz, 1H), 3.92 (dd, J = 12.6, 2.6 Hz, 1H), 3.75 (dd, J = 12.6, 3.0 Hz, 1H), 2.35 (s, 3H); 13C NMR (101 MHz, MeOD-d4) δ 160.13, 156.96, 149.72, 146.56, 142.74, 141.48, 137.81, 130.83, 129.49, 119.98, 110.37, 90.97, 87.97, 75.66, 72.59, 63.38, 21.64; HRMS (TOF, ESI) m/z calcd for C19H21IN5O6S 574.0252 [M + H]+, found 574.0253.
- (Z)-2′,3′,5′-Tri-O-(tert-butyldimethylsilyl)-2-(1-amino-2-tosylvinyl)adenosine (13). Iodovinylsulfone 9 (303 mg, 0.33 mmol) was dissolved in methanolic ammonia (1 M, 12 mL), and the resulting mixture was stirred at rt for 2 h. The volatiles were removed under reduced pressure, and the residue was suspended in a mixture of H2O (30 mL) and EtOAc (50 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered, and evaporated to give 13 (256 mg, 96%) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 7.85 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 7.6 Hz, 2H), 6.9 (s, 2H), 6.27 (s, 1H), 6.04 (d, J = 3.6 Hz, 1H), 5.62 (s, 2H), 4.32 (t, J = 4.0 Hz, 1H), 4.29 (t, J = 4.6 Hz, 1H), 4.13–4.11 (m, 1H), 4.01 (dd, J = 11.6, 2.8 Hz, 1H), 3.81 (dd, J = 11.6, 2.0 Hz, 1H), 2.39 (s, 3H), 0.96 (s, 9H), 0.92 (s, 9H), 0.79 (s, 9H), 0.16 (s, 3H), 0.14 (s, 3H), 0.09 (s, 3H), 0.07 (s, 3H), −0.05 (s, 3H), −0.18 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 154.64, 153.41, 149.98, 149.84, 143.04, 141.87, 140.74, 129.62, 126.37, 120.19, 92.90, 88.32, 84.73, 77.02, 71.20, 62.16, 26.27, 25.98, 25.75, 21.64, 18.72, 18.24, 17.96, −4.14, −4.65, −4.67, −4.93, −5.18, −5.23; HRMS (TOF, ESI) m/z calcd for C37H65N6O6SSi3 805.3989 [M + H]+, found 805.3971.
- (Z)-2-(1-Amino-2-tosylvinyl)-2′-deoxyadenosine (14). Iodovinylsulfone 10 (100 mg, 0.18 mmol) was dissolved in methanolic ammonia (1 M, 5 mL), and the resulting mixture was stirred at rt for 2h. The volatiles were evaporated at reduced pressure and co-evaporated with acetonitrile (3 × 5 mL), yielding an off-white solid, which was suspended in 10% MeOH in CH2Cl2. The off-white precipitate was removed by vacuum filtration, and the mother liquor was evaporated at reduced pressure to give 14 (56 mg, 70%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.45 (s, 1H), 7.80 (d, J = 8.3 Hz, 2H), 7.55 (s, 2H), 7.39 (d, J = 7.6 Hz, 2H), 6.98 (s, 2H), 6.40 (t, J = 6.8 Hz, 1H), 6.00 (s, 1H), 5.34 (s, 1H), 4.93 (s, 1H), 4.40 (s, 1H), 3.87–2.82 (m, 1H), 3.59–3.49 (m, 2H), 2.70–2.63 (m, 1H), 2.37 (s, 3H), 2.30 (ddd, J = 13.6, 6.4, 3.6 Hz, 1H); 13C NMR (101 MHz, MeOD-d4) δ 156.75, 154.60, 152.29, 150.71, 144.65, 143.07, 142.23, 130.66, 127.00, 120.50, 91.72, 89.22, 85.62, 72.47, 63.12, 41.34, 21.46; HRMS (TOF, ESI) m/z calcd for C19H23N6O5S 447.1445 [M + H]+, found 447.1445.
- (Z)-2-(1-Amino-2-tosylvinyl)adenosine (15). Treatment of 11 (150 mg, 0.26 mmol) with methanolic ammonia (1 M, 10 mL), as described for 14, gave 15 (85 mg, 70%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (s, 1H), 7.86–7.74 (m, 2H), 7.57 (s, 2H), 7.43–7.34 (m, 2H), 7.06 (s, 1H), 6.98 (s, 2H), 6.00 (s, 1H), 5.94 (d, J = 5.8 Hz, 1H), 5.48 (d, J = 6.2 Hz, 1H), 5.21 (d, J = 5.0 Hz, 1H), 5.02 (t, J = 5.5 Hz, 1H), 4.52 (q, J = 5.8 Hz, 1H), 4.14 (td, J = 5.0, 3.5 Hz, 1H), 3.93 (q, J = 3.9 Hz, 1H), 3.64 (dt, J = 11.9, 4.9 Hz, 1H), 3.55 (dt, J = 11.9, 4.8 Hz, 1H), 2.37 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 13C NMR (101 MHz, DMSO-d6) δ 155.39, 152.37, 150.23, 149.45, 142.81, 141.78, 140.90, 129.70, 125.63, 119.32, 90.73, 87.05, 85.52, 73.95, 70.28, 61.29, 54.93, 20.99; HRMS (TOF, ESI) m/z calcd for C19H23N6O6S 463.1394 [M + H]+, found 463.1383.
- 3′,5′-Di-O-(tert-butyldimethylsilyl)-2-(2-tosylacetyl)-2′-deoxyadenosine (16). Step a. Treatment of iodovinylsulfone 8 (200 mg, 0.25 mmol) with methanolic ammonia (1 M, 10 mL), as described for 13, gave (Z)-3′,5′-Di-O-(tert-butyldimethylsilyl)-2-(1-amino-2-tosylvinyl)-2′-deoxyadenosine 12 (160 mg, 95%) as an off-white solid of sufficient purity to be used directly in the next step. Step b. CH3CO2H/H2O (1:1, 0.5 mL) was added to a stirring solution of 12 (150 mg, 0.22 mmol) in MeOH (5 mL) at rt. The resulting solution was stirred at rt for 8.0 h. The volatiles were removed under reduced pressure, and the residue was suspended in mixture of H2O (20 mL) and EtOAc (30 mL). The organic layer was washed with brine (20 mL), dried over Na2SO4, filtered, and concentrated to dryness under vacuum. The residue was purified by column chromatography (40 → 50% EtOAc/hexane) to give 16 (129 mg, 86%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.34 (s, 1H), 7.77 (d, J = 8.4 Hz, 2H), 7.26 (d, J = 8.0 Hz, 2H), 6.69 (s, 2H),6.42 (t, J = 6.2 Hz, 1H), 5.10 (s, 2H), 4.62–4.57 (m, 1H), 4.03 (q, J = 3.2 Hz, 1H), 3.89 (dd, J = 11.2, 3.6 Hz, 1H), 3.80 (dd, J = 11.2, 2.8 Hz, 1H), 2.54–2.46 (m, 2H), 2.36 (s, 3H), 0.93 (s, 9H), 0.92 (s, 9H), 0.12 (s, 3H), 0.12 (s, 3H), 0.11 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 187.75, 155.82, 153.54, 148.75, 145.09, 141.63, 136.42, 129.75, 128.77, 121.08, 88.12, 84.44, 71.70, 62.80, 62.69, 42.16, 26.09, 25.89, 21.72, 18.55, 18.14, −4.46, −4.66, −5.25, −5.35; HRMS (TOF, ESI) m/z calcd for C31H50N5O6SSi2 676.3015 [M + H]+, found 676.3001.
- 2′,3′,5′-Tri-O-(tert-butyldimethylsilyl)-2-(2-tosylacetyl)adenosine (17). Treatment of 13 (250 mg, 0.31 mmol) with CH3CO2H/H2O (1:1, 1.0 mL), as described for 16 (Step b), gave 17 (213 mg, 85%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.46 (s, 1H), 7.76 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 6.78 (s,2H), 5.98 (d, J = 4.0 Hz, 1H), 5.38 (d, J = 14.0 Hz, 1H), 4.76 (d, J = 14.0 Hz, 1H), 4.37 (t, J = 4.4 Hz, 1H), 4.32 (t, J = 4.4 Hz, 1H), 4.18–4.15 (m, 1H), 4.05 (dd, J = 11.6, 3.0 Hz, 1H), 3.84 (dd, J = 11.6, 2.4 Hz, 1H), 2.35 (s, 3H), 0.98 (s, 9H), 0.94 (s, 9H), 0.81 (s, 9H), 0.18 (s, 3H), 0.16 (s, 3H), 0.12 (s, 3H), 0.10 (s, 3H), −0.10 (s, 3H), −0.19 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 187.62, 155.98, 153.54, 148.95, 145.14, 141.93, 136.40, 129.75, 128.78, 121.22, 88.25, 85.18, 77.06, 71.54, 62.64, 62.37, 26.26, 25.98, 25.75, 21.70, 18.70, 18.24, 17.96, −4.15, −4.58, −4.65, −4.72, −5.19, −5.23; HRMS (TOF, ESI) m/z calcd for C37H64N5O7SSi3 806.3829 [M + H]+, found 806.3833.
- 2-(2-Tosylacetyl)-2′-deoxyadenosine (18). Method A. TBAF [330 µL, 0.33 mmol (1.0 M in THF)] was added to a stirring solution of 16 (101 mg, 0.15 mmol) in THF (5 mL) at 0 °C, and the resulting mixture was stirred at rt for 1 h. The volatiles were evaporated, and the residue was column-chromatographed (5 → 10% MeOH/CH2Cl2) to give 18 (58 mg, 87%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.56 (s, 1H), 7.73 (d, J = 8.4 Hz, 2H), 7.68 (s, 2H), 7.38 (d, J = 8.0 Hz, 2H), 6.33 (t, J = 6.8 Hz, 1H), 5.37 (d, J = 4.0 Hz, 1H), 5.30 (d, J = 2.4 Hz, 2H), 4.94 (t, J = 5.6 Hz, 1H), 4.44–3.38 (m, 1H), 3.88 (q, J = 4.4 Hz, 1H), 3.63–3.56 (m 1H), 3.55–3.49 (m 1H), 2.73–2.66 (m, 1H), 2.35 (s, 3H), 2.32–2.27 (m, 1H); 13C NMR (101 MHz, MeOD-d4) δ 189.15, 157.05, 154.60, 150.04, 146.60, 143.82, 137.78, 130.72, 129.58, 121.66, 89.53, 86.28, 72.62, 63.26, 54.81, 41.64, 21.50; HRMS (TOF, ESI) m/z calcd for C19H22N5O6S 448.1285 [M + H]+, found 448.1284.
Method B. Treatment of 14 (67 mg, 0.15 mmol) with CH3CO2H/H2O (1:1, 0.5 mL), as described for 16 (Step b), gave 18 (56 mg, 84%).
- 2-(2-Tosylacetyl)adenosine (19). Method A. Treatment of 17 (202 mg, 0.25 mmol) in THF (10 mL) with TBAF [830 µL, 0.83 mmol (1.0 M in THF)], as described for 18, gave 19 (102 mg, 88%) as a white solid. 1H NMR (400 MHz, MeOD-d4) δ 8.52 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 6.00 (d, J = 5.6 Hz, 1H), 4.63 (t, J = 5.6 Hz, 1H), 4.35 (t, J = 4.4 Hz, 1H), 4.16 (q, J = 3.2 Hz, 1H), 3.91 (dd, J = 12.4, 2.8 Hz, 1H), 3.79 (dd, J = 12.6, 3.4 Hz, 1H), 2.31 (s, 3H); 13C NMR (101 MHz, MeOD-d4) δ 189.07, 157.11, 154.66, 150.28, 146.65, 143.96, 137.70, 130.73, 129.62, 121.79, 90.59, 87.38, 76.07, 71.93, 62.86, 21.49; HRMS (TOF, ESI) m/z calcd for C19H22N5O7S 464.1234 [M + H]+, found 464.1233.
Method B. Treatment of 15 (69.5 mg, 0.15 mmol) with CH3CO2H/H2O (1:1, 1.0 mL), as described for 16 (Step b), gave 19 (57 mg, 82%).
- (E)-2-(1-Propanethio-2-tosylvinyl)adenosine (20). Et3N (9.0 μL, 6.6 mg, 0.065 mmol) and PrSH (4.8 μL, 4.1 mg, 0.054 mmol) were sequentially added to a stirred solution of 11 (31 mg, 0.054 mmol) in MeOH (2 mL) at ambient temperature. After 24 h, the volatiles were evaporated, and the residue was purified by silica gel column chromatography [MeOH/DCM (dichloromethane); 2 → 5%] to give 20 (17 mg, 60%) as a white solid. 1H NMR (400 MHz, MeOD-d4) δ 8.41 (s, 1H), 8.05 (s, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 7.7 Hz, 2H), 6.01 (d, J = 5.9 Hz, 1H), 4.73 (t, J = 5.5 Hz, 1H), 4.33 (dd, J = 5.1, 3.4 Hz, 1H), 4.13 (q, J = 3.2 Hz, 1H), 3.86 (dd, J = 12.4, 2.9 Hz, 1H), 3.77–3.73 (m, 1H), 2.73–2.68 (m, 2H), 2.46 (s, 3H), 1.26–1.20 (m, 2H), 0.67 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, MeOD-d4) δ 157.14, 157.02, 150.64, 146.44, 145.63, 142.94, 141.93, 136.90, 130.76, 130.26, 119.80, 90.81, 87.65, 75.62, 72.33, 63.21, 39.16, 23.17, 21.59, 13.32; HRMS (TOF, ESI) m/z calcd for C22H28N5O6S2 522.1476 [M + H]+, found 522.1477.
- (E/Z)-2-(1-Diisopropylamino-2-tosylvinyl)adenosine (21). (Me2CH)2NH (17 μL, 12.3 mg, 0.12 mmol) was added to a stirred solution of 11 (30 mg, 0.052 mmol) in MeOH (3 mL) at ambient temperature. After 30 min, the volatiles were evaporated, and the residue was purified by silica gel column chromatography (MeOH/DCM; 2 → 5%) to give 21 (E/Z, ~46:54; 21 mg, 74%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 0.46H), 8.41 (s, 0.54H), 7.59 (d, J = 8.4 Hz, 2H), 7.50 (s, 0.92H), 7.48 (s, 1.08H) 7.35 (d, J = 8.0 Hz, 0.92H), 7.28 (d, J = 8.0 Hz, 1.08H), 5.89 (d, J = 6.4 Hz, 0.46H), 5.83 (d, J = 6.4 Hz, 0.54H), 5.53 (dd, J = 6.0, 2.8 Hz, 0.46H), 5.40 (d, J = 6.4 Hz, 0.54H) 5.25 (dt, J = 7.9, 3.5 Hz, 2H), 4.97 (s, 0.54H), 4.94 (s, 0.46H), 4.59–4.51 (m, 1H), 4.14 (qd, J = 5.0, 3.3, 2.2 Hz, 1H), 4.02 (q, J = 3.2 Hz, 0.54H), 3.95 (q, J = 3.2 Hz, 0.46H) 3.71–3.47 (m, 3.46H), 3.25 (d, J = 6.4 Hz, 0.54H), 2.36 (s, 3H), 1.16 (br s, 12H). 13C NMR (101 MHz, DMSO) δ 155.81, 155.72, 154.11, 153.76, 149.31, 149.22, 143.70, 143.63, 141.01, 140.99, 139.96, 139.87, 129.30, 129.00, 126.05, 118.46, 118.45, 93.58, 93.40, 87.42, 87.09, 86.11, 85.97, 73.96, 73.80, 70.90, 70.76, 61.72, 53.21, 21.01, 20.96, 20.20, 19.58; HRMS (TOF, ESI) m/z calcd for C25H35N6O6S 547.2333 [M + H]+, found 547.2330.
- 2-(2-Benzyl-2-tosylacetyl)adenosine (22). Aqueous NaOH solution (1 M, 130 µL, 0.13 mmol) was added to a stirred solution of 19 (30 mg, 0.065 mmol) in MeOH (2 mL) at rt. After 30 min, BnBr (15.4 µL, 22.3 mg, 0.13 mmol) was added, and the resulting mixture was stirred for 24 h. The reaction mixture was then neutralized with dil. HCl to pH ~7, and the volatiles were evaporated. The residue was column-chromatographed (5 → 10% MeOH/DCM) to give 22 (27 mg, 75%) as a 50:50 mixture of diastereomers. 1H NMR (400 MHz, MeOD-d4) δ 8.48 (s, 0.5H), 8.46 (s, 0.5H), 7.67 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.29–7.06 (m, 7.5H), 6.52–6.45 (m, 0.5H), 5.98 (t, J = 5.2 Hz, 1H), 4.61 (t, J = 5.2 Hz, 1H), 4.36–4.32 (m, 1H), 4.19 (“q”, J = 3.2 Hz, 0.5H), 4.16 (“q”, J = 3.2 Hz, 0.5H), 3.94–3.89 (m, 1H), 3.80–3.76 (m, 1H), 3.52–3.42 (m, 2H), 2.25 (s, 1.5H), 2.22 (s, 1.5H); 13C NMR (101 MHz, MeOD-d4) δ 192.47, 192.26, 156.89, 155.13, 154.99, 150.12, 150.06, 146.92, 144.05, 144.02, 137.65, 137.62, 137.60, 137.57, 136.16, 136.09, 130.69, 130.48, 130.36, 130.05, 130.03, 129.63, 127.93, 127.90, 121.66, 90.71, 90.65, 87.50, 87.46, 75.98, 75.90, 72.11, 72.02, 70.39, 63.07, 62.97, 32.72, 32.44, 21.46, 21.44; HRMS (TOF, ESI) m/z calcd for C26H28N5O7S 554.1704 [M + H]+, found 554.1704.
- 2-(2-Allyl-2-tosylacetyl)adenosine (23). Aqueous NaOH solution (1 M, 130 µL, 0.13 mmol) was added to a stirred solution of 19 (30 mg, 0.065 mmol) in MeOH (2 mL) at rt. After 30 min, allyl bromide (11.2 µL 15.7 mg, 0.13 mmol) was added, and the resulting mixture was stirred for 24 h. The reaction mixture was then neutralized with dil. HCl to pH~7, and the volatiles were evaporated. The residue was column-chromatographed (5 → 10% MeOH/DCM) to give 23 (22.3 mg, 68%) as a 50:50 mixture of diastereomers in the form of a white solid. 1H NMR (400 MHz, MeOD-d4) δ 1H NMR (400 MHz, MeOD-d4) δ 8.52 (s, 0.5H), 8.51 (s, 0.5H), 7.69–7.58 (m, 2H), 7.28–7.17 (m, 2H), 6.23 (ddd, J = 14.4, 10.4, 4.4 Hz, 1H), 6.04 (d, J = 5.6 Hz, 0.5H), 6.00 (d, J = 5.6 Hz, 0.5H), 5.82–5.70 (m, 1H), 5.12 (q, J = 1.6 Hz, 0.5H), 5.10 (q, J = 1.6 Hz, 0.5H), 5.09 (t, J = 1.2 Hz, 1H), 4.98 (t, J = 1.2 Hz, 1H), 4.65 (dt, J = 6.4, 5.2 Hz, 1H), 4.35 (ddd, J = 5.2, 3.6, 1.6 Hz, 1H), 4.19 (“q”, J = 3.2 Hz, 0.5H), 4.17 (“q”, J = 3.2 Hz, 0.5H), 3.92 (“ddd”, J = 12.4, 7.6, 2.8 Hz, 1H), 3.82–3.77 (m, 1H), 2.99–2.85 (m, 2H), 2.25 (s, 1.5H), 2.22 (s, 1.5H); 13C NMR (101 MHz, MeOD-d4) δ 192.40, 192.22, 157.04, 155.29, 155.14, 150.30, 150.19, 146.91, 144.07, 143.97, 136.16, 136.08, 133.98, 133.95, 133.94, 133.91, 130.65, 130.46, 130.33, 121.72, 118.65, 118.63, 90.64, 90.59, 87.55, 87.52, 76.07, 76.03, 72.11, 72.06, 68.70, 68.61, 63.03, 62.95, 31.14, 31.05, 30.95, 30.86, 21.44, 21.43; HRMS (TOF, ESI) m/z calcd for C22H26N5O7S 504.1547 [M + H]+, found 504.1545.
- 2′-Deoxyadenosine-2-carboxylic acid 5′-O-triphosphate (24). (MeO)3PO (1.0 mL; dried over 3A molecular sieves) was added to the flame-dried flask containing 8-(β-keto)sulfone 18 [30 mg, 0.067 mmol; dried in vacuum (40 °C, over P2O5)] and proton sponge (21.5 mg, 0.10 mmol), and the resulting solution was stirred at 0 °C for 5 min under an Ar atmosphere. Freshly distilled POCl3 (8.1 μL, 13.4 mg, 0.087 mmol) was then added, and stirring was continued for 1.0 h at 0 °C. The mixture of tributylammonium pyrophosphate (TBAPP; 0.5 M/dimethylformamide (DMF); 670 μL, 0.34 mmol) and Bu3N (49.7 mg, 0.27 mmol) was added and stirred for another 30 min at 0 °C. The reaction mixture was quenched by adjusting pH to 7.5–7.8 with triethylammonium bicarbonate (TEAB) buffer (2 M, several drops). The residue was dissolved in water (5 mL) and was extracted with EtOAc (3 × 5 mL). The water layer was evaporated and co-evaporated (three times) with a mixture of EtOH/H2O (1:1, 5 mL). The residue was chromatographed on a DEAE-Sephadex A-25 column with TEAB (0.1 → 0.6 M), and the appropriate fractions (TLC, Rf 0.28; i-PrOH/H2O/NH4OH, 5:2:3) were evaporated in vacuum and co-evaporated three times with a mixture of EtOH/H2O (1:1, 10 mL) to remove excess of TEAB salt to give 24 as a triethylammonium salt, which was then converted to sodium salt 24 (11.0 mg, 25%) with Dowex-Na+. 1H NMR (400 MHz, D2O) δ 8.56 (s, 1H), 6.61 (s, 1H), 4.89 (s, 1H), 4.32–4.13 (m, 3H), 2.85–2.74 (m, 1H), 2.69–2.58 (m, 1H); 31P NMR (162 MHz, D2O) δ −10.89 (d, J = 19.8 Hz), −11.44 (d, J = 20.1 Hz), −23.24 (t, J = 19.8 Hz); HRMS (TOF, ESI) m/z calcd for C11H15N5O14P3 533.9834 [M − H]−, found 533.9830.
- Adenosine-2-carboxylic acid 5′-O-triphosphate (25). Method A: Treatment of 19 (31 mg, 0.067 mmol) with (MeO)3PO (1.0 mL), proton sponge (21.5 mg, 0.10 mmol), and freshly distilled POCl3 (8.1 μL, 13.4 mg, 0.087 mmol), followed by a mixture of tributylammonium pyrophosphate (TBAPP; 0.5 M/dimethylformamide (DMF); 670 μL, 0.34 mmol) and Bu3N (49.7 mg, 0.27 mmol), as described for 24, gave 25 (9.6 mg, 22%) as a triethylammonium salt.
Method B. (MeO)3PO (1.0 mL; dried over 3A molecular sieves) was added to the flame-dried flask containing 29 [40 mg, 0.12 mmol; dried in vacuum (40 °C, over P2O5)] and proton sponge (38.6 mg, 0.18 mmol), and the resulting solution was stirred at 0 °C for 5 min under an Ar atmosphere. Freshly distilled POCl3 (14.6 μL, 24.1 mg, 0.16 mmol) was then added, and stirring was continued for 1.0 h at 0 °C. The mixture of TBAPP (0.5 M/DMF; 1.2 mL, 0.6 mmol) and Bu3N (115 μL, 89.5 mg, 0.48 mmol) was added and stirred for another 30 min at 0 °C. The reaction mixture was quenched by adjusting pH to 7.5–7.8 with TEAB buffer (2 M, several drops). The residue was dissolved in water (5 mL) and was extracted with EtOAc (3 × 5 mL). The water layer was evaporated and co-evaporated (three times) with a mixture of EtOH/H2O (1:1, 5 mL). The residue was dissolved in MeOH (2 mL). Aqueous NaOH (1 M, 120 μL) was added, and the reaction mixture was stirred rt for 3 h. The reaction mixture was quenched by adjusting pH to 7.0 with HCl (1 M). The residue was chromatographed on a DEAE-Sephadex A-25 column with TEAB (0.1 → 0.6 M), and the appropriate fractions (TLC, Rf 0.31; i-PrOH/H2O/NH4OH, 5:2:3) were evaporated in vacuum and co-evaporated three times with a mixture of EtOH/H2O (1:1, 10 mL) to remove excess of TEAB salt to give 25 as a triethylammonium salt. 1H NMR (400 MHz, D2O) δ 8.63 (s, 1H), 6.23 (s, 1H), 4.75 (s, 1H), 4.62 (d, J = 4.7 Hz, 1H), 4.40 (s, 1H), 4.29 (dd, J = 19.6, 13.3 Hz, 2H); 31P NMR (162 MHz, D2O) δ −5.57 (d, J = 19.4 Hz), −10.90 (d, J = 19.1 Hz), −21.34 (t, J = 19.2 Hz); HRMS (TOF, ESI) m/z calcd for C11H15N5O15P3 549.9783 [M − H]−, found 549.9792.
- 2-(Methoxycarbonyl)adenosine (29). A mixture of 28 [50] (100 mg, 0.34 mmol) and NaOMe (5.4 M; 95 μL, 0.51 mmol) in MeOH (10 mL) was stirred at rt for 15 h. After neutralization with Dowex 50 (H+) and filtration of resin, the volatiles were removed at reduced pressure. The residue was dissolved in a mixture of MeOH/H2O (10 mL; 1:1) and 1.0 M HCl (340 μL, 0.34 mmol) was added. The mixture was stirred at rt for 2 h. After neutralization with 1.0 M NaOH, the volatiles were removed at reduced pressure, and the residue was column-chromatographed (10 → 20% MeOH/DCM) to give 29 (89 mg, 80%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (s, 1H), 7.71 (s, 2H), 5.93 (d, J = 6.4 Hz, 1H), 5.47 (d, J = 6.0 Hz, 1H), 5.22 (d, J = 4.7 Hz, 1H), 5.05 (dd, J = 6.4, 5.2 Hz, 1H), 4.62–4.56 (m, 1H), 4.15 (td, J = 4.8, 2.8 Hz, 1H), 3.96 (q, J = 3.6 Hz, 1H), 3.84 (s, 3H), 3.71–3.64 (m, 1H), 3.56 (ddd, J = 12.0, 6.4, 4.0 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 164.47, 156.15, 150.61, 149.47, 141.85, 120.12, 87.41, 86.08, 73.84, 70.73, 61.67, 52.57. HRMS (TOF, ESI) m/z calcd for C12H16N5O6 326.1095 [M + H]+, found 326.1012.
- Incorporation of the nucleotide analog 24 by Pol β
The incorporation of 24 by Pol β was examined with the substrate containing a 1 nt-gap with a 5′-phosphate at the downstream primer. The substrate was constructed by annealing the 5′-end 32P-labeled upstream primer and downstream primer with a template at the ratio of 1:2:2. The upstream primer was 5′-end labeled with 32P using T4 polynucleotide kinase according to the protocol provided by New England Biolabs (Ipswich, MA, USA). Pol β incorporation of 24 was measured by incubating a 50 nM substrate with increasing concentrations of Pol β (5–100 nM) in the presence of 50 μM of the nucleotide analog at 37 °C for 30 min. The reaction mixture was assembled in a 10 µL reaction buffer containing 50 mM Tris-HCl, pH 7.5, 50 mM KCl, 0.1 mM EDTA, 0.1 mg/mL bovine serum albumin, and 0.01% Nonidet P-40 in the presence of 5 mM MgCl2. The enzyme reaction was stopped using 2x stopping buffer containing 95% formamide and 10 mM EDTA, 0.05% (w/v) bromophenol blue and 0.05% (w/v) xylene cyanol, followed by incubation at 95 °C for 5 min. Substrates and products were separated by a 15% 7M urea-denaturing polyacrylamide gel and detected by a PhosphorImager. All experiments were repeated at least three times independently.
4. Conclusions
We have developed two probes attached to the C2 position of adenosine and 2′-deoxyadenosine that are mechanistically different. The 2-(β-iodovinyl)sulfone probe efficiently reacts with nucleophiles, such as amines or thiols, via a conjugated addition–elimination pathway and can be efficiently converted to 2-(β-keto)sulfones. The 2-(β-keto)sulfone probe instead reacts with electrophiles such as alkyl halides. The aryl ring in sulfone group can be modified to allow for further bioconjugation and/or crosslinking. Adenine nucleosides with a β-ketosulfone group at the C2 position, during conversion to their 5′-triphosphate, undergo an unexpected conversion to 2-carboxylic acid nucleotides. The 2-carboxylic acid of 2′-deoxyadenosine-5′-triphosphate is efficiently incorporated by human DNA polymerase into a double-stranded DNA oligomer containing a one-nucleotide gap.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30061358/s1; copies of 1H, 13C, and 31P NMR spectra.
Author Contributions
Conceptualization, S.F.W.; methodology, A.H.H., Y.L. and S.F.W.; investigation, A.H.H., R.F. and P.S.T.; resources, Y.L. and S.F.W.; writing—original draft preparation, A.H.H., P.S.T., Y.L. and S.F.W.; writing—review and editing, A.H.H., Y.L. and S.F.W.; supervision, S.F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH grant R03ES035200 (Y.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
A.H.H. is a recipient of the FIU Presidential Fellowship. A.H.H. and P.S.T. are recipients of the FIU Dissertation Year Fellowship (DYF).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liang, Y.; Wen, Z.; Cabrera, M.; Howlader, A.H.; Wnuk, S.F. Purines. In Science of Synthesis: Houben-Weyl Methods of Molecular Transformations: Knowledge Updates 2020/1; Christmann, M., Huang, Z., Jiang, X., Li, J.J., Oestreich, M., Petersson, E.J., Schaumann, E., Wang, M., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2020; pp. 195–384. [Google Scholar]
- Li, Y.; Butt, M.; Bao, W.; Xie, R.; Chen, G. Progress in the Functionalization of Purines and Purine Nucleosides by Minisci Reactions Over the Past 50 Years. ChemCatChem 2025, 17, e202401550. [Google Scholar] [CrossRef]
- Sengupta, S.; Das, P. Application of diazonium chemistry in purine modifications: A focused review. J. Heterocycl. Chem. 2022, 59, 5–21. [Google Scholar] [CrossRef]
- Kumamoto, H.; Tanaka, H.; Tsukioka, R.; Ishida, Y.; Nakamura, A.; Kimura, S.; Hayakawa, H.; Kato, K.; Miyasaka, T. First Evident Generation of Purin-2-yllithium: Lithiation of an 8-Silyl-Protected 6-Chloropurine Riboside as a Key Step for the Synthesis of 2-Carbon-Substituted Adenosines. J. Org. Chem. 1999, 64, 7773–7780. [Google Scholar] [CrossRef]
- Nair, V.; Turner, G.A.; Buenger, G.S.; Chamberlain, S.D. New methodologies for the synthesis of C-2 functionalized hypoxanthine nucleosides. J. Org. Chem. 1988, 53, 3051–3057. [Google Scholar] [CrossRef]
- Van Aerschot, A.A.; Mamos, P.; Weyns, N.J.; Ikeda, S.; De Clercq, E.; Herdewijn, P.A. Antiviral activity of C-alkylated purine nucleosides obtained by cross-coupling with tetraalkyltin reagents. J. Med. Chem. 1993, 36, 2938–2942. [Google Scholar] [CrossRef]
- Cain, R.; Salimraj, R.; Punekar, A.S.; Bellini, D.; Fishwick, C.W.G.; Czaplewski, L.; Scott, D.J.; Harris, G.; Dowson, C.G.; Lloyd, A.J.; et al. Structure-Guided Enhancement of Selectivity of Chemical Probe Inhibitors Targeting Bacterial Seryl-tRNA Synthetase. J. Med. Chem. 2019, 62, 9703–9717. [Google Scholar] [CrossRef]
- Liang, Y.; Wnuk, S. Transition Metal-Catalyzed C–H Functionalization of Nucleoside Bases. In Transition-Metal-Catalyzed C-H Functionalization of Heterocycles; Kumar, A., Punniyamurthy, T., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 631–655. [Google Scholar]
- Li, Y.; Zhou, Y.; Zhou, D.; Jiang, Y.; Butt, M.; Yang, H.; Que, Y.; Li, Z.; Chen, G. Regioselective Homolytic C2–H Borylation of Unprotected Adenosine and Adenine Derivatives via Minisci Reaction. J. Am. Chem. Soc. 2024, 146, 21428–21441. [Google Scholar] [CrossRef]
- Ingall, A.H.; Dixon, J.; Bailey, A.; Coombs, M.E.; Cox, D.; McInally, J.I.; Hunt, S.F.; Kindon, N.D.; Teobald, B.J.; Willis, P.A.; et al. Antagonists of the Platelet P2T Receptor: A Novel Approach to Antithrombotic Therapy. J. Med. Chem. 1999, 42, 213–220. [Google Scholar] [CrossRef]
- Springthorpe, B.; Bailey, A.; Barton, P.; Birkinshaw, T.N.; Bonnert, R.V.; Brown, R.C.; Chapman, D.; Dixon, J.; Guile, S.D.; Humphries, R.G.; et al. From ATP to AZD6140: The discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg. Med. Chem. Lett. 2007, 17, 6013–6018. [Google Scholar] [CrossRef]
- Sharif, E.U.; Kalisiak, J.; Lawson, K.V.; Miles, D.H.; Newcomb, E.; Lindsey, E.A.; Rosen, B.R.; Debien, L.P.P.; Chen, A.; Zhao, X.; et al. Discovery of Potent and Selective Methylenephosphonic Acid CD73 Inhibitors. J. Med. Chem. 2021, 64, 845–860. [Google Scholar] [CrossRef]
- Cachatra, V.; Martins, A.; Oliveira, M.C.; Oliveira, M.C.; Gano, L.; Paulo, A.; López, Ó.; Fernández-Bolaños, J.G.; Contino, M.; Colabufo, N.A.; et al. Purine nucleosides as selective inhibitors of butyrylcholinesterase—A multidisciplinary study. Org. Biomol. Chem. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Šinkevičiūtė, U.; Dvořáková, M.; Tichý, M.; Gurská, S.; Ječmeňová, K.; Poštová Slavětínská, L.; Džubák, P.; Hajdúch, M.; Hocek, M. Synthesis and Biological Activity of 2,6-Disubstituted 7-Deazapurine Ribonucleosides. Eur. J. Org. Chem. 2025, 28, e202401187. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Aghazadeh Tabrizi, M.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A(3) Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Szczepaniak, K.; Mieczkowski, A.; Kierozalska, A.; Pavlović Saftić, D.; Głąbała, K.; Przygodzki, T.; Stańczyk, L.; Karolczak, K.; Watała, C.; Rao, H.; et al. Synthesis and evaluation of adenosine derivatives as A(1), A(2A), A(2B) and A(3) adenosine receptor ligands containing boron clusters as phenyl isosteres and selective A(3) agonists. Eur. J. Med. Chem. 2021, 223, 113607. [Google Scholar] [CrossRef]
- Abiru, T.; Miyashita, T.; Watanabe, Y.; Yamaguchi, T.; Machida, H.; Matsuda, A. Nucleosides and nucleotides. 107. 2-(Cycloalkylalkynyl)adenosines: Adenosine A2 receptor agonists with potent antihypertensive effects. J. Med. Chem. 1992, 35, 2253–2260. [Google Scholar] [CrossRef]
- Kim, G.; Jarhad, D.B.; Lee, G.; Kim, G.; Hou, X.; Yu, J.; Lee, C.S.; Warnick, E.; Gao, Z.-G.; Ahn, S.Y.; et al. Structural Modification and Biological Evaluation of 2,8-Disubstituted Adenine and Its Nucleosides as A2A Adenosine Receptor Antagonists: Exploring the Roles of Ribose at Adenosine Receptors. J. Med. Chem. 2024, 67, 10490–10507. [Google Scholar] [CrossRef]
- Krömer, M.; Brunderová, M.; Ivancová, I.; Poštová Slavětínská, L.; Hocek, M. 2-Formyl-dATP as Substrate for Polymerase Synthesis of Reactive DNA Bearing an Aldehyde Group in the Minor Groove. ChemPlusChem 2020, 85, 1164–1170. [Google Scholar] [CrossRef]
- Duan, H.-C.; Zhang, C.; Song, P.; Yang, J.; Wang, Y.; Jia, G. C2-methyladenosine in tRNA promotes protein translation by facilitating the decoding of tandem m2A-tRNA-dependent codons. Nat. Commun. 2024, 15, 1025. [Google Scholar] [CrossRef]
- Ferguson, L.; Madieh, N.S.; Vaideanu, A.; Schatzlein, A.; Festa, J.; Singh, H.; Wells, G.; Bhakta, S.; Brucoli, F. C2-linked alkynyl poly-ethylene glycol(PEG) adenosine conjugates as water-soluble adenosine receptor agonists. Chem. Biol. Drug Des. 2023, 101, 340–349. [Google Scholar] [CrossRef]
- Matyašovský, J.; Perlíková, P.; Malnuit, V.; Pohl, R.; Hocek, M. 2-Substituted dATP Derivatives as Building Blocks for Polymerase-Catalyzed Synthesis of DNA Modified in the Minor Groove. Angew. Chem. Int. Ed. 2016, 55, 15856–15859. [Google Scholar] [CrossRef]
- Parker, W.B.; Shaddix, S.C.; Chang, C.-H.; White, E.L.; Rose, L.M.; Brockman, R.W.; Shortnacy, A.T.; Montgomery, J.A.; Secrist, J.A.; Bennett, L.L. Effects of 2-Chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenine on K562 Cellular Metabolism and the Inhibition of Human Ribonucleotide Reductase and DNA Polymerases by Its 5′-Triphosphate. Cancer Res. 1991, 51, 2386–2394. [Google Scholar] [PubMed]
- Chollet, A.; Kawashima, E. DNA containing the base analogue 2-aminoadenine: Preparation, use as hybridization probes and cleavage by restriction endonucleases. Nucleic Acids Res. 1988, 16, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kutyavin, I.V. Use of Base-Modified Duplex-Stabilizing Deoxynucleoside 5′-Triphosphates To Enhance the Hybridization Properties of Primers and Probes in Detection Polymerase Chain Reaction. Biochemistry 2008, 47, 13666–13673. [Google Scholar] [CrossRef] [PubMed]
- Matyašovský, J.; Pohl, R.; Hocek, M. 2-Allyl- and Propargylamino-dATPs for Site-Specific Enzymatic Introduction of a Single Modification in the Minor Groove of DNA. Chem. Eur. J. 2018, 24, 14938–14941. [Google Scholar] [CrossRef]
- Matyašovský, J.; Hocek, M. 2-Substituted 2′-deoxyinosine 5′-triphosphates as substrates for polymerase synthesis of minor-groove-modified DNA and effects on restriction endonuclease cleavage. Org. Biomol. Chem. 2020, 18, 255–262. [Google Scholar] [CrossRef]
- Liang, Y.; Suzol, S.H.; Wen, Z.; Artiles, A.G.; Mathivathanan, L.; Raptis, R.G.; Wnuk, S.F. Uracil Nucleosides with Reactive Group at C5 Position: 5-(1-Halo-2-sulfonylvinyl)uridine Analogues. Org. Lett. 2016, 18, 1418–1421. [Google Scholar] [CrossRef]
- Wen, Z.; Suzol, S.H.; Peng, J.; Liang, Y.; Snoeck, R.; Andrei, G.; Liekens, S.; Wnuk, S.F. Antiviral and Cytostatic Evaluation of 5-(1-Halo-2-sulfonylvinyl)- and 5-(2-Furyl)uracil Nucleosides. Arch. Pharm. 2017, 350, 1700023. [Google Scholar] [CrossRef]
- Suzol, S.H.; Howlader, A.H.; Wen, Z.; Ren, Y.; Laverde, E.E.; Garcia, C.; Liu, Y.; Wnuk, S.F. Pyrimidine Nucleosides with a Reactive (β-Chlorovinyl)sulfone or (β-Keto)sulfone Group at the C5 Position, Their Reactions with Nucleophiles and Electrophiles, and Their Polymerase-Catalyzed Incorporation into DNA. ACS Omega 2018, 3, 4276–4288. [Google Scholar] [CrossRef]
- Howlader, H.; Suzol, S.H.; Blanco, K.; Martin-Rafa, L.; Laverde, E.E.; Liu, Y.; Wnuk, S.F. Purine Nucleosides with a Reactive (β-Iodovinyl)sulfone or a (β-Keto)sulfone Group at the C8 Position and Their Polymerase-Catalyzed Incorporation into DNA. Asian J. Org. Chem. 2022, 11, e202100764. [Google Scholar] [CrossRef]
- Tanaka, M.; Kozakai, R.; Saito, Y.; Saito, I. Stabilization of DNA duplex by 2-substituted adenine as a minor groove modifier. Bioorg. Med. Chem. Lett. 2011, 21, 7021–7024. [Google Scholar] [CrossRef]
- Grünewald, C.; Kwon, T.; Piton, N.; Förster, U.; Wachtveitl, J.; Engels, J.W. RNA as scaffold for pyrene excited complexes. Bioorg. Med. Chem. 2008, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Purdy, D.F. Synthetic approaches to new doubly modified nucleosides: Congeners of cordycepin and related 2′-deoxyadenosine. Tetrahedron 1991, 47, 365–382. [Google Scholar] [CrossRef]
- Zhang, Y.; Vessally, E. Direct halosulfonylation of alkynes: An overview. RSC Adv. 2021, 11, 33447–33460. [Google Scholar] [CrossRef]
- Reddy, R.J.; Kumar, J.J.; Kumari, A.H. Recent trends in the synthesis and applications of β-iodovinyl sulfones: A decade of progress. Org. Biomol. Chem. 2024, 22, 2492–2509. [Google Scholar] [CrossRef]
- Reddy, R.J.; Kumar, J.J.; Kumari, A.H. Unprecedented Reactivity of β-Iodovinyl Sulfones: An Efficient Synthesis of β-Keto Sulfones and β-Keto Thiosulfones. Eur. J. Org. Chem. 2019, 2019, 3771–3775. [Google Scholar] [CrossRef]
- Nair, V.; Augustine, A.; Suja, T.D. CAN Mediated Reaction of Aryl Sulfinates with Alkenes and Alkynes: Synthesis of Vinyl Sulfones, β-Iodovinyl Sulfones and Acetylenic Sulfones. Synthesis 2002, 2002, 2259–2265. [Google Scholar] [CrossRef]
- Sun, Y.; Abdukader, A.; Lu, D.; Zhang, H.; Liu, C. Synthesis of (E)-β-iodo vinylsulfones via iodine-promoted iodosulfonylation of alkynes with sodium sulfinates in an aqueous medium at room temperature. Green Chem. 2017, 19, 1255–1258. [Google Scholar] [CrossRef]
- Katrun, P.; Chiampanichayakul, S.; Korworapan, K.; Pohmakotr, M.; Reutrakul, V.; Jaipetch, T.; Kuhakarn, C. PhI(OAc)2/KI-Mediated Reaction of Aryl Sulfinates with Alkenes, Alkynes, and α,β-Unsaturated Carbonyl Compounds: Synthesis of Vinyl Sulfones and β-Iodovinyl Sulfones. Eur. J. Org. Chem. 2010, 2010, 5633–5641. [Google Scholar] [CrossRef]
- Wan, J.-P.; Hu, D.; Bai, F.; Wei, L.; Liu, Y. Stereoselective Z-halosulfonylation of terminal alkynes using sulfonohydrazides and CuX (X = Cl, Br, I). RSC Adv. 2016, 6, 73132–73135. [Google Scholar] [CrossRef]
- Liu, L.K.; Chi, Y.; Jen, K.-Y. Copper-catalyzed additions of sulfonyl iodides to simple and cyclic alkenes. J. Org. Chem. 1980, 45, 406–410. [Google Scholar] [CrossRef]
- Tsui, G.C.; Glenadel, Q.; Lau, C.; Lautens, M. Rhodium(I)-Catalyzed Addition of Arylboronic Acids to (Benzyl-/Arylsulfonyl)acetonitriles: Efficient Synthesis of (Z)-β-Sulfonylvinylamines and β-Keto Sulfones. Org. Lett. 2011, 13, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Copper-Catalyzed Coupling of Oxime Acetates with Sodium Sulfinates: An Efficient Synthesis of Sulfone Derivatives. Angew. Chem. Int. Ed. 2014, 53, 4205–4208. [Google Scholar] [CrossRef] [PubMed]
- Markitanov, Y.M.; Timoshenko, V.M.; Shermolovich, Y.G. β-Keto sulfones: Preparation and application in organic synthesis. J. Sulphur Chem. 2014, 35, 188–236. [Google Scholar] [CrossRef]
- Nasuhipur, F.; Ghasemi, Z.; Poupon, M.; Dušek, M. POCl(3) mediated one-pot deoxygenative aromatization and electrophilic chlorination of dihydroxy-2-methyl-4-oxo-indeno[1,2-b]pyrroles. RSC Adv. 2023, 13, 17812–17816. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I.; Kolodiazhna, A. Nucleophilic substitution at phosphorus: Stereochemistry and mechanisms. Tetrahedron Asymmetry 2017, 28, 1651–1674. [Google Scholar] [CrossRef]
- Suryakiran, N.; Srikanth Reddy, T.; Suresh, V.; Lakshman, M.; Venkateswarlu, Y. Synthesis of α-iodo β-ketosulfones and α-iodo methylsulfones using iodine monochloride. Tetrahedron Lett. 2006, 47, 4319–4323. [Google Scholar] [CrossRef]
- Suryakiran, N.; Prabhakar, P.; Srikanth Reddy, T.; Chinni Mahesh, K.; Rajesh, K.; Venkateswarlu, Y. Chemoselective mono halogenation of β-keto-sulfones using potassium halide and hydrogen peroxide; synthesis of halomethyl sulfones and dihalomethyl sulfones. Tetrahedron Lett. 2007, 48, 877–881. [Google Scholar] [CrossRef]
- Matsuda, A.; Nomoto, Y.; Ueda, T. Synthesis of 2- and 8-Cyanoadenosines and Their Derivatives: Nucleosides and Nucleotides. XXVII. Chem. Pharm. Bull. 1979, 27, 183–192. [Google Scholar] [CrossRef]
- Ding, T.; Tang, F.; Ni, G.; Liu, J.; Zhao, H.; Chen, Q. The development of isoguanosine: From discovery, synthesis, and modification to supramolecular structures and potential applications. RSC Adv. 2020, 10, 6223–6248. [Google Scholar] [CrossRef]
- Xia, Z.; Kondhare, D.; Budow-Busse, S.; Leonard, P.; Seela, F. 7-Deaza-2’-deoxyisoguanosine, a Noncanonical Nucleoside for Nucleic Acid Code Expansion and New DNA Constructs: Nucleobase Functionalization of Inverse Watson–Crick and Purine–Purine Base Pairs. Bioconjugate Chem. 2024, 35, 1233–1250. [Google Scholar] [CrossRef]
- Hollenstein, M. Deoxynucleoside triphosphates bearing histamine, carboxylic acid, and hydroxyl residues—Synthesis and biochemical characterization. Org. Biomol. Chem. 2013, 11, 5162–5172. [Google Scholar] [CrossRef] [PubMed]
- Franzini, R.M.; Samain, F.; Abd Elrahman, M.; Mikutis, G.; Nauer, A.; Zimmermann, M.; Scheuermann, J.; Hall, J.; Neri, D. Systematic Evaluation and Optimization of Modification Reactions of Oligonucleotides with Amines and Carboxylic Acids for the Synthesis of DNA-Encoded Chemical Libraries. Bioconjugate Chem. 2014, 25, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, V.P.; Vozdvizhenskaya, O.A.; Baryshnikova, M.A.; Pershina, A.G.; Musiyak, V.V.; Matveeva, T.V.; Nevskaya, K.V.; Brikunova, O.Y.; Gruzdev, D.A.; Levit, G.L. Synthesis and Cytotoxic Activity of the Derivatives of N-(Purin-6-yl)aminopolymethylene Carboxylic Acids and Related Compounds. Molecules 2023, 28, 1853. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.M.; Lai, Y.; Xu, M.; Casin, A.H.; Laverde, E.E.; Liu, Y. AP endonuclease 1 prevents trinucleotide repeat expansion via a novel mechanism during base excision repair. Nucleic Acids Res. 2015, 43, 5948–5960. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).