Abstract
Hydrogel delivery systems are popular dosage forms that have a number of advantages, such as ease of use, painlessness, increased efficiency due to prolongation of rheological, swelling and sorption characteristics, regulation of drug release, and stimulus sensitivity. Particular interest is shown in hydrogels of cellulose ether derivatives due to the possibility of obtaining their modified forms to vary the solubility, the degree of prolonged action, and the release of the active substance, as well as their widespread availability, affordability, and the possibility of sourcing raw materials from different sources. Hydroxypropyl methylcellulose (HPMC, “hypromellose”) is one of the most popular cellulose ethers in the production of medicines as a filler, coating and carrier. Research on hydrogel carriers based on polymer complexes and modified forms of HPMC using acrylic, citric, and lactic acids, PVP, chitosan, Na-CMC, and gelatin is of particular interest, as they provide the necessary rheological and swelling characteristics. There is growing interest in medical transdermal hydrogels, films, capsules, membranes, nanocrystals, and nanofibers based on HPMC with the incorporation of biologically active substances (BASs), especially those of plant origin, as antibacterial, wound-healing, antimicrobial, mucoadhesive, anti-inflammatory, and antioxidant agents. The aim of this article is to review modern research and achievements in the field of hydrogel systems based on cellulose ethers, particularly HPMC, analyzing their properties, methods of production, and prospects for application in medicine and pharmacy.
Keywords:
hydrogels; plant extracts; cellulose derivatives; cellulose ethers; drug delivery; release; HPMC; dosage forms; films 1. Introduction
In the field of development of medical drugs and products, among all popular forms of drug delivery, hydrogel delivery systems have a number of advantages. Hydrogel-based drug delivery systems are easy to use, painless, and offer enhanced efficiency due to the prolonged release of the active ingredient. An important distinction of hydrogel systems is their convenience in use. This difference allows them to be considered an alternative to traditional forms. By selecting and combining an active ingredient with a hydrogel base, transdermal systems that have antiviral, antibacterial, and local anesthetic effects are developed [1,2,3,4]. Hydrogels also have the ability to respond to changes in external conditions, such as pH, temperature, electric field, light, ionic strength, magnetic oscillations, etc., which allows them to be classified as intelligent materials with adjustable properties. Additional properties of hydrogels include biodegradability, biocompatibility, and sensitivity to various factors. Biodegradability is currently [1,5,6] an important property for the development of environmentally friendly materials and technologies.
Hydrogels, which are three-dimensional polymer networks crosslinked through physical and chemical interactions, are synthesized from polymers of synthetic origin, semi-synthetic, and natural origin [7,8,9]. Polymers of different origins have both advantages and disadvantages. Synthetic and semi-synthetic polymers, while possessing the ability to polymerize and excellent mechanical properties, cannot surpass natural ones in terms of biocompatibility with the human body, and are also not capable of creating an accessible environment for other cells. Among the wide range of natural polymers, including those of protein, nucleic acid, and polysaccharide origins, this review will focus on cellulose ether derivatives, in particular, HPMC and hydrogels based on them [10,11]. As recent literature reviews show, cellulose derivatives are among the leading materials for pharmaceutical hydrogel production due to their widespread availability in nature, non-toxicity, and low cost.
Hydrogel systems are capable of swelling in water and aqueous solutions, which makes them compatible with the human body. Hydrogels also function as matrices in which biologically active substances can be immobilized, including synthetic and natural compounds, and plant extracts, for biomedical applications and enhancing biocompatibility [7]. Especially promising “green” sources of biologically active substances are plants rich in phenolic compounds, flavonoids, tannins, and others. In addition to biocompatibility and safety, another important property of hydrogels is their ability to prolong the action of a drug. This is particularly significant when developing medications for long-term use and high-toxicity treatments, in cancer therapy, and in the treatment of slow-healing wounds using pain-relieving agents. For instance, studies [12,13,14] have reported the development of drug formulations containing lidocaine and prilocaine microemulsions, which are local anesthetics with prolonged action, targeted delivery, and enhanced drug efficacy, demonstrating the promise of research in this direction. The potential of using cellulose derivatives as drug carriers, mathematical models of drug release, and the feasibility of creating ointment and gel drug formulations with prolonged action have also been demonstrated in [15,16,17,18,19,20]. Previously, the authors of [21,22,23] obtained hydrogels of the anesthetic substances rihlocaine and AK-29. New hydrogel dressings were developed using the radical crosslinking method based on poly-N-vinylpyrrolidone. These dressings contain the plant-derived medicinal substance “Alhidin”, extracted from the plant camelthorn (Alhagi kirghisorum Shrenk.), which grows in Kazakhstan [24]. The authors of [25] also obtained extracts from the plant Tamarix of the Tamaricaceae family, which were subsequently immobilized into polymer matrices in the form of films [26]. Previously, polyphenolic, terpenoid and other biologically active compounds from plants of this genus were found and characterized. Extracts and individual components have shown antioxidant, anti-amnesic, antimicrobial, antifungal, and cytotoxic activity. Using extracts of Tamarix Hispida as the aqueous drug component of HPMC hydrogel, we can obtain hydrogels with the abovementioned properties and use these widely in medicine and pharmacology [27].
The proposed review is a logical continuation of the studies of prolonged systems with biologically active substances based on polymer hydrogels and their compositions of synthetic and natural origin and will be used to study the possibility of creating hydrogel carriers of plant extracts based on HPMC and its compositions.
Among natural polymers for medical purposes, cellulose is advantageously distinguished by its diversity due to its modified forms. The introduction of additional groups into the structure of cellulose allows for varying their solubility, and provides the opportunity for immobilization of medicinal substances and regulation of release. As mentioned above, hydrogels are classified into natural, semi-synthetic, and synthetic based on their origin (Figure 1, [7]). The classification of hydrogels is based on their physical and chemical properties, as well as their structural features.
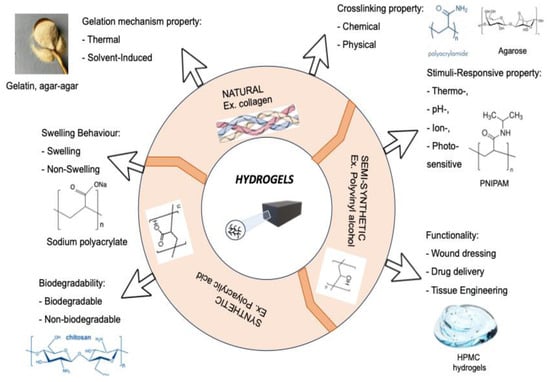
Figure 1.
Classification of hydrogels based on their properties [7].
It should be noted that it is hydrogels based on natural polymers that have been put to use in such an important field as medicine. Hydrogels are used in the production of contact lenses, matrices for cell proliferation, bases for targeted drug delivery, stabilizers for nanoparticles, composite materials, and more [28,29].
2. Cellulose Ether Derivatives as Hydrogel Bases
Cellulose and its derivatives, particularly cellulose ethers [30,31,32], are rightfully considered the favorites among natural raw materials for hydrogels. This is evidenced by the studies of various authors [18,19,29,33,34,35,36]. A significant advantage of cellulose derivatives is their abundance, which allows for a wide range of raw materials to be used for their production, such as various grains and cotton [37]. Cellulose derivatives, as complex esters, find widespread application in the pharmaceutical, food, and cosmetic industries. Among cellulose derivatives, the most widely used are methylcellulose (MC), hydroxypropyl cellulose (HPC), hydroxyethyl cellulose (HEC), and hydroxypropyl methylcellulose (HPMC) [38,39] (Figure 2).
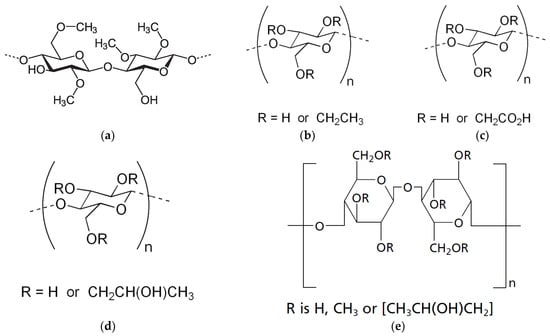
Figure 2.
Cellulose ether derivatives methylcellulose (MC) (a), ethyl cellulose (EC) (b), carboxymethylcellulose (CMC) (c), hydroxypropyl cellulose (HPC) (d), hydroxypropyl methylcellulose (HPMC) (e) [39].
Among the cellulose derivatives mentioned above, the most popular in the production of medicines is HPMC, which, in many literary sources, is called “hypromellose”. Hypromellose is a key component of drug coatings and fillers. This cellulose ether has advantages over other derivatives such as solubility in aqueous media, good swelling capacity, polymer viscosity, biocompatibility and biodegradability [40]. Hypromellose, like chitosan, has the advantage of having a complex-forming ability [41,42]. Polymer complexes and the modification of HPMC are also widely used to obtain hydrogel carriers of medicinal substances and materials with the necessary rheological and swelling characteristics. Thus, a number of studies [43,44,45,46,47] are dedicated to the development and study of the properties of hydrogels of cellulose derivatives crosslinked with acrylic acid and the delivery of such medicinal substances as galantamine hydrobromide and benzophenone-4. The structural and rheological properties of the hydrogel matrix play an important role in the development of drug delivery systems. These properties can be regulated by selecting a crosslinking agent, copolymerization, and altering external conditions such as temperature, ionic strength, and pH. To improve the physical, mechanical, rheological and bioadhesive properties, three-dimensional hydrogels with polyvinylpyrrolidone (PVP) were synthesized, which have swelling and water-retaining capacity for transporting medicinal substances.
In general, the processes for producing hydrogels are quite simple. In pharmaceutical production, hydrogels can be used both with and without the inclusion of a medicinal substance. For example, the medications Hitopran (Prontosan), Purilon, Matopat, Granugel are used in medical practice as wound-healing dressings in the form of hydrogel bandages and do not contain an active ingredient. Medical gels based on cellulose additionally include the following components: antimicrobial preservatives (methylparaben, propylparaben, chlorhexidine gluconate), stabilizers (disodium edetate), dispersing agents (alcohol, glycerin, propylene glycol, sorbitol), and homogenizers. The gelling ability depends on the type and molecular weight of the cellulose derivatives, but the minimum gelling concentration ranges from 4% to 6%. The convenience of using cellulose-derivative-based gels lies in their simple preparation, the absence of additional crosslinking agents, and the use of purified water as the hydrophilic phase. The methods for obtaining HPMC hydrogels are based on both traditional and environmentally friendly alternative methods, which increases their applicability.
3. Methods for Obtaining Hydrogels Based on HPMC
The successful development of hydrogel-based drug delivery systems is determined by the initial stage of creating the hydrogel matrix. This involves using both traditional methods and, increasingly in recent years, innovative techniques employing more environmentally friendly approaches. The search for new approaches to hydrogel synthesis is driven by the fact that hydrogels produced using traditional methods often exhibit low mechanical stability and slow responsiveness to external conditions [48].
The order of mixing components plays an important role in the preparation of gel formulations. Mixing the components above with the gelling agent should be carried out considering their influence on the gelling process. If these ingredients affect the rate and extent of swelling of the gelling agent, they are mixed after gelling. If such an intervention does not occur, the drug and other additives are mixed before the swelling process; in this case, it is also important to consider the impact of swelling time, mixing temperature, and other processing conditions on the physicochemical stability of the formulation and additives. Typically, the following order of mixing is recommended:
- The active ingredients are dissolved or suspended in the hydrophilic phase necessary for preparing the gel.
- Other additives are dissolved in the obtained solution or in a small amount of the hydrophilic phase, accordingly.
- If necessary, the dispersion of the active ingredient is mixed with the solution of additives.
- The gelling agent powder is added to the resulting dispersion solution with gentle stirring and left to swell.
When preparing cellulose-derivative-based hydrogels, temperature and pH of the dispersion are critical parameters, as the gelation mechanism of these polymers depends on temperature, and their optimal stability is pH-dependent. Thus, it is recommended to heat the macromolecule dispersion either before or after the polymer swelling.
Overall, the method for producing most hydrogels based on cellulose derivatives involves dispersing the polymer powder in cold water using mechanical stirring to form a homogeneous, single-phase dispersion. This is followed by heating the dispersion to approximately 60–80 °C and then gradually cooling it to room temperature to form the gel, a process known as the “hot/cold” method (Figure 3) [49,50]. Additionally, there are differences in the pH values of the dispersion medium favorable for gel formation: NaCMC, MC, and HPMC gels form over a wide pH range (4–10), while HPC and HEC form gels at a pH of 6–8 and under alkaline pH conditions, respectively. Another important parameter in the preparation of hydrogels from cellulose derivatives is the swelling duration of the polymer. Typically, a swelling time of about 24–48 h is required to obtain homogeneous gels.
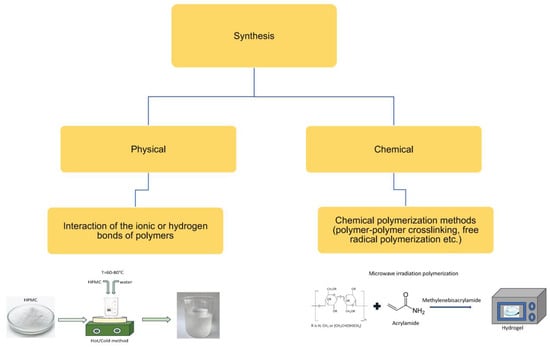
Figure 3.
Method for preparing hydrogels based on HPMC [49,50].
Finally, the removal of entrapped air is also an important consideration in the preparation of HPMC hydrogels as the presence of air bubbles in the gel inevitably affects its transparency. The inclusion of air bubbles in the gel can be minimized by positioning the stirrer at the bottom of the mixing container. Typically, air bubbles are further removed using various methods, including prolonged storage, storage at low temperatures, ultrasonic treatment, or the addition of silicone-based defoamers. Additionally, in large-scale production, vacuum deaerators are used to remove entrapped air. In small-scale production, the preparation of hydrogels by manually or mechanically dispersing the polymer in hot/cold water or a co-solvent is carried out using simple equipment and utensils available in a pharmaceutical laboratory, such as porcelain or glass mortars and pestles, measuring cylinders, magnetic stirrers, and various propellers and mixers. In large-scale production of pharmaceutical cellulose derivatives, various mills, separators, mixers, deaerators, and switches are used. In the pharmaceutical application of HPMC gels, the physical method of production is the most convenient and popular, especially when creating medicinal systems with plant extracts.
4. Important Physicochemical Properties of HPMC and Its Hydrogels
HPMC (hydroxypropyl methylcellulose) is a modified propylene glycol ether of methylcellulose, consisting of methoxy and hydroxypropyl groups along the linear polysaccharide chain (P. Timmins [39]).
Cellulose derivatives, particularly HPMC, are among the widely used natural biodegradable polymers applied in the development of oral drug delivery systems with controlled release [51]. The variation in cellulose derivatives regarding molecular weight, solubility, viscosity, and degree of dissociation allows for their extensive use in various applications. The main property of HPMC, which is used in the creation of immobilized systems and in the mechanism of drug release, is its solubility and swelling of its gels, which allows for a variation in parameters to achieve different degrees of prolongation.
The physicochemical properties of HPMC, such as surface activity, solubility, and gel formation, depend on three factors: the presence of methoxy groups, the presence of hydroxypropyl groups, and molecular weight. HPMC is soluble in cold water due to the targeted alkylation of the hydroxyl group of natural cellulose with methoxy and hydroxypropyl groups [15]. After dissolving in water, it forms a clear viscous gel. Hypromellose also dissolves very well in other solvents, such as ethanol, isopropanol, dichloromethane, as well as in their aqueous solutions at various ratios.
Temperature significantly affects the properties of the gel formed from HPMC. Aqueous solutions of hypromellose exhibit sol-gel thermal transitions at high temperatures, which most often manifest as turbidity of the solution caused by the process of hydrophobic interaction between the methoxyl groups in the hypromellose [52]. For hypromellose, the gelation process begins with the dissolution of the polymer, and with a further increase in temperature, the formation of terminal separated sections and hydrophobic associations of chains occurs. Hydration of hypromellose is highly temperature-dependent; as the temperature increases, HPMC loses hydration water, which leads to a decrease in the relative viscosity of the polymer, and the loss of hydration water contributes to the strengthening of polymer–polymer interactions, such as hydrophobic ones, primarily due to methoxy substituents [53]. Increased hydrophobic interactions lead to increased viscosity, which is the point of gelation. The gelation temperature of hydroxypropyl methylcellulose (HPMC) ranges from 50 °C to 90 °C and depends, like the solidification temperature, on the number of methoxy groups in the molecule [54]. Substitution with ethoxy and dioxy groups contributes to an increase in the gelation temperature. The results of the study [55] showed that the gelation temperature can be reduced to 36 °C by replacing the hydroxypropoxy groups.
As the temperature increases, the gelation process occurs, leading to the precipitation of polymer molecules [56]. The gelation point and the turbidity point are independent of each other; so, the solution may become turbid before reaching the gelation temperature. If a polyelectrolyte is present in the solution, both the turbidity and the gelation temperature decrease [53].
HPMC is not used in materials that require thermal treatment because its decomposition temperature is 170–180 °C, and its melting point is 190–200 °C. Moreover, the decomposition temperature of HPMC depends on the structure of the side chains [57]. The lack of thermal stability prevents the use of further processing methods for the polymer that require its melting. Thus, in [58], attempts were made to improve the polymer’s thermal stability. The development by the chemical company “Dow” (USA) for the extrusion and spray drying process required the use of hot molten polymer. A new product under the brand name “Affinisol HPMC HME” has been proposed, which features a low glass transition temperature, low viscosity, color stability at high temperatures, and extrusion capability. The authors also achieved improved solubility in organic solvents, which allows the polymer to be used in spray drying.
The source of hydroxypropyl methylcellulose (HPMC) is cellulose fibers, which are heated with an acoustic alkaline solution during the etherification process [16]. It is then treated with propylene oxide and methyl chloride. The resulting product has a specific ratio of substituted hydroxypropyl and methyl groups, which, in turn, depends on the ratio of methyl chloride and propylene oxide introduced after alkalization. The ratio of substituted groups affects the viscosity and gelation temperature of aqueous solutions of hypromellose.
The glass transition temperature of the polymer plays an important role in the development of controlled drug delivery systems. It was previously mentioned that hypromellose is widely used as a filler in pharmaceutical formulations and for the development of controlled drug delivery systems. When taking drugs with controlled delivery, some properties affect the release of the active drug. For example, it is known that a decrease in the glass transition temperature of the polymer reduces the mobility of macromolecules within the structure. At the same time, as the temperature increases, the mobility of macromolecules begins to rise, leading to an increased rate of drug transport. In the work of Doelker E. et al. [59], the glass transition temperature of the HPMC polymer was investigated. Various methods, such as thermomechanical analysis, dynamic mechanical analysis, differential scanning calorimetry, and torsion analysis, were used to determine and compare the results. However, different analysis methods show different glass transition temperature results for HPMC. Therefore, it is recommended to conduct research and comparisons using one of the methods. In determining the glass transition temperature, it is important to consider the degree of substitution as well as the molecular weight of the polymer [15]. All the listed properties of HPMC are important in the creation of medicinal hydrogel forms. The nature and structure of the active pharmaceutical ingredient adjust its properties, especially rheological characteristics, swelling properties, solubility, compatibility, uniformity, and physico-mechanical strength.
Hydrogel systems with phytocompounds based on polysaccharides show improved properties such as biocompatibility, controlled release of active substances, mechanical strength, and hydrophilicity. Cellulose ethers have all of the above properties, and HPMC occupies a worthy place. The works summarize research on the production of polysaccharide hydrogels with phytocompounds in various dosage forms using solution casting technologies, with porogenic agents, gas foaming, lyophilization, cryogelation, extrusion, coacervation, emulsification, spray drying and electroforming [60,61]. In [62], cellulose and CMC are mentioned among the popular carriers, but it should be noted that, in recent years, there has been increasing interest in obtaining hydrogel systems of synthetic biologically active substances, phytocompounds with hypromellose (HPMC) [43,63,64,65,66,67,68,69,70,71,72]. The main focus in [65] is on properties such as thermosensitivity, swelling capacity, physical and mechanical properties, and the release of biologically active substances. The high swelling tendency, hydrophilicity, and thermal sensitivity of HPMC made it possible to use it to obtain a “smart” and biocompatible film with a uniform surface. The formation of hydrogen bonds between the NH- and OH-groups of chitosan and HPMC increases the strength, and the degree of swelling reaches the minimum value of the CH:HPMC 40:60 system. Due to chitosan, the hydrogel system acquires pH sensitivity and the phase transition temperature of HPMC increases. The introduction of essential oils into the composition of the CH-HPMC hydrogel increases the thixotropic and rheological properties, and enhances the penetration of fluconazole [66]. In the binary PVP-HPMC gel, improved adhesive properties and rheological characteristics are observed. Bases made from both pure HPMC and the binary PVP-HPMC mixture [43] contribute to the slow release of the model drug benzophenone-4. Additionally, the release of the active substance is further slowed by changes in viscosity, which significantly increases at concentrations above 8%. Upon contact with the skin and intense rubbing, viscosity rapidly decreases, ensuring gel spreadability. The addition of PVP enhances the prolongation of drug action due to the electrostatic interaction between PVP and benzophenone-4. An HPMC-based hydrogel [67] containing PGA microfibers and ofloxacin, formed through blending, exhibits high wound-healing activity, a swelling degree of 531.8–1700%, and elongation within the range of 70–120%. The synergistic antimicrobial effect of the microfibers and the active ingredient is enhanced by the strong swelling capacity of HPMC, which plays a crucial role in the tissue granulation stage and promotes rapid wound healing.
Studies have demonstrated the possibility of obtaining a porous HPMC gel containing carvedilol, dissolved in Tween-20, lactic acid [68], and polysaccharides from Agaricus blazei Murill [69], with controlled release and rheological properties suitable for bone tissue regeneration. The gelation temperature of HPMC was reduced to 37 °C due to the presence of polysaccharides, making it comfortable for the oral mucosa and dental restoration. Additionally, the porosity of the HPMC gel allowed for the regulation of the release of poorly soluble carvedilol. As is well known, the rheological characteristics, swelling properties of HPMC, and gelation temperature are essential for the development of pharmaceutical dosage forms, particularly prolonged-release tablets with controlled drug release [70,71,72].
Researchers have described the kinetics of release of the active drug substance from a controlled delivery system based on hypromellose [73,74]. Various methods of analysis have been used, such us NMR spectroscopy, to characterize the mobility of water in the gel layer of the HPMC polymer [75].
5. Relevance of Pharmaceutical Application of HPMC with BAS
According to the WHO, more than 80% of medicines are of plant origin. Most diseases require the use of medicinal herbs along with synthetic drugs. Enhancing the efficiency of plant extracts and the rational use of natural resources are among the pressing global issues included in the Sustainable Development Goals due to the growing number of patients with skin diseases, trophic ulcers, and purulent wounds of various etiologies, necessitating a range of affordable applicative materials and their production [76,77].
The growing interest in the field of natural compound chemistry research is explained by several factors:
- (1)
- The diversity of both the chemical structures and biological activities of naturally occurring secondary metabolites;
- (2)
- The use of new bioactive natural compounds as biochemical probes;
- (3)
- The development of new and sensitive methods for detecting biologically active natural products;
- (4)
- Improved isolation methods;
- (5)
- Purification and structural characterization of these active components, as well as the demand for natural products [76].
The scientific literature includes studies on the development of wound-healing pharmaceutical formulations based on natural polymers and plant extracts [78,79]. The biological activity of medicinal plants and their extracts is determined by their components, which define their beneficial properties such as antioxidant, wound-healing, anti-inflammatory, antibacterial, anti-tumor, and other effects. Among the natural components of plants, phenolic compounds and secondary metabolites that exhibit the abovementioned activities stand out. An example of the application of natural polymers is the development of a wound-healing dosage form based on alginates. Leading positions in the creation of polymeric dosage forms are held by biopolymers that are biodegradable and typically derived from natural sources, such as plants, algae, animal organisms, and bacteria. Examples of such biopolymers include polysaccharides, lipopolysaccharides, and proteins, which hold a significant share of the pharmaceutical sector [80]. Thus, Klemm D. et al. [81] propose a range of affordable, biodegradable biopolymers with low cost, which can serve as the basis for medicinal products, particularly medical wound-healing dressings. Among biopolymers used as carriers for plant-based medicinal substances, HPMC stands out, as evidenced by data on HPMC-based materials combined with biologically active substances, extracts, and synthetic compounds, demonstrating enhanced effectiveness in wound treatment. Examples of HPMC applications as a drug carrier can be found in the works of many researchers. Below are some examples of soft dosage forms based on HPMC with medicinal substances and plant components (Table 1).

Table 1.
List of HPMC-based formulations with biologically active substances (BAS).
The analysis of studies and data presented in Table 1 shows that the geographic application of HPMC as a carrier of biologically active substances (BASs) is quite extensive. The advantageous properties of HPMC for the creation of pharmaceutical formulations enable the development of soft transdermal systems incorporating biologically active substances (BASs). Among the developed formulations, gel and film forms with HPMC are the most popular [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. The studies on obtaining capsules, biomembranes, nanocrystals, and nanofibers are particularly interesting. The soft biomaterials developed are proposed as antibacterial, wound-healing, antimicrobial, and anti-inflammatory agents for the treatment of burns of varying degrees, as membranes, sorbent materials, and drug delivery carriers. As the data presented in the table show, HPMC plays an important role in hydrogel formulations, providing gelation, moisture retention, controlled release and prolongation of the action of medicinal components.
In the process of wound healing, plant extracts show activity and advantages due to the content of biologically active substances such as flavonoids, terpenoids, fatty acids, phenolic compounds, alkaloids, catechins, quinones and carbohydrates [106,107]. The main disadvantage of plant-based biologically active substances is their short duration of action and instability, which can be solved by the creation of hydrogel dressing materials based on natural polymers. Secondary metabolites of plants exhibit antibacterial, anti-inflammatory, antioxidant and regenerative effects. The stages of the physiological process of wound healing include platelet aggregation, the appearance of a hemostatic effect (hemostasis), the formation of macrophages that cleanse damaged tissue with further tissue growth (inflammatory phase), the stage of re-epithelialization and the formation of granulation tissue with the participation of fibroblasts, keratinocytes and endothelial cells (proliferative phase), and the stage of replacing type III collagen with type I collagen with strengthening of the formed tissue (remodeling). Plant extracts participate in stimulating the process of formation of new blood vessels, the flow of nutrients to damaged tissue, the removal of degradation products, and the formation of granulation tissue and collagen.
Among plant extracts, aloe vera [70,94], henna [83], curcuma [108], Araucaria heterophylla [109], and others are popular in wound treatment and healing. These medicinal plants are used in the preparation of dosage forms with cellulose ethers, and with HPMC for the treatment of wounds and burns with the properties necessary for treatment. For wound healing, the most commonly used compounds are flavonoids—rutin, quercetin, kaempferol, phenolic compounds and terpenoids—tannins, anthocyanins, ellagic acid, and alkaloids—berberine, embelin, etc.
The anti-inflammatory activity of plant extracts is due to the presence of flavonoids (curcumin, quercetin, quercetin-3,7-di-O-α-L-rhamnopyranoside, Epigallocatechin-3-gallate, 5-Hydroxy-7,8-dimethoxyflavanone, 6,7-Dimethoxycoumarin, liquiritigenin, isoflavone, etc.), terpenes (8-o-acetylharpagide, schizanol, stigmasterol, 7-B-hydroxysitosterol, marinoid D, maslinic acid, oleanolic acid, glycyrrhetinic acid etc.), essential oils (eugenol, linalool, camphor, borneol, thymol, citral, menthol, γ-terpinene, thymoquinone, carvone, α-terpineol, etc.), alkaloids (colchicine, sinomenine, capsaicin, berberine), and phenolic compounds (10-Shogaol, 4-hydroxybenzoic acid, cis-mellitoside, trans-mellitoside, salicin, ferulic acid, dihydromellitoside, etc.) [110].
Plant extracts can be used individually or in combination as antibacterial components of medicines with well-known antibacterial drugs, providing a synergistic effect [111]. The antimicrobial properties are due to the presence of natural compounds such as allicin, piperine, curcumin, eugenol, chlorogenic acid, quercetin, carvacrol, thymol, etc.
The mechanisms of action of plant secondary metabolites (PSMs) on microbial cells include disrupting the structure and function of the bacterial cytoplasmic membrane, preventing complex formation with membrane proteins, inhibiting enzyme synthesis, blocking the synthesis and functions of DNA and RNA, and increasing the coagulation of cytoplasmic components [112]. Antimicrobial action based on the destruction of the membrane of microbial cells is exhibited by flavonoids, terpenes and terpenoids, essential oils. For alkaloids, the literature describes effects on key stages of the pathogenesis process, such as inhibiting the production of staphylococcal α-hemolysin (e.g., capsaicin from Capsium L.).
It is important to note that the extraction of plant biologically active substances is carried out through the preparation of decoctions, infusions and alcohol tinctures. In the study [111] of the antibacterial activity of plant extracts using the dual reporter system Dualrep2, it was shown that alcoholic extracts exhibit greater activity compared to aqueous ones, and that alcoholic extracts are better obtained from dried raw materials than from fresh ones.
Medicinal systems based on HPMC and its compositions are obtained both with individual synthetic biologically active substances and on the basis of plant extracts individually and in combination with known drugs. The Aloe vera plant (Aloe barbadensis) is rich in polyphenolic compounds with antioxidant activity and is abundant in vitamins. The anthraquinones and saponins in aloe gel exhibit antimicrobial activity against Gram-positive bacteria such as Shigella flexneri and Streptococcus pyogenes [113]. The presence of various classes of natural compounds in Aloe vera has enabled its use in an HPMC-based gel as a burn treatment for skin wound healing [87]. Aloe vera gel is an excellent and accessible alternative to antimicrobial agents among plant-based sources with a rich composition of phytometabolites. In the wound-dressing material with Aloe vera gel, HPMC is included into the composite base as the main film-forming and moisture-retaining component [94]. The similarity of the nature of HPMC and the active compounds of Aloe vera, such as polysaccharides (glucomannans), glycoproteins, amino acids, flavonoids, provides the fibrous material with strength and heat resistance due to the formation of bonds between these components. HPMC also facilitates the dispersion of Aloe vera in the polymer mixture, thus ensuring its uniform distribution.
For the treatment of burn infections caused by Gram-positive bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa, a composition based on cellulose ether derivatives, extracts of henna (Lawsonia inermis L.) and chamomile (Matricaria chamomilla L.) was proposed [83]. The antibacterial effect of the composition is due to terpenoids, phenolic compounds of the alcohol extract of chamomile, and the presence of such active components of henna as Lawson (2-hydroxy-1,4-naphthoquinone), tannins. Also, the presence of a powerful antioxidant gallic acid, flavonoids, mucilagin, and mannitol contributes to an important stage, skin healing, in the treatment of burn wounds. The base of HPMC and CMC provides controlled release of active components by a diffusion mechanism.
6. Conclusions
The main types of hydrogels of cellulose ethers and their methods of synthesizing, classification, uses and chemical properties of hydrogel forms with BAS are reviewed in this article. Hydrogels of cellulose ethers have become a popular material for thorough research and practical use in a variety of fields due to their capacity to absorb and hold large amounts of liquid and due to its economical availability. Cellulose-based products are the most eco-friendly and biodegradable; thus, as hydrogels, when they reach the aquatic environment, they have the unique ability to maintain a three-dimensional structure. Hydroxypropyl methylcellulose is a good material for drug transfer as tablets, ointments, gels, films with prolonged effect.
In the therapeutic treatment of wounds of various etiologies, antibacterial, anti-inflammatory and antioxidant effects are important for the active principle, which can be characteristic of both individual synthetic drugs and the BAC of plant extracts. In recent years, there has been a trend to search for natural sources of biologically active substances that meet the requirements for wound treatment and can be a worthy alternative to well-known drugs, especially antimicrobial agents, to which resistance has already developed. The advantage of using plant extracts is that phytocomponents can act at several stages in the wound-healing process, from hemostasis to remodeling, which is possible due to phytometabolites such as flavonoids, terpenes, tannins, polysaccharides, terpenoids, phenolic compounds, alkaloids, essential oils, saponins. The similarity in nature and structure between phytometabolites and cellulose ether derivatives makes them promising for the development of biocompatible, biodegradable, effective, and controlled wound dressings, gels, films, fibers, and membranes.
To sum up, hydrogels of hydroxypropyl methylcellulose with plant extracts with biological active substances are of great interest to researchers as regards biomedical applications in the form of ointments, gels, and films for local antibacterial, antioxidant activities. A review of the literature showed that there is currently insufficient research on the biodegradation of drug delivery systems based on cellulose derivatives with biologically active substances, which is a broad field for further research.
Author Contributions
Conceptualization, A.A. and S.Z.; methodology, Z.A.; software, D.O. and Z.M.; validation, N.S., D.O. and E.D.; formal analysis, A.N. and E.D.; investigation, A.A., S.Z. and Z.A.; resources, S.Z.; data curation, Z.M., N.S. and A.N.; writing—original draft preparation, A.A.; writing—review and editing, S.Z.; visualization, N.S.; supervision, Z.A.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic Kazakhstan, grant number AP22684531.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPMC | Hydroxypropyl Methylcellulose |
| Na-CMC | Sodium Carboxymethyl Cellulose |
| PVP | Polyvinylpyrrolidone |
| MC | Methylcellulose |
| HPC | Hydroxypropyl Cellulose |
| HEC | Hydroxyethyl Cellulose |
| PAA | Polyacrylic Acid |
References
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Duan, J.J.; Zhang, L.N. Robust and smart hydrogels based on natural polymers. Chin. J. Polym. Sci. 2017, 35, 1165–1180. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Pillai, L.V.; Patel, K.P.; Desai, A.R.; Shukla, M.R.; Desai, D.T.; Patel, H.P.; Ranch, K.M.; Shah, S.A.; Shah, D.O. Lidocaine tripotassium phosphate complex laden microemulsion for prolonged local anaesthesia: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 185, 110632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dang, M.; Zhang, W.; Lei, Y.; Zhou, W. Sustained delivery of prilocaine and lidocaine using depot microemulsion system: In vitro, ex vivo and in vivo animal studies. Drug Dev. Ind. Pharm. 2020, 46, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Michida, N.; Hayashi, M.; Hori, T. Comparison of event related potentials with and without hypnagogic imagery. Psychiatry Clin. Neurosci. 1998, 52, 145–147. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Sadeghi, E. Effect of Strong Electrolyte Containing Gelling Aids on the Sol-Gel Transition Temperature of Hypromellose 2910. Master’s Thesis, University of New Mexico, Albuquerque, NM, USA, 2018. [Google Scholar]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- De Sousa Moraes, P.R.F.; Saska, S.; Barud, H.; De Lima, L.R.; Da Conceicao Amaro Martins, V.; De Guzzi Plepis, A.M.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial cellulose/collagen hydrogel for wound healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Zhumagalieva, S.N.; Kudaibergenova, B.M.; Beisebekov, M.K.; Abilov, Z.A. Properties of bentonite and local anesthetics composition. J. Appl. Polym. Sci. 2007, 106, 1601–1605. [Google Scholar] [CrossRef]
- Zhumagalieva, S.N.; Beisebekov, M.K.; Abilov, Z.A. Immobilization of 2,5-dimethyl-4-benzoyl-oxypiperidine succinate over polyacrylic acid (PAA) gels. I. Study of interaction between linear and network PAA with succinate of 2,5-dimethyl-4-benzoyl-oxypiperidine. J. Appl. Polym. Sci. 2005, 96, 1183–1186. [Google Scholar] [CrossRef]
- Zhumagalieva, S.N.; Beisebekov, M.K.; Abilov, Z.A. Immobilization of 2,5-dimethyl-4-benzoyl-oxypiperldine succinate over polyacrylic acid (PAA) gels. II. Study of quantitative characteristics of immobilization of succinate of 2,5-dimethyl-4-benzoyl-oxypiperidine over PAA. J. Appl. Polym. Sci. 2005, 96, 1187–1192. [Google Scholar] [CrossRef]
- Temirkhanova, G.; Burasheva, G.; Abilov, Z.; Irmukhametova, G.; Mun, A.; Beksultanov, Z.; Myktybaeva, Z.; Shnaukshta, V. Creation of polymer hydrogel dressings with herbal medicinal substsnce “Alkhydin” and their properties. Eurasian Chem.-Technol. J. 2017, 19, 57–62. [Google Scholar] [CrossRef]
- Zhumagaliyeva, S.N.; Amanzholkyzy, A.; Sultanova, N.A.; Abilov, Z.A. Use of ultrasound for extraction of biologically active substances from tamarix hispida willd. Chem. Plant Raw Mater. 2021, 3, 283–289. [Google Scholar] [CrossRef]
- Zhumagaliyeva, S.N.; Abdikarim, G.G.; Berikova, A.B.; Abilov, Z.A.; Koetz, J.; Kopbayeva, M.T. Study of the Mechanical Properties of Gelatin Films with Natural Compounds of Tamarix hispida. Eurasian Chem.-Technol. J. 2023, 25, 165–171. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Kang, Y.; Wang, A. Synthesis, swelling and responsive properties of a new composite hydrogel based on hydroxyethyl cellulose and medicinal stone. Compos. Part B Eng. 2011, 42, 809–818. [Google Scholar] [CrossRef]
- Kryazhev, D.V.; Smirnov, V.F.; Mochalova, A.E.; Smirnova, O.N.; Zakharova, E.A.; Zotov, K.A.; Smirnova, L.A. Stability of synthetic and natural polymer composite materials to the action of micromyccetes under natural conditions. Bull. N.I. Lobachevsky Nizhny Novgorod Univ. 2010, 2, 536–540. [Google Scholar]
- Li, X.; Wan, C.; Tao, T.; Chai, H.; Huang, Q.; Chai, Y.; Wu, Y. An overview of the development status and applications of cellulose-based functional materials. Cellulose 2024, 31, 61–99. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Zhao, Y.; Peng, Z. Effects of cellulose nanocrystal polymorphs and initial state of hydrogels on swelling and drug release behavior of alginate-based hydrogels. Polym. Bull. 2020, 77, 4401–4416. [Google Scholar] [CrossRef]
- Gupta, Y.; Khan, M.S.; Bansal, M.; Singh, M.K.; Pragatheesh, K.; Thakur, A. A review of carboxymethyl cellulose composite-based hydrogels in drug delivery applications. Results Chem. 2024, 10, 101695. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mäder, K.; Siepmann, F. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.H. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef]
- Selvaraj, S.; Chauhan, A.; Dutta, V.; Verma, R.; Rao, S.K.; Radhakrishnan, A.; Ghotekar, S. A state-of-the-art review on plant-derived cellulose-based green hydrogels and their multifunctional role in advanced biomedical applications. Int. J. Biol. Macromol. 2024, 265 Pt 2, 130991. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2010, 57, 533–546. [Google Scholar] [CrossRef]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as wound healing materials. Wound Heal. Biomater. 2016, 2, 261–287. [Google Scholar]
- Karimian, A.; Parsian, H.; Majidinia, M.; Rahimi, M.; Mir, S.M.; Samadi Kafil, H.; Shafiei-Irannejad, V.; Kheyrollah, M.; Ostadi, H.; Yousefi, B. Nanocrystalline cellulose: Preparation, physicochemical properties, and applications in drug delivery systems. Int. J. Biol. Macromol. 2019, 133, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pan, P.; Svirskis, D.; Waterhouse, G.I.N.; Wu, Z. Hydroxypropyl Methylcellulose Bioadhesive Hydrogels for Topical Application and Sustained Drug Release: The Effect of Polyvinylpyrrolidone on the Physicomechanical Properties of Hydrogel. Pharmaceutics 2023, 15, 2360. [Google Scholar] [CrossRef]
- dos Santos Carvalho, J.D.; Rabelo, R.S.; Hubinger, M.D. Thermo-rheological properties of chitosan hydrogels with hydroxypropyl methylcellulose and methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Qureshi, U.F. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 400–410. [Google Scholar]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl Methylcellulose-Based Hydrogel Copolymeric for Controlled Delivery of Galantamine Hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Lamberti, G.; Cascone, S.; Cafaro, M.M.; Titomanlio, G.; d’Amore, M.; Barba, A.A. Measurements of water content in hydroxypropyl-methyl-cellulose based hydrogels via texture analysis. Carbohydr. Polym. 2013, 92, 765–768. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Ghorbani, S.; Eyni, H.; Bazaz, S.R.; Nazari, H.; Asl, L.S.; Zaferani, H.; Kiani, V.; Mehrizi, A.A.; Soleimani, M. Hydrogels Based on Cellulose and its Derivatives: Applications, Synthesis, and Characteristics. Polym. Sci. Ser. A 2018, 60, 707–722. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Ettehadi, S.; Amini, M.; Sabzi, M. Synthesis and characterization of hydroxypropyl methylcellulose-g-poly(acrylamide)/LAPONITE® RD nanocomposites as novel magnetic- and pH-sensitive carriers for controlled drug release. RSC Adv. 2015, 5, 44516–44523. [Google Scholar] [CrossRef]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Hoboken, NJ, USA, 2010; 363p. [Google Scholar]
- Haque, A.; Morris, E.R. Thermogelation of methylcellulose. Part I: Molecular structures and processes. Carbohydr. Polym. 1993, 22, 161–173. [Google Scholar] [CrossRef]
- Mitchell, K.; Ford, J.L.; Armstrong, D.J.; Elliott, P.N.C.; Rostron, C.; Hogan, J.E. The influence of additives on the cloud point, disintegration and dissolution of hydroxypropylmethylcellulose gels and matrix tablets. Int. J. Pharm. 1990, 66, 233–242. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Joshi, S.C. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Sarkar, N. Thermal Gelation Properties of Methyl and Hydroxypropyl Methylcellulose. J. Appl. Polym. Sci. 1979, 24, 1073–1087. [Google Scholar] [CrossRef]
- Jani, R.; Patel, D. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. Asian J. Pharm. Sci. 2014, 10, 292–305. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A New Extrudable Form of Hypromellose: AFFINISOLTM HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Doelker, E. Cellulose derivatives. Adv. Polym. Sci. 1993, 107, 198–265. [Google Scholar]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Platon, I.V.; Lazar, M.M.; Dinu, M.V.; Aprotosoaie, A.C. Trends in polysaccharide-based hydrogels and their role in enhancing the bioavailability and bioactivity of phytocompounds. Carbohydr. Polym. 2024, 334, 122033. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.; Lee, M.; Kwon, S. Controlled release of quercetin from HPMC/gellan gum hydrogel for inhibiting melanogenesis in murine melanoma cells. Korean J. Chem. Eng. 2023, 40, 337–343. [Google Scholar] [CrossRef]
- Abou-Taleb, H.A.; Mohamed, M.S.; Zayed, G.M.; Abdelaty, L.N.; Makki, M.A.; Abdel-Aleem, H.L.; El-Mokhtar, M.A.; Hetta, H.F.; Abdullah, N.; Saddik, M.S. HPMC-Zein Film-forming Gel Loaded with 5-Fluorouracil Coupled with CO2 Laser Dermabrasion for Managing Stable Vitiligo. AAPS PharmSciTech 2024, 25, 225. [Google Scholar] [CrossRef]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Cesarino, I.; Costa, C.M.; Lanceros-Méndez, S.; Pawlicka, A.; Silva, M.M. Thermo-sensitive chitosan–cellulose derivative hydrogels: Swelling behaviour and morphologic studies. Cellulose 2014, 21, 4531–4544. [Google Scholar] [CrossRef]
- Mu, A.M.; Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, I.; St, C.; Mitu, M.A.; Lupuliasa, D. Chitosan/HPMC-based hydrogels containing essential oils for topical delivery of fluconazole: Preliminary studies. Farmacia 2018, 66, 248–256. [Google Scholar]
- Agubata, C.O.; Okereke, C.; Nzekwe, I.T.; Onoja, R.I.; Obitte, N.C. Development and evaluation of wound healing hydrogels based on a quinolone, hydroxypropyl methylcellulose and biodegradable microfibres. Eur. J. Pharm. Sci. 2016, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Mu, H.; Genina, N. Bespoke hydroxypropyl methylcellulose-based solid foams loaded with poorly soluble drugs by tunable modular design. Carbohydr. Polym. 2025, 357, 123397. [Google Scholar] [CrossRef]
- Silva, G.D.A.; Campelo, M.D.S.; Lima, A.B.N.; Mattos, A.L.A.; De Sousa, V.C.; Dutra, P.G.P.; Leal, L.K.A.M.; Ricardo, N.M.P.S.; Ribeiro, M.E.N.P. Rheological and osteogenic effect of Agaricus blazei Murill polysaccharides on composite hydrogels based on HPMC/graphene oxide—A preliminary study. Mater. Today Commun. 2024, 40, 109986. [Google Scholar] [CrossRef]
- Knarr, M.; Rogers, T.L.; Petermann, O.; Adden, R. Investigation and rank-ordering of hydroxypropyl methylcellulose (HPMC) properties impacting controlled release performance. J. Drug Deliv. Sci. Technol. 2025, 104, 106425. [Google Scholar] [CrossRef]
- Ellakwa, T.E.; Abu-Khadra, A.S.; Ellakwa, D.E.S. Influence of physico-chemical properties of hydroxypropyl methylcellulose on quetiapine fumarate release from sustained release matrix tablets. BMC Chem. 2024, 18, 219. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Feng, Y.; Wang, H.; Xu, Y.; Yin, T.; Zhang, Y.; He, H.; Gou, J.; Tang, X. Further enhancement of the sustained-release properties and stability of direct compression gel matrix bilayer tablets by controlling the particle size of HPMC and drug microencapsulation. Powder Technol. 2024, 448, 120256. [Google Scholar] [CrossRef]
- Kim, C.-J. Release kinetics of coated, donut-shaped tablets for water soluble drugs. Eur. J. Pharm. Sci. 1999, 7, 237–242. [Google Scholar] [CrossRef]
- Elena Campos-Aldrete, M. Influence of the viscosity grade and the particle size of HPMC on metronidazole release from matrix tablets. Eur. J. Pharm. Biopharm. 1997, 43, 173–178. [Google Scholar] [CrossRef]
- Melia, C.D.; Rajabi-Siahboomi, A.R.; Hodsdon, A.C.; Adler, J.; Mitchell, J.R. Structure and behaviour of hydrophilic matrix sustained release dosage forms: 1. The origin and mechanism of formation of gas bubbles in the hydrated surface layer. Int. J. Fharmaceutics 1993, 100, 263–269. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Obradovic, J.; Wondraczek, H.; Fardim, P.; Lassila, L.; Navard, P. Preparation of three-dimensional cellulose objects previously swollen in a DMAc/LiCl solvent system. Cellulose 2014, 21, 4029–4038. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Kouchak, M.; Rezaee, S.; Moshabeh, N.; Handali, S. Preparation and evaluation of matrix containing lidocaine and prilocaine for using in transdermal films. J. Rep. Pharm. Sci. 2019, 8, 270–276. [Google Scholar]
- Bagheri, M.; Shokoohinia, Y.; Pourmanouchehri, Z.; Jalilian, F.; Khaledian, S.; Mirzaie, S.; Behbood, L. Formulation and evaluation of the novel herbal antibacterial gel to the treatment of cutaneous burn infections. J. Rep. Pharm. Sci. 2021, 10, 93–100. [Google Scholar]
- Wu, Y.C.; Wu, G.X.; Huang, H.H.; Kuo, S.M. Liposome-encapsulated farnesol accelerated tissue repair in third-degree burns on a rat model. Burns 2019, 45, 1139–1151. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Morales, S.; Okamoto, Y.; Chan, H.K. Topical application of bacteriophages for treatment of wound infections. Transl. Res. 2020, 220, 153–166. [Google Scholar] [CrossRef]
- Saddik, M.S.; Alsharif, F.M.; El-Mokhtar, M.A.; Al-Hakkani, M.F.; El-Mahdy, M.M.; Farghaly, H.S.; Abou-Taleb, H.A. Biosynthesis, Characterization, and Wound-Healing Activity of Phenytoin-Loaded Copper Nanoparticles. AAPS PharmSciTech 2020, 21, 175. [Google Scholar] [CrossRef]
- Kumari, S.; Harjai, K.; Chhibber, S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J. Infect. Dev. Ctries. 2010, 4, 367–377. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity and in Vivo Evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef] [PubMed]
- Mohd Noor, A.; Bin Bai, S. Mechanical properties and water vapour permeability of film from haruan (channa striatus) and fusidic acid spray for wound dressing and wound healing. Pak. J. Pharm. Sci. 2010, 23, 155–159. [Google Scholar]
- Huang, T.W.; Lu, H.T.; Ho, Y.C.; Lu, K.Y.; Wang, P.; Mi, F.L. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mater. Sci. Eng. C 2021, 118, 111396. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; Da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef]
- Uslu, I.; Aytimur, A. Production and characterization of poly(vinyl alcohol)/poly(vinylpyrrolidone) iodine/poly(ethylene glycol) electrospun fibers with (hydroxypropyl)methyl cellulose and aloe vera as promising material for wound dressing. J. Appl. Polym. Sci. 2012, 124, 3520–3524. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou B sen Valenzuela-Medina, D.; Du, W.X.; Gregorski, K.S.; Williams, T.G.; Wood, D.F.; Glenn, G.M.; Orts, W.J. Solution blow spun poly(lactic acid)/hydroxypropyl methylcellulose nanofibers with antimicrobial properties. Eur. Polym. J. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Das, S.; De, A.; Das, B.; Mukherjee, B.; Samanta, A. Development of gum odina-gelatin based antimicrobial loaded biodegradable spongy scaffold: A promising wound care tool. J. Appl. Polym. Sci. 2021, 138, 50057. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- Nochos, A.; Douroumis, D.; Bouropoulos, N. In vitro release of bovine serum albumin from alginate/HPMC hydrogel beads. Carbohydr. Polym. 2008, 74, 451–457. [Google Scholar] [CrossRef]
- Kundu, J.; Patra, C.; Kundu, S.C. Design, fabrication and characterization of silk fibroin-HPMC-PEG blended films as vehicle for transmucosal delivery. Mater. Sci. Eng. C 2008, 28, 1376–1380. [Google Scholar] [CrossRef]
- Ghosal, K.; Ray, S.D. Alginate/hydrophobic HPMC (60M) particulate systems: New matrix for site-specific and controlled drug delivery. Braz. J. Pharm. Sci. 2011, 47, 833–844. [Google Scholar] [CrossRef]
- Natori, N.; Shibano, Y.; Hiroki, A.; Taguchi, M.; Miyajima, A.; Yoshizawa, K.; Kawano, Y.; Hanawa, T. Preparation and Evaluation of Hydrogel Film Containing Tramadol for Reduction of Peripheral Neuropathic Pain. J. Pharm. Sci. 2023, 112, 132–137. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, J.; Sang, Y.; Xu, X.; Shang, Q. Insulin-loaded hydroxypropyl methyl cellulose-co-polyacrylamide-co-methacrylic acid hydrogels used as rectal suppositories to regulate the blood glucose of diabetic rats. Int. J. Biol. Macromol. 2019, 121, 1346–1353. [Google Scholar] [CrossRef]
- Corredor-Chaparro, M.Y.; Vargas-Riveros, D.; Mora-Huertas, C.E. Hypromellose–Collagen hydrogels/sesame oil organogel based bigels as controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 75, 103637. [Google Scholar] [CrossRef]
- Abazari, M.; Akbari, T.; Hasani, M.; Sharifikolouei, E.; Raoufi, M.; Foroumadi, A.; Sharifzadeh, M.; Firoozpour, L.; Khoobi, M. Polysaccharide-based hydrogels containing herbal extracts for wound healing applications. Carbohydr. Polym. 2022, 294, 119808. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Nishadani, D.K.S.; Gunathilake, T.M.S.U.; Ching, Y.C.; Noothalapati, H. Carboxymethyl cellulose hydrogel for pH-responsive drug release of curcumin. Iran. Polym. J. 2024, 33, 1449–1467. [Google Scholar] [CrossRef]
- Younis, N.A.; Hemdan, A.; Zafer, M.M.; Abd-Elsalam, W.H.; Abouelatta, S.M. Standardization and quantitative analysis of Araucaria heterophylla extract via an UPLC-MS/MS method and its formulation as an antibacterial phytonanoemulsion gel. Sci. Rep. 2022, 12, 12557. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Singab, A.N.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Baymiev, A.K.; Chuvatkina, A.K.; Vladimirova, A.A.; Matniyazov, R.T.; Mavzyutov, A.R.; Baymiev, A.K. Analysis of Antibacterial Action Mechanisms of Medicinal Plant Extracts Using Dual Reporter System Dualrep2. Antibiot Khimioter = Antibiot. Chemother. 2023, 68, 11–16. [Google Scholar] [CrossRef]
- Nesterovich, V.M.; Belykh, D.A.; Gorokhovets, N.V.; Kurbatov, L.K.; Zamyatnin, A.A.; Ikryannikova, L.N. Secondary metabolites of plants and their possible role in the “age of superbugs”. Biomeditsinskaya Khimiya 2023, 69, 371–382. [Google Scholar] [CrossRef]
- Keerthana, S.; Charumathy, M.; Gangadhar, L.; Anooj, E.S. Comparision of in vitro and ex vivo experimental analysis of aloe vera extracts on the antioxidant and antimicrobial activity. Int. J. Manag. Technol. Eng. 2019, IX, 3531–3540. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).