Gadolinium Complex with Tris-Hydroxypyridinone as an Input for New Imaging Probes: Thermodynamic Stability, Molecular Modeling and Biodistribution
Abstract
1. Introduction
2. Results and Discussion
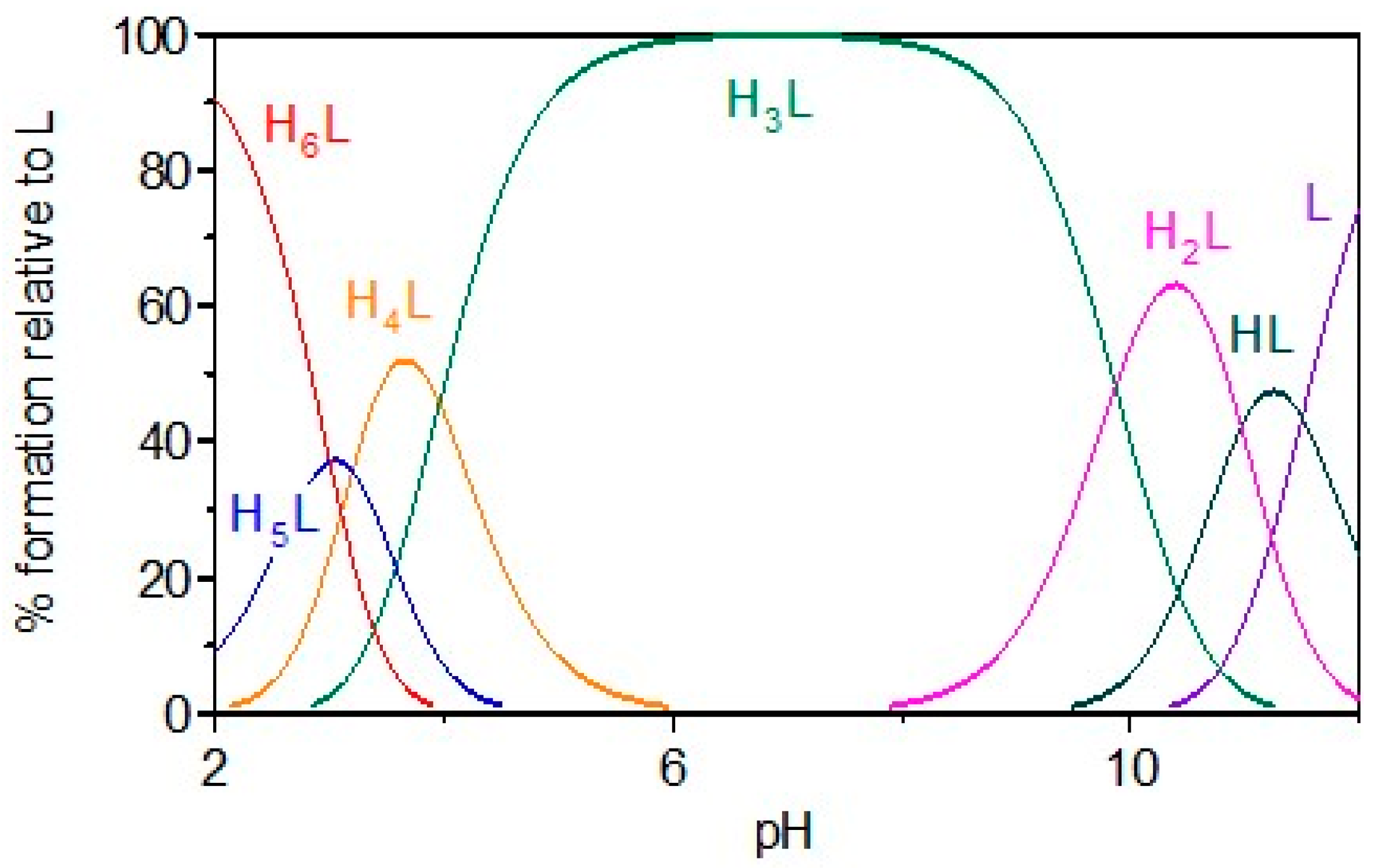
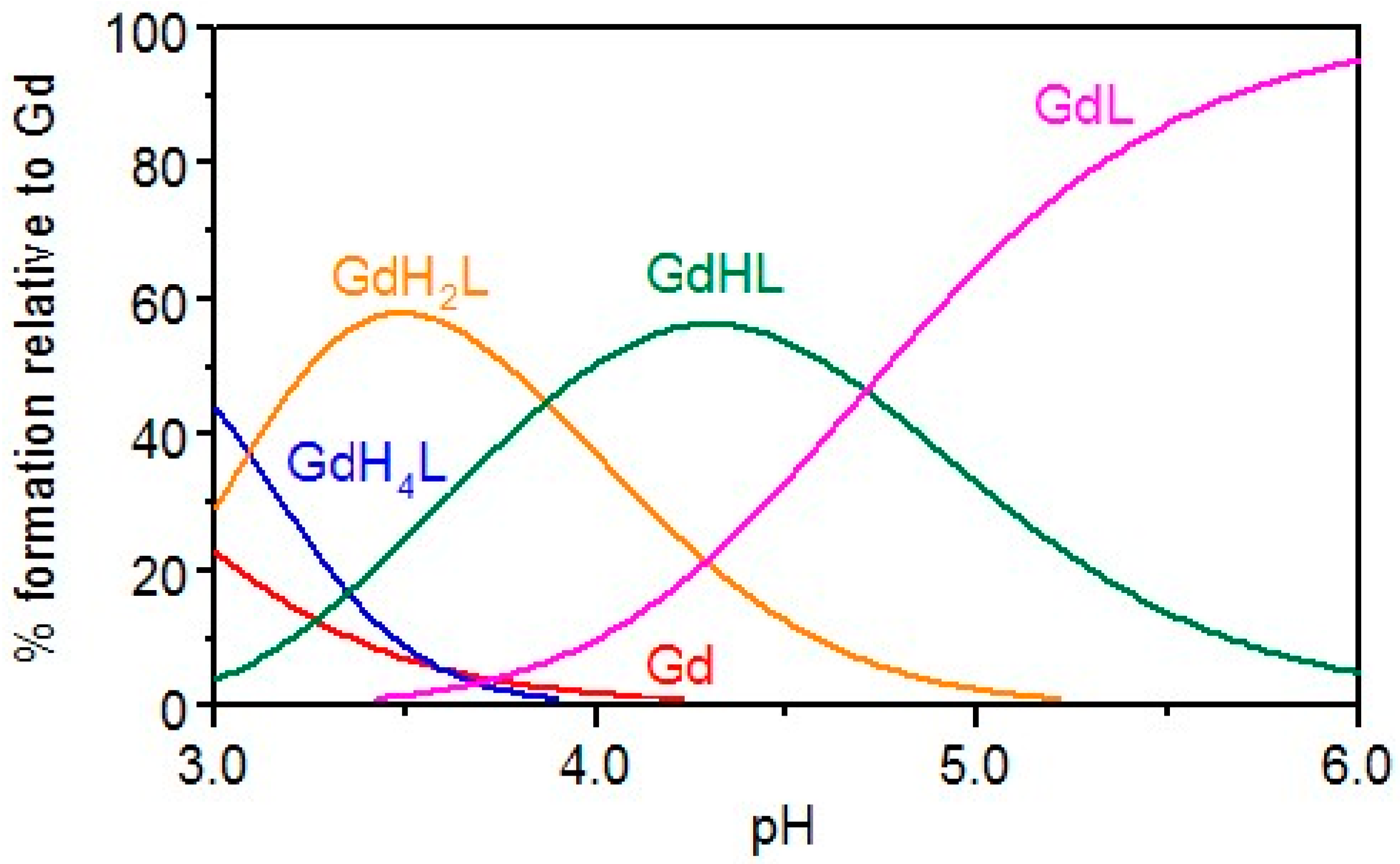
2.1. Gd Chelation Studies
| Compound | log Ki | (m,h,l) | log β (GdmHhLl) |
|---|---|---|---|
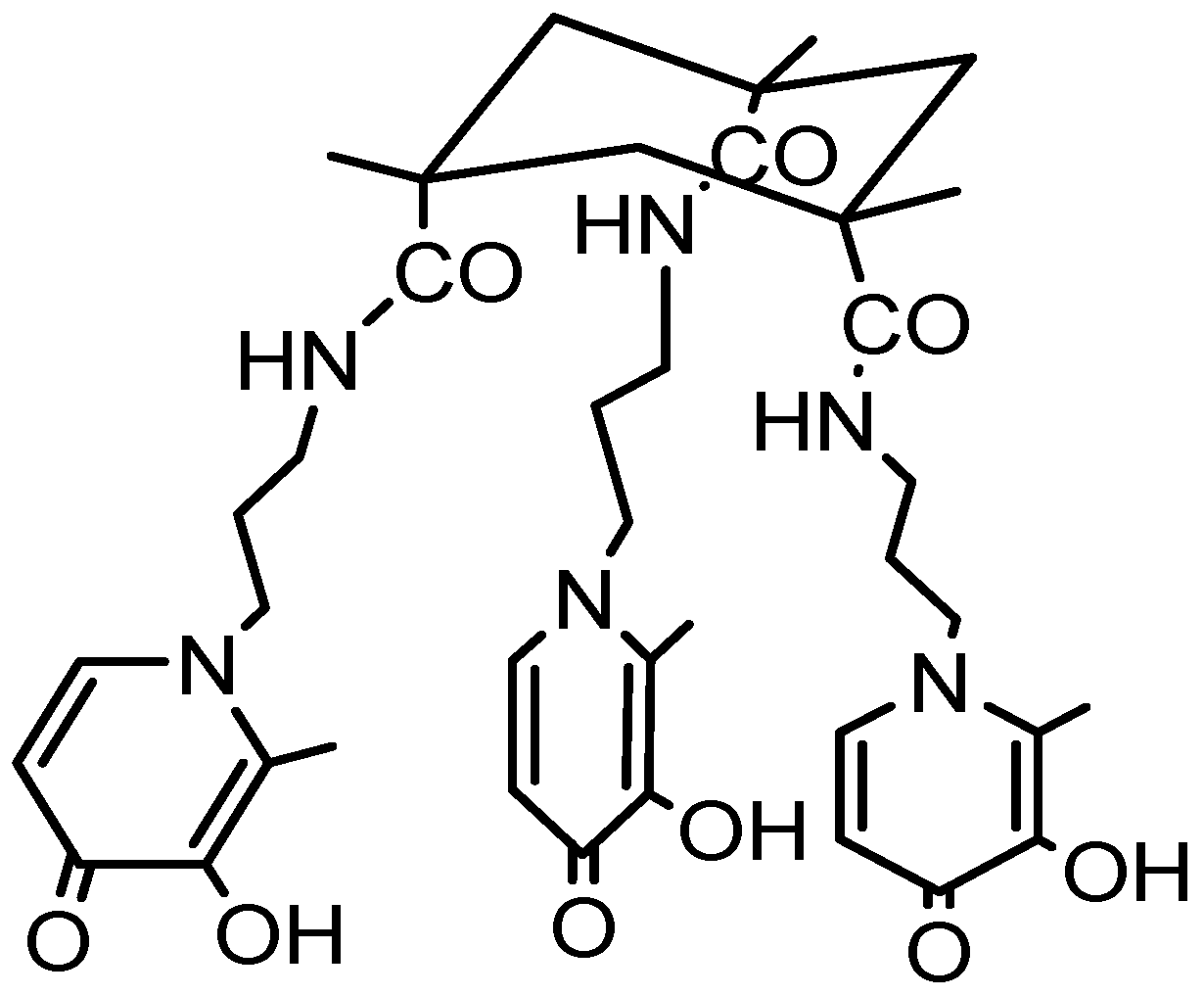
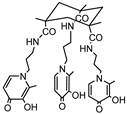 KEMPPr(3,4-HP)3 | 11.5(4) 10.97(5) 9.87(6) 3.96(7) 3.19(8) 3.04(9) | (1,4,1) (1,2,1) (1,1,1) (1,0,1) pGd | 41.35(8) 35.17(7) 31.30(4) 26.59(8) 13.2 d |
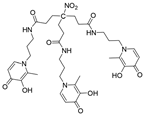 H3L2 e | 9.93(2) 9.75(4) 9.18(5) 4.26(5) 3.11(6) 2.77(7) | (1,4,1) (1,2,1) (1,0,1) pGd | 37.74(4) 30.03(6) 21.22(5) 14.3 |
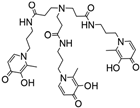 NTP(PrHP)3 | 9.95 f 9.84 f 9.09 f 6.77 f 3.81 f 3.14 f 2.76 f | (1,5,1) (1,4,1) (1,3,1) (1,2,1) (1,1,1) (1,5,2) (1,3,2) pGd | 42.8 g 39.46 g 35.69 g 31.16 g 26.35 g 65.3 g 52.3 g 12.3 |
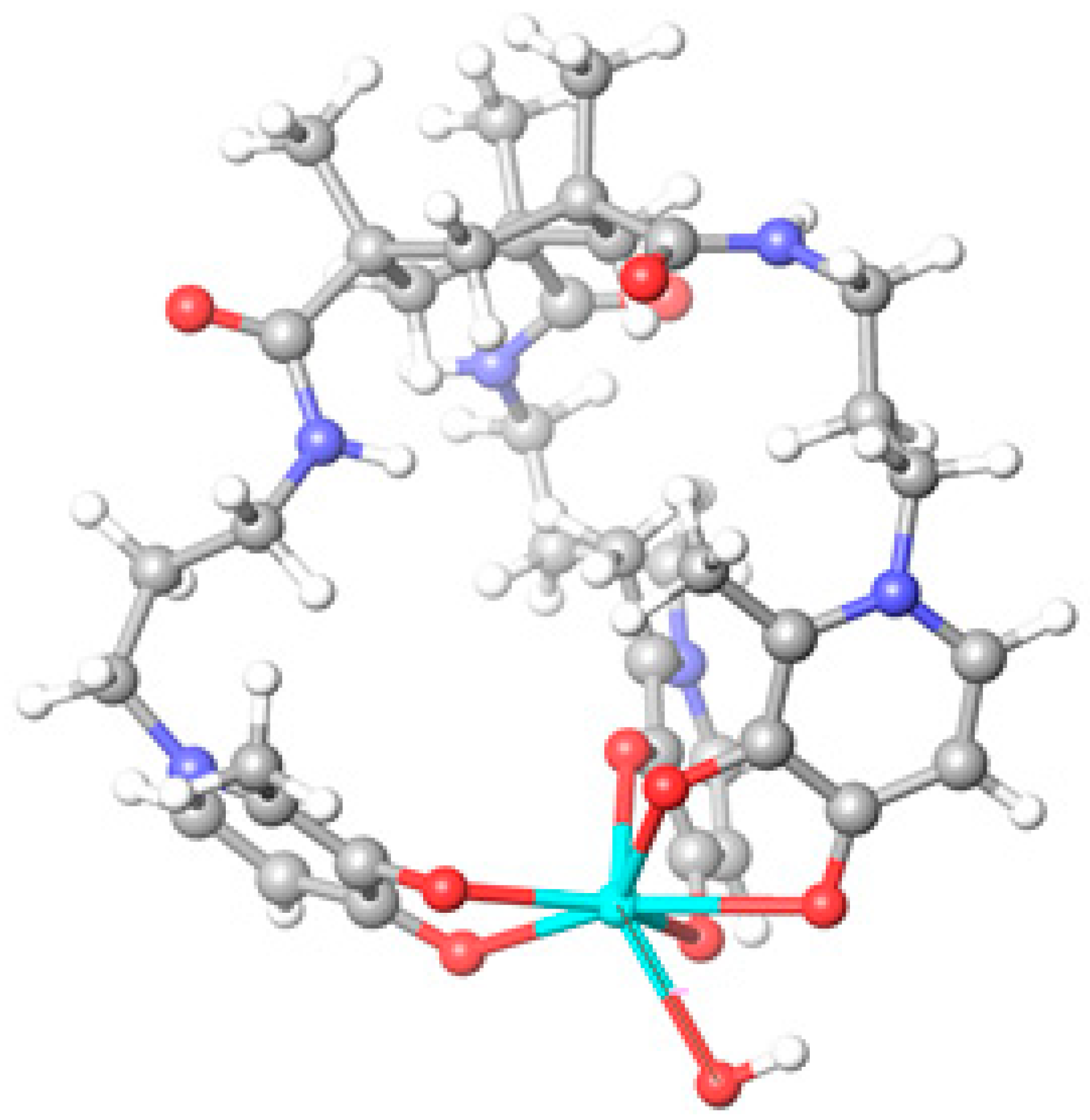
2.2. Molecular Modeling of the Gd Complex
2.3. In Silico Prediction of ADME Properties and Drug Likeness
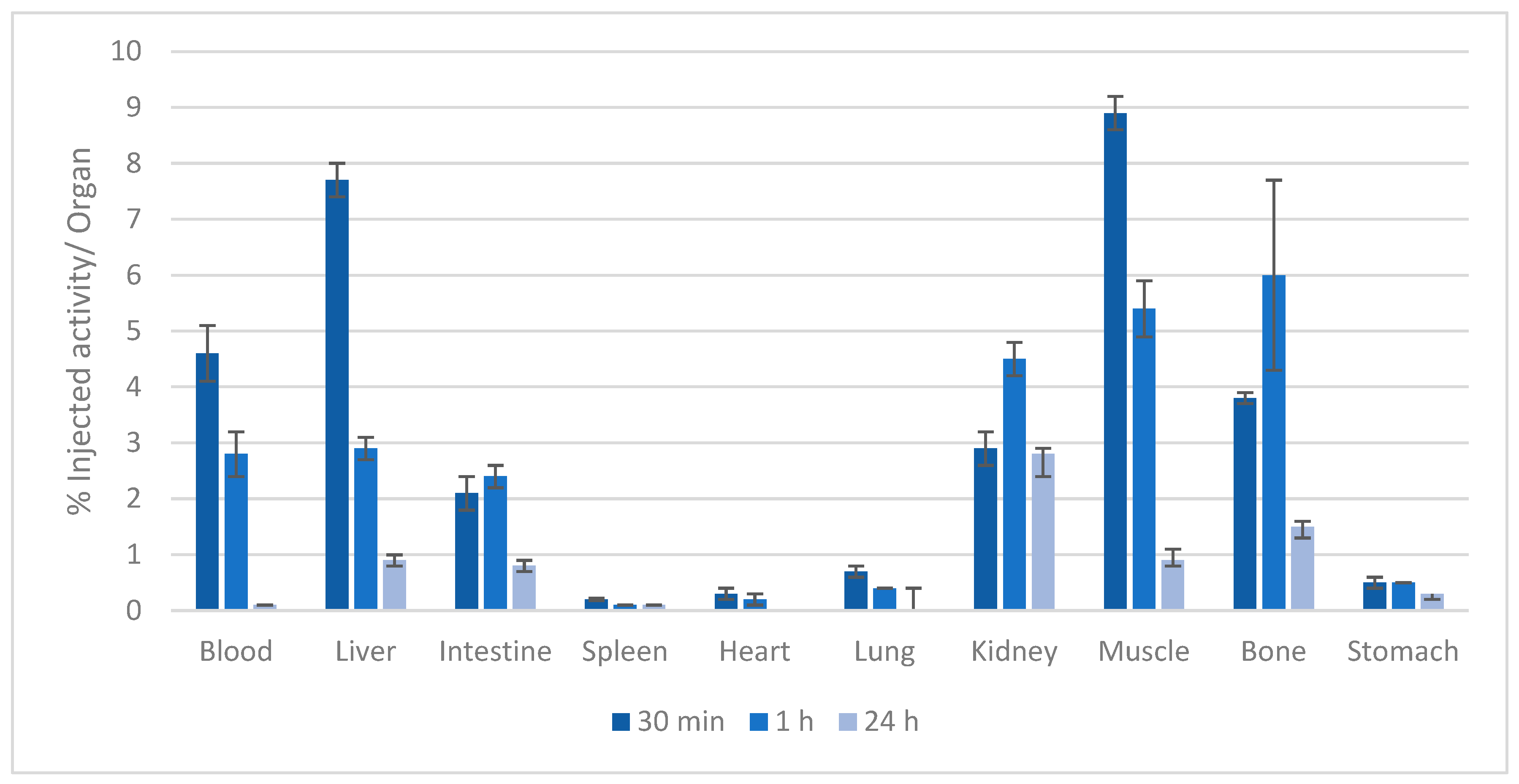
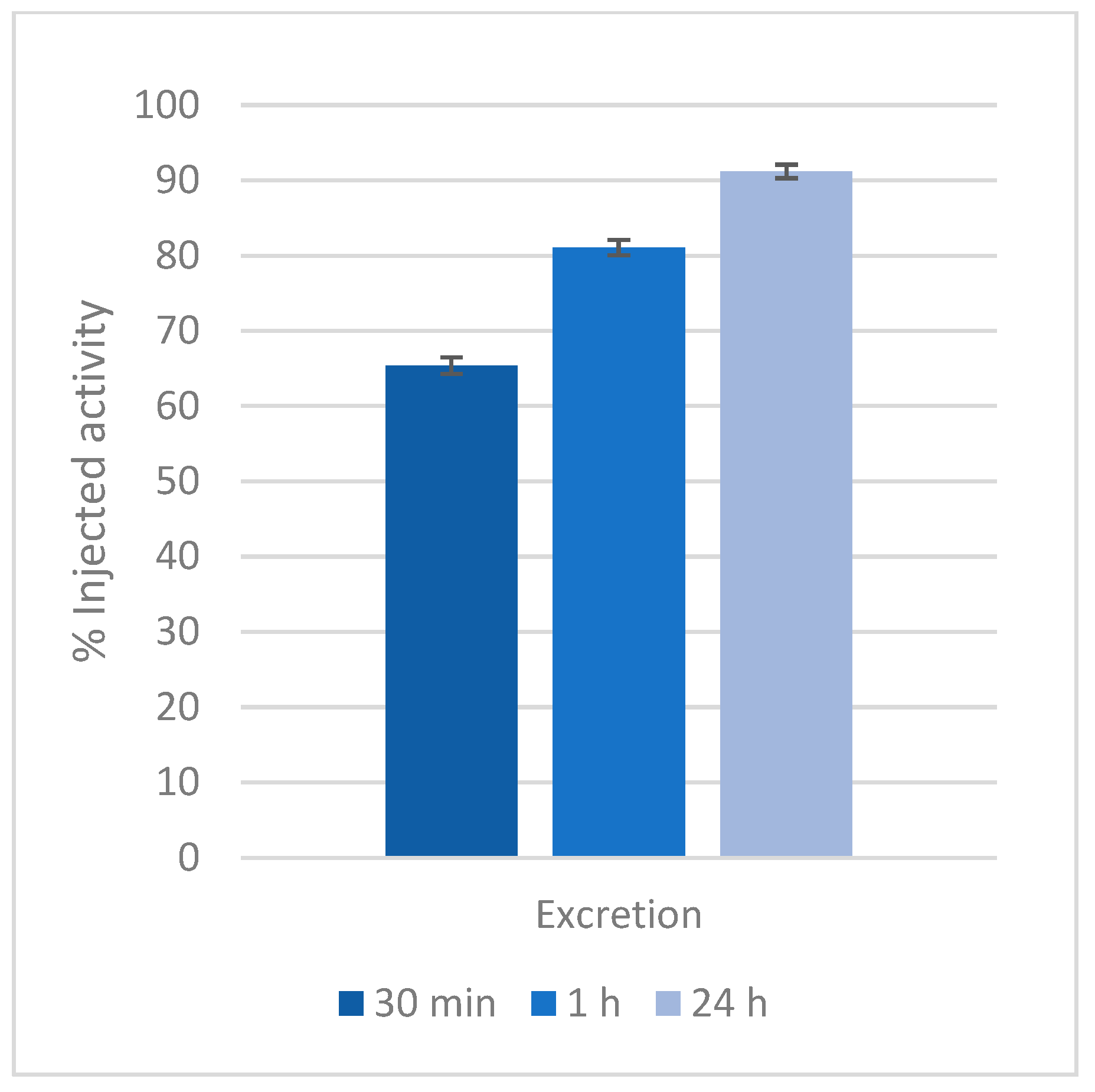
2.4. Biodistribution of the Gd-KEMPPr(3,4-HP)3 Complex
3. Materials and Methods
3.1. Gd Complexation Studies
3.1.1. Materials and Equipment
3.1.2. Potentiometric Measurements
3.1.3. Calculation of Equilibrium Constants
3.2. Molecular Modeling
3.3. Biodistribution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Crich, S.G.; Gianolio, E.; Giovenzana, G.B.; Tei, L.; Terreno, E. High sensitivity lanthanide(III) based probes for MR-medical imaging. Coord. Chem. Rev. 2006, 250, 1562–1579. [Google Scholar] [CrossRef]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The Interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Abramova, L.; Gaab, G.; Turabelidze, G.; Patel, P.; Arduino, M.; Hess, T.; Kallen, A.; Jhung, M. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002–2006. J. Am. Med. Assoc. 2007, 297, 1542–1544. [Google Scholar] [CrossRef][Green Version]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Uzal-Varela, R.; Rodríguez-Rodríguez, A.; Wang, H.; Esteban-Gómez, D.; Brandariz, I.; Gale, E.M.; Caravan, P.; Platas-Iglesias, C. Prediction of Gd(III) complex stability. Coord. Chem. Rev. 2022, 467, 214606. [Google Scholar] [CrossRef]
- Prybylski, J.P.; Semelka, R.C.; Jay, M. The stability of gadolinium-based contrast agents in human serum: A re-analysis of literature data and association with clinical outcomes. Magn. Reson. Imag. 2017, 38, 145–151. [Google Scholar] [CrossRef]
- Werner, E.J.; Avedano, S.; Botta, M.; Hay, B.P.; Moore, E.G.; Aime, S.; Raymond, K.N. Highly soluble tris-hydroxypyridonate Gd(III) complexes with increased hydration number, fast water exchange, slow electronic relaxation, and high relaxivity. J. Am. Chem. Soc. 2007, 129, 1870–1871. [Google Scholar] [CrossRef]
- Pierre, V.C.; Melchior, M.; Doble, D.M.J.; Raymond, K.N. Toward optimized high relaxivity MRI agents: Thermodynamic selectivity of hydroxypyridonate /catecholate ligands. Inorg. Chem. 2004, 43, 8520–8525. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Martins, A.F.; Melchior, A.; Marques, S.M.; Chaves, S.; Villette, S.; Petoud, S.; Zanonato, P.L.; Tolazzi, M.; Bonnet, C.S.; et al. New tris-3,4-HOPO lanthanide complexes as potential imaging probes. Complex stability and magnetic properties. Dalton Trans. 2013, 42, 6046–6057. [Google Scholar] [CrossRef]
- Chaves, S.; Gwizdała, K.; Chand, K.; Gano, L.; Pallier, A.; Tóth, É.; Santos, M.A. GdIII and GaIII complexes with a new tris-3,4-HOPO ligand, towards new potential imaging probes: Complex stability, magnetic properties and biodistribution. Dalton Trans. 2022, 51, 6436–6447. [Google Scholar] [CrossRef] [PubMed]
- Grazina, R.; Gano, L.; Sebestik, J.; Santos, M.A. New tripodal hydroxypyridinone based chelating agents for Fe(III), Al(III) and Ga(III): Synthesis, physico-chemical properties and bioevaluation. J. Inorg. Biochem. 2009, 103, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Yang, R.; Schulman, S.G. An operational pH in aqueous dimethylsulfoxide based upon the acidity dependence of the rate of a simple ionic recombination reaction in the lowest excited singlet state. Talanta 2003, 60, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.; Marques, S.M.; Matos, A.M.F.; Nunes, A.; Gano, L.; Tuccinardi, T.; Martinelli, A.; Santos, M.A. New tris(hydroxypyridinones) as iron and aluminium sequestering agents: Synthesis, complexation and in vivo studies. Chem. Eur. J. 2010, 16, 10535–10545. [Google Scholar] [CrossRef]
- Pawlak, Z.; Bates, R.G. Solute-solvent interactions in acid-base dissociation: Nine protonated nitrogen bases in water-DMSO solvents. J. Sol. Chem. 1975, 4, 817–829. [Google Scholar] [CrossRef]
- Malla, B.; Neeraja, R.; Kumar, J.S.; Ramanaiah, M. An eletrometric method for the determination of impact of DMSO-water mixtures on pKa values of salicylic acid derivatives. Int. J. App. Pharm. 2023, 15, 309–314. [Google Scholar] [CrossRef]
- Kalidas, C.; Hefter, G.; Marcus, Y. Gibbs energies of transfer of cations from water to mixed aqueous organic solvents. Chem. Rev. 2000, 100, 819–852. [Google Scholar] [CrossRef]
- Beck, M.T.; Nagypal, I. Chemistry of Complex Equilibria; Ellis Horwood Ltd.: Chichester, UK, 1990; ISBN 13-173063-0. [Google Scholar]
- Raymond, K.N.; Carrano, C.J. Coordination chemistry and microbial iron transport. Acc. Chem. Res. 1979, 12, 183–190. [Google Scholar] [CrossRef]
- Xu, J.; Franklin, S.J.; Whisenhunt, D.W., Jr.; Raymond, K.N. Gadolinium complex of tris[(3-hydroxy-1-methyl-2-oxo-1,2-didehydropyridine-4-carboxamido)ethyl]- amine: A New Class of gadolinium magnetic resonance relaxation agents. J. Am. Chem. Soc. 1995, 117, 7245–7246. [Google Scholar] [CrossRef]
- Puerta, D.T.; Botta, M.; Jocker, C.J.; Werner, E.J.; Avedano, S.; Raymond, K.N.; Cohen, S.M. Tris(pyrone) chelates of Gd(III) as high solubility MRI-CA. J. Am. Chem. Soc. 2006, 128, 2222–2223. [Google Scholar] [CrossRef]
- Werner, E.J.; Datta, A.; Jocher, C.J.; Raymond, K.N. High-relaxivity MRI contrast agents: Where coordination chemistry meets medical imaging. Angew. Chem. Int. Ed. 2008, 47, 8568–8580. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Bauschlicher, C.W. A comparison of the accuracy of different functionals. Chem. Phys. Lett. 1995, 246, 40–44. [Google Scholar] [CrossRef]
- Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H.; Schwerdtfeger, P.; Pitzer, R.M. Accuracy of energy-adjusted quasirelativistic ab initio pseudopotentials. Mol. Phys. 1993, 78, 1211–1224. [Google Scholar] [CrossRef]
- Kuchle, W.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 1994, 100, 7535–7542. [Google Scholar] [CrossRef]
- Konings, M.S.; Dow, W.C.; Love, D.B.; Raymond, K.N.; Quay, S.C.; Rocklage, S.M. Gadolinium complexation by a new DTPA-amide ligand. Amide oxygen coordination. Inorg. Chem. 1990, 29, 1488–1491. [Google Scholar] [CrossRef]
- Junhu, W.; Masashi, T.; Takafumi, K.; Masuo, T. Structure and bonding in some Gd(III) metal complexes studied by three-dimensional X-Ray analysis and 155Gd Mossbauer spectroscopy. J. Rare Earths 2007, 25, 647–653. [Google Scholar] [CrossRef]
- Lipinsky, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Waring, M.J. Defining optimum lipophilicity and molecular weight ranges for drug candidates—Molecular weight dependent lower log D limits based on permeability. Bioorg. Med. Chem. Lett. 2009, 19, 2844–2851. [Google Scholar] [CrossRef]
- Bennion, B.J.; Be, N.A.; McNerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C.; et al. Predicting a drug’s membrane permeability: A computational model validated with in vitro permeability assay data. J. Phys. Chem. B 2017, 121, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From serendipity to rational design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.K.; Misselwitz, B.; Tso, L.; Doble, D.M.J.; Schmitt-Willich, H.; Raymond, K.N. In vivo evaluation of gadolinium hydroxypyridonate chelates: Initial experience as contrast media in magnetic resonance imaging. J. Med. Chem. 2005, 48, 3874–3877. [Google Scholar] [CrossRef]
- Le Fur, M.; Rotile, N.J.; Correcher, C.; Jordan, V.C.; Ross, A.W.; Catana, C.; Caravan, P. Yttrium-86 is a positron emitting surrogate of gadolinium for noninvasive quantification of whole-body distribution of gadolinium-based contrast agents. Angew. Chem. Int. Ed. 2020, 59, 1474–1478. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. Potentiometric titrations using Gran plots: A textbook omission. J. Chem. Ed. 1965, 42, 375–378. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, I.; Gano, L.; Chaves, S.; Santos, M.A. Gadolinium Complex with Tris-Hydroxypyridinone as an Input for New Imaging Probes: Thermodynamic Stability, Molecular Modeling and Biodistribution. Molecules 2025, 30, 1295. https://doi.org/10.3390/molecules30061295
Dias I, Gano L, Chaves S, Santos MA. Gadolinium Complex with Tris-Hydroxypyridinone as an Input for New Imaging Probes: Thermodynamic Stability, Molecular Modeling and Biodistribution. Molecules. 2025; 30(6):1295. https://doi.org/10.3390/molecules30061295
Chicago/Turabian StyleDias, Inês, Lurdes Gano, Sílvia Chaves, and M. Amélia Santos. 2025. "Gadolinium Complex with Tris-Hydroxypyridinone as an Input for New Imaging Probes: Thermodynamic Stability, Molecular Modeling and Biodistribution" Molecules 30, no. 6: 1295. https://doi.org/10.3390/molecules30061295
APA StyleDias, I., Gano, L., Chaves, S., & Santos, M. A. (2025). Gadolinium Complex with Tris-Hydroxypyridinone as an Input for New Imaging Probes: Thermodynamic Stability, Molecular Modeling and Biodistribution. Molecules, 30(6), 1295. https://doi.org/10.3390/molecules30061295








