Synergistic Mechanism of Hydroxyl Regulation and a Polyvinylpyrrolidone Surfactant in Enhancing the Catalytic Oxidation Abilities of BiOBr
Abstract
1. Introduction
2. Results and Discussion
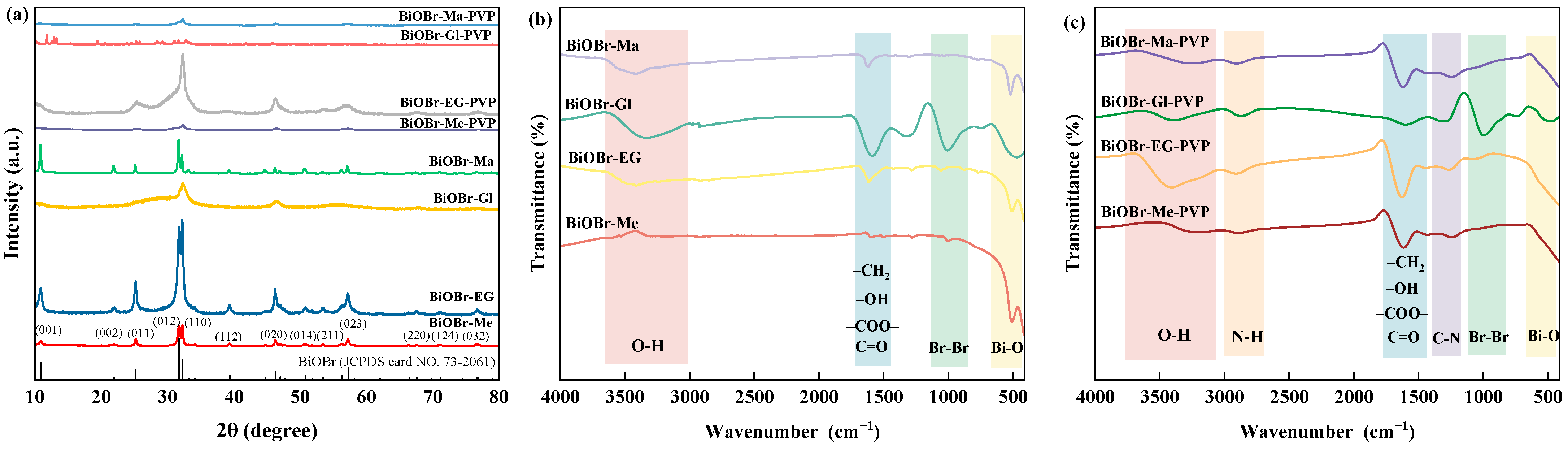
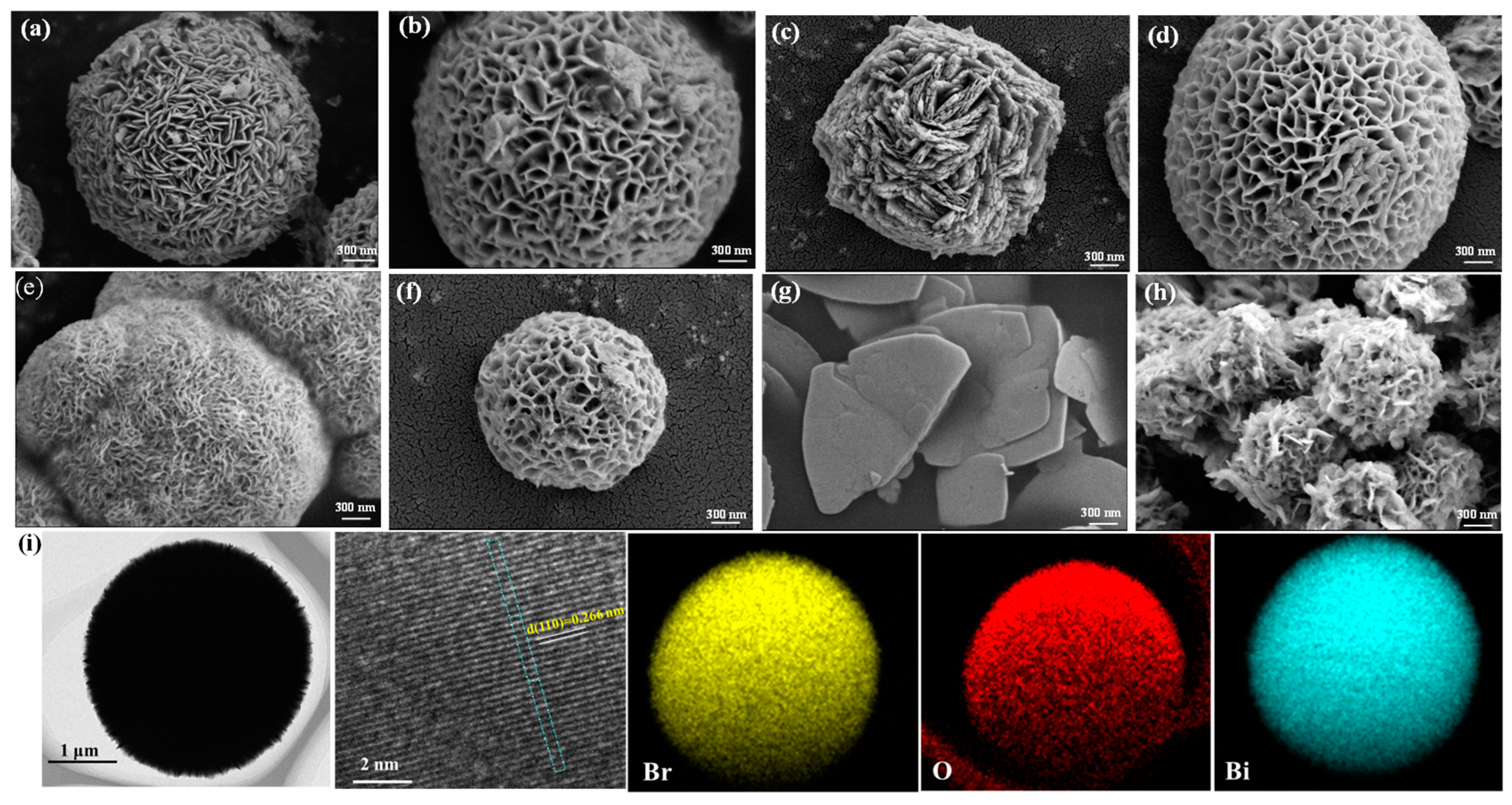
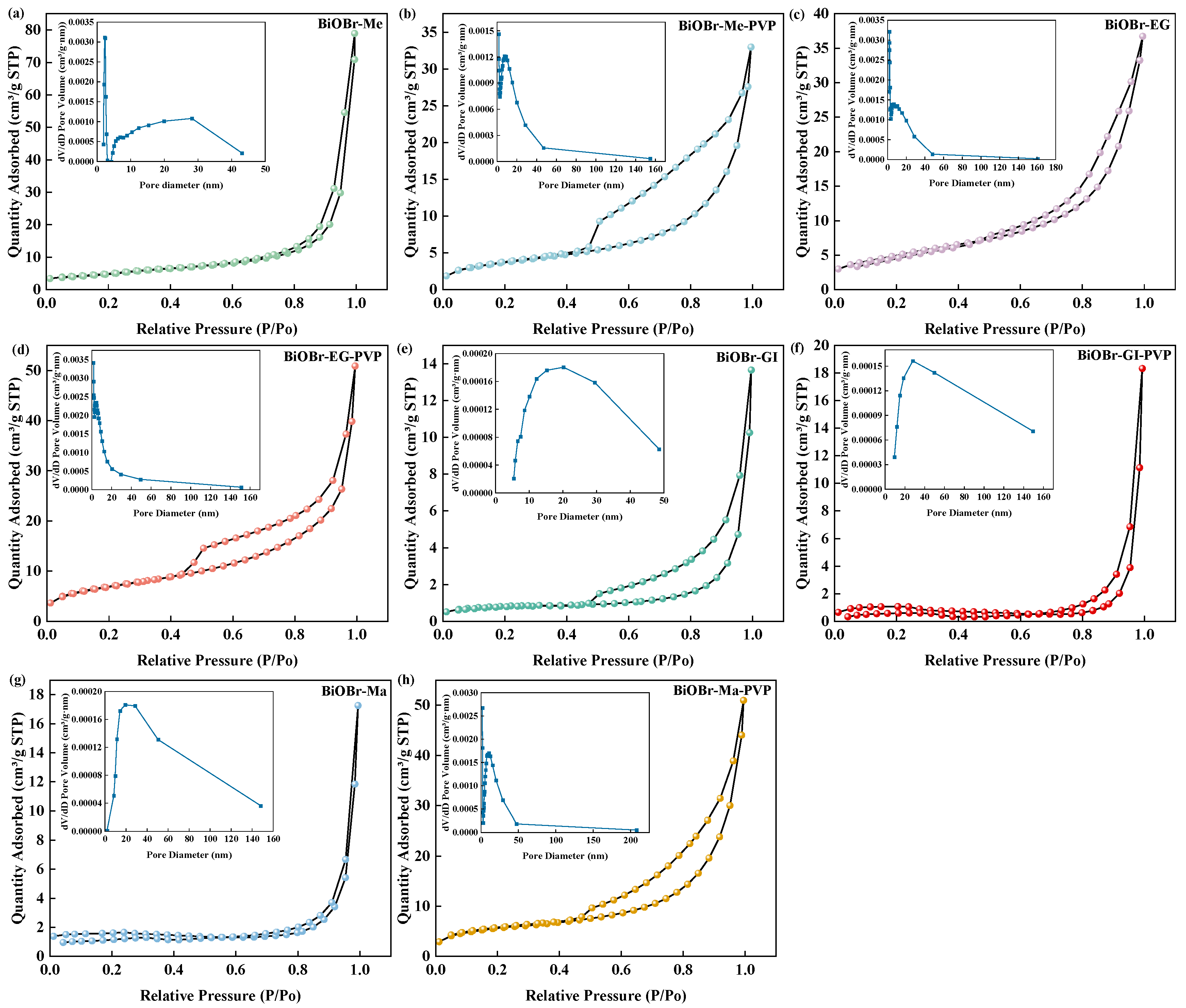
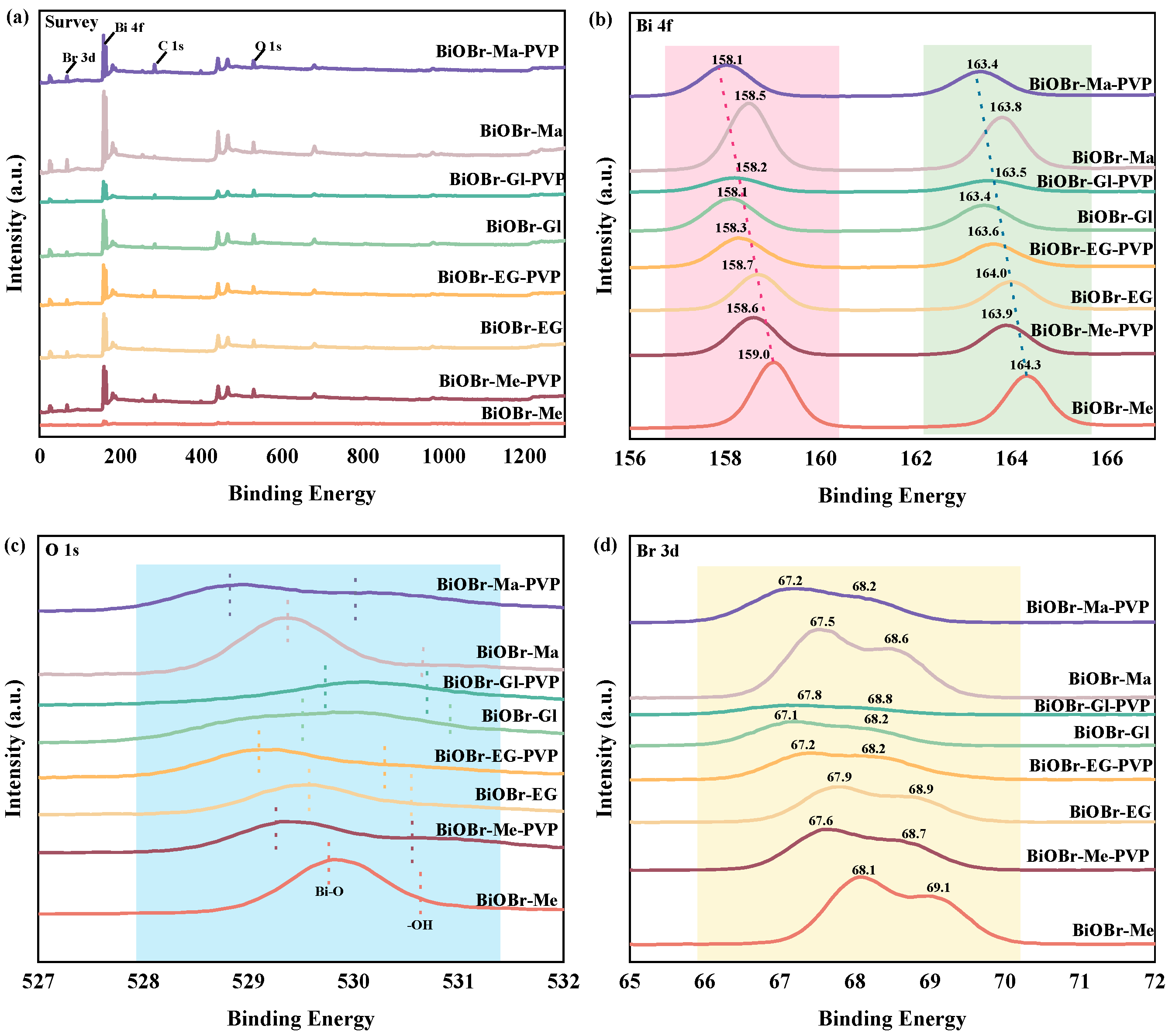
2.1. Morphology and Structure of Photocatalysts
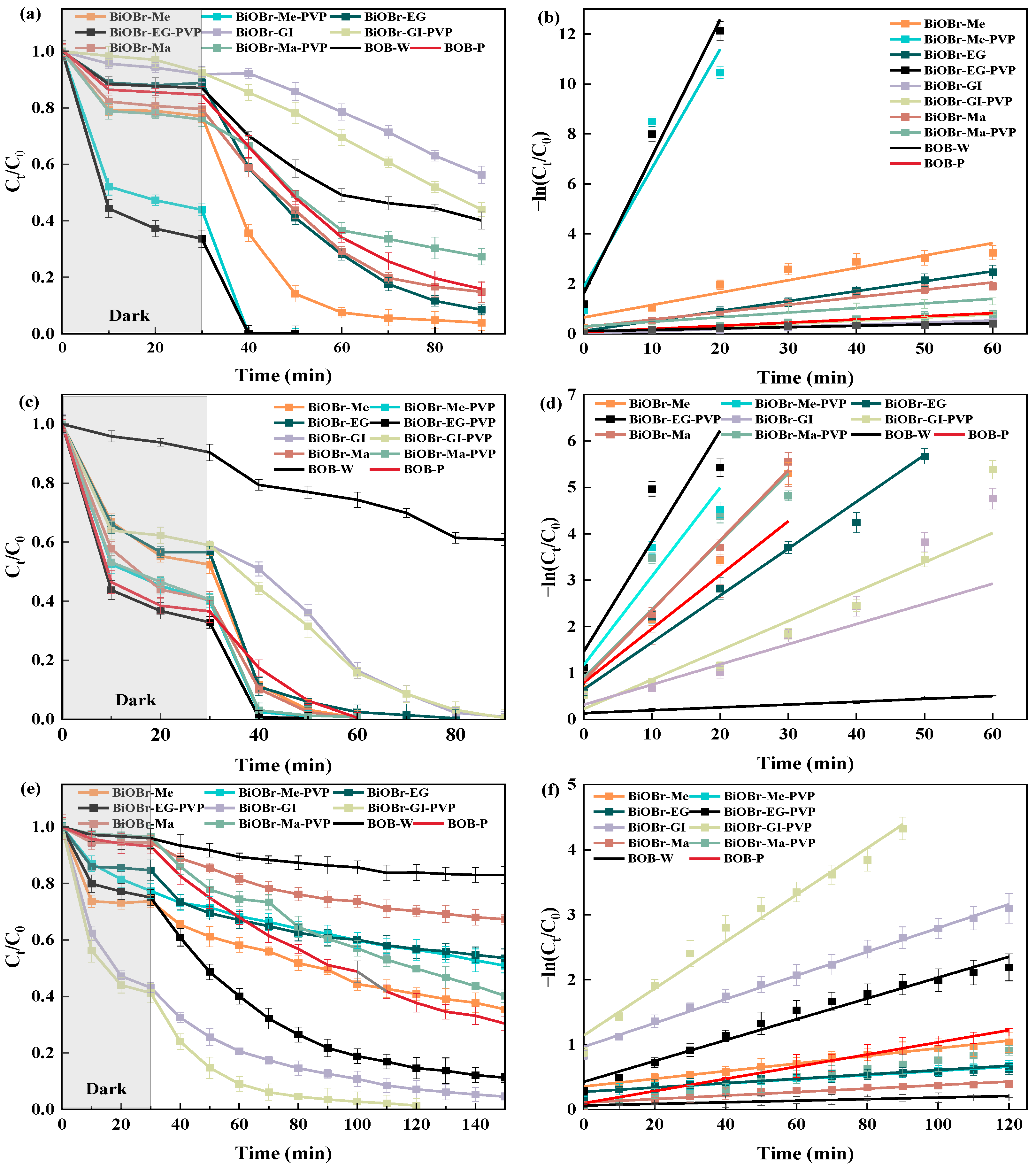
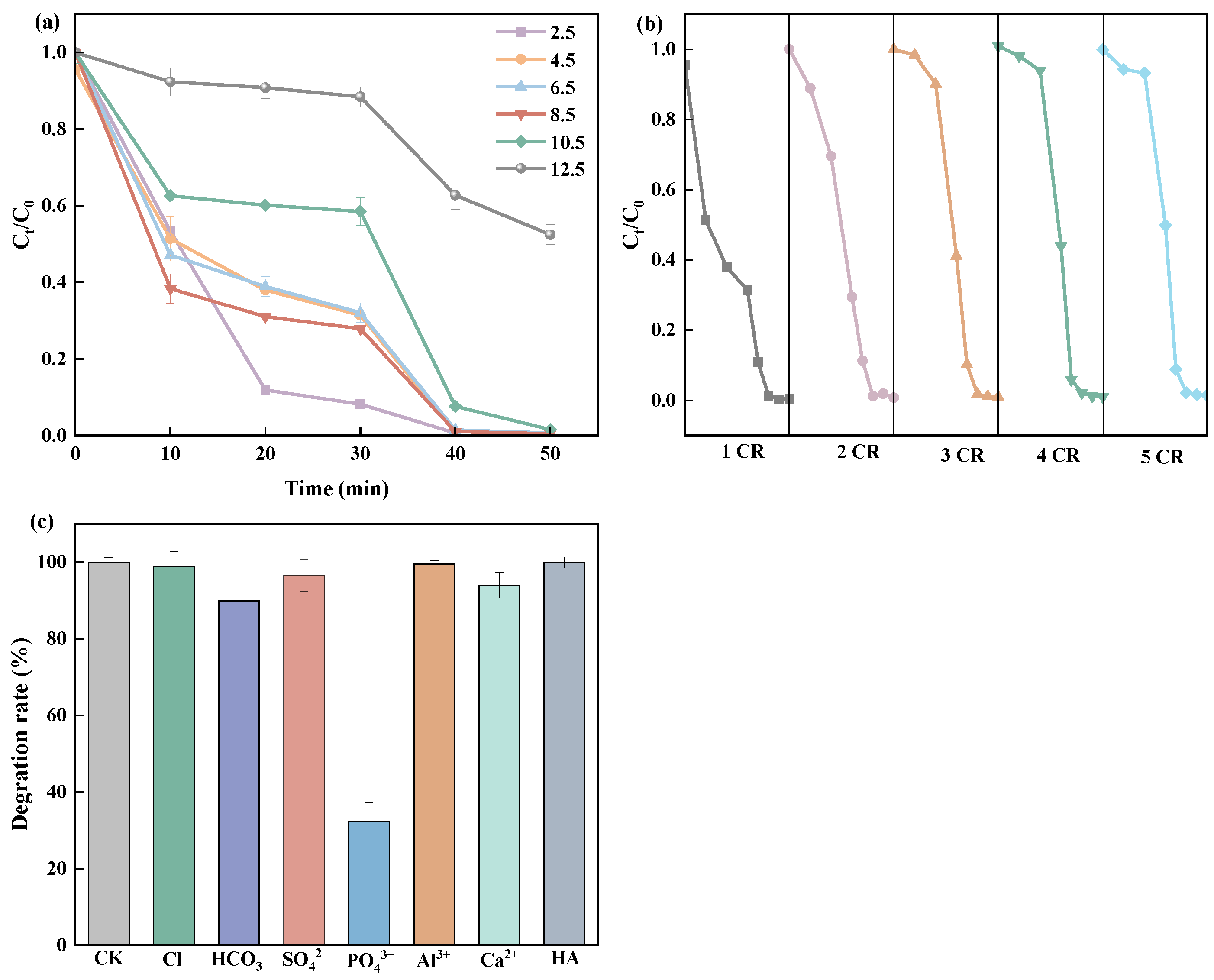
2.2. Photocatalytic Performance of Degradation for Organics
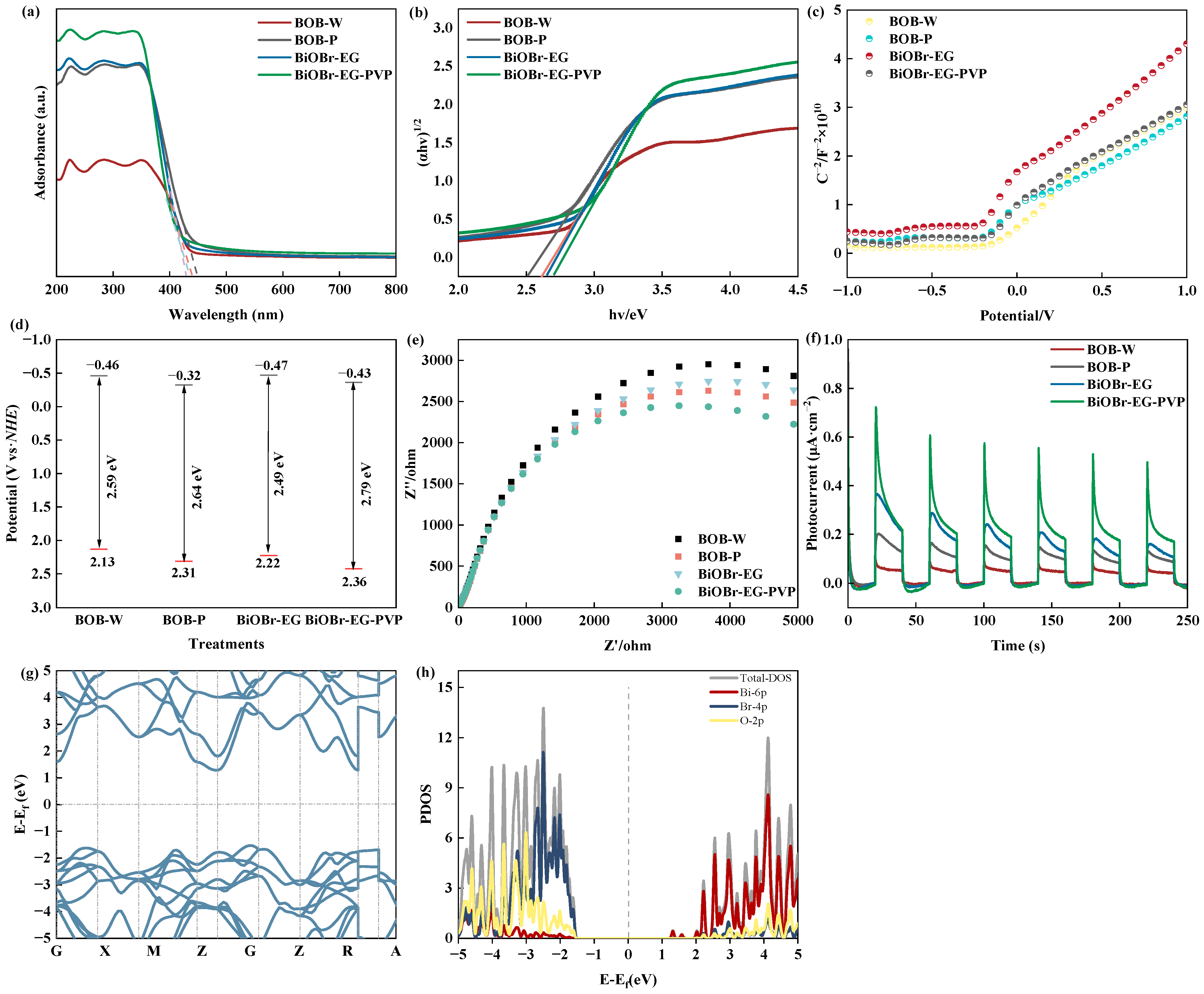
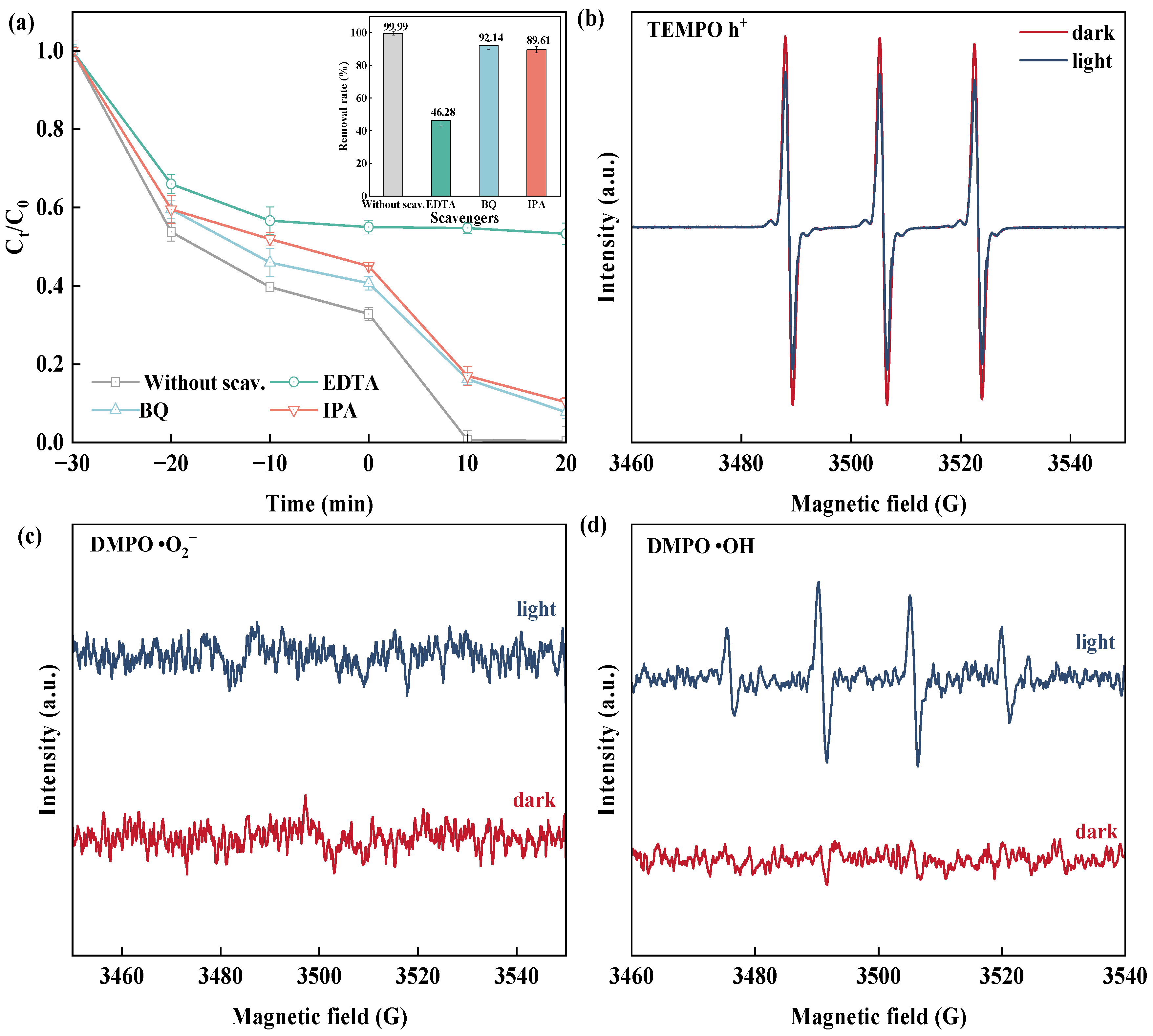
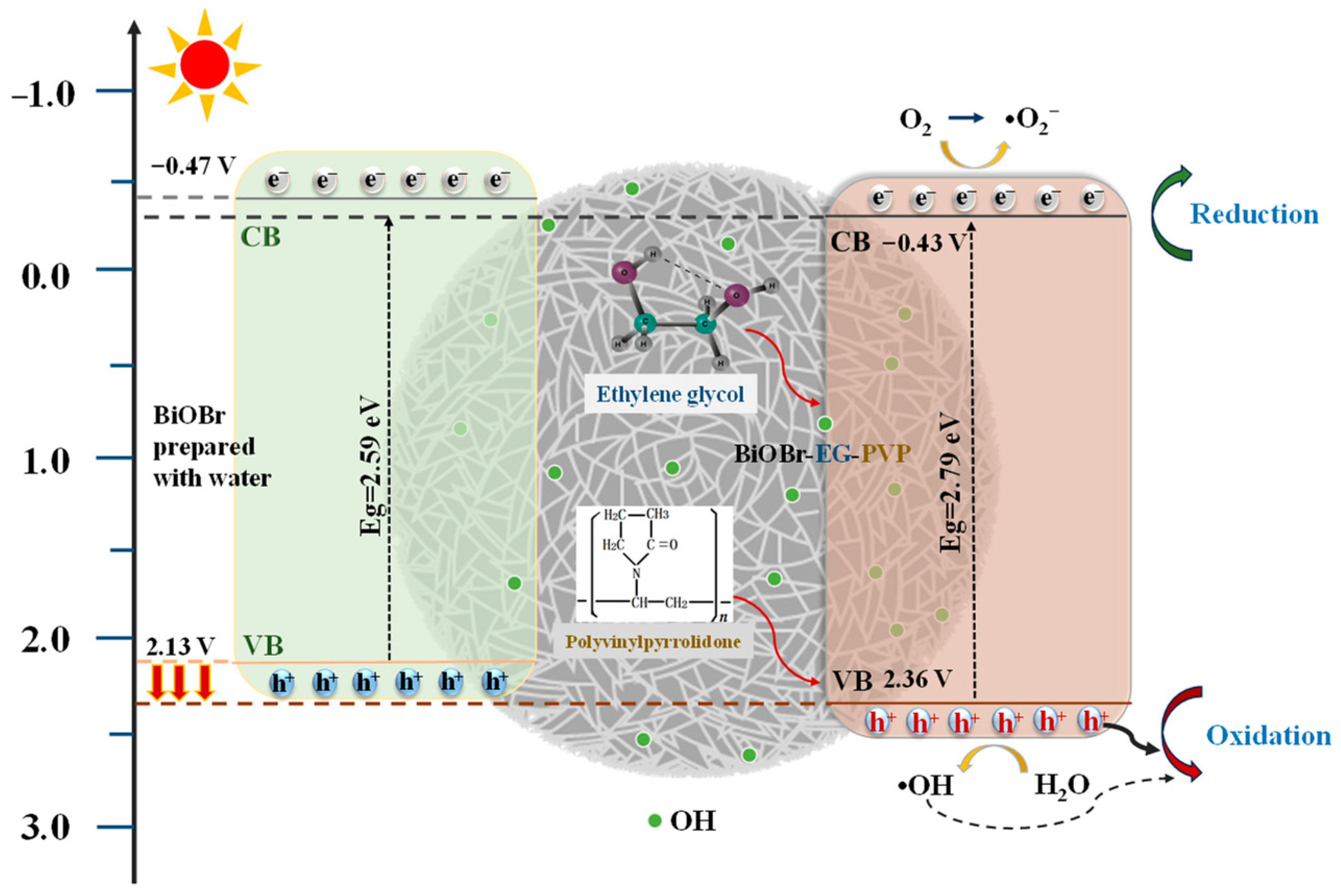
2.3. Photocatalytic Mechanism
2.4. Analysis of Degradation Pathway
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Photocatalysts
3.3. Characterization of Photocatalysts
3.4. Photodegradation Measurements
3.5. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.M.; Zhang, G.P.; Dong, S.H.; Li, N.J.; Xu, Q.F.; Li, H.; Lu, J.M.; Chen, D.Y. Optimizing Adsorption-Redox Sites and Charge Transfer of Ternary Polymer Photocatalyst with P―N Linkage for CO2 Conversion Coupled with Antibiotics Removal. Adv. Funct. Mater. 2024, 34, 2406516. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Onwumere, J.; Pylypchuk, I.V.; Jaworski, A.; Chen, J.H.; Rokicinska, A.; Lindström, M.E.; Kustrowski, P.; Sevastyanova, O.; Slabon, A. LignoPhot: Conversion of hydrolysis lignin into the photoactive hybrid lignin/Bi4O5Br2/BiOBr composite for simultaneous dyes oxidation and Co2+ and Ni2+ recycling. Chemosphere 2021, 279, 130538. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Shao, S.; Fan, S.D.; Tang, W.Q.; Miao, J.W.; Wang, S.; Cao, X.C.; Liu, C.; Ying, G.G.; Chen, Z.B.; et al. Occurrence, distribution, and risk assessment of antibiotics in a typical aquaculture area around the Dongzhai Harbor mangrove forest on Hainan Island. Sci. Total Environ. 2024, 920, 170558. [Google Scholar] [CrossRef]
- Huang, X.L.; Li, G.; Liu, L.H.; He, Y.C.; Su, X.Y.; Pan, Y.W.; Xing, W.N.; Wu, G.Y.; Zhang, M. Boosting peroxymonosulfate activation over Co-N-C@Co9S8 double-shelled nanocages for ciprofloxacin degradation: Insights into catalytic performance, degradation mechanism and routes. Sep. Purif. Technol. 2025, 359, 130662. [Google Scholar] [CrossRef]
- Liang, L.; Cai, S.Q.; Leng, Y.L.; Huang, C.; Liu, Y.Q.; Wang, Y.; Luo, L.; Han, M.; Li, X.H.; Cai, X.H. Intelligent sensing platform based on europium-doped carbon dots for dual-functional detection of ciprofloxacin/Ga3+ and its tracking in vivo. J. Hazard. Mater. 2025, 483, 136622. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K. Recent advances in removal of pharmaceutical pollutants in wastewater using metal oxides and carbonaceous materials as photocatalysts: A review. RSC Appl. Interfaces 2024, 1, 34–429. [Google Scholar] [CrossRef]
- Wang, C.L.; Liu, B.; Ren, J.; Javed, M.S.; Han, W.H. Enhanced photocatalytic performance of SnS2/ZnO Z-scheme composite photocatalysts for efficient environmental remediation. Mater. Res. Bull. 2025, 183, 113222. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Spray-Deposited TiO2 layers on aluminum foil for sustainable water remediation. Crystals 2024, 14, 875. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, X.J.; Yuan, M.; Ni, B.J.; Xia, S.Q.; Zhao, J.F. Insights into the removal of sulfamethazine and sulfonamide-resistant bacteria from wastewater by Fe-Mn spinel oxide modified cow manure biochar activated peroxymonosulfate: A nonradical pathway regulated by enhanced adsorption and 3d orbital electron reconstruction. Appl. Catal. B-Environ. Energy 2025, 361, 124652. [Google Scholar]
- Zhang, J.W.; Shao, S.J.; Guo, Q.; Duan, X.P.; Liu, Y.Z.; Jiao, W.Z. Co-removal of phenol and Cr(VI) by high gravity coupled heterogeneous catalytic ozonation-adsorption. Sep. Purif. Technol. 2025, 358, 130297. [Google Scholar] [CrossRef]
- Chen, M.N.; Song, Q.Q.; Li, Z.K.; Bai, W.W.; Xu, M.Y.; Li, X.; Li, W.P.; Nan, H.Y.; Wang, J.; Zhang, Y.T.; et al. COFs functionalized self-cleaning loose nanofiltration membranes for efficient dye/salt separation. Desalination 2025, 593, 118206. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, S.; Kim, B.H.; Kim, D.H. Tetracycline removal via photo-Fenton processes using Fe-based metal-organic frameworks loaded with Bi2S3: Performance evaluation and insights into the charge-transfer mechanism. Appl. Surf. Sci. 2025, 681, 161564. [Google Scholar] [CrossRef]
- Li, C.L.; Zhang, B.H.; Jin, X.Z.; Wang, Y.; Zheng, Y. Excellent integrated adsorption and photocatalysis of a black BiOBr for fast removal dyes: A case of carbon species and oxygen vacancies synergistic effect. Appl. Surf. Sci. 2022, 577, 151920. [Google Scholar] [CrossRef]
- Tie, W.W.; Bhattacharyya, S.S.; Ma, T.C.; Yuan, S.Y.; Chen, M.H.; He, W.W.; Lee, S.H. Improving photoexcited carrier separation through Z-scheme W18O49/BiOBr heterostructure coupling carbon quantum dots for efficient photoelectric response and tetracycline photodegradation. Carbon 2025, 231, 119707. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, S.W.; Song, P.; Li, A.; Jiang, W.; Di, J. Copper porphyrin modified BiOBr/Bi19S27Br3 for efficient CO2 photoreduction. J. Colloid Interf. Sci. 2025, 679, 383–390. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.; Yuan, Y. S-scheme BiOBr/Bi2WO6 with oxygen vacancies for synergistic photodegradation and hydrogen generation: Mechanisms insight and DFT calculations. J. Alloys Compd. 2024, 995, 174755. [Google Scholar] [CrossRef]
- Wang, Y.G.; He, J.; Zhu, Y.M.; Zhang, H.; Yang, C.; Wang, K.Y.; Wu, S.C.; Chueh, Y.L.; Jiang, W. Hierarchical Bi-doped BiOBr microspheres assembled from nanosheets with (001) facet exposed via crystal facet engineering toward highly efficient visible light photocatalysis. Appl. Surf. Sci. 2020, 514, 145927. [Google Scholar] [CrossRef]
- Yue, C.L.; Zhu, L.L.; Qiu, Y.X.; Du, Z.L.; Qiu, J.L.; Liu, F.Q.; Wang, F.H. Recent advances of plasmonic elemental Bi based photocatalysts in environmental remediation and energy conversion. J. Clean. Prod. 2023, 392, 136017. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X.M. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Ou, H.H.; Jin, Y.; Chong, B.; Bao, J.H.; Kou, S.; Li, H.; Li, Y.; Yan, X.Q.; Lin, B.; Yang, G.D. Hydroxyl-Bonded Co Single Atom Site on Boroncarbonitride Surface Realizes Nonsacrificial H2O2 Synthesis in the Near-Infrared Region. Adv. Mater. 2024, 36, 2404851. [Google Scholar] [CrossRef]
- Li, X.F.; Li, K.N.; Ding, D.; Yan, J.T.; Wang, C.L.; Carabineiro, S.; Liu, Y.; Lv, K.L. Effect of oxygen vacancies on the photocatalytic activity of flower-like BiOBr microspheres towards NO oxidation and CO2 reduction. Sep. Purif. Technol. 2023, 309, 123054. [Google Scholar] [CrossRef]
- Li, D.S.; Peng, Q.; Xie, Y.X.; Tian, J.T.; Min, K.; Chen, L.; Sun, W.Z.; Xu, H.J.; Du, Q.Y. Synergistic enhancement of the catalytic performance of BiOBr: The role of surface hydroxyl groups and amorphous FeOOH. J. Alloys Compd. 2024, 970, 172632. [Google Scholar] [CrossRef]
- Han, L.P.; Guo, Y.X.; Lin, Z.; Huang, H.W. 0D to 3D controllable nanostructures of BiOBr via a facile and fast room-temperature strategy. Colloids Surf. A 2020, 603, 125233. [Google Scholar] [CrossRef]
- Cui, T.X.; Su, Y.M.; Fu, X.H.; Zhu, Y.; Zhang, Y.M. The key role of surface hydroxyls on the activity and selectivity in photocatalytic degradation of organic pollutants and NO removal. J. Alloys Compd. 2022, 921, 165931. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, Y.; Mei, H.; Xu, H.P.; Gan, L.; Zhang, R.B. Meso-erythritol-regulated BiOBr nanosheets with surface hydroxyl imprinting sites for considerably improved photocatalytic capability. Appl. Surf. Sci. 2021, 546, 149116. [Google Scholar] [CrossRef]
- Li, Y.S.; Han, D.M.; Wang, Z.H.; Gu, F.B. Double-Solvent-Induced Derivatization of Bi-MOF to Vacancy-Rich Bi4O5Br2: Toward Efficient Photocatalytic Degradation of Ciprofloxacin in Water and HCHO Gas. ACS Appl. Mater. Inter. 2024, 16, 7080–7096. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.L.; Gao, Y.W.; Xing, X.C.; Zhang, X.H.; Li, F.; Hu, C. Detoxification and selective separation of Cr(VI) and As(III) in wastewater based on interfacial coupling in BiOBr with {110} facet under visible-light irradiation. Appl. Catal. B-Environ. 2022, 307, 121192. [Google Scholar] [CrossRef]
- Lu, L.Z.; Zhang, H.Y.; Sun, Z.; Wang, J.H.; Wang, H.L.; Xue, J.B.; Shen, Q.Q.; Li, Q. Creation of robust oxygen vacancies in 2D ultrathin BiOBr nanosheets by irradiation through photocatalytic memory effect for enhanced CO2 reduction. Chem. Eng. J. 2023, 477, 146892. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, Q.W.; Li, X.; Zhu, Y.W.; Fang, G.G. Plasmonic active “hot carriers” facilitating photocatalytic CO2 reduction and 2,4-dichlorophenol oxidation over Bi-deposited BiOBr with abundant oxygen vacancies. Sep. Purif. Technol. 2024, 332, 125775. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, M.; Wang, Y.; Zhang, A.T.; Zhao, X.; Zeng, G.S.; Deng, F. Bandgap engineering of hierarchical network-like SnIn4S8 microspheres through preparation temperature for excellent photocatalytic performance and high stability. Green Energy Environ. 2019, 4, 264–269. [Google Scholar] [CrossRef]
- Xu, M.L.; Li, D.D.; Sun, K.; Jiao, L.; Xie, C.F.; Ding, C.M.; Jiang, H.L. Interfacial microenvironment modulation boosting electron transfer between metal nanoparticles and MOFs for enhanced photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 16372–16376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wang, Z.Y.; Huang, B.B.; Dai, Y.; Zhang, X.Y.; Qin, X.Y. Synthesis of BiOBr-PVP hybrids with enhanced adsorption-photocatalytic properties. Appl. Surf. Sci. 2015, 347, 258–264. [Google Scholar] [CrossRef]
- Wang, Q.L.; Jin, Y.H.; Zhang, Y.F.; Li, Y.X.; Wang, X.X.; Cao, X.Z.; Wang, B.Y. Polyvinyl pyrrolidone-coordinated ultrathin bismuth oxybromide nanosheets for boosting photoreduction of carbon dioxide via ligand-to-metal charge transfer. J. Colloid Interf. Sci. 2022, 606, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.F.; Zhang, M.T.; Zhang, L.; Bingham, P.A.; Li, W.; Kubuki, S. PVP surfactant-modified flower-like BiOBr with tunable bandgap structure for efficient photocatalytic decontamination of pollutants. Appl. Surf. Sci. 2020, 530, 147233. [Google Scholar] [CrossRef]
- Zhao, J.L.; Miao, Z.R.; Zhang, Y.F.; Wen, G.Y.; Liu, L.H.; Wang, X.X.; Cao, X.Z.; Wang, B.Y. Oxygen vacancy-rich hierarchical BiOBr hollow microspheres with dramatic CO2 photoreduction activity. J. Colloid Interf. Sci. 2021, 593, 231–243. [Google Scholar] [CrossRef]
- Liang, C.J.; Ma, J.; Cao, Y.X.; Zhang, T.S.; Yang, C.Y.; Wu, Y.F.; Li, H.M.; Xu, H.; Hua, Y.J.; Wang, C.T. Adsorption of BiOBr microspheres to rhodamine B and its influence on photocatalytic reaction. Chemosphere 2022, 304, 135320. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Wen, H.; Zhong, T.; Huang, H.W.; Lin, Z. Core-shell-like BiOBr@BiOBr homojunction for enhanced photocatalysis. Colloids Surf. A 2022, 644, 128829. [Google Scholar] [CrossRef]
- Cui, S.H.; Zhang, R.; Peng, Y.T.; Gao, X.; Li, Z.; Fan, B.B.; Guan, C.Y.; Beiyuan, J.; Zhou, Y.Y.; Liu, J.; et al. New insights into ball milling effects on MgAl-LDHs exfoliation on biochar support: A case study for cadmium adsorption. J. Hazard. Mater. 2021, 416, 126258. [Google Scholar] [CrossRef]
- Ighnih, H.; Ouachtak, H.; Malekshah, R.E.; Haounati, R.; Jada, A.; Addi, A.A. Synergistic enhancement of pollutant removal from water by using BiOCl/BiOBr heterojunction on clay surface and sunlight irradiation. J. Water Process Eng. 2024, 58, 104766. [Google Scholar] [CrossRef]
- Ni, Q.Q.; Ke, X.; Qian, W.J.; Yan, Z.; Luan, J.D.; Liu, W.G. Insight into tetracycline photocatalytic degradation mechanism in a wide pH range on BiOI/BiOBr: Coupling DFT/QSAR simulations with experiments. Appl. Catal. B-Environ. Energy 2024, 340, 123226. [Google Scholar] [CrossRef]
- Xi, J.H.; Xia, H.; Ning, X.M.; Zhang, Z.; Liu, J.; Mu, Z.J.; Zhang, S.T.; Du, P.Y.; Lu, X.Q. Carbon-Intercalated 0D/2D hybrid of hematite quantum Dots/Graphitic carbon nitride nanosheets as superior catalyst for advanced oxidation. Small 2019, 15, e1902744. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.F.; Xu, Z.F.; Wang, L.; Yu, Y.X.; Mitchell, D.; Cui, D.; Xu, X.; Shi, J.; Sannomiya, T.; Du, Y.; et al. Modulation of photocatalytic properties by strain in 2D BiOBr nanosheets. ACS Appl. Mater. Inter. 2015, 7, 27592–27596. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.L.; Chen, H.W.; Chen, M.; Zhang, P.; Ding, N. Preparation of floating BiOCl0.6I0.4/ZnO photocatalyst and its inactivation of Microcystis aeruginosa under visible light. J. Environ. Sci.-China 2023, 125, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Z.; Lu, Y.; Lu, R.; Hu, Y.D.; Rodriguez, R.D.; Chen, J.J. Constructing BiOBr/TiO2 heterostructure nanotubes for enhanced adsorption/photocatalytic performance. J. Water Process Eng. 2023, 54, 103972. [Google Scholar] [CrossRef]
- Li, X.L.; Mao, X.M.; Zhang, X.C.; Wang, Y.F.; Wang, Y.W.; Zhang, H.; Hao, X.G.; Fan, C.M. Citric acid-assisted synthesis of nano-Ag/BiOBr with enhanced photocatalytic activity. Sci. China Chem. 2015, 58, 457–466. [Google Scholar] [CrossRef]
- Zhang, R.J.; Chilivery, R.; Yao, D.F.; Chen, W.B.; Lu, F.S.; Gao, W.H.; Fang, Y.W.; Zhong, Z.Y.; Song, Y.B. Controlled engineering of tunable 3D-BiOX (X = Cl, Br) hierarchical nanostructures via dopamine-mediated synergetic interactions for efficient visible-light absorption photocatalysis. Appl. Surf. Sci. 2022, 574, 151683. [Google Scholar] [CrossRef]
- Wang, L.; Min, X.P.; Sui, X.Y.; Chen, J.H.; Wang, Y. Facile construction of novel BiOBr/Bi12O17Cl2 heterojunction composites with enhanced photocatalytic performance. J. Colloid Interf. Sci. 2020, 560, 21–33. [Google Scholar] [CrossRef]
- Bao, L.L.; Li, Y.; Xi, Z.; Wang, X.Y.; Afzal, M.; Alarifi, A.; Srivastava, D.; Prakash, O.; Kumar, A.; Jin, J.C. A new 2D Zn(II)-based coordination polymer as photocatalyst for photodegradation of methyl orange in water: Effect of photocatalyst dosage and dye concentration. J. Mol. Struct. 2023, 1292, 136103. [Google Scholar] [CrossRef]
- Rajesh, G.; Kumar, P.S.; Akilandeswari, S.; Rangasamy, G.; Mandal, A.; Shankar, V.U.; Ramya, M.; Nirmala, K.; Thirumalai, K. A synergistic consequence of catalyst dosage, pH solution and reactive species of Fe-doped CdAl2O4 nanoparticles on the degradation of toxic environmental pollutants. Chemosphere 2023, 318, 137919. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, W.Y.; Li, F.T.; Zhao, J.; Liu, R.H.; Liu, S.J.; Li, B. Construction of amorphous TiO2/BiOBr heterojunctions via facets coupling for enhanced photocatalytic activity. J. Hazard. Mater. 2015, 292, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.F.; Wang, K.; Yang, L.; Shi, J.H.; Zhang, Y.; Yang, F.; Yu, X.J.; Yao, B.H. Construction of a novel g-C3N4@Bi/BiOBr ternary heterojunction with Z-scheme mechanism for the efficient photocatalytic removal of ciprofloxacin. Opt. Mater. 2022, 134, 113125. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Wang, X.J.; Wang, Y.H.; Li, S.; Cui, B.L.; Du, Y. Persulfate Activation of Photocatalysts Based on S-Scheme Bi3NbO7/BiOBr0.75I0.25 Nanosheet Heterojunctions for Degradation of Organic Pollutants. Acs Appl. Nano Mater. 2023, 6, 10768–10778. [Google Scholar] [CrossRef]
- Geng, A.B.; Xu, L.J.; Gan, L.; Mei, C.T.; Wang, L.J.; Fang, X.Y.; Li, M.R.; Pan, M.Z.; Han, S.G.; Cui, J.Q. Using wood flour waste to produce biochar as the support to enhance the visible-light photocatalytic performance of BiOBr for organic and inorganic contaminants removal. Chemosphere 2020, 250, 126291. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Duan, L.; Li, H.S.; Zhang, C.; Gao, Q.S.; Zhang, H.L.; Li, S.L.; Li, M.Y. Fast removal of sulfamethoxazole by MIL-101(Fe)-NH2/perylene diimide activated persulfate under visible light. Sep. Purif. Technol. 2025, 358, 130292. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.Y.; Qian, C.X.; He, L.; Chen, K.K.; Zhang, T.Q.; Chen, Z.L.; Ye, M.M. The role of adsorption in photocatalytic degradation of ibuprofen under visible light irradiation by BiOBr microspheres. Chem. Eng. J. 2016, 297, 139–147. [Google Scholar] [CrossRef]
- Liu, H.Y.; Ma, H.X.; Liu, J.L.; Yu, Z.; Tang, S.R.; Sun, J.B.; Wu, S.B.; Wang, L.Z.; Xiao, H.B.; Wang, W.L.; et al. Visible light-induced periodate activation by Bi2WO6@nitrogen-deficient C3N4 composites for efficient degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2025, 357, 130107. [Google Scholar] [CrossRef]
- Hu, W.X.; Li, A.F.; Li, H.P.; Wang, Y.; Fan, Z.K.; Deng, Q.H.; Wang, G.A.; Xia, Y.G.; Hou, W.G. Metal single Atom-Hydroxyl incorporation in poly(heptazine imide) to create active sites for photocatalytic water oxidation. Small 2025, 21, e2408436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.P.; Li, X.X.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; Lu, J.M. Internal electric field and adsorption effect synergistically boost carbon dioxide conversion on cadmium sulfide@covalent triazine frameworks Core-Shell photocatalyst. Adv. Funct. Mater. 2023, 33, 2308553. [Google Scholar] [CrossRef]
- Sun, C.; Ding, Y.L.; Zhao, Y.W.; Deng, Z.H.; Lian, K.; Wang, Z.T.; Cui, J.Z.; Wang, R.F.; Bai, J.B. Perovskite derived oxygen vacancies-rich BiOBr nanosheets for highly efficient photocatalysis. Appl. Surf. Sci. 2025, 682, 161703. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Gu, X.R.; Song, Y.H.; Xie, R.Y.; Zhang, S.Z.; Li, J.Y.; Sheng, S.H.; Zou, H.F. One-step synthesis of oxygen vacancy-rich BiOBr/TiO2 composite: Ultrafast adsorption-photocatalytic performance and mechanism. Chem. Eng. J. 2024, 495, 153261. [Google Scholar] [CrossRef]
- Wang, L.W.; Zhao, Z.J.; Meza, J. Linear-scaling three-dimensional fragment method for large-scale electronic structure calculations. Phys. Rev. B 2008, 77, 165113. [Google Scholar] [CrossRef]
- Jia, W.L.; Fu, J.Y.; Cao, Z.Y.; Wang, L.; Chi, X.B.; Gao, W.G.; Wange, L.W. Fast plane wave density functional theory molecular dynamics calculations on multi-GPU machines. J. Comput. Phys. 2013, 251, 102–115. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Comment on “Generalized gradient approximation made simple”—Reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Ziesche, P.; Kurth, S.; Perdew, J.P. Density functionals from LDA to GGA. Comp. Mater. Sci. 1998, 11, 122–127. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2013, 88, 085117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xuan, B.; Wang, J.; Chen, X.; Zhao, C.; Zhao, L.; Kang, J. Synergistic Mechanism of Hydroxyl Regulation and a Polyvinylpyrrolidone Surfactant in Enhancing the Catalytic Oxidation Abilities of BiOBr. Molecules 2025, 30, 1286. https://doi.org/10.3390/molecules30061286
Zhang Y, Xuan B, Wang J, Chen X, Zhao C, Zhao L, Kang J. Synergistic Mechanism of Hydroxyl Regulation and a Polyvinylpyrrolidone Surfactant in Enhancing the Catalytic Oxidation Abilities of BiOBr. Molecules. 2025; 30(6):1286. https://doi.org/10.3390/molecules30061286
Chicago/Turabian StyleZhang, Yiran, Boyuan Xuan, Jiekai Wang, Xiang Chen, Changwei Zhao, Lixia Zhao, and Jing Kang. 2025. "Synergistic Mechanism of Hydroxyl Regulation and a Polyvinylpyrrolidone Surfactant in Enhancing the Catalytic Oxidation Abilities of BiOBr" Molecules 30, no. 6: 1286. https://doi.org/10.3390/molecules30061286
APA StyleZhang, Y., Xuan, B., Wang, J., Chen, X., Zhao, C., Zhao, L., & Kang, J. (2025). Synergistic Mechanism of Hydroxyl Regulation and a Polyvinylpyrrolidone Surfactant in Enhancing the Catalytic Oxidation Abilities of BiOBr. Molecules, 30(6), 1286. https://doi.org/10.3390/molecules30061286






