Flexible Formation of Nanoparticles: Selectively Self-Assembling with Glycoclusters to Form Nano-Photosensitizers for Multipurpose Bioimaging and Photodynamic Therapy
Abstract
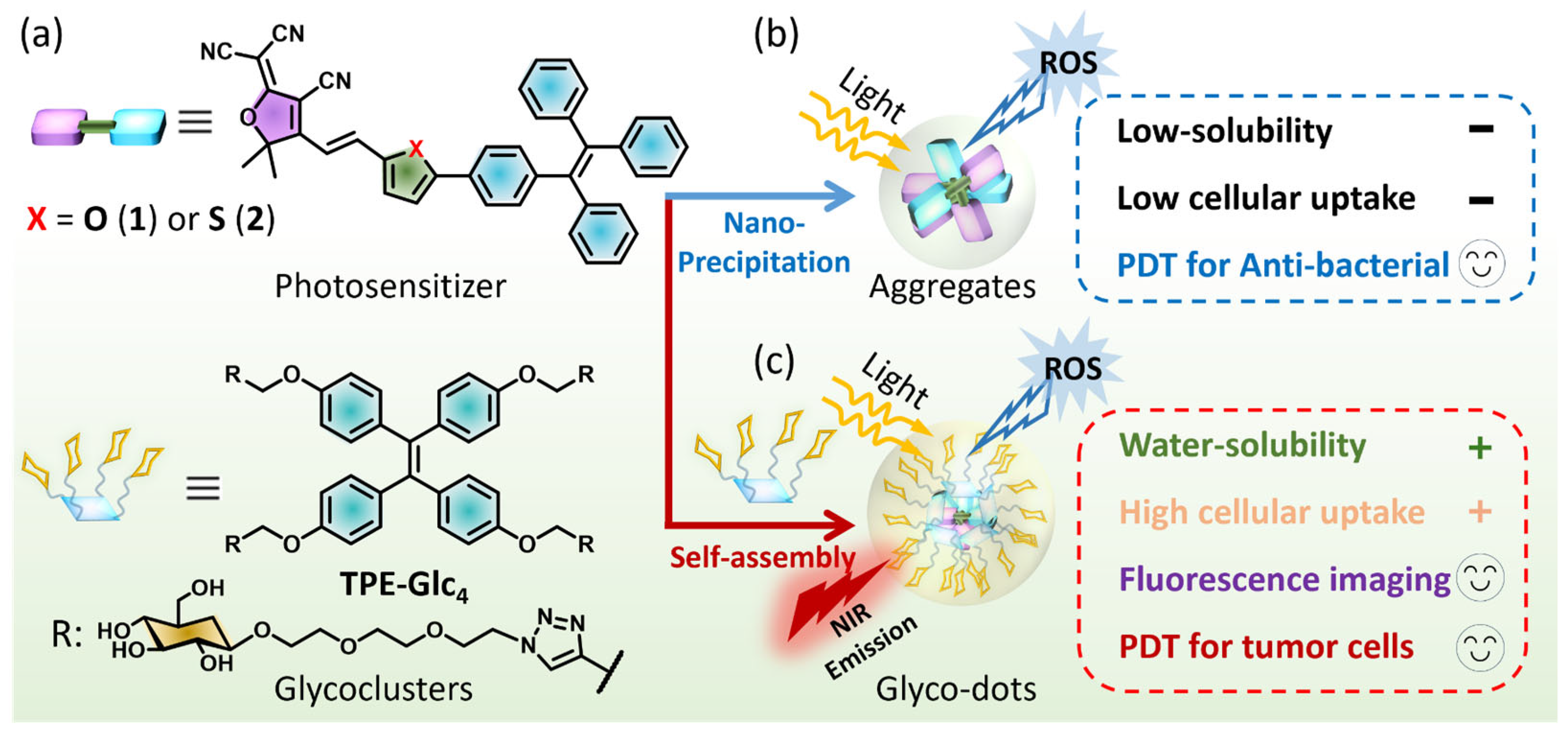
1. Introduction
2. Results and Discussion
2.1. Structure and Photophysical Properties
2.2. Formation of Aggregates and Glyco-Dots
2.3. ROS Generation of Aggregates and Glyco-Dots
2.4. NIR Cell Imaging and Photodynamic Therapy in HepG2 Cells
2.5. Photodynamic Therapy for Anti-Bacterial Studies
3. Materials and Methods
3.1. Synthesis
3.2. UV–Vis Absorption
3.3. Fluorescence Spectroscopy
3.4. Absolute Fluorescence Quantum Yield
3.5. Theoretical Calculations
3.6. Formation of Amorphous Aggregates
3.7. Formation of Glyco-Dots
3.8. Cell Lines and Cell Culture
3.9. In Vitro Dark/Light Cytotoxicity
3.10. Cell Uptake and Imaging
3.11. Bacterial Toxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, M.; Yan, M.; Ye, J.; Li, Y.; Dehaen, W.; Yin, S. Near-infrared boron–dipyrrin (BODIPY) nanomaterials: Molecular design and anti-tumor therapeutics. Coord. Chem. Rev. 2024, 506, 215718. [Google Scholar] [CrossRef]
- Jiang, M.; Yin, Y.; Cai, W.; Zhang, J.; Fan, L.; Yi, Y.; Dai, Y.; Zhou, T.; Liu, J. UV/enzyme dual responsive photosensitizer-loaded 4-(Phenylazo)benzoic Acid-mPEG nanosystem for enhanced photodynamic insecticide efficacy. J. Appl. Polym. Sci. 2021, 138, 50731. [Google Scholar] [CrossRef]
- Liu, B.-K.; Zheng, J.; Wang, H.; Niu, L.-Y.; Yang, Q.-Z. BODIPY-based photosensitizers with simultaneous photodynamic antitumor and antibacterial effects. Mater. Chem. Front. 2023, 7, 5879–5890. [Google Scholar] [CrossRef]
- Xu, F.-Z.; Zhu, L.; Han, H.-H.; Zou, J.-W.; Zang, Y.; Li, J.; James, T.D.; He, X.-P.; Wang, C.-Y. Molecularly engineered AIEgens with enhanced quantum and singlet-oxygen yield for mitochondria-targeted imaging and photodynamic therapy. Chem. Sci. 2022, 13, 9373–9380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Han, H.-H.; Zhu, L.; Xu, F.-Z.; Ma, X.-Y.; Li, J.; James, T.D.; Zang, Y.; He, X.-P.; Wang, C. Long-Wavelength AIE-Based Fluorescent Probes for Mitochondria-Targeted Imaging and Photodynamic Therapy of Hepatoma Cells. ACS Appl. Bio Mater. 2021, 4, 7016–7024. [Google Scholar] [CrossRef]
- Han, H.-H.; Wang, H.-M.; Jangili, P.; Li, M.; Wu, L.; Zang, Y.; Sedgwick, A.C.; Li, J.; He, X.-P.; James, T.D.; et al. The design of small-molecule prodrugs and activatable phototherapeutics for cancer therapy. Chem. Soc. Rev. 2023, 52, 879–920. [Google Scholar] [CrossRef]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Yoon, J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Zhou, S.; Gu, P.; Zhu, X.; Wang, C.; Zhang, Q. Evolution of BODIPY as triplet photosensitizers from homogeneous to heterogeneous: The strategies of functionalization to various forms and their recent applications. Coord. Chem. Rev. 2023, 482, 215074. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, J.; Wu, L.; Xiong, S.; Jiang, W.; Wang, P. Self-assembly strategies of organic small-molecule photosensitizers for photodynamic therapy. Coord. Chem. Rev. 2024, 510, 215863. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Wang, Y.; Wang, J.; Zhou, L.; Cheng, H.-B.; Yoon, J. Activatable nano-photosensitizers for precise photodynamic cancer therapy. Coord. Chem. Rev. 2023, 493, 215324. [Google Scholar] [CrossRef]
- Teng, K.-X.; Niu, L.-Y.; Yang, Q.-Z. Supramolecular Photosensitizer Enables Oxygen-Independent Generation of Hydroxyl Radicals for Photodynamic Therapy. J. Am. Chem. Soc. 2023, 145, 4081–4087. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, J.; Shen, P.; Zhao, Z.; Tang, B.Z. Exploring and leveraging aggregation effects on reactive oxygen species generation in photodynamic therapy. Aggregate 2024, 5, e540. [Google Scholar] [CrossRef]
- Lam, K.W.K.; Chau, J.H.C.; Yu, E.Y.; Sun, F.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Sun, J.; He, X.; Tang, B.Z. An Alkaline Phosphatase-Responsive Aggregation-Induced Emission Photosensitizer for Selective Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2023, 17, 7145–7156. [Google Scholar] [CrossRef]
- Min, X.; Yi, F.; Han, X.-L.; Li, M.; Gao, Q.; Liang, X.; Chen, Z.; Sun, Y.; Liu, Y. Targeted photodynamic therapy using a water-soluble aggregation-Induced emission photosensitizer activated by an acidic tumor microenvironment. Chem. Eng. J. 2022, 432, 134327. [Google Scholar] [CrossRef]
- Tam, L.K.B.; Chu, J.C.H.; He, L.; Yang, C.; Han, K.-C.; Cheung, P.C.K.; Ng, D.K.P.; Lo, P.-C. Enzyme-Responsive Double-Locked Photodynamic Molecular Beacon for Targeted Photodynamic Anticancer Therapy. J. Am. Chem. Soc. 2023, 145, 7361–7375. [Google Scholar] [CrossRef]
- Dong, L.; Shi, X.; Li, H.; Liu, Z.-F.; Teng, K.-X.; Niu, L.-Y.; Hu, Z.; Yang, Q.-Z. Light-Facilitated Reassembly to Generate J-Aggregates of Glucosyl aza-BODIPY with Second Near-Infrared Emission for Bioimaging. ACS Mater. Lett. 2024, 6, 3523–3532. [Google Scholar] [CrossRef]
- Thomas, B.; Yan, K.-C.; Hu, X.-L.; Donnier-Maréchal, M.; Chen, G.-R.; He, X.-P.; Vidal, S. Fluorescent glycoconjugates and their applications. Chem. Soc. Rev. 2020, 49, 593–641. [Google Scholar] [PubMed]
- Xie, H.-N.; Chen, Y.-Y.; Zhu, G.-B.; Han, H.-H.; Hu, X.-L.; Pan, Z.-Q.; Zang, Y.; Xie, D.-H.; He, X.-P.; Li, J.; et al. Targeted delivery of maytansine to liver cancer cells via galactose-modified supramolecular two-dimensional glycomaterial. Chem. Commun. 2022, 58, 5029–5032. [Google Scholar] [CrossRef]
- Fu, Y.; Han, H.-H.; Zhang, J.; He, X.-P.; Feringa, B.L.; Tian, H. Photocontrolled Fluorescence “Double-Check” Bioimaging Enabled by a Glycoprobe–Protein Hybrid. J. Am. Chem. Soc. 2018, 140, 8671–8674. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fu, M.; Liu, L.; Han, H.-H.; Zang, Y.; Chen, G.-R.; Li, J.; He, X.-P.; Vidal, S. Supramolecular Assembly of TPE-Based Glycoclusters with Dicyanomethylene-4H-pyran (DM) Fluorescent Probes Improve Their Properties for Peroxynitrite Sensing and Cell Imaging. Chem. Eur. J. 2020, 26, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo [18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 802503. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, W.; Zhang, P.; Jiang, M.; Chen, Y.; Kwok, R.T.K.; Lee, M.M.S.; Shan, G.; Qi, R.; Zhou, X.; et al. Engineering Sensor Arrays Using Aggregation-Induced Emission Luminogens for Pathogen Identification. Adv. Funct. Mater. 2019, 29, 1805986. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Zhuang, Z.; Wang, B.; Dai, J.; Feng, G.; Lou, X.; Xia, F.; Zhao, Z.; Tang, B.Z. Effective Therapy of Drug-Resistant Bacterial Infection by Killing Planktonic Bacteria and Destructing Biofilms with Cationic Photosensitizer Based on Phosphindole Oxide. Small 2022, 18, 2200743. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.-L.; Li, W.-J.; Hu, Y.; Sun, L.-L.; Dong, L.; Xing, J.; Gong, J.; Gong, X.; Han, H.-H. Flexible Formation of Nanoparticles: Selectively Self-Assembling with Glycoclusters to Form Nano-Photosensitizers for Multipurpose Bioimaging and Photodynamic Therapy. Molecules 2025, 30, 1274. https://doi.org/10.3390/molecules30061274
He K-L, Li W-J, Hu Y, Sun L-L, Dong L, Xing J, Gong J, Gong X, Han H-H. Flexible Formation of Nanoparticles: Selectively Self-Assembling with Glycoclusters to Form Nano-Photosensitizers for Multipurpose Bioimaging and Photodynamic Therapy. Molecules. 2025; 30(6):1274. https://doi.org/10.3390/molecules30061274
Chicago/Turabian StyleHe, Kai-Li, Wen-Jia Li, Yu Hu, Lu-Lu Sun, Lei Dong, Jing Xing, Jin Gong, Xiaoming Gong, and Hai-Hao Han. 2025. "Flexible Formation of Nanoparticles: Selectively Self-Assembling with Glycoclusters to Form Nano-Photosensitizers for Multipurpose Bioimaging and Photodynamic Therapy" Molecules 30, no. 6: 1274. https://doi.org/10.3390/molecules30061274
APA StyleHe, K.-L., Li, W.-J., Hu, Y., Sun, L.-L., Dong, L., Xing, J., Gong, J., Gong, X., & Han, H.-H. (2025). Flexible Formation of Nanoparticles: Selectively Self-Assembling with Glycoclusters to Form Nano-Photosensitizers for Multipurpose Bioimaging and Photodynamic Therapy. Molecules, 30(6), 1274. https://doi.org/10.3390/molecules30061274





