In Situ Formation of FeNi Nanoparticles on Polypyrrole Hydrogel for Efficient Electrocatalytic Nitrate Reduction to Ammonia
Abstract
1. Introduction
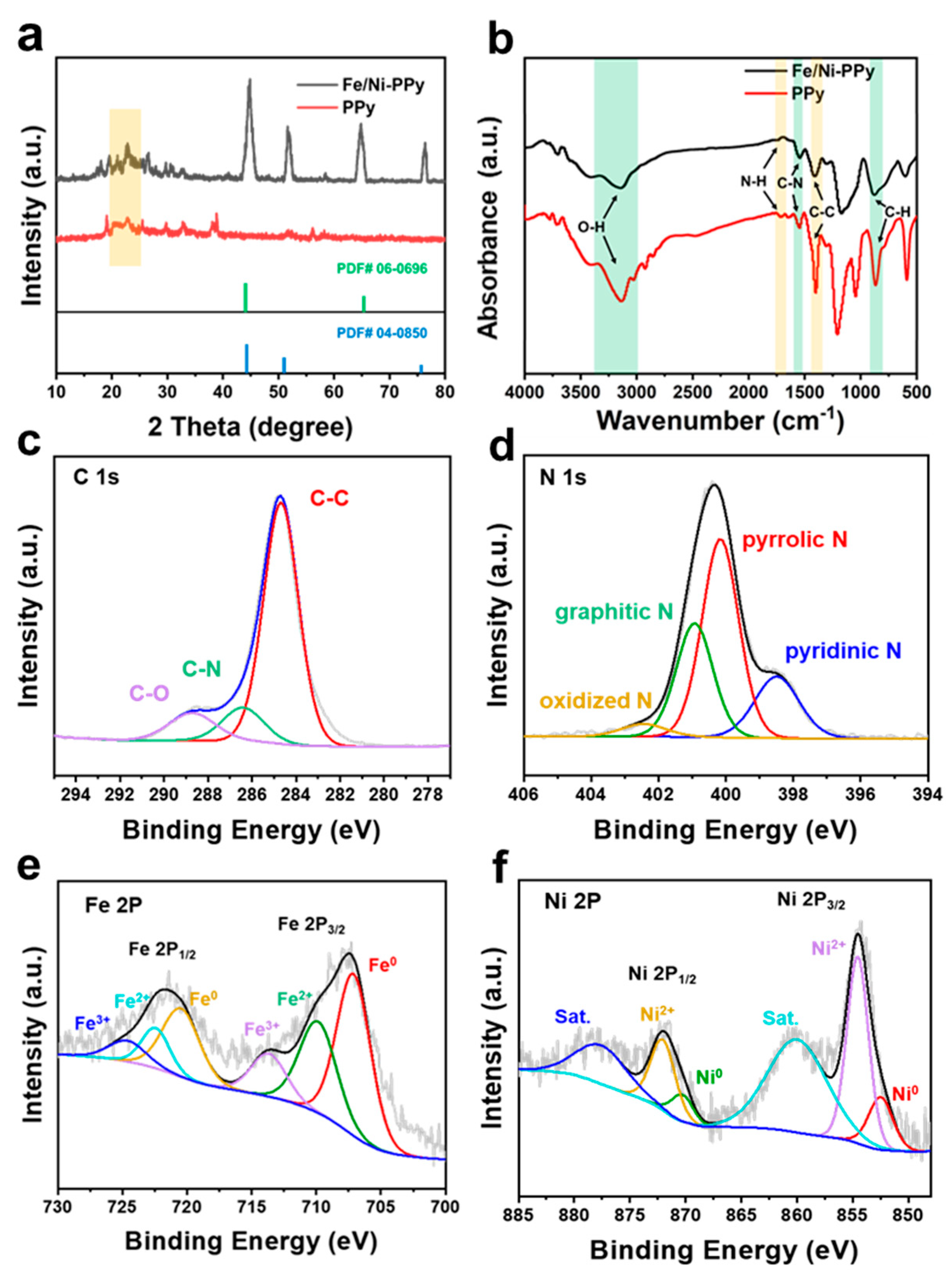
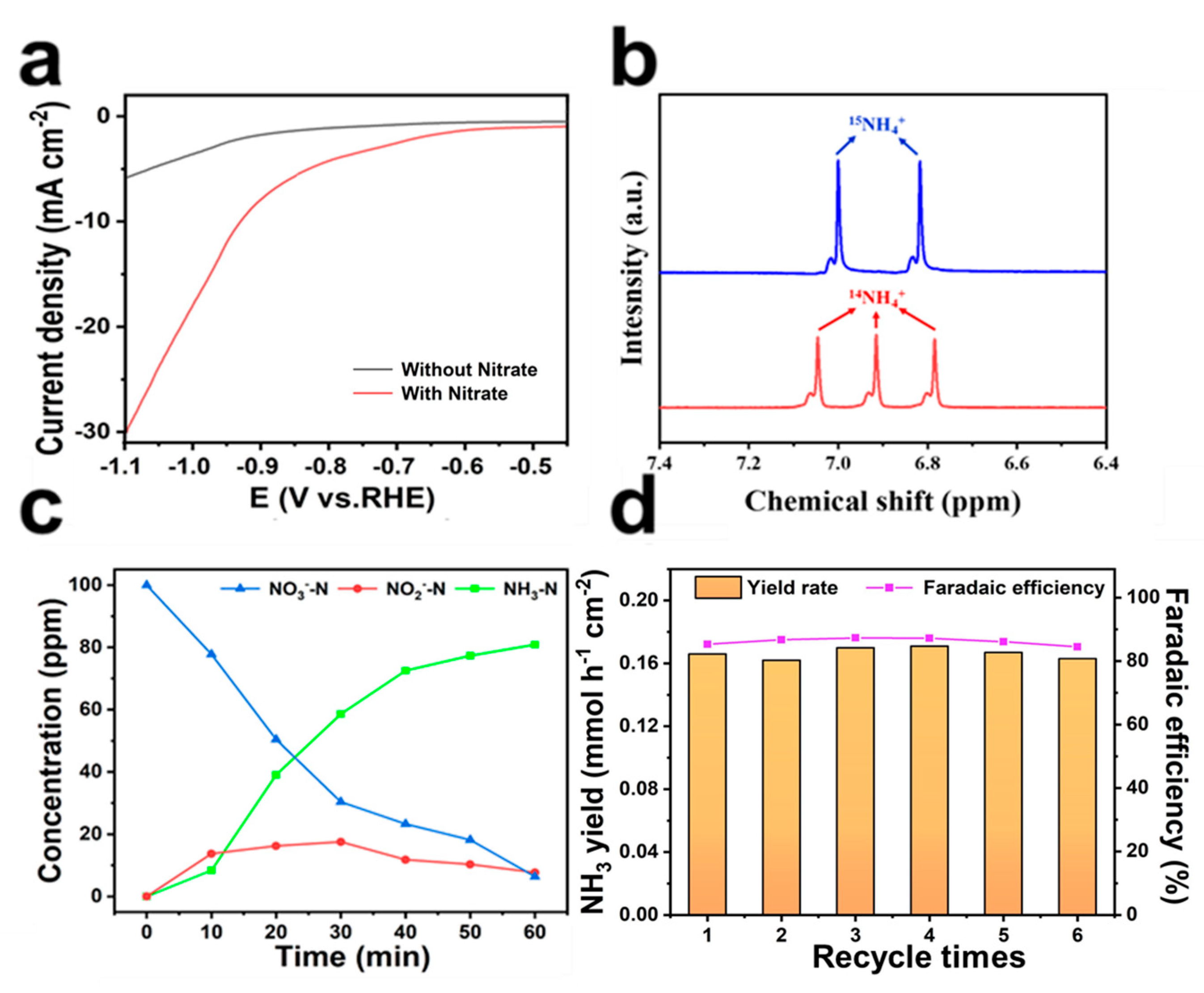
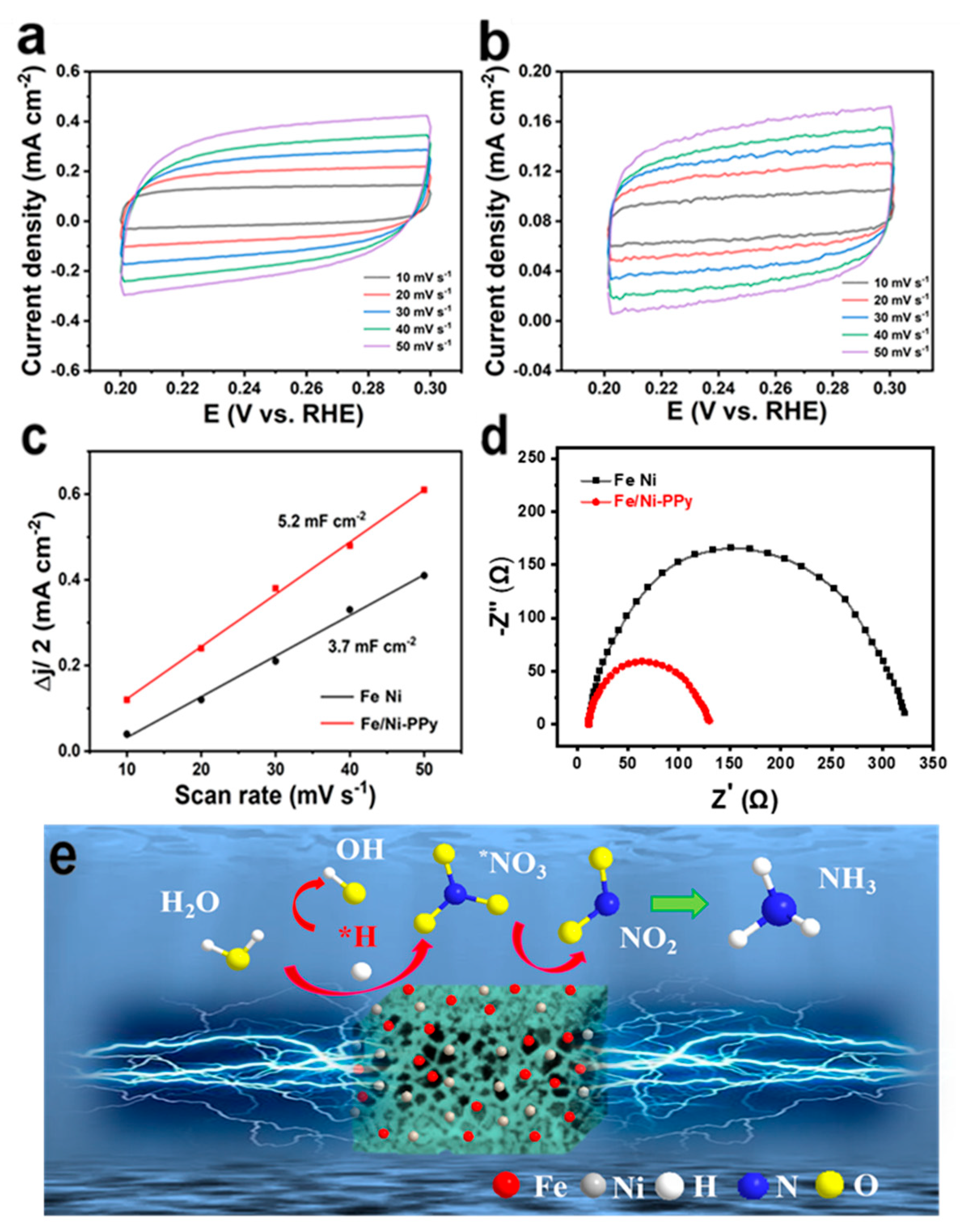
2. Results and Discussion
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of the Catalysts
3.3. Material Characterizations
3.4. Electrocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yüzbaşıoğlu, A.E.; Avşar, C.; Gezerman, A.O. The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Curr. Res. Green Sustain. Chem. 2022, 5, 100307. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Cayuela, M.L.; Sánchez-Monedero, M.A.; Wang, Q. Compost biochemical quality mediates nitrogen leaching loss in a greenhouse soil under vegetable cultivation. Geoderma 2020, 358, 113984. [Google Scholar] [CrossRef]
- Xu, Z.; Zhong, S.; Yu, Y.; Wang, Y.; Cheng, H.; Du, D.; Wang, C. Rhus typhina L. triggered greater allelopathic effects than Koelreuteria paniculata Laxm under ammonium fertilization. Sci. Hortic. 2023, 309, 111703. [Google Scholar] [CrossRef]
- Ren, Y.; Li, S.; Yu, C.; Zheng, Y.; Wang, C.; Qian, B.; Wang, L.; Fang, W.; Sun, Y.; Qiu, J. NH3 Electrosynthesis from N2 Molecules: Progresses, Challenges, and Future Perspectives. J. Am. Chem. Soc. 2024, 146, 6409–6421. [Google Scholar] [CrossRef]
- Wei, W.; Hu, X.; Yang, S.; Wang, K.; Zeng, C.; Hou, Z.; Cui, H.; Liu, S.; Zhu, L. Denitrifying halophilic archaea derived from salt dominate the degradation of nitrite in salted radish during pickling. Food Res. Int. 2022, 152, 110906. [Google Scholar] [CrossRef]
- Guo, X.; Du, H.; Qu, F.; Li, J. Recent progress in electrocatalytic nitrogen reduction. J. Mater. Chem. A 2019, 7, 3531–3543. [Google Scholar] [CrossRef]
- Dai, H.; Liu, L.; Zhao, H.; Zhou, P.; Ying, Y.; Yin, M.; Wang, X.; Fan, W.; Bai, H. Efficient electrocatalytic reduction of nitrate to ammonia using Cu–CeO2 solid solution. Int. J. Hydrogen Energy 2024, 73, 257–264. [Google Scholar] [CrossRef]
- Estudillo-Wong, L.A.; Santillán-Díaz, G.; Arce-Estrada, E.M.; Alonso-Vante, N.; Manzo-Robledo, A. Electroreduction of NOxz species in alkaline medium on Pt nanoparticles. Electrochim. Acta 2013, 88, 358–364. [Google Scholar] [CrossRef]
- Yao, J.; Yan, J. Efficient nitrate-to-ammonia transformation through a direct eight-electron reduction. Sci. China Chem. 2020, 63, 1737–1739. [Google Scholar] [CrossRef]
- Tang, C.; Qiao, S.-Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Jia, R.; Zhang, C.; Zhang, B. Electrochemical synthesis of nitric acid from air and ammonia through waste utilization. Natl. Sci. Rev. 2019, 6, 730–738. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Peng, W.; Luo, M.; Xu, X.; Jiang, K.; Peng, M.; Chen, D.; Chan, T.-S.; Tan, Y. Spontaneous Atomic Ruthenium Doping in Mo2CTX MXene Defects Enhances Electrocatalytic Activity for the Nitrogen Reduction Reaction. Adv. Energy Mater. 2020, 10, 2001364. [Google Scholar] [CrossRef]
- Sun, L.; Liu, B. Mesoporous PdN Alloy Nanocubes for Efficient Electrochemical Nitrate Reduction to Ammonia. Adv. Mater. 2023, 35, 2207305. [Google Scholar] [CrossRef]
- Han, E.; Li, L.; Gao, T.; Pan, Y.; Cai, J. Nitrite determination in food using electrochemical sensor based on self-assembled MWCNTs/AuNPs/poly-melamine nanocomposite. Food Chem. 2024, 437, 137773. [Google Scholar] [CrossRef]
- Wu, H.; Xie, R.; Hao, Y.; Pang, J.; Gao, H.; Qu, F.; Tian, M.; Guo, C.; Mao, B.; Chai, F. Portable smartphone-integrated AuAg nanoclusters electrospun membranes for multivariate fluorescent sensing of Hg2+, Cu2+ and l-histidine in water and food samples. Food Chem. 2023, 418, 135961. [Google Scholar] [CrossRef]
- Gibert, O.; Abenza, M.; Reig, M.; Vecino, X.; Sánchez, D.; Arnaldos, M.; Cortina, J.L. Removal of nitrate from groundwater by nano-scale zero-valent iron injection pulses in continuous-flow packed soil columns. Sci. Total Environ. 2022, 810, 152300. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Iarchuk, A.; Dutta, A.; Broekmann, P. Novel Ni foam catalysts for sustainable nitrate to ammonia electroreduction. J. Hazard. Mater. 2022, 439, 129504. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Q.; Liao, P.; Duan, W.; Liang, S.; Yan, Z.; Feng, C. Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production. Small 2020, 16, 2004526. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, Y.; Guo, H.; Xie, F.; Cong, Y.; Kuang, M.; Yang, J. Tandem catalysis in electrocatalytic nitrate reduction: Unlocking efficiency and mechanism. Interdiscip. Mater. 2024, 3, 245–269. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Carvalho, O.Q.; Marks, R.; Nguyen, H.K.K.; Vitale-Sullivan, M.E.; Martinez, S.C.; Árnadóttir, L.; Stoerzinger, K.A. Role of Electronic Structure on Nitrate Reduction to Ammonium: A Periodic Journey. J. Am. Chem. Soc. 2022, 144, 14809–14818. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Sun, S.; Dai, C.; Zhao, P.; Xi, S.; Ren, Y.; Tan, H.R.; Lim, P.C.; Lin, M.; Diao, C.; Zhang, D.; et al. Spin-related Cu-Co pair to increase electrochemical ammonia generation on high-entropy oxides. Nat. Commun. 2024, 15, 260. [Google Scholar] [CrossRef]
- Ma, X.; Zhong, J.; Huang, W.; Wang, R.; Li, S.; Zhou, Z.; Li, C. Tuning the d-band centers of bimetallic FeNi catalysts derived from layered double hydroxides for selective electrocatalytic reduction of nitrates. Chem. Eng. J. 2023, 474, 145721. [Google Scholar] [CrossRef]
- Estudillo-Wong, L.A.; Guerrero-Barajas, C.; Vázquez-Arenas, J.; Alonso-Vante, N. Revisiting Current Trends in Electrode Assembly and Characterization Methodologies for Biofilm Applications. Surfaces 2023, 6, 2–28. [Google Scholar] [CrossRef]
- Gao, D.; Fabiano, S. Conductive hydrogels put electrons in charge. Science 2024, 384, 509–510. [Google Scholar] [CrossRef]
- Li, P.; Sun, W.; Li, J.; Chen, J.-P.; Wang, X.; Mei, Z.; Jin, G.; Lei, Y.; Xin, R.; Yang, M.; et al. N-type semiconducting hydrogel. Science 2024, 384, 557–563. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, X.; Yan, Y.; Zhou, J. A Sustainable Dual Cross-Linked Cellulose Hydrogel Electrolyte for High-Performance Zinc-Metal Batteries. Nano Micro Lett. 2024, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, G.; Liu, X.; Zhu, B.; Chai, X.; Zhang, Q.; Liu, J.; He, C. Superhydrophilic Phytic-Acid-Doped Conductive Hydrogels as Metal-Free and Binder-Free Electrocatalysts for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2019, 58, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Li, R.; Li, H.; Shu, Y.; Li, Y.; Qiu, S.; He, C.; Yang, Y. Phytic acid assisted fabrication of graphene/polyaniline composite hydrogels for high-capacitance supercapacitors. Compos. Part B 2018, 155, 132–137. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Fang, Z.; Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 2021, 14, 3522–3531. [Google Scholar] [CrossRef]
- Shan, A.; Idrees, A.; Zaman, W.Q.; Mohsin, A.; Abbas, Z.; Stadler, F.J.; Lyu, S. Synthesis of CaCO3 supported nano zero-valent iron-nickel nanocomposite (nZVI-Ni@CaCO3) and its application for trichloroethylene removal in persulfate activated system. Environ. Res. 2024, 245, 118050. [Google Scholar] [CrossRef]
- Muthusamy, T.; Sethuram Markandaraj, S.; Shanmugam, S. Nickel nanoparticles wrapped in N-doped carbon nanostructures for efficient electrochemical reduction of NO to NH3. J. Mater. Chem. A 2022, 10, 6470–6474. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Zhang, Y.; Wang, Q. NiFe Alloy Nanoparticles with hcp Crystal Structure Stimulate Superior Oxygen Evolution Reaction Electrocatalytic Activity. Angew. Chem. Int. Ed. 2019, 58, 6099–6103. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-Healing, Wet-Adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Zhong, D.; Qiao, Q.; Zeng, H. Efficient removal of Cr(VI) by chitosan cross-linked bentonite loaded nano-zero-valent iron composite: Performance and mechanism. J. Hazard. Mater. 2024, 480, 136183. [Google Scholar] [CrossRef]
- Vigneshwaran, J.; Jose, J.; Thomas, S.; Gagliardi, A.; Narayan, R.L.; Jose, S.P. PPy-PdO modified MXene for flexible binder-free electrodes for asymmetric supercapacitors: Insights from experimental and DFT investigations. Chem. Eng. J. 2024, 487, 150555. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Alkayal, N.S.; Bashal, A.H.; Alharbi, K.H.; Alharbi, W. Chitosan-Strontium Oxide Nanocomposite: Preparation, Characterization, and Catalytic Potency in Thiadiazoles Synthesis. Polymers 2022, 14, 2827. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, B.; Yu, J.; Yan, Z.; Liu, R.; Yu, C.; Zhao, Z.; Zhang, H.; Yao, F.; Li, J. A polypyrrole-dopamine/poly(vinyl alcohol) anisotropic hydrogel for strain sensor and bioelectrodes. Chem. Eng. J. 2024, 486, 150182. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.-C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2024, 37, 2413073. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Hsu, Y.-I.; Uyama, H. Smart versatile hydrogels tailored by metal-phenolic coordinating carbon and polypyrrole for soft actuation, strain sensing and writing recognition. Chem. Eng. J. 2024, 493, 152671. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Yan, B.; Zhang, F.; Shi, Y.; Guo, X. Single Cu atoms confined in N-doped porous carbon networks by flash nanocomplexation as efficient trifunctional electrocatalysts for Zn-air batteries and water splitting. Compos. Part B 2023, 253, 110575. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B 2020, 263, 118344. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, S.; Gao, L.; Chen, A.; Jiao, M.; Cui, H.; Zhou, Z. Boosting bifunctional electrocatalytic activity in S and N co-doped carbon nanosheets for high-efficiency Zn–air batteries. J. Mater. Chem. A 2020, 8, 4386–4395. [Google Scholar] [CrossRef]
- Tang, S.; Yu, C.; Liu, X.; Fu, D.; Liao, W.; Xu, F.; Zhong, W. 2-Methylimidazole assisted synthesis of nanocrystalline shell reinforced PPy hydrogel with high mechanical and electrochemical performance. Chem. Eng. J. 2022, 430, 133033. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Cao, A.; Wei, Y.; Wang, H.; Li, X.; Jiang, Z.; Waterhouse, G.I.N.; Lu, S.; Zang, S.-Q. Interfacial hydrogen bonding-involved electrocatalytic ammonia synthesis on OH-terminated MXene. Appl. Catal. B 2023, 328, 122473. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from Cation-Nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Gong, Z.; Zhong, W.; He, Z.; Liu, Q.; Chen, H.; Zhou, D.; Zhang, N.; Kang, X.; Chen, Y. Regulating surface oxygen species on copper (I) oxides via plasma treatment for effective reduction of nitrate to ammonia. Appl. Catal. B 2022, 305, 121021. [Google Scholar] [CrossRef]
- Huang, C.-C.; Pourzolfaghar, H.; Huang, C.-L.; Liao, C.-P.; Li, Y.-Y. FeNi nanoalloy-carbon nanotubes on defected graphene as an excellent electrocatalyst for lithium-oxygen batteries. Carbon 2024, 222, 118973. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Zhang, J.; Jiao, Y.; Zhu, Q.; Wang, J.; Chen, Y.; Li, X. Regulating Ni microchemical state for enhanced C-C/H bonds adsorption and activation towards methylcyclohexane steam reforming. Chem. Eng. J. 2025, 506, 160152. [Google Scholar] [CrossRef]
- Ma, L.; Xu, F.; Zhang, L.; Nie, Z.; Xia, K.; Guo, M.; Li, M.; Ding, X. Breaking the linear correlations for enhanced electrochemical nitrogen reduction by carbon-encapsulated mixed-valence Fe7(PO4)6. J. Energy Chem. 2022, 71, 182–187. [Google Scholar] [CrossRef]
- Qiao, H.; Han, Y.; Yao, L.; Xu, X.; Ma, J.; Wen, B.; Hu, J.; Huang, H. Coating zero valent iron onto hollow carbon spheres as efficient electrocatalyst for N2 fixation and neutral Zn-N2 battery. Chem. Eng. J. 2023, 464, 142628. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Li, Y.; Yu, L.; Chen, F.; Li, L. Conductive polymer protection strategy to promote electrochemical nitrate reduction to ammonia in highly acidic condition over Cu-based catalyst. Chem. Eng. J. 2024, 481, 148596. [Google Scholar] [CrossRef]
- Luo, H.; Li, S.; Wu, Z.; Liu, Y.; Luo, W.; Li, W.; Zhang, D.; Chen, J.; Yang, J. Modulating the Active Hydrogen Adsorption on Fe–N Interface for Boosted Electrocatalytic Nitrate Reduction with Ultra-Long Stability. Adv. Mater. 2023, 35, 2304695. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, M.; Wang, S.; Wang, Y.; Zhou, J.; Hao, F.; Liu, F.; Yan, Y.; Meng, X.; Guo, L.; et al. Atomic Scale Cooperativity of Alloy Nanostructures for Efficient Nitrate Electroreduction to Ammonia in Neutral Media. Adv. Funct. Mater. 2024, 2420153. [Google Scholar] [CrossRef]
- Kou, M.; Yuan, Y.; Zhao, R.; Wang, Y.; Zhao, J.; Yuan, Q.; Zhao, J. Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis. Molecules 2024, 29, 2261. [Google Scholar] [CrossRef]
- Deng, K.; Lian, Z.; Wang, W.; Yu, J.; Mao, Q.; Yu, H.; Wang, Z.; Wang, L.; Wang, H. Hydrogen spillover effect tuning the rate-determining step of hydrogen evolution over Pd/Ir hetero-metallene for industry-level current density. Appl. Catal. B Environ. Energy 2024, 352, 124047. [Google Scholar] [CrossRef]
- Niu, Z.; Fan, S.; Li, X.; Wang, P.; Liu, Z.; Wang, J.; Bai, C.; Zhang, D. Bifunctional copper-cobalt spinel electrocatalysts for efficient tandem-like nitrate reduction to ammonia. Chem. Eng. J. 2022, 450, 138343. [Google Scholar] [CrossRef]
- Mondal, S.; Dilly Rajan, K.; Patra, L.; Rathinam, M.; Ganesh, V. Sulfur Vacancy-Induced Enhancement of Piezocatalytic H2 Production in MoS2. Small 2025, 2411828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kohler, H.; Schwotzer, M.; Guth, U. Stability improvement of layered Au,Pt-YSZ mixed-potential gas sensing electrodes by cathodic polarization: Studies by steady state and dynamic electrochemical methods. Sens. Actuators B 2021, 342, 130065. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Li, D.; Jiang, T.; Chen, Q.; Mao, C.; Li, L.; Jiang, D.; Mao, B. Carbon dots-boosted active hydrogen for efficient electrocatalytic reduction of nitrate to ammonia. J. Alloys Compd. 2025, 1014, 178694. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Huang, Y.; Tang, M.; Zou, X.; Pan, Z.; Huo, X.; Wu, J.; Lin, C.; Sun, Z.; et al. Electrochemical Nitrate Reduction to Ammonia on CuCo Nanowires at Practical Level. Adv. Funct. Mater. 2024, 34, 2405179. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, L.; Ma, T.; Zhang, J.-J.; Wu, H.; Su, J.; Song, Y.; Zhu, H.; Liu, Q.; Zhu, M.; et al. Direct Synthesis of Ammonia from Nitrate on Amorphous Graphene with Near 100% Efficiency. Adv. Mater. 2023, 35, 2211856. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yan, P.; Guo, Q.; Zhang, D.; Mao, C.; Yuan, Q.; Sun, H.; Liu, M.; Liu, Y.; Mao, B. In Situ Formation of FeNi Nanoparticles on Polypyrrole Hydrogel for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Molecules 2025, 30, 1271. https://doi.org/10.3390/molecules30061271
Li L, Yan P, Guo Q, Zhang D, Mao C, Yuan Q, Sun H, Liu M, Liu Y, Mao B. In Situ Formation of FeNi Nanoparticles on Polypyrrole Hydrogel for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Molecules. 2025; 30(6):1271. https://doi.org/10.3390/molecules30061271
Chicago/Turabian StyleLi, Lixia, Paihao Yan, Qinkai Guo, Dongxu Zhang, Chunliang Mao, Quan Yuan, Hongtao Sun, Mingze Liu, Yanhong Liu, and Baodong Mao. 2025. "In Situ Formation of FeNi Nanoparticles on Polypyrrole Hydrogel for Efficient Electrocatalytic Nitrate Reduction to Ammonia" Molecules 30, no. 6: 1271. https://doi.org/10.3390/molecules30061271
APA StyleLi, L., Yan, P., Guo, Q., Zhang, D., Mao, C., Yuan, Q., Sun, H., Liu, M., Liu, Y., & Mao, B. (2025). In Situ Formation of FeNi Nanoparticles on Polypyrrole Hydrogel for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Molecules, 30(6), 1271. https://doi.org/10.3390/molecules30061271







