Isolation of Carrot Chromoplasts and Assessment of Their Carotenoid Content and Bioaccessibility
Abstract
1. Introduction
2. Results and Discussion
2.1. Optical Microscopy
2.2. Isolation of Carrot Chromoplasts
2.3. Carotenoid Content
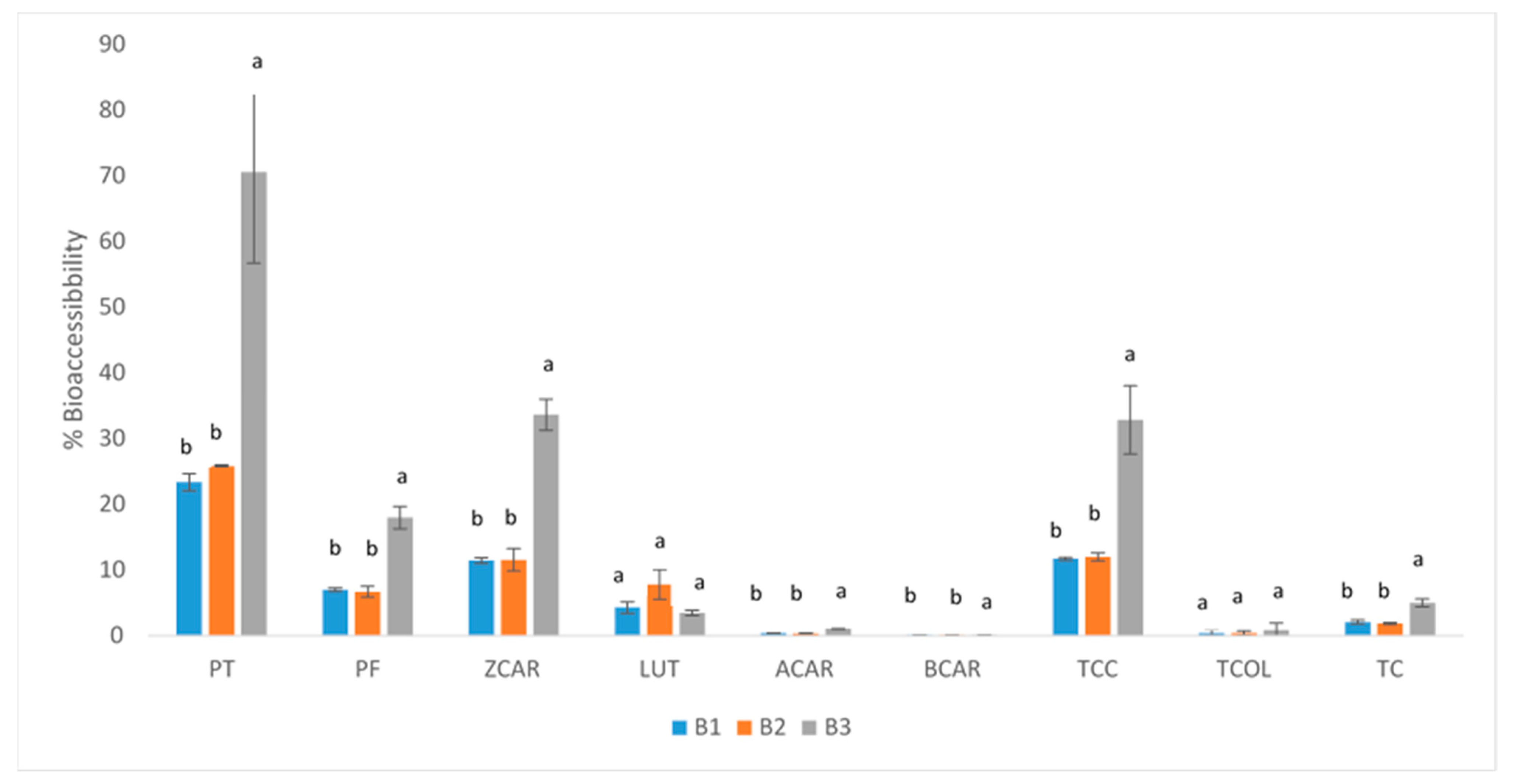
2.4. Bioaccessibility and Carotenoid Bioaccesible Content
3. Materials and Methods
3.1. Samples
3.2. Chemicals
3.3. Isolation of Carrot Chromoplasts
3.4. Light Microscopy
3.5. Simulated In Vitro Digestion Method
3.6. Carotenoid Content
3.7. Bioaccessibility and Carotenoid Bioaccessible Content
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| %BIO | Bioaccessibility percentage |
| CBC | Carotenoid Bioaccessible Content |
| BCAR | β-carotene |
| ACAR | α-carotene |
| ZCAR | ζ-carotene |
| LUT | Lutein |
| PT | Phytoene |
| PF | Phytofluene |
| TCC | Total Colourless Carotenoids |
| TCOL | Total Coloured Carotenoids |
| TC | Total Carotenoids |
References
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of Chromoplast Morphology on Carotenoid Bioaccessibility of Carrot, Mango, Papaya, and Tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Killeit, U. European Database of Carotenoid Levels in Food. Dtsch. Leb. 2021, 117, 912. [Google Scholar]
- Song, H.; Lu, Q.; Song, T.; Gao, C.; Zhu, W.; Guo, X. Study on the Mechanism of Carotenoid Production and Accumulation in Orange Red Carrot (Daucus carota L.). Sci. Hortic. 2024, 327, 112825. [Google Scholar] [CrossRef]
- Chevalier, W.; Moussa, S.A.; Ottoni, M.M.N.; Dubois-Laurent, C.; Huet, S.; Aubert, C.; Desnoues, E.; Navez, B.; Cottet, V.; Chalot, G.; et al. Evaluation of Pedoclimatic Factors and Cultural Practices Effects on Carotenoid and Sugar Content in Carrot Root. Eur. J. Agron. 2022, 140, 126577. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yang, Y.; Fei, Z.; Yuan, H.; Fish, T.; Thannhauser, T.W.; Mazourek, M.; Kochian, L.V.; Wang, X.; Li, L. Proteomic Analysis of Chromoplasts from Six Crop Species Reveals Insights into Chromoplast Function and Development. J. Exp. Bot. 2013, 64, 949–961. [Google Scholar] [CrossRef]
- Esquivel, P.; Schweiggert, R.M.; Chacón-Ordóñez, T.; Steingass, C.B.; Carle, R.; Jiménez, V.M. Carotenoid Assembly in Fruits and Vegetables. Food Chem. Funct. Anal. 2019, 2019, 51–67. [Google Scholar] [CrossRef]
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.C. Chromoplast Differentiation: Current Status and Perspectives. Plant Cell Physiol. 2010, 51, 1601–1611. [Google Scholar] [CrossRef]
- Carrillo, C.; Buvé, C.; Panozzo, A.; Grauwet, T.; Hendrickx, M. Role of Structural Barriers in the in Vitro Bioaccessibility of Anthocyanins in Comparison with Carotenoids. Food Chem. 2017, 227, 271–279. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Carle, R. Carotenoid Deposition in Plant and Animal Foods and Its Impact on Bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef]
- Jeffery, J.; Holzenburg, A.; King, S. Physical Barriers to Carotenoid Bioaccessibility. Ultrastructure Survey of Chromoplast and Cell Wall Morphology in Nine Carotenoid-Containing Fruits and Vegetables. J. Sci. Food Agric. 2012, 92, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, H. Chromoplast Biogenesis and Carotenoid Accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Angaman, D.M.; Petrizzo, R.; Hernández-Gras, F.; Romero-Segura, C.; Pateraki, I.; Busquets, M.; Boronat, A. Precursor Uptake Assays and Metabolic Analyses in Isolated Tomato Fruit Chromoplasts. Plant Methods 2012, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Berry, H.M.; Nogueira, M.; Drapal, M.; Almeida, J.; Perez-Fons, L.; Enfissi, E.M.A.; Fraser, P.D. Isolation and Characterization of Sub-Plastidial Fractions from Carotenoid Rich Fruits, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 671, ISBN 9780323913539. [Google Scholar]
- Vásquez-Caicedo, A.L.; Heller, A.; Neidhart, S.; Carle, R. Chromoplast Morphology and β-Carotene Accumulation during Postharvest Ripening of Mango Cv. “Tommy Atkins” . J. Agric. Food Chem. 2006, 54, 5769–5776. [Google Scholar] [CrossRef]
- Kim, J.E.; Rensing, K.H.; Douglas, C.J.; Cheng, K.M. Chromoplasts Ultrastructure and Estimated Carotene Content in Root Secondary Phloem of Different Carrot Varieties. Planta 2010, 231, 549–558. [Google Scholar] [CrossRef]
- Liedvogel, B. Isolation of Membranous Chromoplast from Daffodil Flowers. Methods Enzymol. 1987, 148, 5–10. [Google Scholar]
- Benítez-González, A.M.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Vicario, I.M.; Meléndez-Martínez, A.J. Towards More Sustainable Cooking Practices to Increase the Bioaccessibility of Colourless and Provitamin A Carotenoids in Cooked Carrots. Food Funct. 2024, 15, 8835–8847. [Google Scholar] [CrossRef]
- Lu, S.; Li, L. Carotenoid Metabolism: Biosynthesis, Regulation, and Beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H.; Zeng, Y.; Xu, Q. Plastids and Carotenoid Accumulation. In Carotenoids in Nature. Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 279–293. [Google Scholar]
- Coulon, D.; Bréhélin, C. Isolation of Plastoglobules for Lipid Analyses. Methods Mol. Biol. 2021, 2295, 321–335. [Google Scholar] [CrossRef]
- Oleszkiewicz, T.; Klimek-Chodacka, M.; Milewska-Hendel, A.; Zubko, M.; Stróż, D.; Kurczyńska, E.; Boba, A.; Szopa, J.; Baranski, R. Unique Chromoplast Organisation and Carotenoid Gene Expression in Carotenoid-Rich Carrot Callus. Planta 2018, 248, 1455–1471. [Google Scholar] [CrossRef]
- Pyke, K. Plastid Biogenesis and Differentiation. Top. Curr. Genet. 2007, 19, 1–28. [Google Scholar] [CrossRef]
- Lundquist, P.K. Chromoplast Differentiation: A Central Role for Plastoglobule Lipid Droplets Comes into Focus. New Phytol. 2023, 237, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of Phytoene and Phytofluene Is Superior to Other Carotenoids from Selected Fruit and Vegetable Juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.-D. Study of the Time-Course of Cis/Trans (Z/E) Isomerization of Lycopene, Phytoene, and Phytofluene from Tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef]
- Benítez-González, A.M.; Aguilera-Velázquez, J.R.; Bautista Palomas, J.; Meléndez-Martínez, A.J. Evaluation of Carrot and Agroindustrial Residues for Obtaining Tenebrio Molitor (Yellow Mealworm) Powder Enriched in Bioaccessible Provitamin A and Colourless Carotenoids. LWT 2024, 214, 117011. [Google Scholar] [CrossRef]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary Fat Composition, Food Matrix and Relative Polarity Modulate the Micellarization and Intestinal Uptake of Carotenoids from Vegetables and Fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef]
- Zumbado-Chinchilla, C.; Arroyo-Esquivel, L.; Cortés-Muñoz, M.; Incer-González, A.I.; Esquivel, P.E. Effect of Lipid Addition on Carotenoid Bioaccessibility in a Dairy-Based Papaya (Carica papaya) Beverage. ACS Food Sci. Technol. 2024, 4, 586–594. [Google Scholar] [CrossRef]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Comparative Study of the Bioaccessibility of the Colorless Carotenoids Phytoene and Phytofluene in Powders and Pulps of Tomato: Microstructural Analysis and Effect of Addition of Sunflower Oil. Food Funct. 2018, 9, 5016–5023. [Google Scholar] [CrossRef]
- Yan, X.; Huang, J.; Huang, L.; Luo, C.; Li, Z.; Xu, P.; Tan, K.; Cheong, K.L.; Tan, K. Effects of Dietary Lipids on Bioaccessibility and Bioavailability of Natural Carotenoids. LWT 2024, 200, 116171. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Karamalegkos, A.A.; Mapelli-Brahm, P.; Ezcurra, M.; Meléndez-Martínez, A.J. Phytoene and Phytoene-Rich Microalgae Extracts Extend Lifespan in C. Elegans and Protect against Amyloid-β Toxicity in an Alzheimer’s Disease Model. Antioxidants 2024, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Perazzoli, G.; Luque, C.; León-Vaz, A.; Gómez-Villegas, P.; Rengel, R.; Molina-Márquez, A.; Morón-Ortiz, Á.; Mapelli-Brahm, P.; Prados, J.; Melguizo, C.; et al. Preliminary Assessment of the Protective and Antitumor Effects of Several Phytoene-Containing Bacterial and Microalgal Extracts in Colorectal Cancer. Molecules 2024, 29, 5003. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Steingass, C.B.; Heller, A.; Esquivel, P.; Carle, R. Characterization of Chromoplasts and Carotenoids of Red- and Yellow-Fleshed Papaya (Carica papaya L.). Planta 2011, 234, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W.; Bierer, T.L.; Gugger, E.T. Absorption and Transport of Carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 76–85. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Alminger, M.; Lemmens, L.; Colle, I.; Knockaert, G.; Moelants, K.; Van Loey, A.; Hendrickx, M. In Vitro Approaches to Estimate the Effect of Food Processing on Carotenoid Bioavailability Need Thorough Understanding of Process Induced Microstructural Changes. Trends Food Sci. Technol. 2010, 21, 607–618. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Hernanz, D.; Vicario, I.M. Simultaneous Determination of Dietary Isoprenoids (Carotenoids, Chlorophylls and Tocopherols) in Human Faeces by Rapid Resolution Liquid Chromatography. J. Chromatogr. A 2019, 1583, 63–72. [Google Scholar] [CrossRef]


| Carotenoid | CC | CBC | % BIO |
|---|---|---|---|
| phytoene | 6.723 ± 0.005 | 4.276 ± 0.431 | 63.603 ± 9.669 |
| phytofluene | 5.202 ± 0.284 | 1.782 ± 0.081 | 34.256 ± 6.872 |
| TCC | 11.925 ± 0.279 | 6.058 ± 0.512 | 50.769 ± 3.107 |
| ζ-carotene | 4.804 ± 0.200 | 0.499 ± 0.013 | 10.387 ± 1.322 |
| lutein | 1.372 ± 0.114 | 0.312 ± 0.014 | 22.740 ± 5.120 |
| α-carotene | 56.241 ± 1.279 | 1.134 ± 0.127 | 2.016 ± 0.244 |
| β-carotene | 56.710 ± 2.792 | 0.781 ± 0.103 | 1.378 ± 0.193 |
| TCOL | 119.127 ± 3.757 | 2.726 ± 0.258 | 2.293 ± 0.289 |
| TC | 131.052 ± 3.478 | 8.785 ± 0.770 | 6.703 ± 0.766 |
| Carotenoid | Band 1 (B1) | Band 2 (B2) | Band 3 (B3) |
|---|---|---|---|
| phytoene | 1.509 ± 0.056 (3.48%) a | 1.589 ± 0.180 (2.88%) a | 1.574 ± 0.024 (3.08%) a |
| phytofluene | 3.782 ± 0.066 (8.71%) a | 4.135 ± 0.422 (7.49%) a | 3.993 ± 0.048 (7.81%) a |
| TCC | 5.291 ± 0.122 (12.19%) a | 5.725 ± 0.602 (10.37%) a | 5.567 ± 0.072 (10.89%) a |
| ζ-carotene | 1.533 ± 0.043 (3.53%) a | 1.706 ± 0.201 (3.10%) a | 1.458 ± 0.011 (2.85%) a |
| lutein | 0.740 ± 0.079 (1.70%) a | 0.539 ± 0.107 (0.97%) ab | 0.392 ± 0.006 (0.77%) b |
| α-carotene | 19.374 ± 3.165 (44.62%) a | 25.430 ± 3.800 (46.06%) a | 20.720 ± 0.465 (40.52%) a |
| β-carotene | 16.478 ± 2.524 (37.95%) a | 21.812 ± 3.074 (39.51%) a | 22.993 ± 0.528 (44.97%) a |
| TCOL | 38.125 ± 5.652 (87.81%) a | 49.487 ± 7.183 (89.63%) a | 45.563 ± 1.010 (89.11%) a |
| TC | 43.416 ± 5.531 a | 55.211 ± 7.785 a | 51.130 ± 1.082 a |
| Carotenoid | Band 1 (B1) | Band 2 (B2) | Band 3 (B3) |
|---|---|---|---|
| phytoene | 0.353 ± 0.033 (40.47%) a | 0.410 ± 0.044 (41.10%) a | 1.113 ± 0.236 (43.38%) b |
| phytofluene | 0.264 ± 0.005 (29.45%) a | 0.273 ± 0.007 (27.47%) a | 0.717 ± 0.076 (28.16%) b |
| TCC | 0.617 ± 0.028 (69.19%) a | 0.683 ± 0.038 (68.57%)a | 1.830 ± 0.312 (71.54%) b |
| ζ-carotene | 0.175 ± 0.011 (19.08%) a | 0.195 ± 0.006 (19.58%) a | 0.491 ± 0.038 (19.30%) b |
| lutein | 0.031 ± 0.003 (3.23%) a | 0.041 ± 0.004(4.10%) a | 0.013 ± 0.001 (0.54%) b |
| α-carotene | 0.066 ± 0.002 (7.53%) a | 0.075 ± 0.001 (7.53%) a | 0.212 ± 0.004 (8.37%) b |
| β-carotene | 0.002 ± 0.001 (0.24%) a | 0.002 ± 0.001 (0.21%) a | 0.006 ± 0.001 (0.25%) b |
| TCOL | 0.275 ± 0.017 (30.81%)a | 0.313 ± 0.010 (31.43%) a | 0.722 ± 0.032 (28.46%) b |
| TC | 0.892 ± 0.011 a | 0.996 ± 0.028 a | 2.552 ± 0.345 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-González, A.M.; Gómez-Gómez, L.; Ahrazem, O.; Esquivel, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Isolation of Carrot Chromoplasts and Assessment of Their Carotenoid Content and Bioaccessibility. Molecules 2025, 30, 1267. https://doi.org/10.3390/molecules30061267
Benítez-González AM, Gómez-Gómez L, Ahrazem O, Esquivel P, Stinco CM, Meléndez-Martínez AJ. Isolation of Carrot Chromoplasts and Assessment of Their Carotenoid Content and Bioaccessibility. Molecules. 2025; 30(6):1267. https://doi.org/10.3390/molecules30061267
Chicago/Turabian StyleBenítez-González, Ana M., Lourdes Gómez-Gómez, Oussama Ahrazem, Patricia Esquivel, Carla M. Stinco, and Antonio J. Meléndez-Martínez. 2025. "Isolation of Carrot Chromoplasts and Assessment of Their Carotenoid Content and Bioaccessibility" Molecules 30, no. 6: 1267. https://doi.org/10.3390/molecules30061267
APA StyleBenítez-González, A. M., Gómez-Gómez, L., Ahrazem, O., Esquivel, P., Stinco, C. M., & Meléndez-Martínez, A. J. (2025). Isolation of Carrot Chromoplasts and Assessment of Their Carotenoid Content and Bioaccessibility. Molecules, 30(6), 1267. https://doi.org/10.3390/molecules30061267









