A Molecular Dynamics Study of the Solvation Properties of Sugars in Supercritical Carbon Dioxide
Abstract
1. Introduction
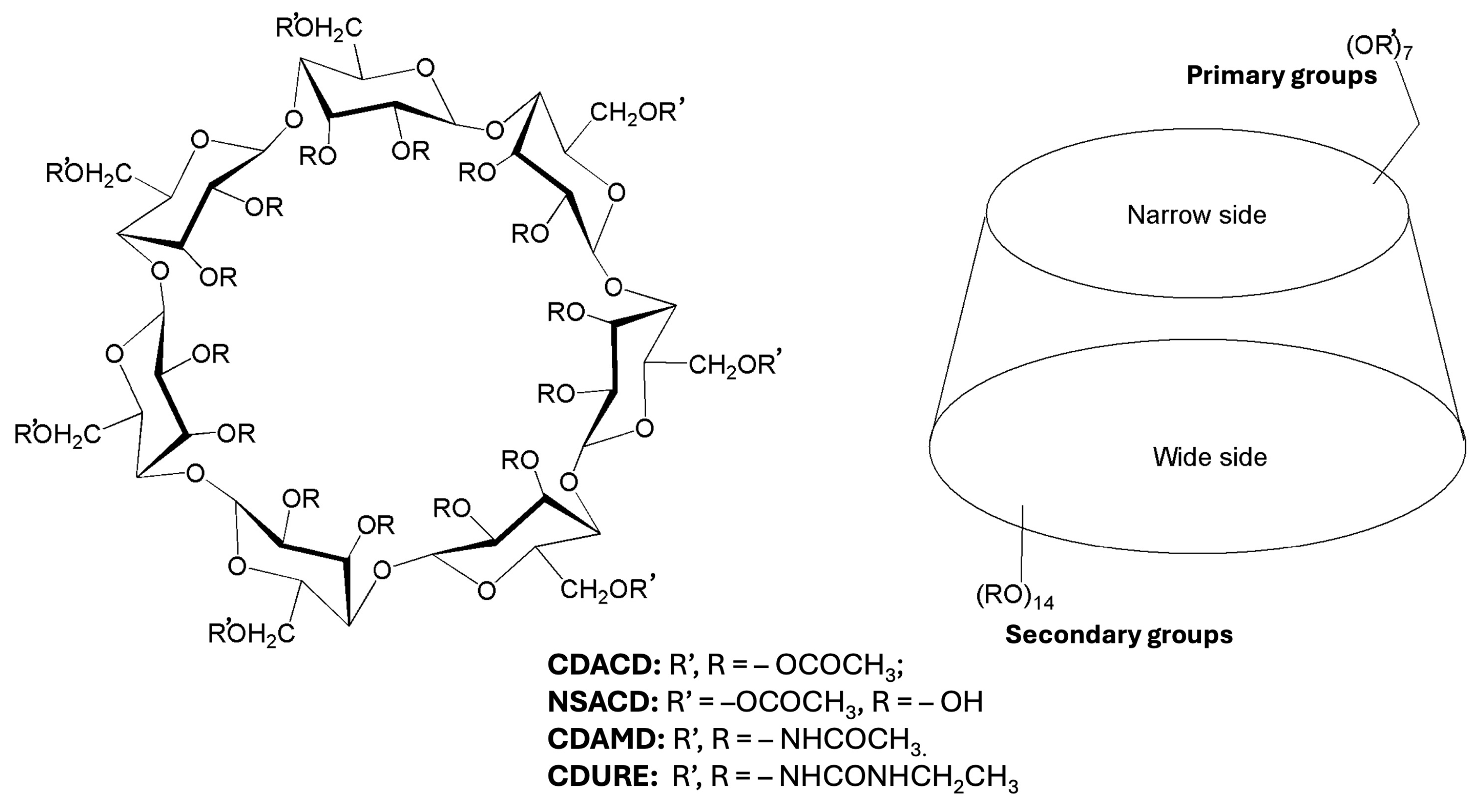
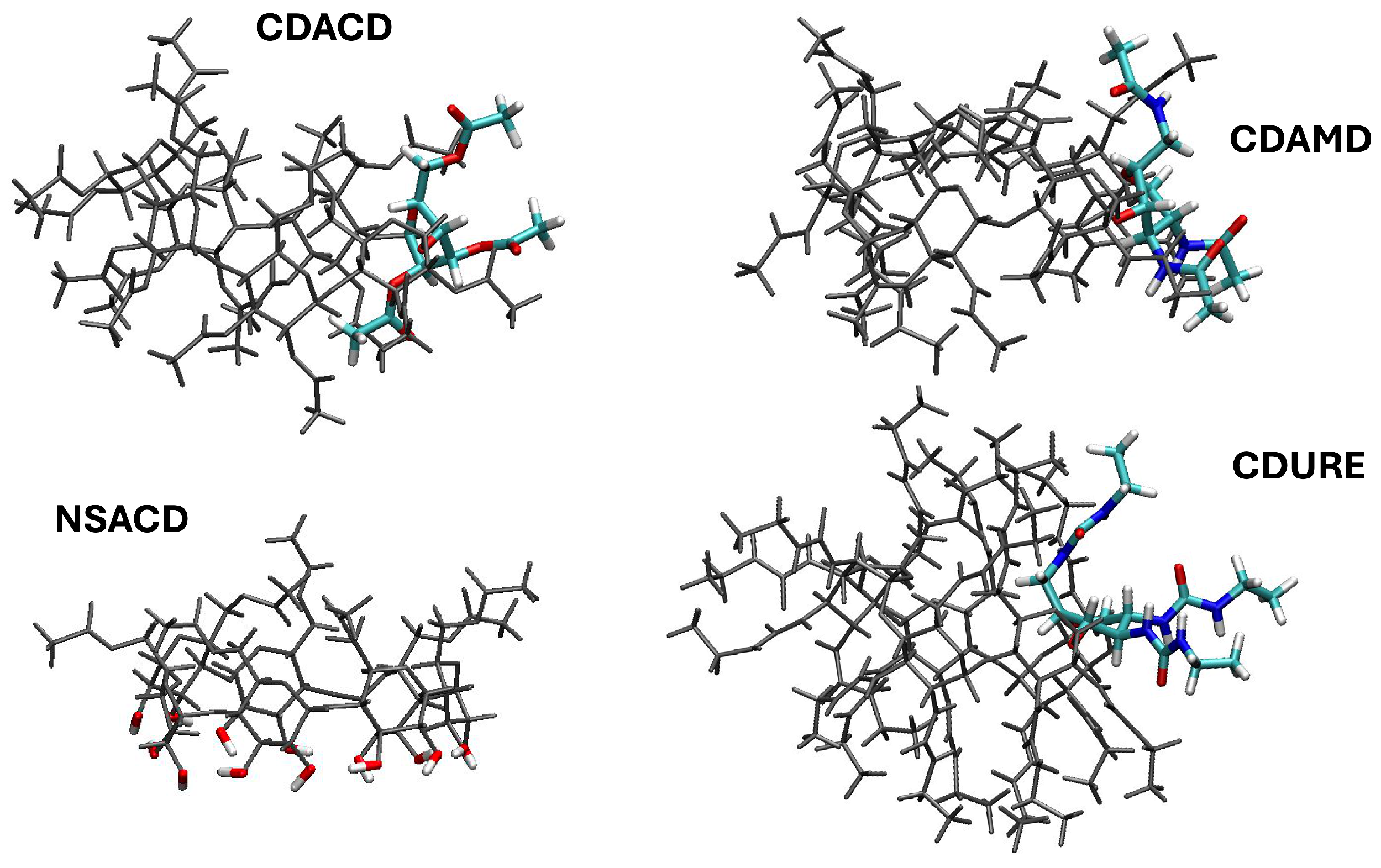
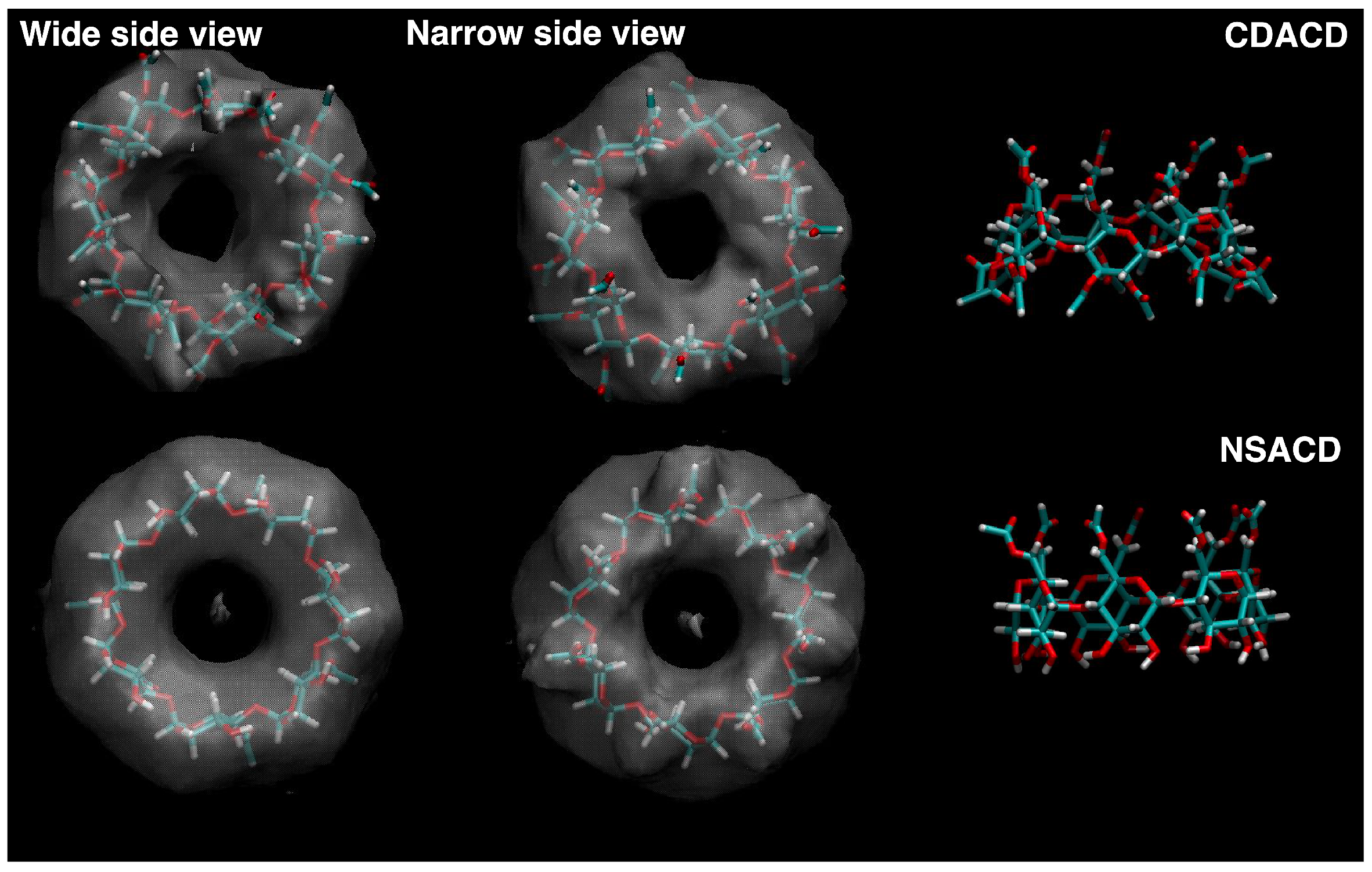
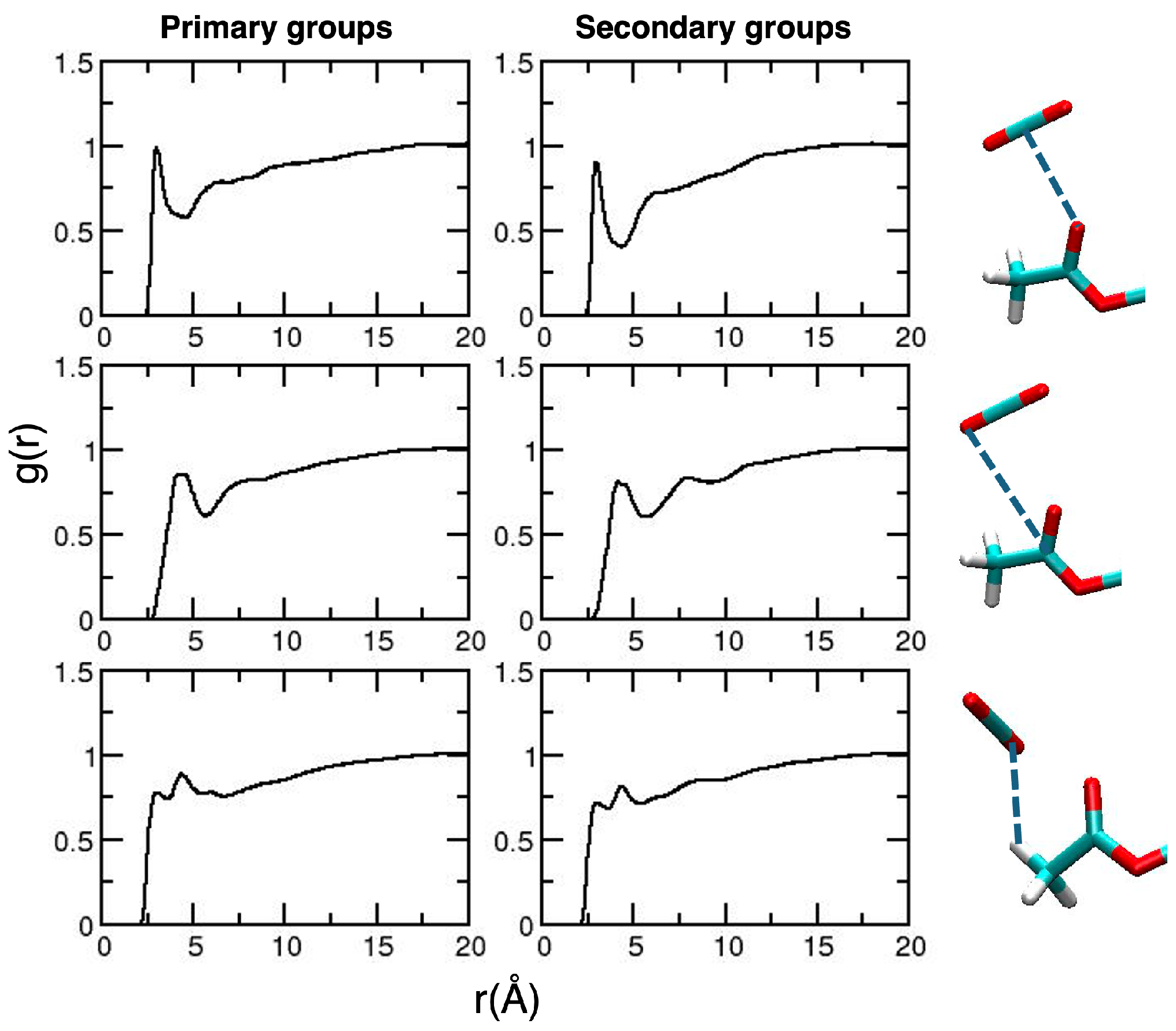
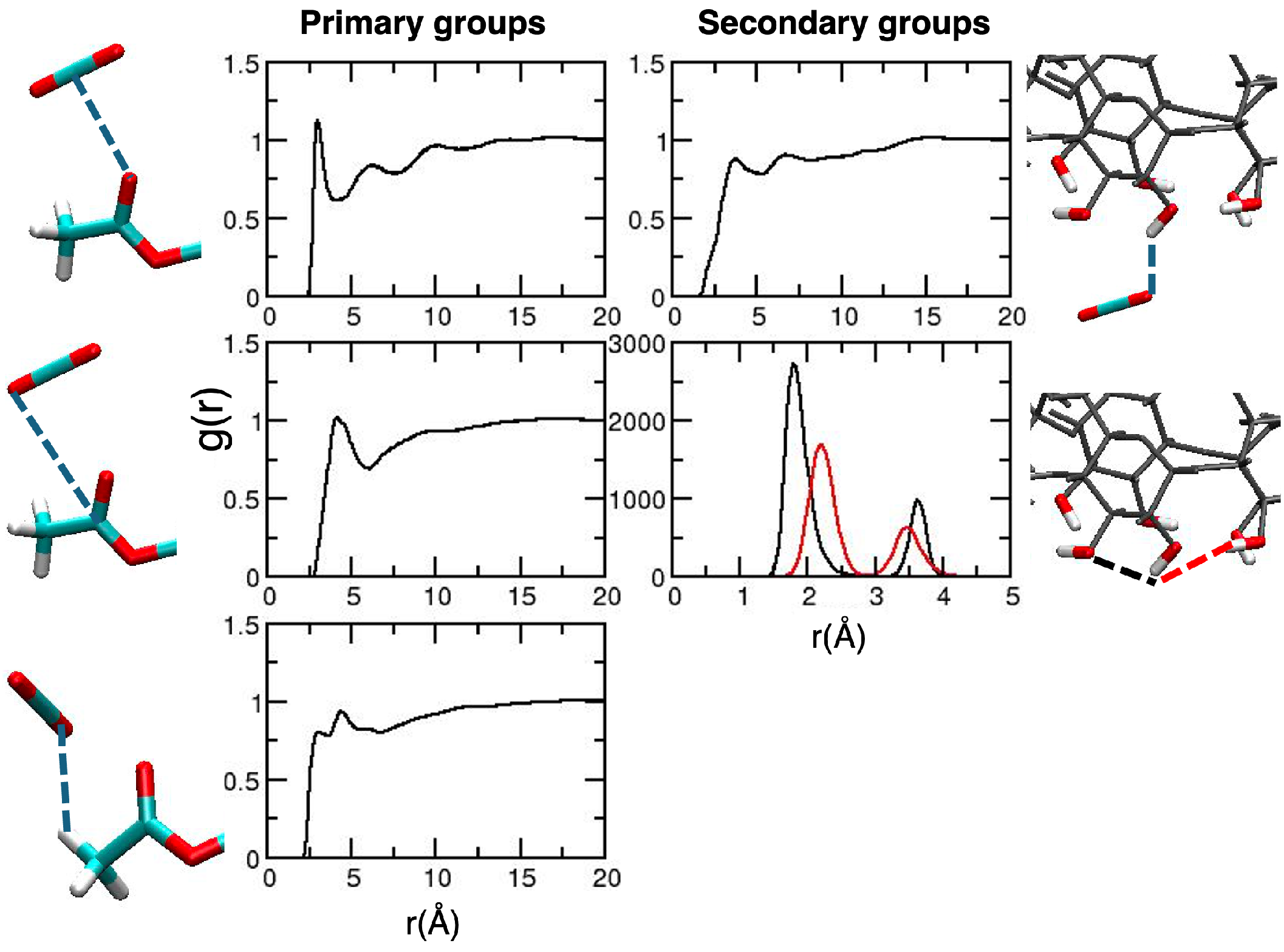
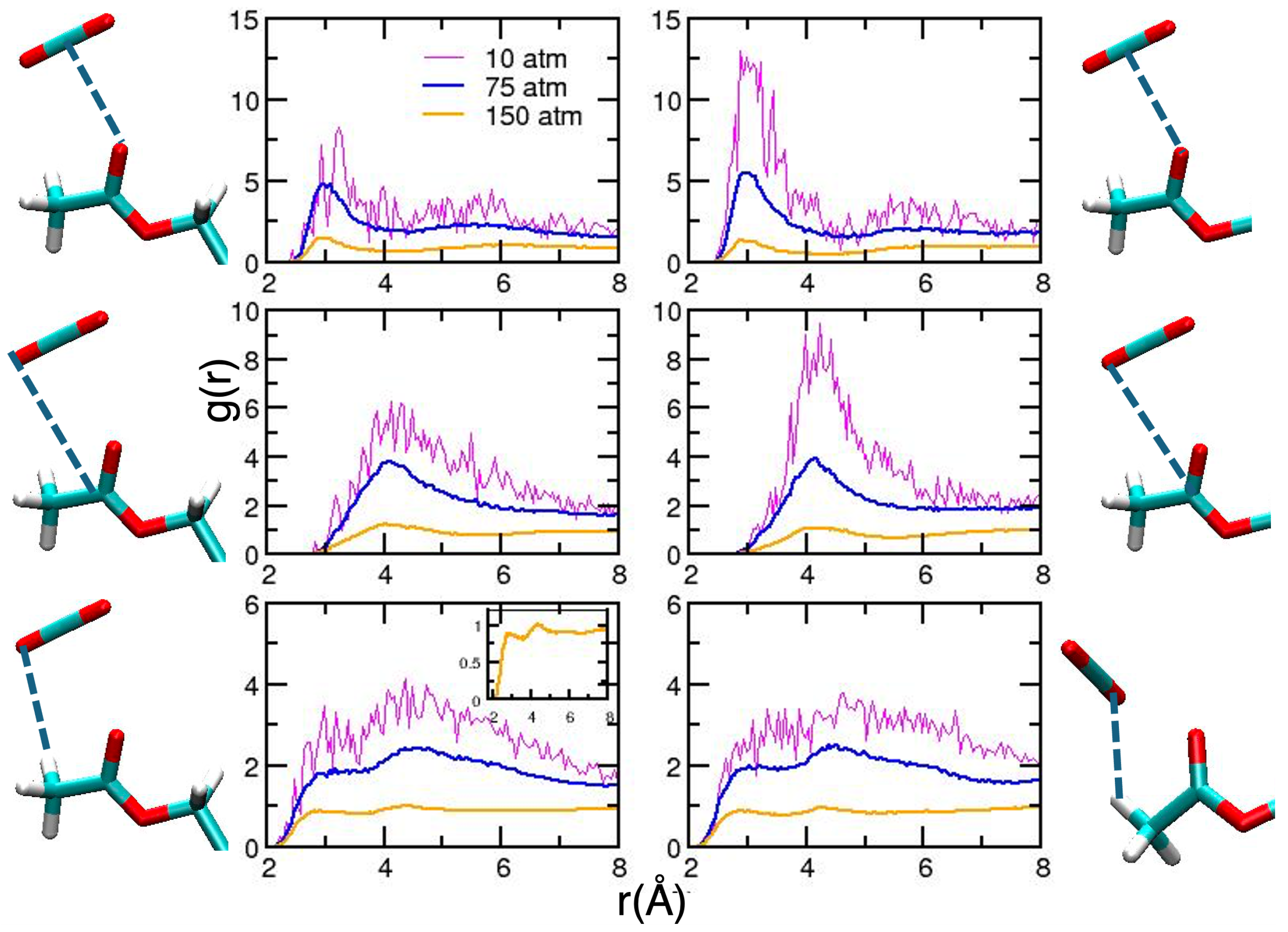
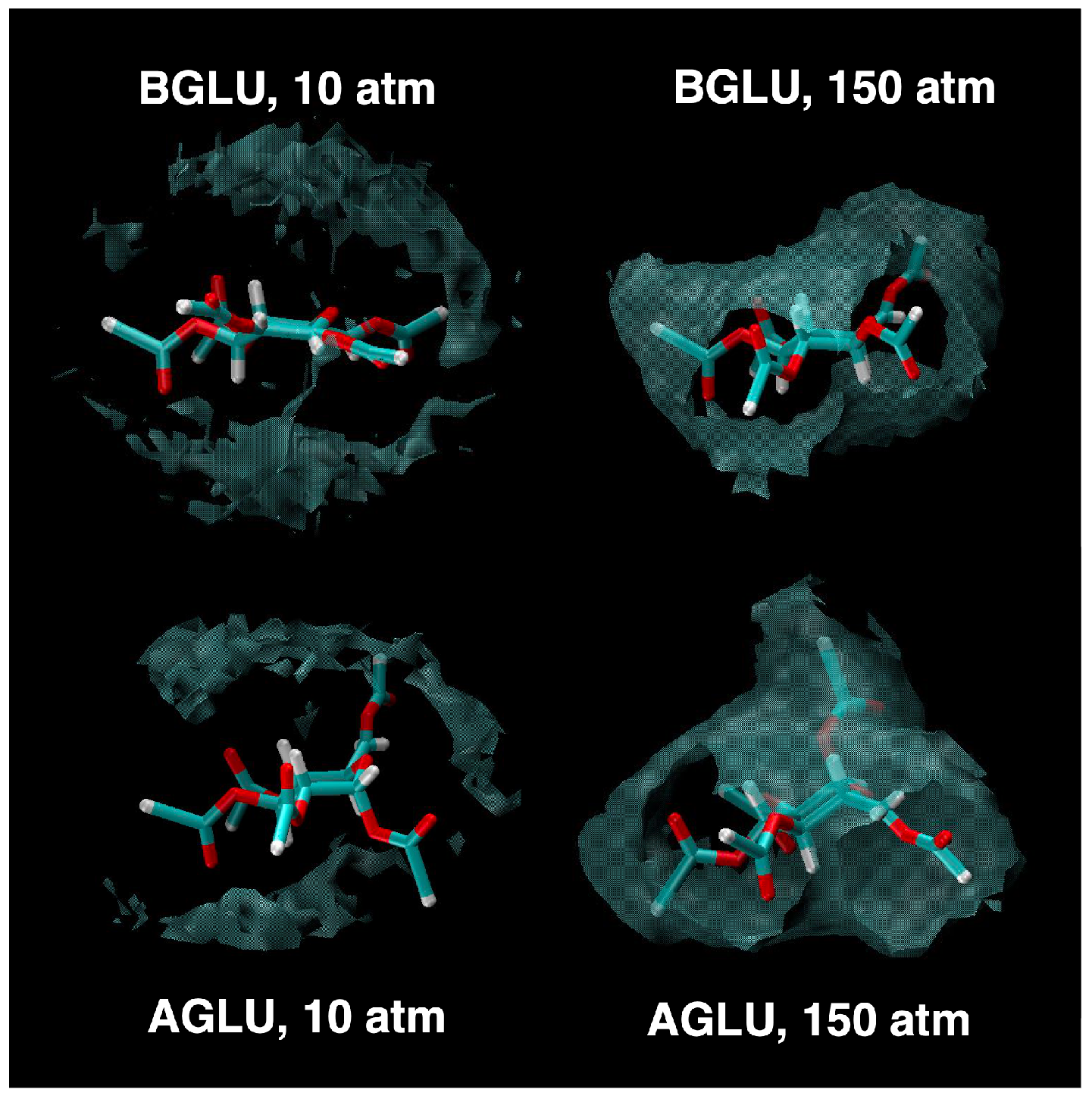
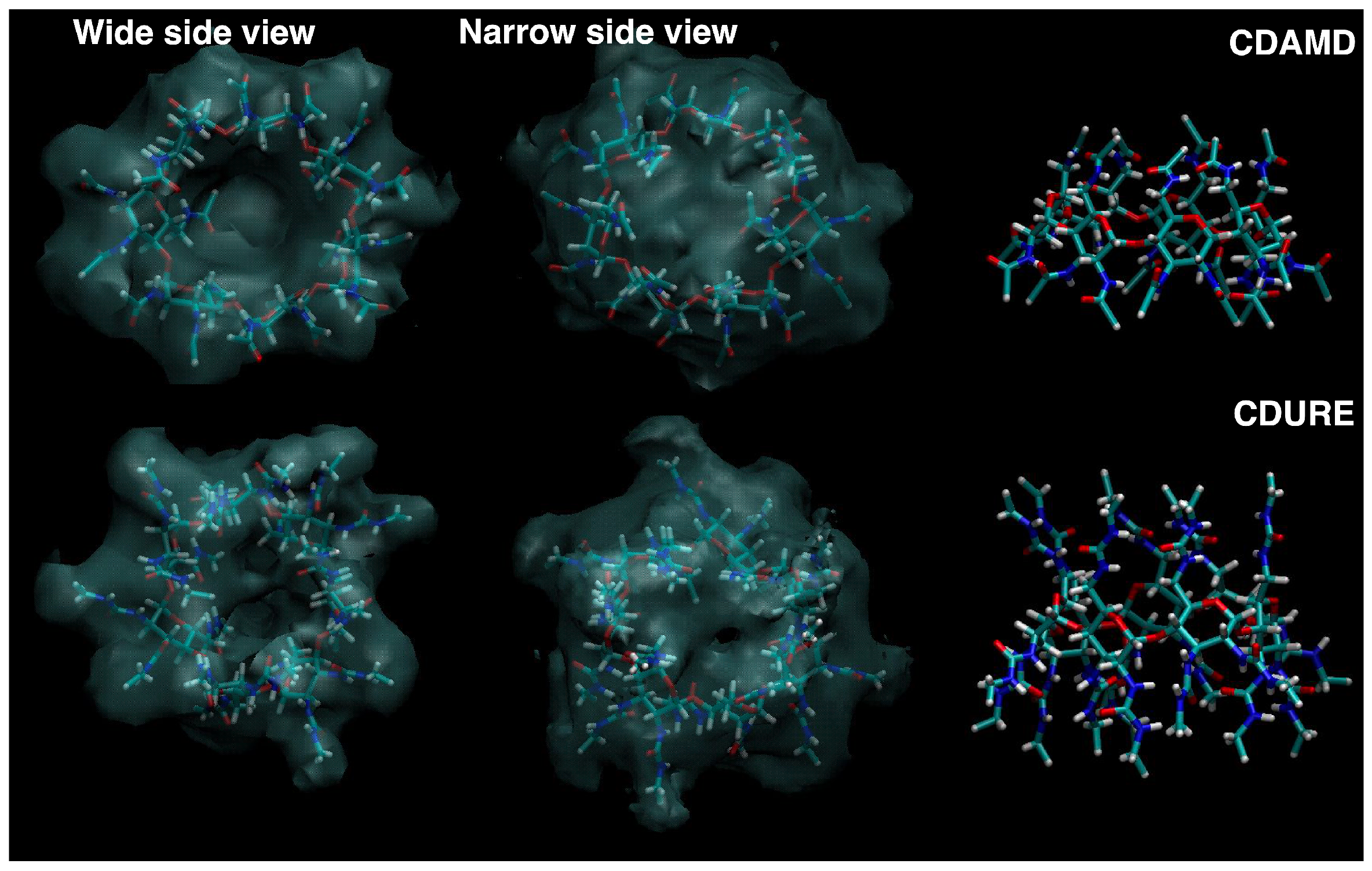
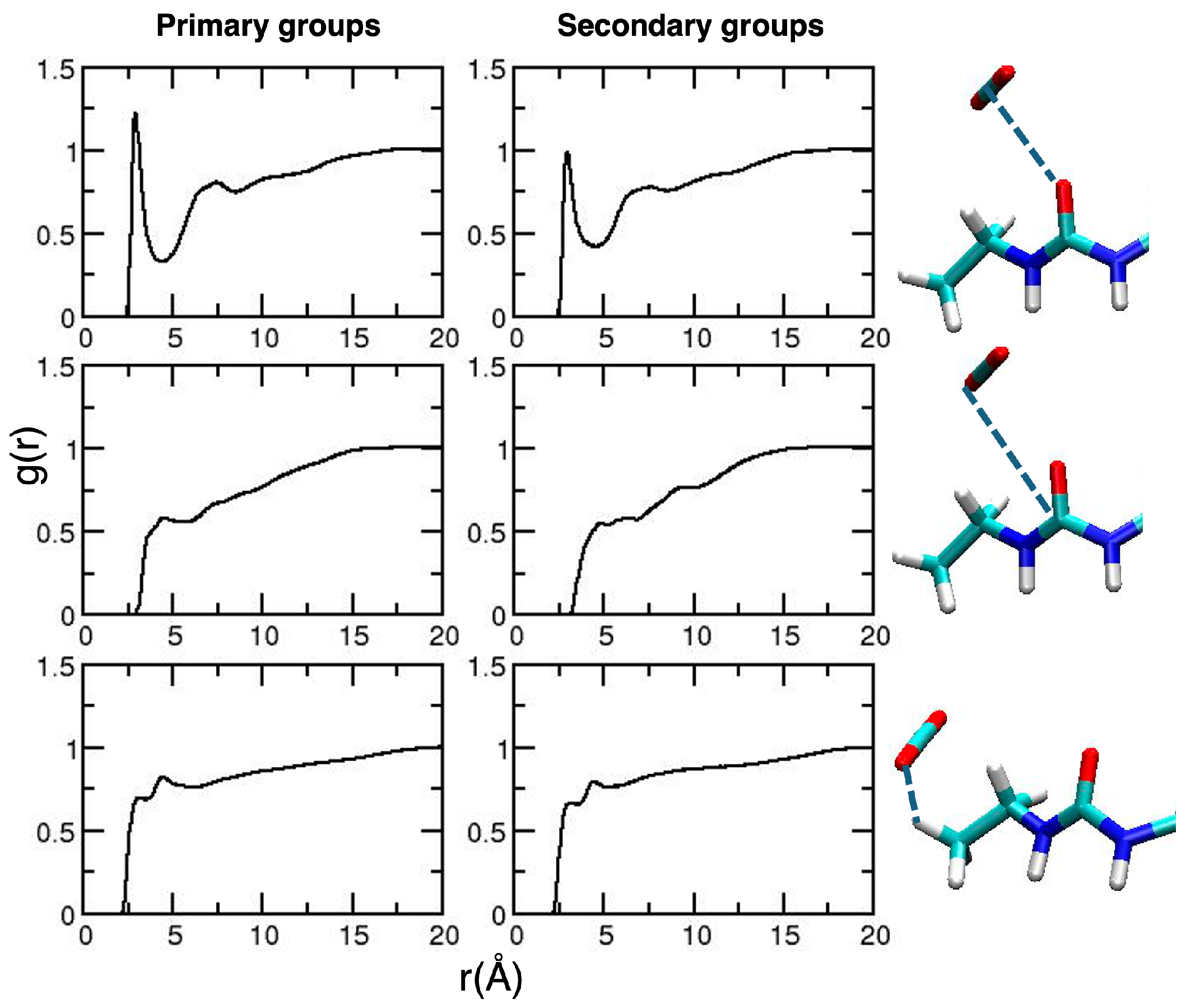
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Goos, E.; Riedel, U.; Zhao, L.; Blum, L. Phase diagrams of CO2 and CO2–N2 gas mixtures and their application in compression processes. Energy Procedia 2011, 4, 3778–3785. [Google Scholar] [CrossRef]
- Eckert, C.; Knutson, B.; Debenedetti, P. Supercritical fluids as solvents for chemical and materials processing. Nature 1996, 383, 313–318. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 1878–1895. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.P.; Foster, N.R. Solubilities of solid mixtures in supercritical carbon dioxide: A review. J. Supercrit. Fluids 2000, 17, 111–134. [Google Scholar] [CrossRef]
- Harada, K.H.; Koizumi, A. Environmental and biological monitoring of persistent fluorinated compounds in Japan and their toxicities. Environ. Health Prev. Med. 2009, 14, 7–19. [Google Scholar] [CrossRef]
- Nelson, M.R.; Borkman, R.F. Ab Initio Calculations on CO2 Binding to Carbonyl Groups. J. Phys. Chem. A 1998, 102, 7860–7863. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Ingrosso, F.; Ruiz-López, M.F. Modeling Solvation in Supercritical CO2. ChemPhysChem 2017, 18, 2560–2572. [Google Scholar] [CrossRef]
- Raveendran, P.; Wallen, S.L. Sugar Acetates as Novel, Renewable CO2-philes. J. Am. Chem. Soc. 2002, 124, 7274–7275. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Blatchford, M.A.; Hurrey, M.L.; White, P.S.; Wallen, S.L. Crystallization and processing of carbohydrates using carbon dioxide. Green Chem. 2005, 7, 129–131. [Google Scholar] [CrossRef]
- Potluri, V.K.; Hamilton, A.D.; Karanikas, C.F.; Bane, S.E.; Xu, J.; Beckman, E.J.; Enick, R.M. The high CO2-solubility of per-acetylated α-, β-, and γ-cyclodextrin. Fluid Phase Equilibria 2003, 211, 211–217. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Hu, D.; Sun, S.; Yuan, P.Q.; Zhao, L.; Liu, T. Exploration of CO2-Philicity of Poly(vinyl acetate-co-alkyl vinyl ether) through Molecular Modeling and Dissolution Behavior Measurement. J. Phys. Chem. B 2015, 119, 12490–12501. [Google Scholar] [CrossRef]
- Gong, H.; Gui, W.; Zhang, H.; Lv, W.; Xu, L.; Li, Y.; Dong, M. Molecular dynamics study on the dissolution behaviors of poly(vinyl acetate)-polyether block copolymers in supercritical CO. J. Appl. Polym. Sci. 2021, 138, 50151. [Google Scholar] [CrossRef]
- Ivanova, G.I.; Vão, E.R.; Temtem, M.; Aguiar-Ricardo, A.; Casimiro, T.; Cabrita, E.J. High-pressure NMR characterization of triacetyl-beta-cyclodextrin in supercritical carbon dioxide. Magn. Reson. Chem. 2009, 47, 133–141. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Filardo, G.; Blasi, M.D.; Galia, A.; Ponchel, A.; Bricout, H.; Sayede, A.; Monflier, E. Peracetylated β-cyclodextrin as solubilizer of arylphosphines in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 36, 173–181. [Google Scholar] [CrossRef]
- Ganapathy, H.S.; Woo, M.H.; Gal, Y.S.; Lim, K.T. Inclusion Complex Formation of Water- Soluble Drug, Captopril, and Peracetylated-β-Cyclodextrin in Supercritical CO2 for Controlled Release Applications. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2007; Volume 342, pp. 489–492. [Google Scholar] [CrossRef]
- Young Lee, M.; Subban Ganapathy, H.; Taek Lim, K. Controlled drug release applications of the inclusion complex of peracetylated-β-cyclodextrin and water-soluble drugs formed in supercritical carbon dioxide. J. Phys. Chem. Solids 2010, 71, 630–633. [Google Scholar] [CrossRef]
- Galia, A.; Navarre, E.C.; Scialdone, O.; Filardo, G.; Monflier, E. Complexation of phosphine ligands with peracetylated-β-cyclodextrin in supercritical carbon dioxide: Effect of temperature and cosolvent on the equilibrium constant. J. Supercrit. Fluids 2009, 49, 154–160. [Google Scholar] [CrossRef]
- Ingrosso, F.; Altarsha, M.; Dumarçay, F.; Kevern, G.; Barth, D.; Marsura, A.; Ruiz-López, M.F. Driving forces controlling host–guest recognition in supercritical carbon dioxide solvent. Chem. Eur. J. 2016, 22, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.A.; Su, R.; Wang, J.W.; Fan, Y.; Jiang, L.; Li, X.; Cao, J. Synthesis and characterization of a host‚Äìguest complex based on acetylated-β-cyclodextrin and its application in improving the viscosity of supercritical carbon dioxide. Fuel 2024, 363, 130837. [Google Scholar] [CrossRef]
- Demirbaş, A. Supercritical fluid extraction and chemicals from biomass with supercritical fluids. Energy Convers. Manag. 2001, 42, 279–294. [Google Scholar] [CrossRef]
- Arumugham, T.; AlYammahi, J.; Rambabu, K.; Hassan, S.W.; Banat, F. Supercritical CO2 pretreatment of date fruit biomass for enhanced recovery of fruit sugars. Sustain. Energy Technol. Assess. 2022, 52, 102231. [Google Scholar] [CrossRef]
- Bushnaq, H.; Krishnamoorthy, R.; Abu-Zahra, M.; Hasan, S.W.; Taher, H.; Alomar, S.Y.; Ahmad, N.; Banat, F. Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation. Processes 2022, 10, 257. [Google Scholar] [CrossRef]
- Prado, J.M.; Lachos-Perez, D.; Forster-Carneiro, T.; Rostagno, M.A. Sub- and supercritical water hydrolysis of agricultural and food industry residues for the production of fermentable sugars: A review. Food Bioprod. Process. 2016, 98, 95–123. [Google Scholar] [CrossRef]
- Moreira, B.P.; Draszewski, C.P.; Rosa, N.C.; Tres, M.V.; Zabot, G.L.; Pereira, F.C.; Abaide, E.R.; Castilhos, F. Integrated rice bran processing by supercritical CO2 extraction and subcritical water hydrolysis to obtain oil, fermentable sugars, and platform chemicals. J. Supercrit. Fluids 2023, 192, 105786. [Google Scholar] [CrossRef]
- Gong, T.; Liu, S.; Wang, H.; Zhang, M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, J.; Lee, H.S.; Wulandari, S.; Murali, V.; Kim, J.; Park, Y.K.; Ha, J.M.; Jae, J. Highly efficient hydrolysis of cellulose to sugars using supercritical CO2 as a green acid catalyst and solvent. Chem. Eng. J. 2024, 493, 152336. [Google Scholar] [CrossRef]
- Alinia, R.; Zabihi, S.; Esmaeilzadeh, F.; Kalajahi, J.F. Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for sugar production. Biosyst. Eng. 2010, 107, 61–66. [Google Scholar] [CrossRef]
- Haines, A.H.; Steytler, D.C.; Rivett, C. Solubility dependence of peracylated d-glucopyranoses in supercritical carbon dioxide on the structure of their acyl moieties. J. Supercrit. Fluids 2008, 44, 21–24. [Google Scholar] [CrossRef]
- Kobayashi, K.; Firoozabadi, A. Effect of Branching on Mutual Solubility of Alkane-CO2 Systems by Molecular Simulations. J. Phys. Chem. B 2022, 126, 8300–8308. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.H.; Tapriyal, D.; Enick, R.M.; Hamilton, A.D. Fiber Formation by Highly CO2-Soluble Bisureas Containing Peracetylated Carbohydrate Groups. Angew. Chem. Int. Ed. 2007, 46, 3284–3287. [Google Scholar] [CrossRef]
- Menuel, S.; Wagner, M.; Barth, D.; Marsura, A. Supercritical CO2 improved phosphine imide reaction on peracetylated β-cyclodextrin. Tetrahedron Lett. 2005, 46, 3307–3309. [Google Scholar] [CrossRef]
- Nasri, S.; Lestoquoy, M.; Ponchel, A.; Monflier, E.; Menuel, S. Mechanochemical synthesis of β-cyclodextrin urea derivatives under reactive CO2 atmosphere by Staudinger aza-Wittig reaction. RSC Mechanochem. 2024, 1, 228–234. [Google Scholar] [CrossRef]
- Raveendran, P.; Wallen, S.L. Cooperative C–H⋯O Hydrogen Bonding in CO2: Lewis Base Complexes: Implications for Solvation in Supercritical CO2. J. Am. Chem. Soc. 2002, 124, 12590–12599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Hao, J.; Fujita, S.-i.; Arai, M.; Wu, Z.; Zhao, F. Theoretical study on interaction between CO2 and carbonyl compounds: Influence of CO2 on infrared spectroscopy and activity of CO. J. Supercrit. Fluids 2010, 54, 9–15. [Google Scholar] [CrossRef]
- Chang, H.; Yang, C.; Li, X.; Gao, W.; Wei, W.; Liu, G.; Pang, X.; Qiao, Y. Ab initio analysis of the interaction of CO2 with acetylated d-glucopyranose derivatives. C. R. Chim. 2015, 18, 935–944. [Google Scholar] [CrossRef]
- Liu, J.F.; Yang, H.J.; Wang, W.; Li, Z. Solubilities of Amide Compounds in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2008, 53, 2189–2192. [Google Scholar] [CrossRef]
- Coelho, J.P.; Naydenov, G.P.; Yankov, D.S.; Stateva, R.P. Experimental Measurements and Correlation of the Solubility of Three Primary Amides in Supercritical CO2: Acetanilide, Propanamide, and Butanamide. J. Chem. Eng. Data 2013, 58, 2110–2115. [Google Scholar] [CrossRef]
- Cooper, A.I. Recent Developments in Materials Synthesis and Processing Using Supercritical CO2. Adv. Mat. 2001, 13, 1111–1114. [Google Scholar] [CrossRef]
- Azofra, L.M.; Altarsha, M.; Ruiz-López, M.F.; Ingrosso, F. A theoretical investigation of the CO2-philicity of amides and carbamides. In Proceedings of the 8th Congress on Electronic Structure: Principles and Applications (ESPA 2012): A Conference Selection from Theoretical Chemistry Accounts, Barcelona, Spain, 26–29 June 2012; Novoa, J.J., Ruiz López, M.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 33–41. [Google Scholar] [CrossRef]
- Altarsha, M.; Ingrosso, F.; Ruiz-Lopez, M.F. A New Glimpse into the CO2-Philicity of Carbonyl Compounds. ChemPhysChem 2012, 13, 3397–3403. [Google Scholar] [CrossRef]
- Skarmoutsos, I.; Samios, J. Solvation structure and dynamics of favipiravir in supercritical CO2. A molecular dynamics investigation. J. Mol. Liq. 2023, 392, 123512. [Google Scholar] [CrossRef]
- Cézard, C.; Trivelli, X.; Aubry, F.; Djedaïni-Pilard, F.; Dupradeau, F.Y. Molecular dynamics studies of native and substituted cyclodextrins in different media: 1. Charge derivation and force field performances. Phys. Chem. Chem. Phys. 2011, 13, 15103–15121. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Gubica, T. Application of Molecular Dynamics Simulations in the Analysis of Cyclodextrin Complexes. Int. J. Mol. Sci. 2021, 22, 9422. [Google Scholar] [CrossRef]
- Altarsha, M.; Ingrosso, F.; Ruiz-Lopez, M.F. Cavity Closure Dynamics of Peracetylated β-Cyclodextrins in Supercritical Carbon Dioxide. J. Phys. Chem. B 2012, 116, 3982–3990. [Google Scholar] [CrossRef]
- Antipova, M.L.; Odintsova, E.G.; Petrenko, V.E. Behavior of β-cyclodextrin/naproxen inclusion complex in supercritical carbon dioxide. Computer simulation. J. Mol. Liq. 2024, 407, 125234. [Google Scholar] [CrossRef]
- Maia-Obi, L.P.; Vidinha, P.; Ferraz, H.G.; Bazito, R.C. Non-Inclusion Complexation of Peracetylated β-Cyclodextrin with Ibuprofen in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2021, 169, 105098. [Google Scholar] [CrossRef]
- Harata, K. X-Ray crystal structure of the permethylated β-cyclodextrin complex with m-iodophenol. A stabilized skew-boat pyranose conformation in the distorted macrocyclic ring. J. Chem. Soc. Chem. Commun. 1988, 928–929. [Google Scholar] [CrossRef]
- Huerta, E.H.; Gutiérrez-Flores, J.; Gómez-Balderas, R.; Garza, J.; Ramos, E.; Vargas, R. Conformations of α-cyclodextrin and its role on the stability of inclusion complexes in aqueous solution. J. Mol. Liq. 2024, 414, 126267. [Google Scholar] [CrossRef]
- Suárez, D.; Díaz, N. Conformational and entropy analyses of extended molecular dynamics simulations of α-, β- and γ-cyclodextrins and of the β-cyclodextrin/nabumetone complex. Phys. Chem. Chem. Phys. 2017, 19, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. The C-H⋯O hydrogen bond: Structural implications and supramolecular design. Acc. Chem. Res. 1996, 29, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Yu, Z.; Batista, E.R.; Yang, P.; Perez, D. Acceleration of Solvation Free Energy Calculation via Thermodynamic Integration Coupled with Gaussian Process Regression and Improved Gelman-Rubin Convergence Diagnostics. J. Chem. Theor. Comput. 2024, 20, 2570–2581. [Google Scholar] [CrossRef]
- Case, D.; Betz, R.; Cerutti, D.; Cheatham, T., III; Darden, T.; Duke, R.; Giese, T.; Gohlke, H.; Goetz, A.; Homeyer, N.; et al. Amber16; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Basma, M.; Sundara, S.; Calgan, D.; Vernali, T.; Woods, R.J. Solvated ensemble averaging in the calculation of partial atomic charges. J. Comput. Chem. 2001, 22, 1125–1137. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Woods, R.J. Solvent interactions determine carbohydrate conformation. Proc. Natl. Acad. Sci. USA 2001, 98, 10541–10545. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Woods, R.J. Quantum Mechanical Study of the Nonbonded Forces in Water–Methanol Complexes. J. Phys. Chem. A 2001, 105, 4150–4155. [Google Scholar] [CrossRef][Green Version]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins Struct. Funct. Bioinform. 2006, 65, 712–725. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve And Critical Properties as Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]



| Interaction Energy (kcal/mol) | |
|---|---|
| CDACD | −174.63 ± 28 |
| CDAMD | −191.91 ± 40 |
| CDURE | −264.63 ± 59 |
| AGLU (150 atm) | −50.00 ± 16 |
| BGLU (150 atm) | −40.47 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, A.; Ingrosso, F. A Molecular Dynamics Study of the Solvation Properties of Sugars in Supercritical Carbon Dioxide. Molecules 2025, 30, 1256. https://doi.org/10.3390/molecules30061256
Lambert A, Ingrosso F. A Molecular Dynamics Study of the Solvation Properties of Sugars in Supercritical Carbon Dioxide. Molecules. 2025; 30(6):1256. https://doi.org/10.3390/molecules30061256
Chicago/Turabian StyleLambert, Alexandrine, and Francesca Ingrosso. 2025. "A Molecular Dynamics Study of the Solvation Properties of Sugars in Supercritical Carbon Dioxide" Molecules 30, no. 6: 1256. https://doi.org/10.3390/molecules30061256
APA StyleLambert, A., & Ingrosso, F. (2025). A Molecular Dynamics Study of the Solvation Properties of Sugars in Supercritical Carbon Dioxide. Molecules, 30(6), 1256. https://doi.org/10.3390/molecules30061256







