Role of Furfural and 5-Methyl-2-furfural in Glucose-Induced Inhibition of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Formation in Chemical Models and Pork Patties
Abstract
1. Introduction
2. Results and Discussion
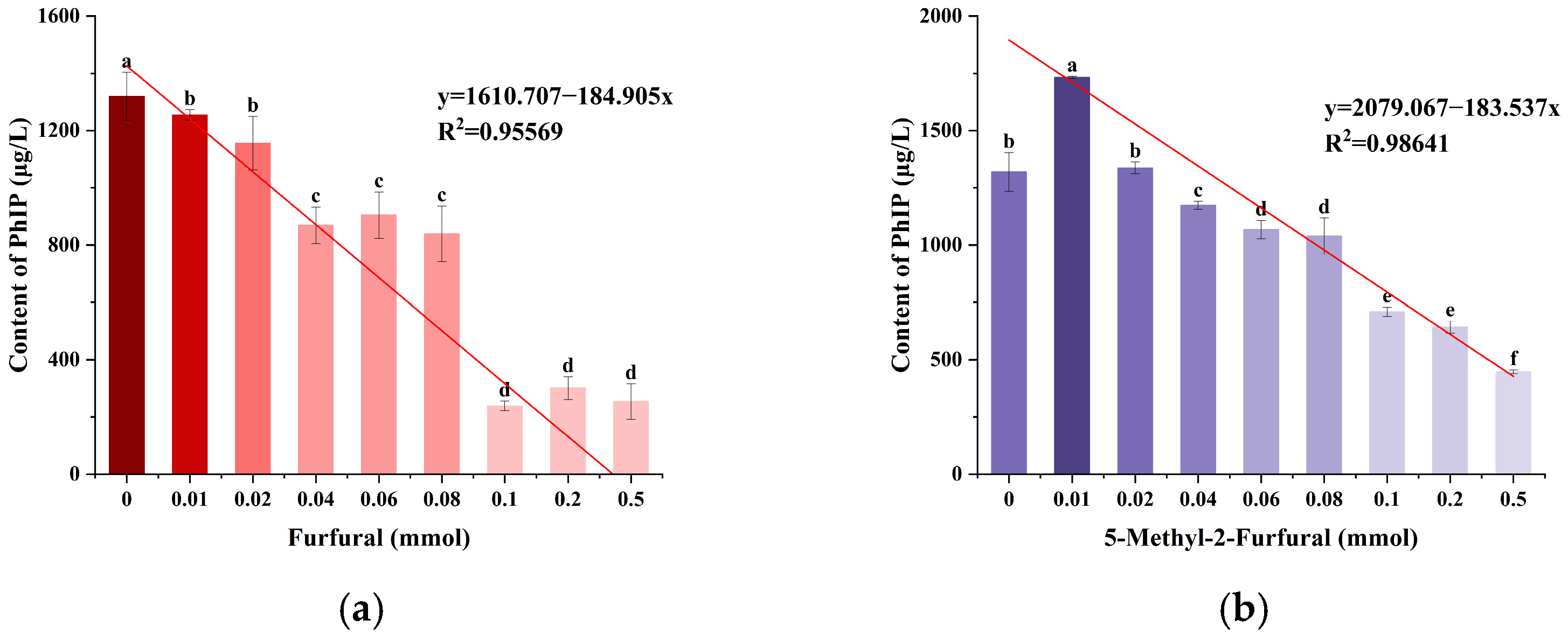
2.1. Effects of Furfural and 5-Methyl-2-furfural on the Formation of PhIP in the Chemical Models
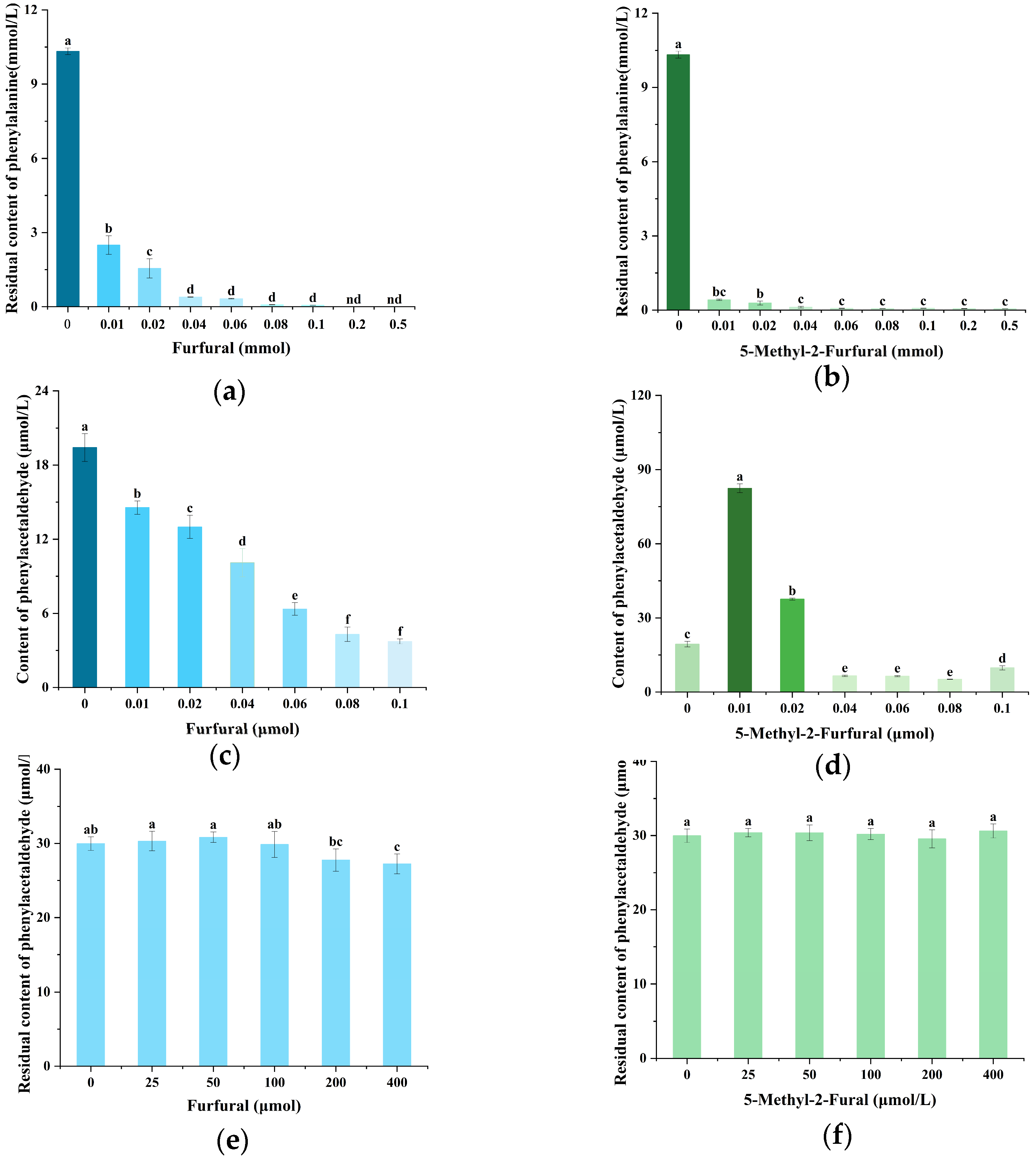
2.2. Effects of Furfural and 5-Methyl-2-Furfural on PhIP and Its Precursors in the Chemical Models
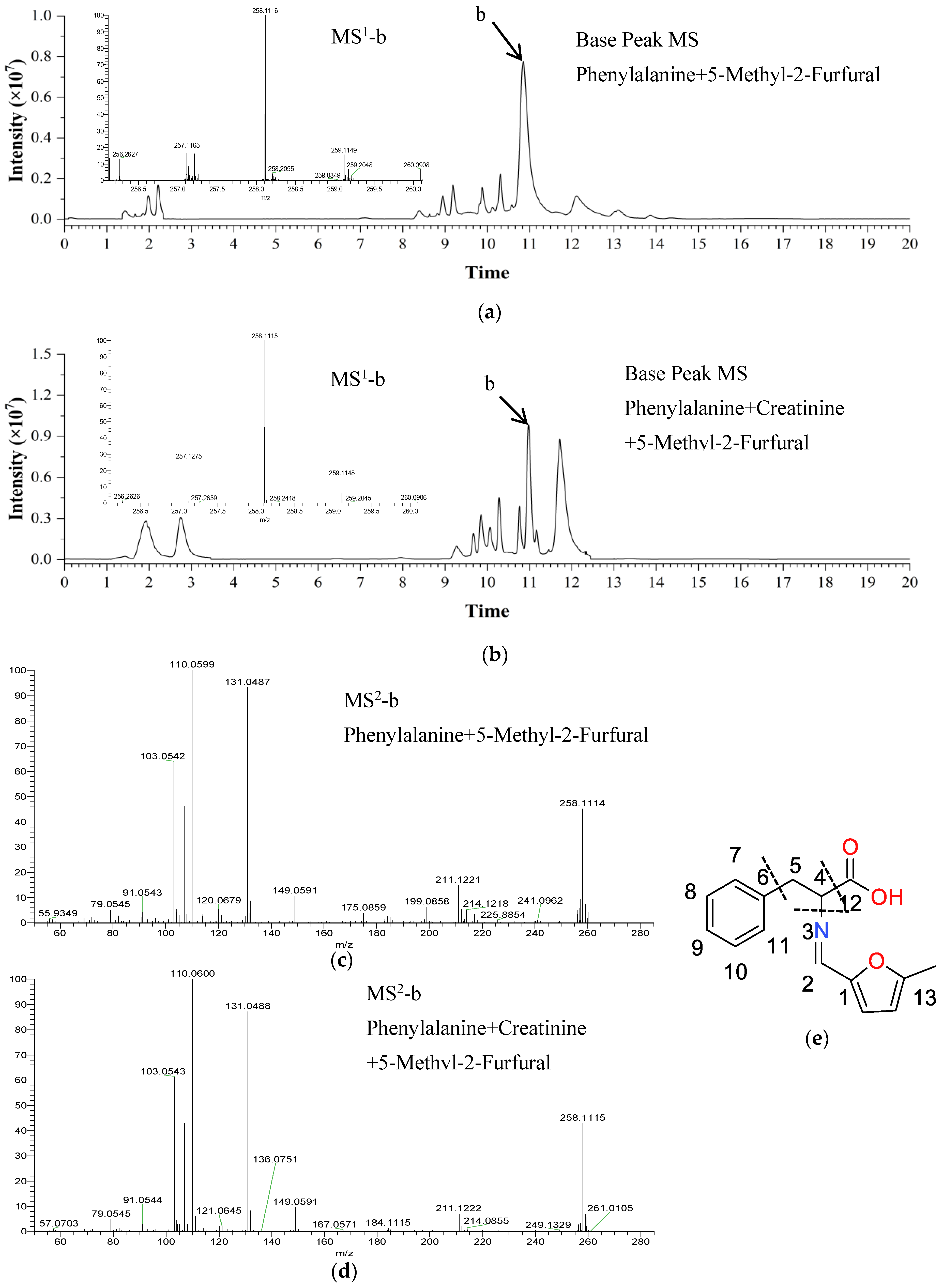
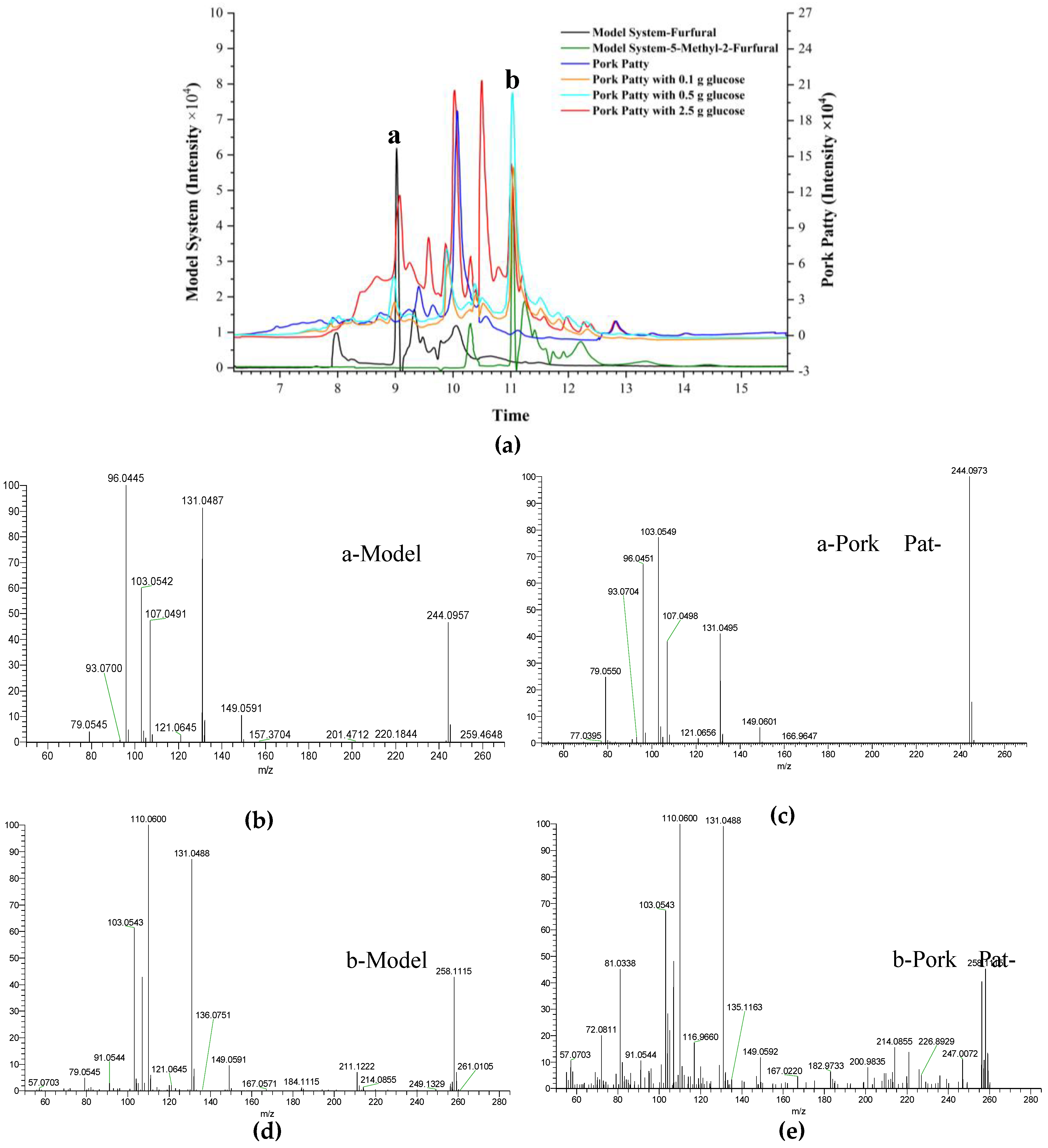
2.3. Identification of Reaction Products in Chemical Models
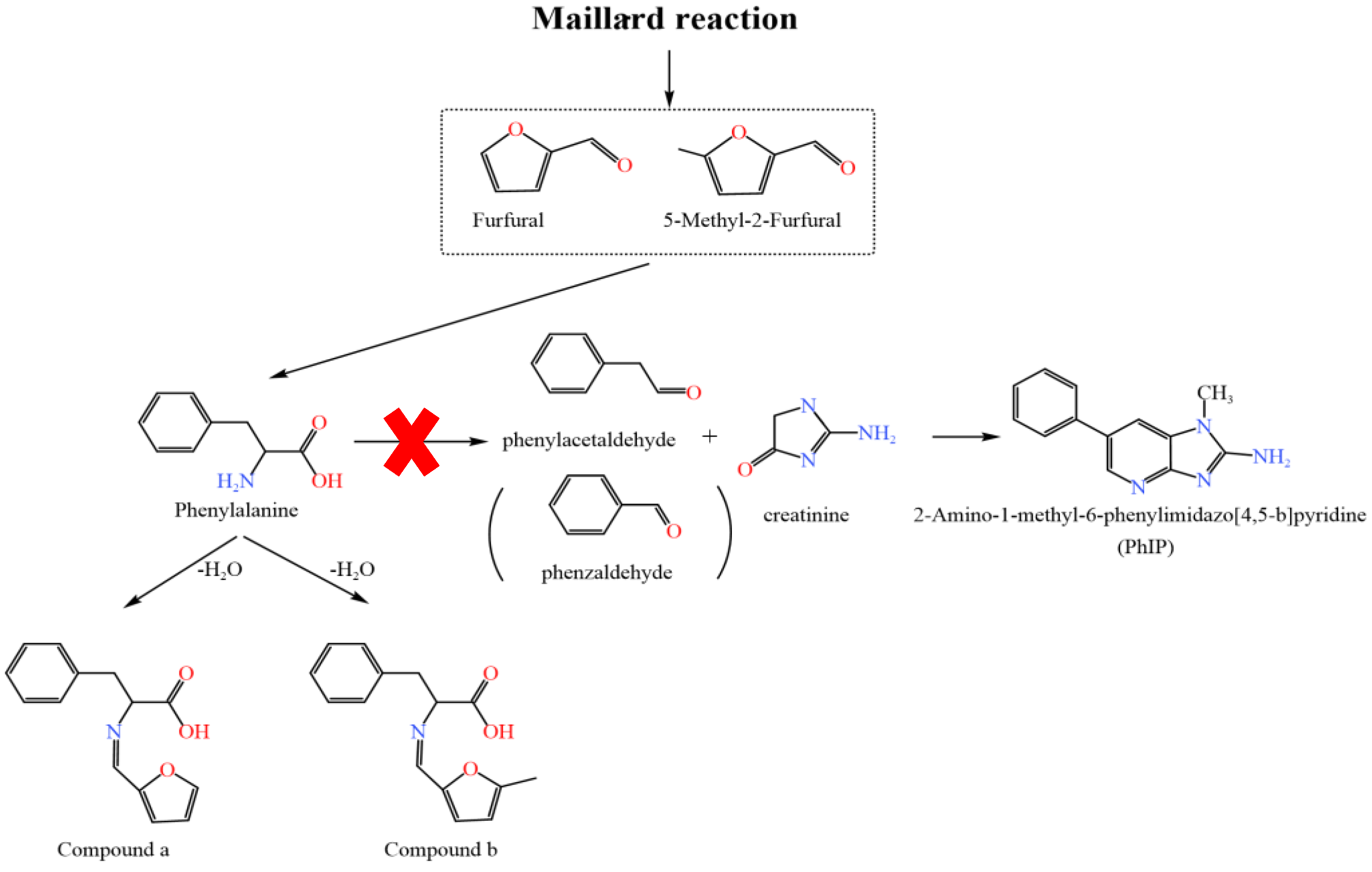
2.4. Reaction Pathways of Furfural and 5-Methyl-2-Furfural Affecting the Formation of PhIP
2.5. Verification of the Reaction Pathway in Roasted Pork Patties
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Model Systems
3.3. HPLC Analysis of Furfural and 5-Methyl-2-furfural
3.4. LC-MS/MS Analysis of PhIP
3.5. HPLC Analysis of Creatinine
3.6. HPLC Analysis of Phe
3.7. GC-MS Analysis of Phenylacetaldehyde
3.8. UPLC-HRMS Separation and Identification of Reactants
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; Bekhit, A.E.-D.; Biancolillo, A.; Wold, J.P. Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Appl. Sci. 2020, 10, 6802. [Google Scholar] [CrossRef]
- Xiong, K.; Li, M.-M.; Chen, Y.-Q.; Hu, Y.-M.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Prot. 2024, 87, 100338–100356. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Q.; Xu, Y.; Li, C.; Bai, W.; Zeng, X.; Wu, Q.; Xu, H.; Deng, J. Effect and mechanism of polyphenols containing m-dihydroxyl structure on 2-amino-1-methyl-6-phenylimidazole [4,5-b] pyridine (PhIP) formation in chemical models and roast pork patties. Food Chem. X 2024, 23, 101672–101685. [Google Scholar] [CrossRef]
- Wang, H.; Chu, X.; Du, P.; He, H.; He, F.; Liu, Y.; Wang, W.; Ma, Y.; Wen, L.; Wang, Y.; et al. Unveiling heterocyclic aromatic amines (HAAs) in thermally processed meat products: Formation, toxicity, and strategies for reduction—A comprehensive review. Food Chem. X 2023, 19, 100833–100850. [Google Scholar] [CrossRef] [PubMed]
- Bula, M.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K. Formation of heterocyclic aromatic amines in relation to pork quality and heat treatment parameters. Food Chem. 2019, 276, 511–519. [Google Scholar] [CrossRef]
- Jeong, K.J.; Seo, J.K.; Ahamed, Z.; Su Lee, Y.; Yang, H.S. Paprika extract as a natural antioxidant in cold-stored pork patties: Effect on oxidative stability and heterocyclic amines inhibition. Food Chem. X 2023, 20, 100936–100945. [Google Scholar] [CrossRef] [PubMed]
- Elbir, Z.; Oz, F. Determination of creatine, creatinine, free amino acid and heterocyclic aromatic amine contents of plain beef and chicken juices. J. Food Sci. Technol. 2020, 58, 3293–3302. [Google Scholar] [CrossRef]
- Shin, H.S.; Strasburg, G.M.; Ustunol, Z. Influence of Different Unifloral Honeys on Heterocyclic Aromatic Amine Formation and Overall Mutagenicity in Fried Ground-beef Patties. J. Food Sci. 2003, 68, 810–815. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Chu, J.; Sun, C.; Lin, S. Vegetable extracts: Effective inhibitors of heterocyclic aromatic amines and advanced glycation end products in roasted Mackerel. Food Chem. 2023, 412, 135559–135568. [Google Scholar] [CrossRef]
- Jing, M.L.; Jiang, Q.Q.; Zhu, Y.M.; Fan, D.M.; Wang, M.F.; Zhao, Y.L. Effect of acrolein, a lipid oxidation product, on the formation of the heterocyclic aromatic amine 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in model systems and roasted tilapia fish patties. Food Chem. X 2022, 14, 100315–100322. [Google Scholar] [CrossRef]
- Farshi, P.; Amamcharla, J.; Getty, K.; Smith, J.S. Effect of Immersion Time of Chicken Breast in Potato Starch Coating Containing Lysine on PhIP Levels. Foods 2024, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Hölzl-Armstrong, L.; Moody, S.; Kucab, J.E.; Zwart, E.P.; Bellamri, M.; Luijten, M.; Turesky, R.J.; Stratton, M.R.; Arlt, V.M.; Phillips, D.H. Mutagenicity of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (N–OH-PhIP) in human TP53 knock-in (Hupki) mouse embryo fibroblasts. Food Chem. Toxicol. 2021, 147, 111855–111868. [Google Scholar] [CrossRef]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W.; Schilter, B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef]
- Kimura, S.; Kawabe, M.; Yu, A.; Morishima, H.; Fernandez-Salguero, P.; Hammons, G.; Ward, J.; Kadlubar, F.; Gonzalez, F. Carcinogenesis of the food mutagen PhIP in mice is independent of CYP1A2. Carcinogenesis 2003, 24, 583–587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reng, Q.; Zhu, L.L.; Feng, L.; Li, Y.J.; Zhu, Y.X.; Wang, T.T.; Jiang, F. Dietary meat mutagens intake and cancer risk: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Consumption of Thermally Processed Meat Containing Carcinogenic Compounds (Polycyclic Aromatic Hydrocarbons and Heterocyclic Aromatic Amines) versus a Risk of Some Cancers in Humans and the Possibility of Reducing Their Formation by Natural Food Additives—A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 4781. [Google Scholar] [CrossRef] [PubMed]
- Prasath, S.T.; Navaneethan, C. Systematic analysis of the role of different foods on breast, lung, and prostate cancer incidence. Food Chem. Adv. 2024, 4, 100733–100741. [Google Scholar] [CrossRef]
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2012, 20, 150–160. [Google Scholar] [CrossRef]
- Oz, F.; Kaya, M. Heterocyclic Aromatic Amines in Meat. J. Food Process Preserv. 2011, 35, 739–753. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Kim, J.H.; Kim, H.W.; Oh, D.H.; Jeong, J.W.; Hur, S.J. Main mechanisms for carcinogenic heterocyclic amine reduction in cooked meat by natural materials. Meat Sci. 2022, 183, 108663. [Google Scholar] [CrossRef]
- Guo, J.; Koopmeiners, J.S.; Walmsley, S.J.; Villalta, P.W.; Yao, L.; Murugan, P.; Tejpaul, R.; Weight, C.J.; Turesky, R.J. The Cooked Meat Carcinogen 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine Hair Dosimeter, DNA Adductomics Discovery, and Associations with Prostate Cancer Pathology Biomarkers. Chem. Res. Toxicol. 2022, 35, 703–730. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Cooked Meat Products: A Review. Polycycl. Aromat. Compd. 2018, 40, 1557–1567. [Google Scholar] [CrossRef]
- Murkovic, M.; Weber, H.-J.; Geiszler, S.; Frohlich, K.; Pfannhauser, W. Formation of the food associated carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in model systems. Food Chem. 1999, 65, 233–237. [Google Scholar] [CrossRef]
- Deng, P.; Xue, C.; He, Z.; Wang, Z.; Qin, F.; Oz, E.; Chen, J.; El Sheikha, A.F.; Proestos, C.; Oz, F.; et al. Synergistic Inhibitory Effects of Selected Amino Acids on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in both Benzaldehyde– and Phenylacetaldehyde–Creatinine Model Systems. J. Agric. Food. Chem. 2022, 70, 10858–10871. [Google Scholar] [CrossRef]
- Lai, Y.W.; Lee, Y.T.; Cao, H.; Zhang, H.L.; Chen, B.H. Extraction of heterocyclic amines and polycyclic aromatic hydrocarbons from pork jerky and the effect of flavoring on formation and inhibition. Food Chem. 2023, 402, 134291. [Google Scholar] [CrossRef] [PubMed]
- Hasnol, N.D.; Jinap, S.; Sanny, M. Effect of different types of sugars in a marinating formulation on the formation of heterocyclic amines in grilled chicken. Food Chem. 2014, 145, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Q.; Wang, Z.; Chen, Q.; Sun, F.; Xu, M.; Kong, B. Influence of different ratios of sucrose and green tea leaves on heterocyclic aromatic amine formation and quality characteristics of smoked chicken drumsticks. Food Control 2022, 133, 108613. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic Aromatic Amines in Cooked Meat Products: Causes, Formation, Occurrence, and Risk Assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Skog, K.; Jägerstad, M. Effects of glucose on the formation of PhIP in a model system. Carcinogenesis 1991, 12, 2297–2300. [Google Scholar] [CrossRef]
- Skog, K.; Jägerstad, M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems. Mutat. Res-Fund. Mol. M. 1990, 230, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, B.; Niu, Z.; Zhang, Y.; Gao, J.; Shi, L.; Wang, S.; Wang, S. Role of alpha-Dicarbonyl Compounds in the Inhibition Effect of Reducing Sugars on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. J. Agric. Food. Chem. 2017, 65, 10084–10092. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, W.; Bao, Y.; Zhu, Y.; Zhang, Y.; Lu, Y.; Peng, Z.; Zhou, G. Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism. Foods 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, X.; Bao, Y.; Zhu, Y.; Zhang, Y.; Li, J.; Peng, Z. Inhibitory effects of hyperoside and quercitrin from Zanthoxylum bungeanum Maxim. leaf on 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine formation by trapping phenylacetaldehyde. Eur. Food Res. Technol. 2021, 248, 25–34. [Google Scholar] [CrossRef]
- Han, T.; Wang, T.; Hou, H.; Wang, Z.; Xiao, T.; Gai, S.; Wang, M.; Liu, D. Raw to charred: Changes of precursors and intermediates and their correlation with heterocyclic amines formation in grilled lamb. Meat Sci. 2022, 195, 108999–109007. [Google Scholar] [CrossRef]
- Cheng, K.W.; Chen, F.; Wang, M. Inhibitory activities of dietary phenolic compounds on heterocyclic amine formation in both chemical model system and beef patties. Mol. Nutr. Food Res. 2007, 51, 969–976. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, S.; Wang, M.; Chen, J.; Zheng, Z.-P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016, 7, 1057–1066. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, X.; Sun, F.; Liu, H.; Chen, Q.; Kong, B. Inhibitory effects of hydrocolloids on the formation of heterocyclic aromatic amines in smoked chicken drumsticks and the underlying mechanism. Food Hydrocoll. 2022, 133, 107940–107949. [Google Scholar] [CrossRef]
- De Moura Neves-Gonçalves, T.; Pinto, E.; Viegas, O.; Braga, A.R.C.; de Souza Mesquita, L.M.; Ferreira, I.M.P.L.V.O.; García-Jares, C.; De Rosso, V.V.; Domene, S.M.Á. Evaluation of the potential of annatto seed powder to reduce the formation of heterocyclic amines in charcoal-grilled and pan-fried beef patties. Food Chem. 2025, 462, 462–475. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, H.; Lv, X.; Lin, Q.; Chen, B.-H.; Lai, Y.-W.; Chen, L.; Teng, H.; Cao, H. The evaluation of catechins reducing heterocyclic aromatic amine formation: Structure-activity relationship and mechanism speculation. Curr. Res. Food Sci. 2024, 8, 10727–10737. [Google Scholar] [CrossRef]
- Seung-Eun, M.; Han-Seung, S. Inhibition of mutagenic 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) formation using various food ingredients in a model systems. Food Sci. Biotechnol. 2013, 22, 323–329. [Google Scholar] [CrossRef]
- Murkovic, M. Formation of heterocyclic aromatic amines in model systems. J. Chromator. B 2004, 802, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.G., Jr. Organic Chemistry, 7th ed.; China Machine Press: Beijing, China, 2011; pp. 842–843. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zheng, Z.; Liu, D.; Sun, S.; Zhao, X.; Lv, L.; Xie, D.; Han, Z.; He, J. Role of Furfural and 5-Methyl-2-furfural in Glucose-Induced Inhibition of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Formation in Chemical Models and Pork Patties. Molecules 2025, 30, 1254. https://doi.org/10.3390/molecules30061254
Qin Y, Zheng Z, Liu D, Sun S, Zhao X, Lv L, Xie D, Han Z, He J. Role of Furfural and 5-Methyl-2-furfural in Glucose-Induced Inhibition of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Formation in Chemical Models and Pork Patties. Molecules. 2025; 30(6):1254. https://doi.org/10.3390/molecules30061254
Chicago/Turabian StyleQin, Yuexia, Zhuyu Zheng, Di Liu, Shuhua Sun, Xiaolei Zhao, Lei Lv, Dengyu Xie, Zhonghui Han, and Jinxing He. 2025. "Role of Furfural and 5-Methyl-2-furfural in Glucose-Induced Inhibition of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Formation in Chemical Models and Pork Patties" Molecules 30, no. 6: 1254. https://doi.org/10.3390/molecules30061254
APA StyleQin, Y., Zheng, Z., Liu, D., Sun, S., Zhao, X., Lv, L., Xie, D., Han, Z., & He, J. (2025). Role of Furfural and 5-Methyl-2-furfural in Glucose-Induced Inhibition of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Formation in Chemical Models and Pork Patties. Molecules, 30(6), 1254. https://doi.org/10.3390/molecules30061254







