Research Progress on the Enhancement of Immobilized Enzyme Catalytic Performance and Its Application in the Synthesis of Vitamin E Succinate
Abstract
1. Introduction
1.1. Overview of Lipases
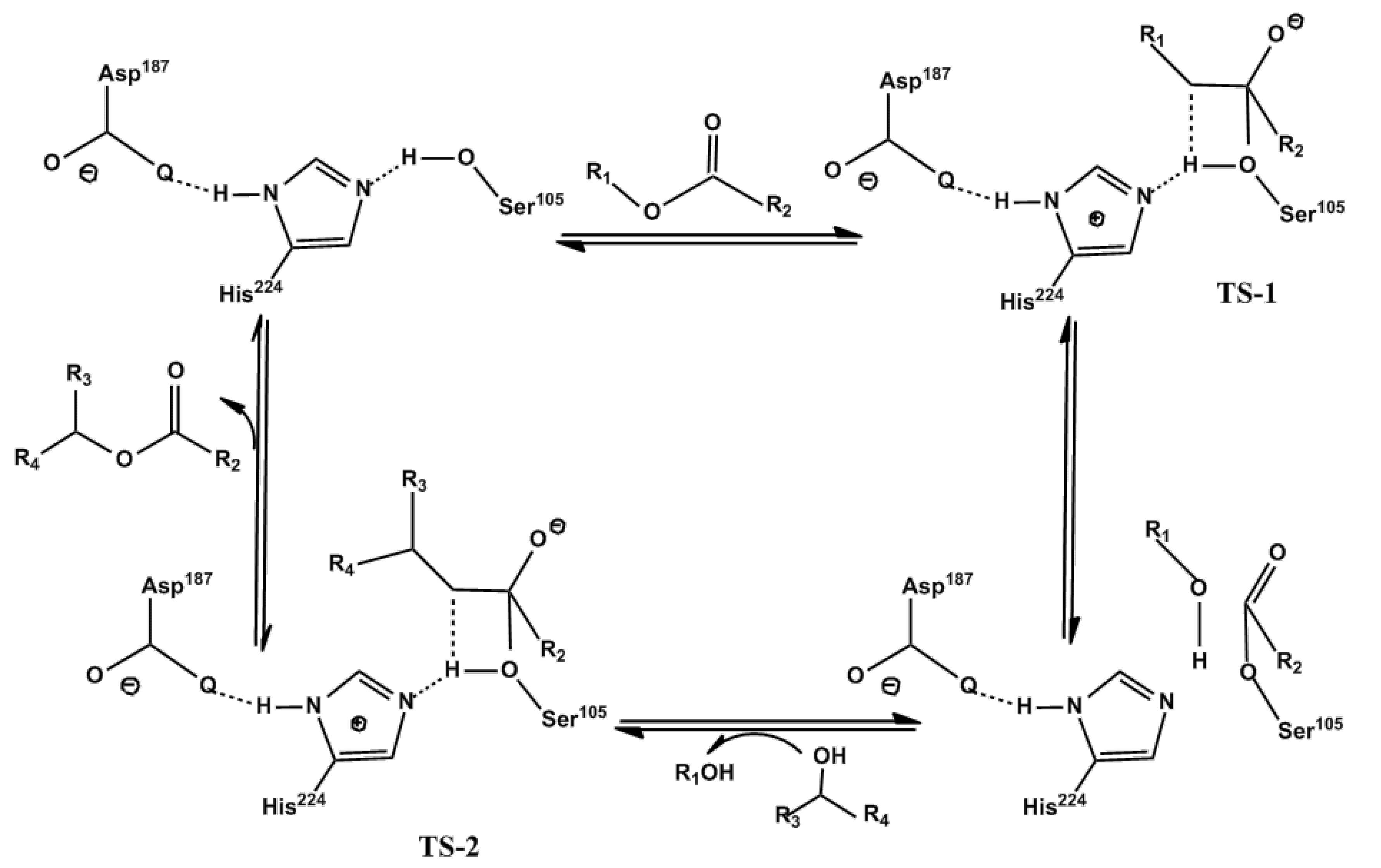
1.2. Lipase Catalytic Mechanism
1.3. Application of Lipase and Its Existing Problems
- (1)
- Applications in the food industry
- (2)
- Application in the chemical industry
- (3)
- Application in textiles
- (4)
- Application in medicine and health
- (5)
- Application in environmental protection
2. Immobilized Lipase
2.1. Application of Immobilized Lipase in the Synthesis of Vitamin E Succinate
2.2. Preparation Method of Immobilized Lipase
2.3. Carriers for Immobilized Lipases
3. Nanomaterial Immobilized Lipases
3.1. Nanoporous Materials-Metal-Organic Frameworks (MOFs)
3.2. Organic Polymer Nanomaterials
3.3. Preparation Method of Nanostructured Enzyme Catalyst
- (1)
- Methods based on surface fixation of nanomaterials
- (2)
- Methods based on the assembly of nanomaterials
- (3)
- Methods based on in situ synthesis of block copolymers
4. Research Progress on the Directional Immobilization of Biological Enzymes in Nanomaterials
| Immobilized Vectors | Enzyme Species | Fields of Application | Modifiers | Performance | References | |
|---|---|---|---|---|---|---|
| Free Enzymes | Immobilized Enzymes | |||||
| CNT-Ni | Lipase | Biocatalysis | PyBA4-(1-pyrene)butyric acid | It was inactivated after 40 °C and 24 h | The activity at 40 °C and 24 h was 80.2% of the initial activity | [104] |
| CNTs | Laccase | Biocatalysis for the degradation of phenolic compounds such as hydroquinone | 0.3 mol/L HNO3 oxidation | The activity was 32% after 50 °C and 4 h | The fixation rate is 96%; the activity was between 54.1% and 84.5% after 50 °C and 4 h | [105] |
| CNT | Lactate dehydrogenase | Biocatalysis | Nitric acid and sulfuric acid | Activity at 70 °C is only 6% of the initial activity | At 70 °C, the activity was 24.0~33.0% of the initial activity | [105] |
| MWCNT | Cellulase | Hydrolyzed cellulase | N-hydroxysuccinimide, APTES | _ | The fixation rate was 85%, and the enzyme activity remained at 75% after 6 cycles | [106] |
| GO | Pectinase | Food industry | Sodium alginate | 15 d completely inactivated | The 15 d enzyme activity was 78.7% of the initial activity | [107] |
| GO-NZ | Laccase | Destaining of the dye | APTES | 4 d activity less than 60% | The activity of immobilized laccase remained above 93.0% on the 4th day. The activity of 5 cycles was 95.0% of the initial activity | [108] |
| GO | Lysozyme | Biocatalysis | Sodium alginate | _ | Maintain the adsorption capacity at 80% after 4 cycles | [109] |
| rGO | Horseradish peroxidase | Environmental governance | _ | _ | 10 cycles of activity still maintain 70% of the initial activity | [110] |
| Fe3O4NPs | Lipase | Biocatalyst, green pharmaceutical esters | Chitosan | The 30-day activity is only 31% of the initial activity | The 30-day activity was 95% of the initial activity | [111] |
| Fe3O4 | Laccase | Environmental and catalytic fields | APTES | It is almost inactivated at 25 °C and 20 d | The fixation rate was 76.2~84.4%; cycle 11 times, the activity is about 71%; At 25 °C, the activity of 20 d is about 85% | [112] |
| Fe3O4 | Lysozyme | Enzyme activity studies | Butanetetracarboxylic acid | 40 °C, 180 min activity 45.0%; inactivated after 40 days; 49.9% activity at 60 | The activity at 40 °C and 180 min was 66.6%. The activity is about 60% after 45 days; the activity at 60 °C was 73.9%; 4 cycles of reuse are still 75% active | [113] |
| Fe3O4@SiO2 | Horseradish peroxidase | Environmental remediation and removal of organic pollutants | Polydopamine | The activity was 38.1% at 4 °C and 30 d | 70.0% of the activity was recycled for 4 times, and 30.0% was reused for 8 times; the activity was 80.3% at 4 °C and 30 d | [114] |
5. Vitamin E Succinate
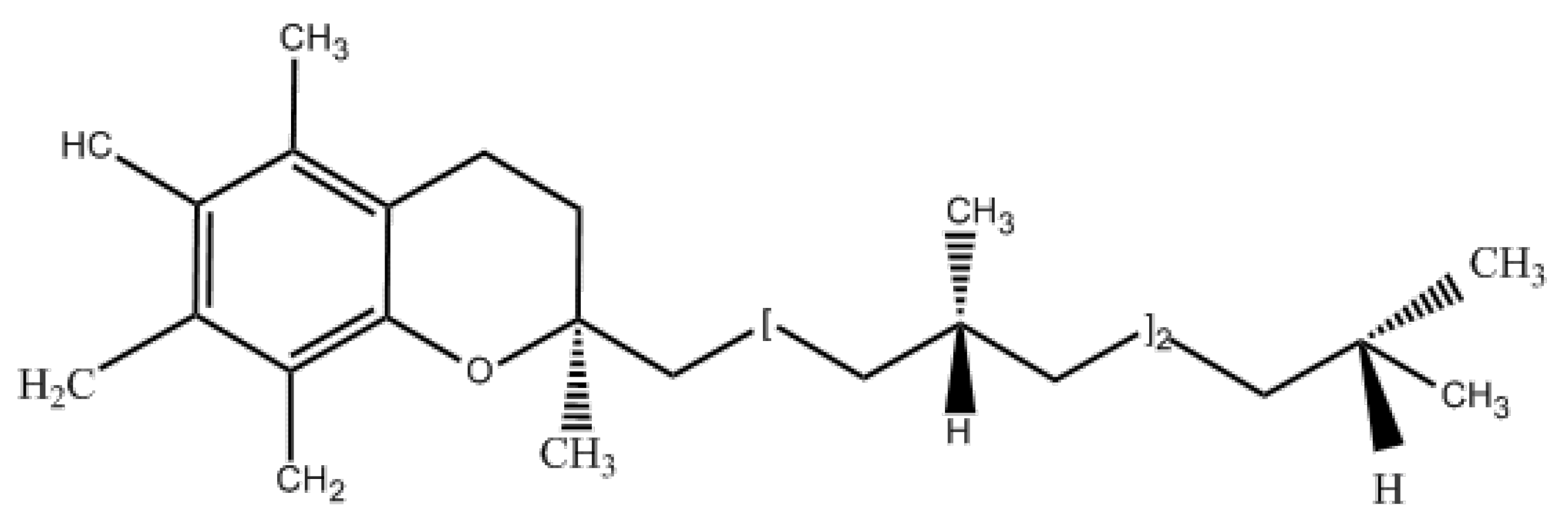
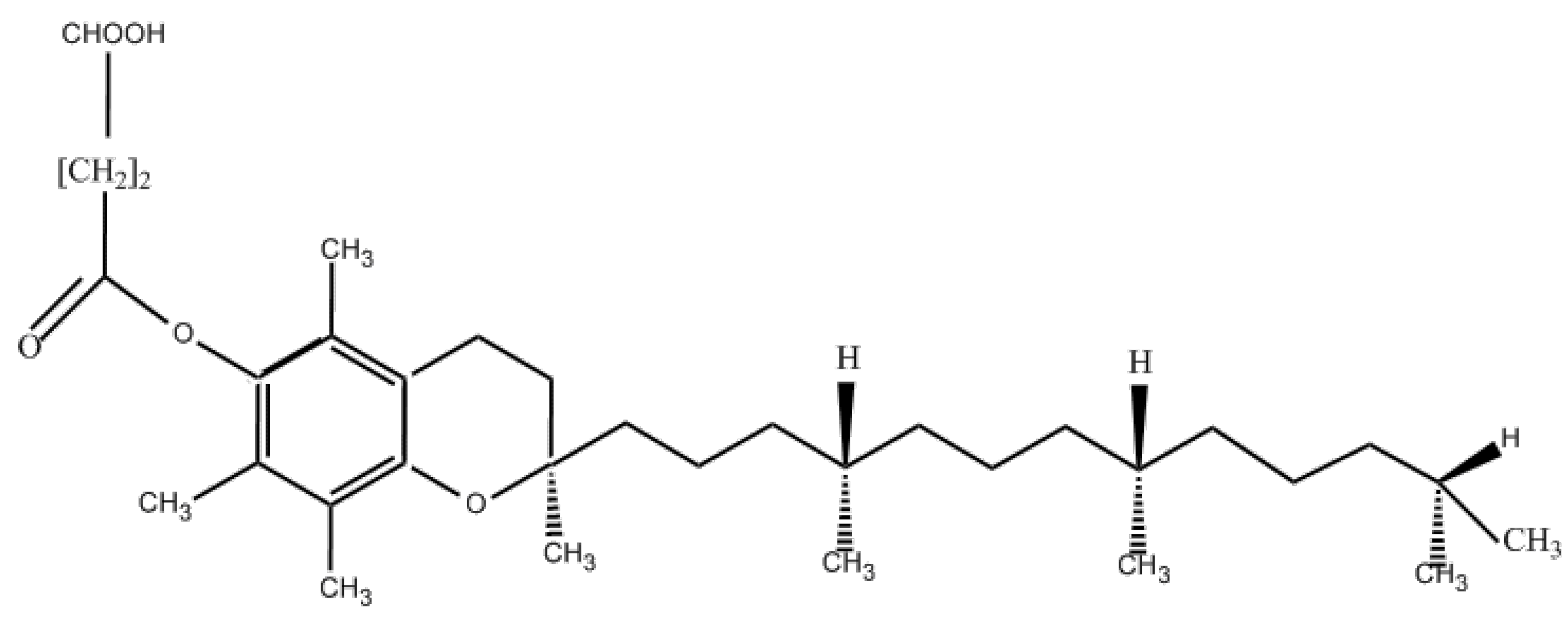
5.1. Physical and Chemical Properties and Uses of Vitamin E Succinate
5.2. Preparation Method of Vitamin E Succinate
6. Improvement in Catalytic Performance of Immobilized Lipase and Research Significance and Prospect of Synthesis of Vitamin E Succinate
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B-Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Parfait, A.N.A.; Jorel, S.A.; Mario, D.A.; Lorenzo, B.; Giangiacomo, B.; Fabrizio, G.; Armelle, D.T.; Gabriel, A.A.; Jules-Roger, K.; Maria, D.; et al. Hydroethanolic plant extracts from Cameroon positively modulate enzymes relevant to carbohydrate/lipid digestion and cardio-metabolic diseases. Food Funct. 2019, 10, 6533–6542. [Google Scholar]
- Xinnan, M.; Wenrui, H.; Yongqing, S.; Juan, H.; Jiacong, W.; Lei, W.; Yun, W. Novel Recyclable UCST-Type Immobilized Glucose Isomerase Biocatalyst with Excellent Performance for Isomerization of Glucose to Fructose. J. Agric. Food Chem. 2022, 70, 13959–13968. [Google Scholar]
- Ren, X.; Liang, Q.; Ma, H. Effects of sweeping frequency ultrasound pretreatment on the hydrolysis of zein: Angiotensin-converting enzyme inhibitory activity and thermodynamics analysis. J. Food Sci. Technol. 2018, 55, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Xing, H.; Wang, Z.; Ding, W.; Zhu, P.; Liu, B.; Ma, H. Establishment of an Enzymatic Membrane Reactor for Angiotensin-Converting Enzyme Inhibitory Peptides Preparation from Wheat Germ Protein Isolates. J. Eng. 2016, 39, 296–305. [Google Scholar] [CrossRef]
- Puchalvert, D.U.; Zoppolo, F.; Bentura, M.; Castilla, A.; Savio, E.; Giordano, S.R.; Irazoqui, G. Feasibility of a stereoselective synthesis of [11C](S,S)-S-adenosylmethionine ([11C](S,S)-SAM) catalyzed by an immobilized enzyme. Process Biochem. 2025, 149, 137–143. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2005, 39, 235–251. [Google Scholar] [CrossRef]
- Zheng, G.-W.; Xu, J.-H. New opportunities for biocatalysis: Driving the synthesis of chiral chemicals. Curr. Opin. Biotechnol. 2011, 22, 784–792. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Ma, H.; Dong, Y.; Fu, J.; Lai, X.; Yagoub, A.E.-G.A.; Peng, W.; Ni, H. Antioxidant and lipase inhibitory activities of Camellia pollen extracts: The effect of composition and extraction solvents. All Life 2022, 15, 1304–1314. [Google Scholar] [CrossRef]
- He, W.-S.; Li, L.; Zhao, J.; Xu, H.; Rui, J.; Cui, D.; Li, H.; Zhang, H.; Liu, X. Candida sp. 99-125 lipase-catalyzed synthesis of ergosterol linolenate and its characterization. Food Chem. 2018, 280, 286–293. [Google Scholar] [CrossRef]
- Zan, X.; Cui, F.; Sun, J.; Zhou, S.; Song, Y. Novel Dual-Functional Enzyme Lip10 Catalyzes Lipase and Acyltransferase Activities in the Oleaginous Fungus Mucor circinelloides. J. Agric. Food Chem. 2019, 67, 13176–13184. [Google Scholar] [CrossRef] [PubMed]
- He, W.-S.; Hu, D.; Wang, Y.; Chen, X.-Y.; Jia, C.-S.; Ma, H.-L.; Feng, B. A novel chemo-enzymatic synthesis of hydrophilic phytosterol derivatives. Food Chem. 2016, 192, 557–565. [Google Scholar] [CrossRef] [PubMed]
- WenSen, H.; Yuying, S.; Zhishuo, L.; Haonan, Y.; Junjie, L.; Qingzhi, W.; Chen, T.; Bin, Z. Enhanced antioxidant capacity of lipoic acid in different food systems through lipase-mediated esterification with phytosterols. J. Sci. Food Agric. 2022, 102, 7115–7125. [Google Scholar]
- Chen, G.; Khan, I.M.; He, W.; Li, Y.; Jin, P.; Campanella, O.H.; Zhang, H.; Huo, Y.; Chen, Y.; Yang, H.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- Burckhard, S.; Szostak, J.W. Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature 2007, 448, 828–831. [Google Scholar]
- He, W.-S.; Cui, D.-D.; Zhang, Y.-L.; Liu, Y.; Yin, J.; Chen, G.; Jia, C.-S.; Feng, B. Highly Efficient Synthesis of Phytosterol Linolenate Catalyzed by Candida Rugosa Lipase through Transesterification. Food Sci. Technol. Res. 2017, 23, 525–533. [Google Scholar] [CrossRef]
- Yihang, L.; Qingzhi, W.; Chen, L.; Haonan, Y.; Li, J.; Litao, Z.; Fayong, G.; Chen, T.; Tao, H.; WenSen, H. Improved antioxidant activity of rutin via lipase-mediated esterification with oleic acid. J. Sci. Food Agric. 2023, 103, 3489–3500. [Google Scholar]
- Tupikina, E.Y. Non-covalent interactions in the glutathione peroxidase active center and their influence on the enzyme activity. Org. Biomol. Chem. 2022, 20, 5551–5557. [Google Scholar] [CrossRef]
- Bo, L.; Lina, Z.; Hongjian, C.; Dewei, S.; Boxin, D.; Jinwei, L.; Yuanfa, L.; Fei, W. Inactivation of Lipase and Lipoxygenase of Wheat Germ with Temperature-Controlled Short Wave Infrared Radiation and Its Effect on Storage Stability and Quality of Wheat Germ Oil. PLoS ONE 2016, 11, e0167330. [Google Scholar]
- Emad, K.; Mohamed, A.I.A.; Faisal, M.M.; Wei, W.; Frederick, S.; Xingguo, W. Lipid-soluble vitamins from dairy products: Extraction, purification, and analytical techniques. Food Chem. 2021, 373, 131436. [Google Scholar]
- Zhang, H.; Zhou, J.; Zheng, X.; Zhang, Z.; Wang, Z.; Tan, X. Characterization of a Desiccation Stress Induced Lipase Gene from Brassica napus L. J. Agric. Sci. Technol. 2016, 18, 1129–1141. [Google Scholar]
- Chen, Z.; Li, Y.; Wang, L.; Liu, S.; Wang, K.; Sun, J.; Xu, B. Evaluation of the possible non-thermal effect of microwave radiation on the inactivation of wheat germ lipase. J. Food Process Eng. 2017, 40, e12506. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Y.; Hassane Hamadou, A.; Shen, Q.; Xu, B. Tempering–preservation treatment inactivated lipase in wheat bran and retained phenolic compounds. Int. J. Food Sci. Technol. 2021, 57, 2104–2112. [Google Scholar] [CrossRef]
- Huang, C.; Lin, Z.; Zhang, Y.; Liu, Z.; Tang, X.; Li, C.; Lin, L.; Huang, W.; Ye, Y. Structure-guided preparation of fuctional oil rich in 1,3-diacylglycerols and linoleic acid from Camellia oil by combi-lipase. J. Sci. Food Agric. 2022, 103, 108–117. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, S.; Wang, L.; Xu, B. Study of the interfacial activity of wheat germ lipase. Qual. Assur. Saf. Crops Foods 2019, 11, 491–498. [Google Scholar] [CrossRef]
- Miranda, K.; Baeza-Jimenez, R.; Noriega-Rodriguez, J.A.; Garcia, H.S.; Otero, C. Optimization of structured diacylglycerols production containing ω-3 fatty acids via enzyme-catalysed glycerolysis of fish oil. Eur. Food Res. Technol. 2013, 236, 435–440. [Google Scholar] [CrossRef]
- Ren, R.; Yang, X.; Xu, J.; Zhang, K.; Zhang, M.; Liu, G.; Yao, X.; Lou, L.; Xu, J.; Zhu, L.; et al. Genome-wide identification and analysis of promising GDSL-type lipases related to gummy stem blight resistance in watermelon (Citrullus lanatus). Sci. Hortic. 2021, 289, 110461. [Google Scholar] [CrossRef]
- Nelson, A.A.; Adenike, A.Z.; Oladele, A.M. Characterization of a purified novel Aureobasidium pullulans NAC8 lipase and covalent-immobilization for use in the biodegradation of oil-contaminated wastewater. Int. J. Biol. Macromol. 2025, 304, 140781. [Google Scholar]
- Suresh, J.; Azelee, N.I.W.; Illias, R.M.; Toemen, S. Evaluation of hybrid catalyst with lipase immobilized on potassium oxide-carbon beads for biodiesel production. Energy 2025, 318, 134787. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Peponi, L.; Marcos-Fernández, Á.; Kenny, J.M.; Martínez-Richa, A. Synthesis, characterization and hydrolytic degradation of polyester-urethanes obtained by lipase biocatalysis. Polym. Degrad. Stab. 2014, 108, 188–194. [Google Scholar] [CrossRef]
- Berhanu, A.; Amare, G. Microbial Lipases and Their Industrial Applications: Review. Biotechnol. 2012, 11, 100–118. [Google Scholar]
- Gotor, V. Biocatalysis Applied to the Preparation of Pharmaceuticals. Org. Process Res. Dev. 2002, 6, 420–426. [Google Scholar] [CrossRef]
- Yuan, X.; Liao, J.H.; Du, G.J.; Hou, Y.; Hu, S.Q. Immobilization β-glucosidase from Dictyoglomus thermophilum on UiO-66-NH2: An efficient catalyst for enzymatic synthesis of kinsenoside via reverse hydrolysis reaction. Int. J. Biol. Macromol. 2024, 282 Pt 5, 137330. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, M.; Kaluzna, I.A.; Müller, M.; Stewart, J.D. Regio and enantioselective reduction of t-butyl 6-chloro-3,5-dioxohexanoate with baker’s yeast. Tetrahedron Asymmetry 2004, 15, 2825–2828. [Google Scholar] [CrossRef]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Rathod, V.K. Ultrasound assisted enzyme catalyzed hydrolysis of waste cooking oil under solvent free condition. Ultrason. Sonochemistry 2016, 32, 60–67. [Google Scholar] [CrossRef]
- Rathi, P.; Saxena, R.K.; Gupta, R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 2001, 37, 187–192. [Google Scholar] [CrossRef]
- Ellaiah, P.; Srinivasulu, B.; Adinarayana, K. A review on microbial alkaline proteases. J. Sci. Ind. Res. 2002, 61, 690–704. [Google Scholar]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag. 2016, 47, 98–104. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Chang, S.W.; Lee, G.C.; Shaw, J.F. Candida rugosa lipase LIP1-catalyzed transesterification to produce human milk fat substitute. J. Agric. Food Chem. 2006, 54, 5175–5181. [Google Scholar] [CrossRef]
- Su, F.; Peng, C.; Li, G.-L.; Xu, L.; Yan, Y.-J. Biodiesel production from woody oil catalyzed by Candida rugosa lipase in ionic liquid. Renew. Energy 2016, 90, 329–335. [Google Scholar] [CrossRef]
- Ivić, J.T.; Milosavić, N.; Dimitrijević, A.; Jankulović, M.G.; Bezbradica, D.; Kolarski, D.; Veličković, D. Synthesis of medium-chain length capsinoids from coconut oil catalyzed by Candida rugosa lipases. Food Chem. 2017, 218, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, X.; Wu, S.; Jiang, L.; Huang, H. Synthesis of vitamin E succinate by interfacial activated Candida rugosa lipase encapsulated in sol-gel materials. Chin. J. Catal. 2013, 34, 1608–1616. [Google Scholar] [CrossRef]
- Vorlová, S.; Bornscheuer, U.T.; Gatfield, I.; Hilmer, J.M.; Bertram, H.J.; Schmid, R.D. Enantioselective hydrolysis of d,l-menthyl benzoate to L-(−)-menthol by recombinant Candida rugosa lipase LIP1. Adv. Synth. Catal. 2002, 344, 1152–1155. [Google Scholar] [CrossRef]
- Jiang, X.-j.; Hu, Y.; Jiang, L.; Gong, J.-h.; Huang, H. Synthesis of vitamin E succinate from Candida rugosa lipase in organic medium. Chem. Res. Chin. Univ. 2013, 29, 223–226. [Google Scholar] [CrossRef]
- Qu, W.; Sehemu, R.M.; Zhang, T.; Song, B.; Yang, L.; Ren, X.; Ma, H. Immobilized enzymolysis of corn gluten meal under triple-frequency ultrasound. Int. J. Food Eng. 2018, 14, 20170347. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.K.; Miao, W.J.; Wu, Q.F.; Liu, Y.X.; Sun, Y.; Gao, C. Thermal versus Microwave Inactivation Kinetics of Lipase and Lipoxygenase from Wheat Germ. J. Food Process Eng. 2016, 39, 247–255. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Ma, A.; Ding, Z.; Huo, S.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef] [PubMed]
- Sumitra, D.; Rene, C.L.; Sriramulu, R.Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar]
- Zhang, Y.; Feng, T.; Ni, X.; Xia, J.; Suo, H.; Yan, L.; Zou, B. Immobilized lipase based on SBA-15 adsorption and gel embedding for catalytic synthesis of isoamyl acetate. Food Biosci. 2024, 60, 104427. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Shinji, S.; Yuping, L.; Tetsu, Y.; Rie, W.; Masaaki, K.; Koei, K. Immobilization of Pseudomonas cepacia lipase onto electrospun polyacrylonitrile fibers through physical adsorption and application to transesterification in nonaqueous solvent. Biotechnol. Lett. 2010, 32, 1059–1062. [Google Scholar]
- Li, Y.; Zhou, G.; Qiao, W.; Wang, Y. Immobilization of Porcine pancreas lipase on fiber-like SBA-15 mesoporous material. Mater. Sci. Eng. B 2009, 162, 120–126. [Google Scholar] [CrossRef]
- Adi, W.; Aviad, A.; Christina, D.; Dorith, T. Glycerol triacetate as solvent and acyl donor in the production of isoamyl acetate with Candida antarctica lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.; Zhu, S.; Jiang, B.; Gao, S.; Zhang, Y.; Wang, H.; Xu, S.; Liu, Y.; An, Y. Embedding inulin fructotransferase from Arthrobacter aurescens into novel curdlan-based mesoporous silica microspheres for efficient production of Difructose Anhydride III. Food Chem. 2019, 299, 125128. [Google Scholar] [CrossRef]
- Monier, M.; Wei, Y.; Sarhan, A.A. Evaluation of the potential of polymeric carriers based on photo-crosslinkable chitosan in the formulation of lipase fromCandida rugosa immobilization. J. Mol. Catalysis. B Enzym. 2009, 63, 93–101. [Google Scholar] [CrossRef]
- Raman, J.K.; Seng, C.E.; Pogaku, R. Physical and stability characteristics ofBurkholderia cepacia lipase encapsulated in κ-carrageenan. J. Mol. Catalysis. B Enzym. 2008, 58, 78–83. [Google Scholar]
- Chiou, S.H.; Wu, W.T. Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 2004, 25, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Lin, Y.-H.; Chen, C.-Y.; Chang, J.-S. Characterization of Burkholderia lipase immobilized on celite carriers. J. Taiwan Inst. Chem. Eng. 2009, 40, 359–363. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Diao, X.; Luo, G.; Dai, Y. Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugosa lipase in SBA-15. Bioresour. Technol. 2010, 101, 3830–3837. [Google Scholar] [CrossRef]
- Sumaira, S.; Shazia, K.; Jahangir, K.; Razia, B.; Abid, S.; Tariq, A.; Majid, A.; Alamri, A.S.; Sameeh, M.Y.; Faten, Z.F. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar]
- Wang, F.; Owusu-Fordjour, M.; Xu, L.; Ding, Z.; Gu, Z. Immobilization of Laccase on Magnetic Chelator Nanoparticles for Apple Juice Clarification in Magnetically Stabilized Fluidized Bed. Front. Bioeng. Biotechnol. 2020, 8, 589. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene oxide as a matrix for enzyme immobilization. Langmuir: ACS J. Surf. Colloids 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Sun, Y.; Sun, M.; Duan, J.; Tian, Y.; Du, D.; Li, M. Cascade Amplifying Electrochemical Bioanalysis for Zearalenone Detection in Agricultural Products: Utilizing a Glucose–Fenton–HQ System on Bimetallic–ZIF@CNP Nanocomposites. Foods 2024, 13, 3192. [Google Scholar] [CrossRef]
- Bu, Q.; Yu, F.; Cai, J.; Bai, J.; Xu, J.; Wang, H.; Lin, H.; Long, H. Preparation of sugarcane bagasse-derived Co/Ni/N/MPC nanocomposites and its application in H2O2 detection. Ind. Crops Prod. 2024, 211, 118218. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wu, J.Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Qiu, W.-Y.; Yan, J.-K. Quaternized curdlan/pectin polyelectrolyte complexes as biocompatible nanovehicles for curcumin. Food Chem. 2019, 291, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Sadalage, P.S.; Dar, M.A.; Bhor, R.D.; Bhalerao, B.M.; Kamble, P.N.; Paiva-Santos, A.C.; Nimbalkar, M.S.; Sonawane, K.D.; Pai, K.; Patil, P.S.; et al. Optimization of biogenic synthesis of biocompatible platinum nanoparticles with catalytic, enzyme mimetic and antioxidant activities. Food Biosci. 2022, 50, 102024. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Shi, J.; Zou, X.; Huang, X.; Tahir, H.E. Preparation of conducting polyaniline/protoporphyrin composites and their application for sensing VOCs. Food Chem. 2018, 276, 291–297. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Male, K.B.; Hrapovic, S.; Luong, J.H. Cellulose nanocrystal/gold nanoparticle composite as a matrix for enzyme immobilization. ACS Appl. Mater. Interfaces 2009, 1, 1383–1386. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, N.; Dong, S.; Huang, M.; Yang, L.; Wu, X.; Liu, Z.; Jiang, J.; Zou, Y. Plasmonic ELISA Based on Nanospherical Brush-Induced Signal Amplification for the Ultrasensitive Naked-Eye Simultaneous Detection of the Typical Tetrabromobisphenol A Derivative and Byproduct. J. Agric. Food Chem. 2018, 66, 2996–3002. [Google Scholar] [CrossRef]
- Xia, J.; Liu, F.; Yan, L.; Suo, H.; Qian, J.; Zou, B. Simultaneous determination of tert-butylhydroquinone, butylated hydroxyanisole and phenol in plant oil by metalloporphyrin-based covalent organic framework electrochemical sensor. J. Food Compos. Anal. 2023, 122, 105486. [Google Scholar]
- Wang, S.; Liang, N.; Hu, X.; Li, W.; Guo, Z.; Zhang, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe 2 O 4 -reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Shi, Y.; Rong, X.; Chen, C.; Wu, M.; Takai, Y.; Qiu, X.; Wang, C.-C.; Shimasaki, Y.; Oshima, Y. Effects of ZIF-8 Nanoparticles on the Survival, Development, and Locomotor Activity of Early-life-stages of Zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 211–216. [Google Scholar]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Murugesan, A.; Shoaib, M.; Sheng, W.; Chen, Q. Functionalized metal-organic frameworks with biomolecules for sensing and detection applications of food contaminants. Crit. Rev. Food Sci. Nutr. 2024, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Zhai, X.; Shen, T.; Shi, J.; He, Y.; et al. A novel sustainable biomass-based fluorescent probe for sensitive detection of salicylic acid in rice. Food Chem. 2024, 434, 137260. [Google Scholar] [CrossRef]
- Murugavelu, M.; Sharma, A.S.; Devaraj, S.; Huanhuan, L.; Quansheng, C. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar]
- Kang, L.; Liang, Q.; Abdul, Q.; Rashid, A.; Ren, X.; Ma, H. Preparation technology and preservation mechanism of γ-CD-MOFs biaological packaging film loaded with curcumin. Food Chem. 2023, 420, 136142. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal-organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Lin, T.; Tan, Z.; Zhong, C.; Jia, S. Mesoporous Metal-Organic Framework with Well-Defined Cruciate Flower-Like Morphology for Enzyme Immobilization. ACS Appl. Mater. Interfaces 2017, 9, 10587–10594. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Yu, F. Facile preparation of Fe3O4@MOF core-shell microspheres for lipase immobilization. J. Taiwan Inst. Chem. Eng. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Bai, S.; Shao, X.; Jiang, L.; Li, Q. Immobilized lipase in bio-based metal-organic frameworks constructed by biomimetic mineralization: A sustainable biocatalyst for biodiesel synthesis. Colloids Surf. B Biointerfaces 2020, 188, 110812. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe 3 O 4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, B.; Mu, Y.; Ma, H.; Qu, W. Functional Magnetic Nanoparticles for Highly Efficient Cholesterol Removal. J. Food Sci. 2018, 83, 122–128. [Google Scholar] [CrossRef]
- Danial, M.; Klok, H.-A.; Norde, W.; Stuart, M.A.C. Complex coacervate core micelles with a lysozyme-modified corona. Langmuir 2007, 23, 8003–8009. [Google Scholar] [CrossRef]
- Jia, H.; Zhu, G.; Vugrinovich, B.; Kataphinan, W.; Reneker, D.H.; Wang, P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol. Prog. 2002, 18, 1027–1032. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Z.-M. Immobilization of lipase on chemically modified bimodal ceramic foams for olive oil hydrolysis. Chem. Eng. J. 2008, 144, 103–109. [Google Scholar] [CrossRef]
- Say, R.; Keçili, R.; Biçen, Ö.; Şişman, F.Y.; Hür, D.; Denizli, A.; Ersöz, A. A novel nanoprotein particle synthesis: Nanolipase. Process Biochem. 2011, 46, 1688–1692. [Google Scholar] [CrossRef]
- Velonia, K.; Rowan, A.E.; Nolte, R.J. Lipase polystyrene giant amphiphiles. J. Am. Chem. Soc. 2002, 124, 4224–4225. [Google Scholar] [CrossRef]
- Ming, Y.; Jun, G.; Zheng, L.; Pingkai, O. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 2006, 128, 11008–11009. [Google Scholar]
- Yan, M.; Liu, Z.; Lu, D.; Liu, Z. Fabrication of single carbonic anhydrase nanogel against denaturation and aggregation at high temperature. Biomacromolecules 2007, 8, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Heredia, K.L.; Bontempo, D.; Ly, T.; Byers, J.T.; Halstenberg, S.; Maynard, H.D. In situ preparation of protein—“Smart” polymer conjugates with retention of bioactivity. J. Am. Chem. Soc. 2005, 127, 16955–16960. [Google Scholar] [CrossRef]
- Xie, W.; Xiong, J.; Xiang, G. Enzyme immobilization on functionalized monolithic CNTs-Ni foam composite for highly active and stable biocatalysis in organic solvent. Mol. Catal. 2020, 483, 110714. [Google Scholar] [CrossRef]
- Zaboli, M.; Raissi, H.; Zaboli, M.; Farzad, F.; Torkzadeh-Mahani, M. Stabilization of d-lactate dehydrogenase diagnostic enzyme via immobilization on pristine and carboxyl-functionalized carbon nanotubes, a combined experimental and molecular dynamics simulation study. Arch. Biochem. Biophys. 2019, 661, 178–186. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Dai, X.-Y.; Kong, L.-M.; Wang, X.-L.; Zhu, Q.; Chen, K.; Zhou, T. Preparation, characterization and catalytic behavior of pectinase covalently immobilized onto sodium alginate/graphene oxide composite beads. Food Chem. 2018, 253, 185–193. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Clean Laccase immobilized nanobiocatalysts (graphene oxide—Zeolite nanocomposites): From production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B Environ. 2020, 268, 118443. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Chen, S.; Huang, Y.; He, J. Adsorption of lysozyme by alginate/graphene oxide composite beads with enhanced stability and mechanical property. Mater. Sci. Eng. C 2018, 89, 25–32. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Wu, E.; Li, Y.; Huang, Q.; Yang, Z.; Wei, A.; Hu, Q. Laccase immobilization on amino-functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere 2019, 233, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Khorshidi, A.; Shojaei, A.F.; Rezaei, S.; Faramarzi, M.A. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018, 114, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Chen, Q.; Li, Y.; Liu, E.; Li, D. Immobilized lysozyme onto 1,2,3,4-butanetetracarboxylic (BTCA)-modified magnetic cellulose microsphere for improving bio-catalytic stability and activities. Enzym. Microb. Technol. 2019, 131, 109425. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, X.; Zhang, X.; Wu, J.; Chai, C.; Ma, D.; Chen, Q.; Xiang, D.; Ge, W. Immobilized lignin peroxidase on Fe3O4@SiO2@polydopamine nanoparticles for degradation of organic pollutants. Int. J. Biol. Macromol. 2019, 138, 433–440. [Google Scholar] [CrossRef]
- Xia, J.; Zou, B.; Zhou, R.; Idowu, O.A. Lipase nanogel catalyzed synthesis of Vitamin E succinate in non-aqueous phase. J. Sci. Food Agric. 2020, 101, 3186–3192. [Google Scholar]
- Hu, G.; Cheng, W.; Gu, M.; Hang, L.; Yang, W.; Liu, T.; Li, W.; Shi, X.; Liu, M.; Tian, J. Vitamin e succinate-glycol chitosan modified copper ferrite nanocomposites for lung cancer: Targeted oxidative stress regulation induces cuproptosis and ferroptosis. Chem. Eng. J. 2024, 493, 152408. [Google Scholar] [CrossRef]
- Dong, L.-F.; Jameson, V.J.A.; Tilly, D.; Cerny, J.; Mahdavian, E.; Marín-Hernández, A.; Hernández-Esquivel, L.; Rodríguez-Enríquez, S.; Stursa, J.; Witting, P.K.; et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J. Biol. Chem. 2011, 286, 3717–3728. [Google Scholar] [CrossRef]
- Wang, X.F.; Birringer, M.; Dong, L.F.; Veprek, P.; Low, P.; Swettenham, E.; Stantic, M.; Yuan, L.-H.; Zobalova, R.; Wu, K.; et al. A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res. 2007, 67, 3337–3344. [Google Scholar] [CrossRef]
- Prasad, K.N.; Kumar, B.; Yan, X.D.; Hanson, A.J.; Cole, W.C. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: A review. J. Am. Coll. Nutr. 2003, 22, 108–117. [Google Scholar] [CrossRef]
- Torres, P.; Reyes-Duarte, D.; López-Cortés, N.; Ferrer, M.; Ballesteros, A.; Plou, F.J. Acetylation of vitamin E by Candida antarctica lipase B immobilized on different carriers. Process Biochem. 2007, 43, 145–153. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Chen, M.; Zhang, Y.; Nian, B.; Hu, Y. Ionic liquid modification reshapes the substrate pockets of lipase to boost its stability and activity in Vitamin E succinate synthesis. J. Sci. Food Agric. 2023, 104, 2669–2678. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Zhang, G.; Xia, J.; Zeng, Z.; Yu, P.; Deng, Q.; Gong, D. Green synthesis of polydopamine functionalized magnetic mesoporous biochar for lipase immobilization and its application in interesterification for novel structured lipids production. Food Chem. 2022, 379, 132148. [Google Scholar] [CrossRef]
| Classification | Enzyme Immobilization Method | |||
|---|---|---|---|---|
| Adsorption | Embedding | Covalent Bonding | Cross-Linking | |
| Advantage | Simple method, Little loss of activity, Cheap and fast | Large amount of immobilized enzyme, No need for extraction or purification, Low loss of activity | Strong bonding properties, Excellent stability | Strongly binds to lipase, Good stability in aqueous solution |
| Disadvantage | Leaks easily, Binds non-specifically | Methodological complexity, Mass transfer limitations, Leakage | Increased cost, Decreased activity | May be inactive, Lack of mechanical properties, Difficult to control size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.; Lu, Q.; Zhang, L.; Kong, F.; Zhang, Y.; Lin, Z.; Ni, X.; Zhang, X.; Zhao, Y.; Zou, B. Research Progress on the Enhancement of Immobilized Enzyme Catalytic Performance and Its Application in the Synthesis of Vitamin E Succinate. Molecules 2025, 30, 1241. https://doi.org/10.3390/molecules30061241
Qu L, Lu Q, Zhang L, Kong F, Zhang Y, Lin Z, Ni X, Zhang X, Zhao Y, Zou B. Research Progress on the Enhancement of Immobilized Enzyme Catalytic Performance and Its Application in the Synthesis of Vitamin E Succinate. Molecules. 2025; 30(6):1241. https://doi.org/10.3390/molecules30061241
Chicago/Turabian StyleQu, Liang, Qiongya Lu, Liming Zhang, Fanzhuo Kong, Yuyang Zhang, Zhiyuan Lin, Xing Ni, Xue Zhang, Yani Zhao, and Bin Zou. 2025. "Research Progress on the Enhancement of Immobilized Enzyme Catalytic Performance and Its Application in the Synthesis of Vitamin E Succinate" Molecules 30, no. 6: 1241. https://doi.org/10.3390/molecules30061241
APA StyleQu, L., Lu, Q., Zhang, L., Kong, F., Zhang, Y., Lin, Z., Ni, X., Zhang, X., Zhao, Y., & Zou, B. (2025). Research Progress on the Enhancement of Immobilized Enzyme Catalytic Performance and Its Application in the Synthesis of Vitamin E Succinate. Molecules, 30(6), 1241. https://doi.org/10.3390/molecules30061241






