Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation
Abstract
1. Introduction
2. g-C3N4 in Photocatalytic Antibiotic Degradation
2.1. Advantages of g-C3N4 in Photocatalytic Antibiotic Degradation
2.2. Strategies to Enhance Photocatalytic Capability of g-C3N4
3. g-C3N4-Based S-Scheme Heterojunctions
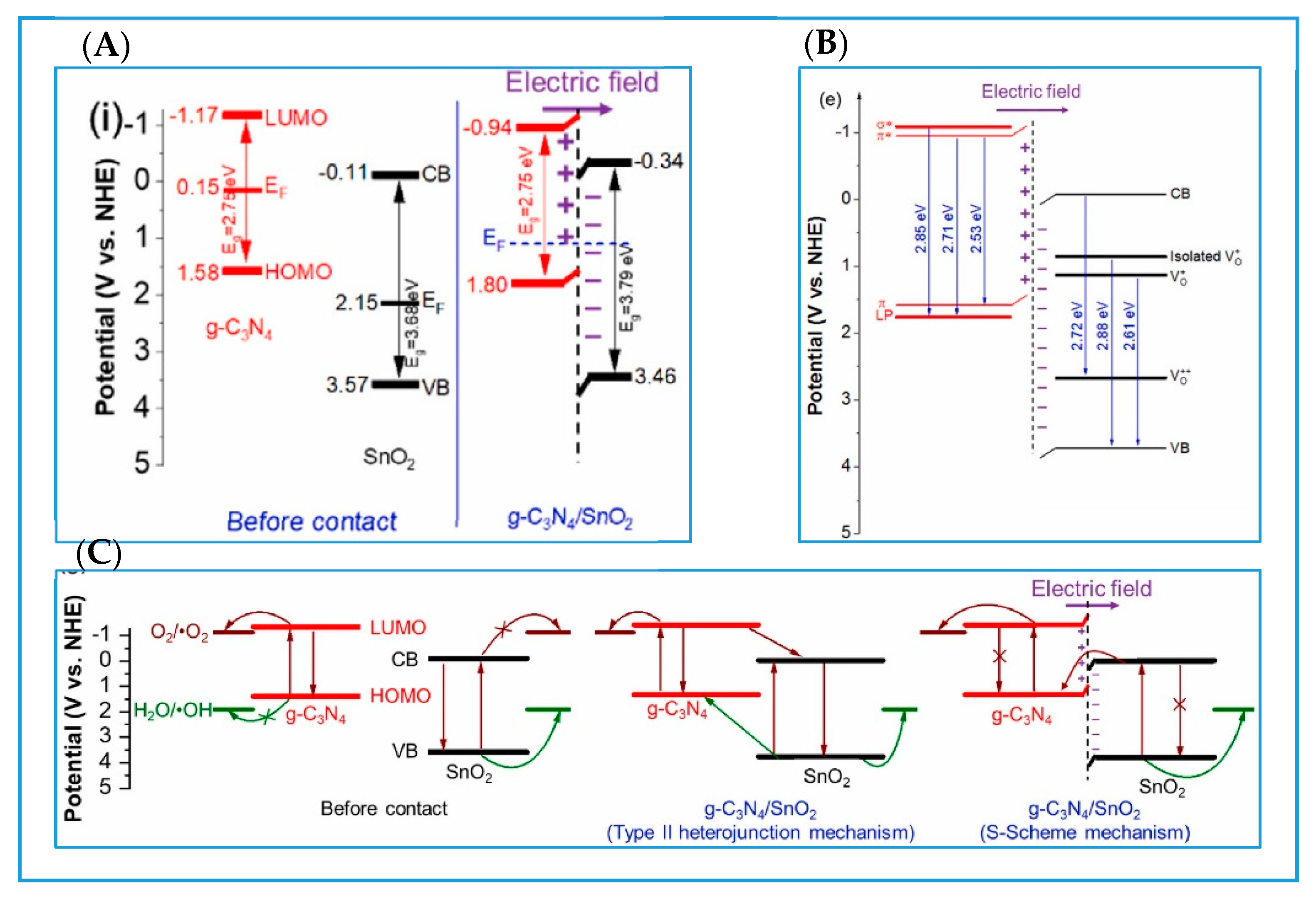
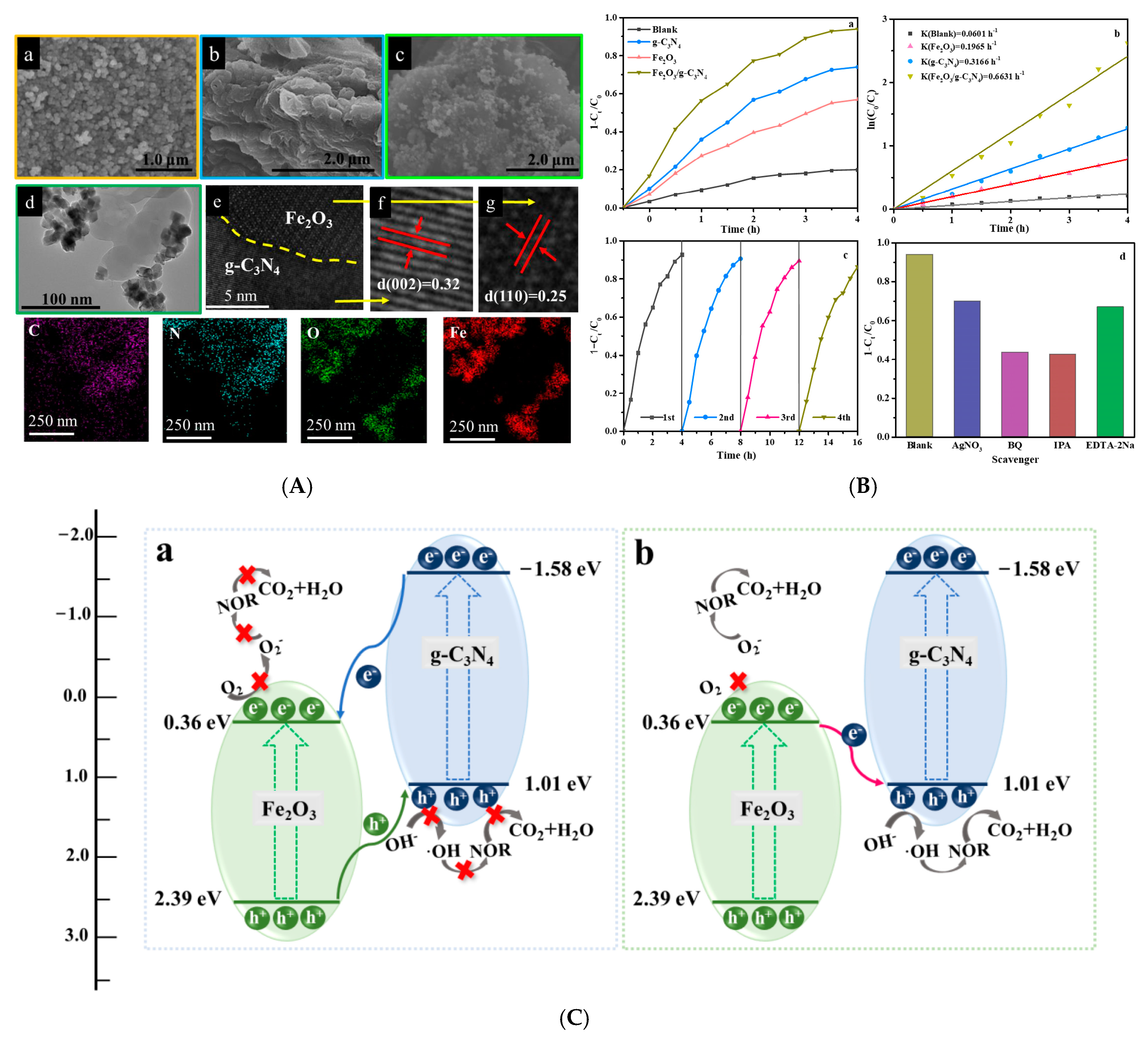
3.1. g-C3N4/Metal Oxide S-Scheme Heterojunctions
3.1.1. g-C3N4/TiO2 S-Scheme Heterojunctions
3.1.2. g-C3N4/Other Metal Oxide S-Scheme Heterojunctions
3.2. g-C3N4/Multicomponent Metal Oxide S-Scheme Heterojunctions
3.3. g-C3N4/Magnetic Oxide S-Scheme Heterojunctions
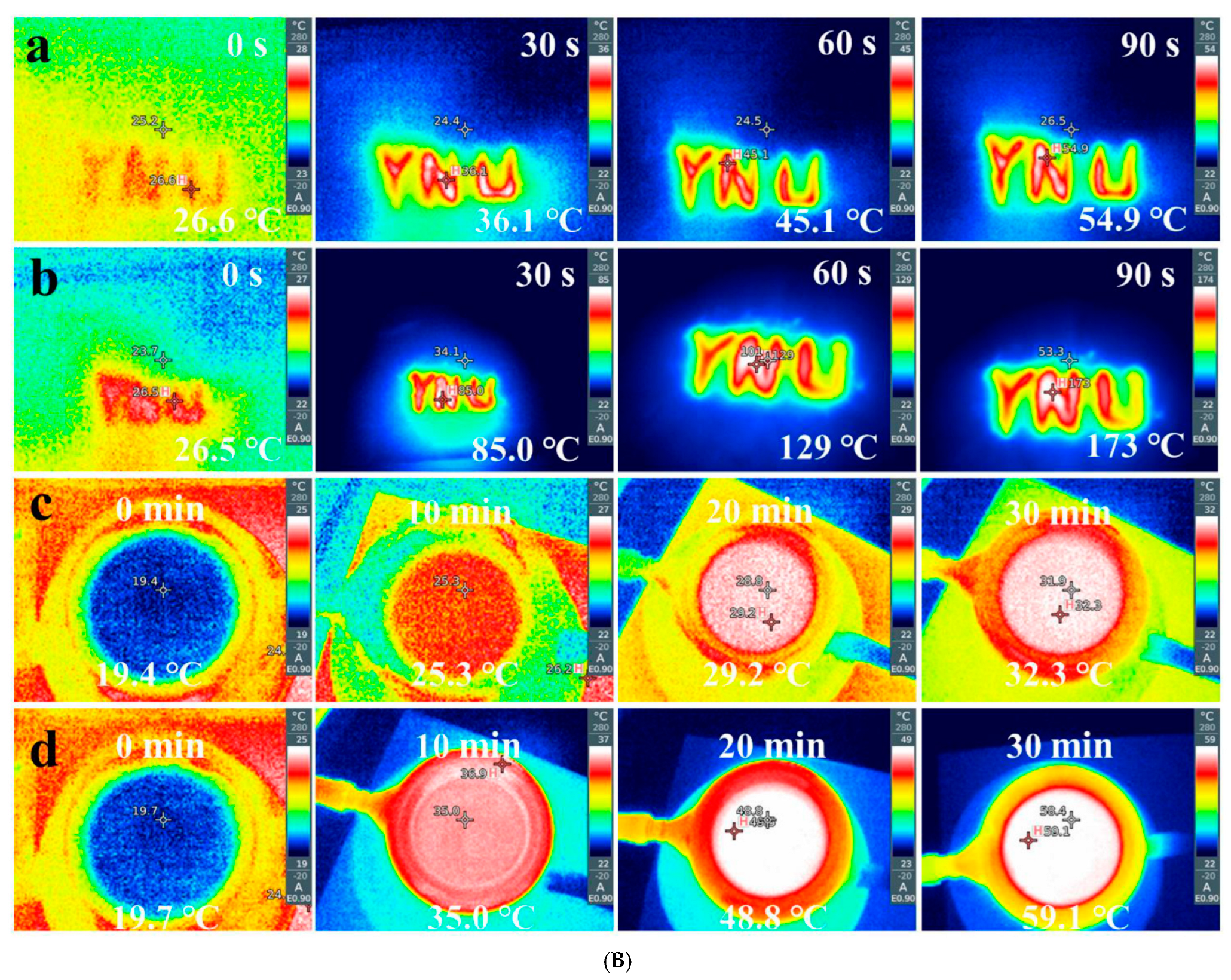
3.4. g-C3N4/Multicomponent Magnetic Oxide S-Scheme Heterojunctions
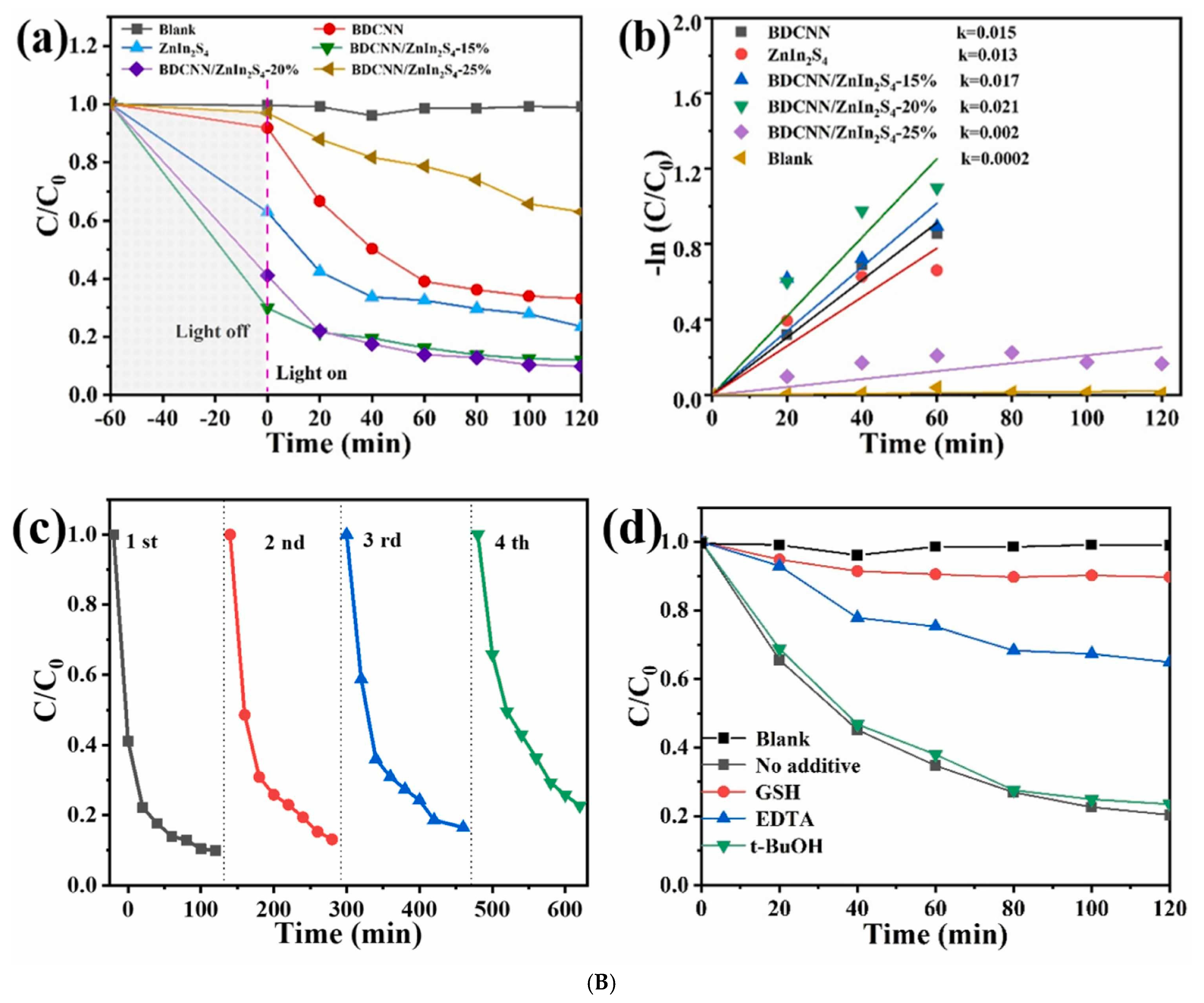
3.5. g-C3N4/Metal Sulfide S-Scheme Heterojunctions
3.6. g-C3N4/Multicomponent Metal Sulfide S-Scheme Heterojunctions
4. Preparation Method
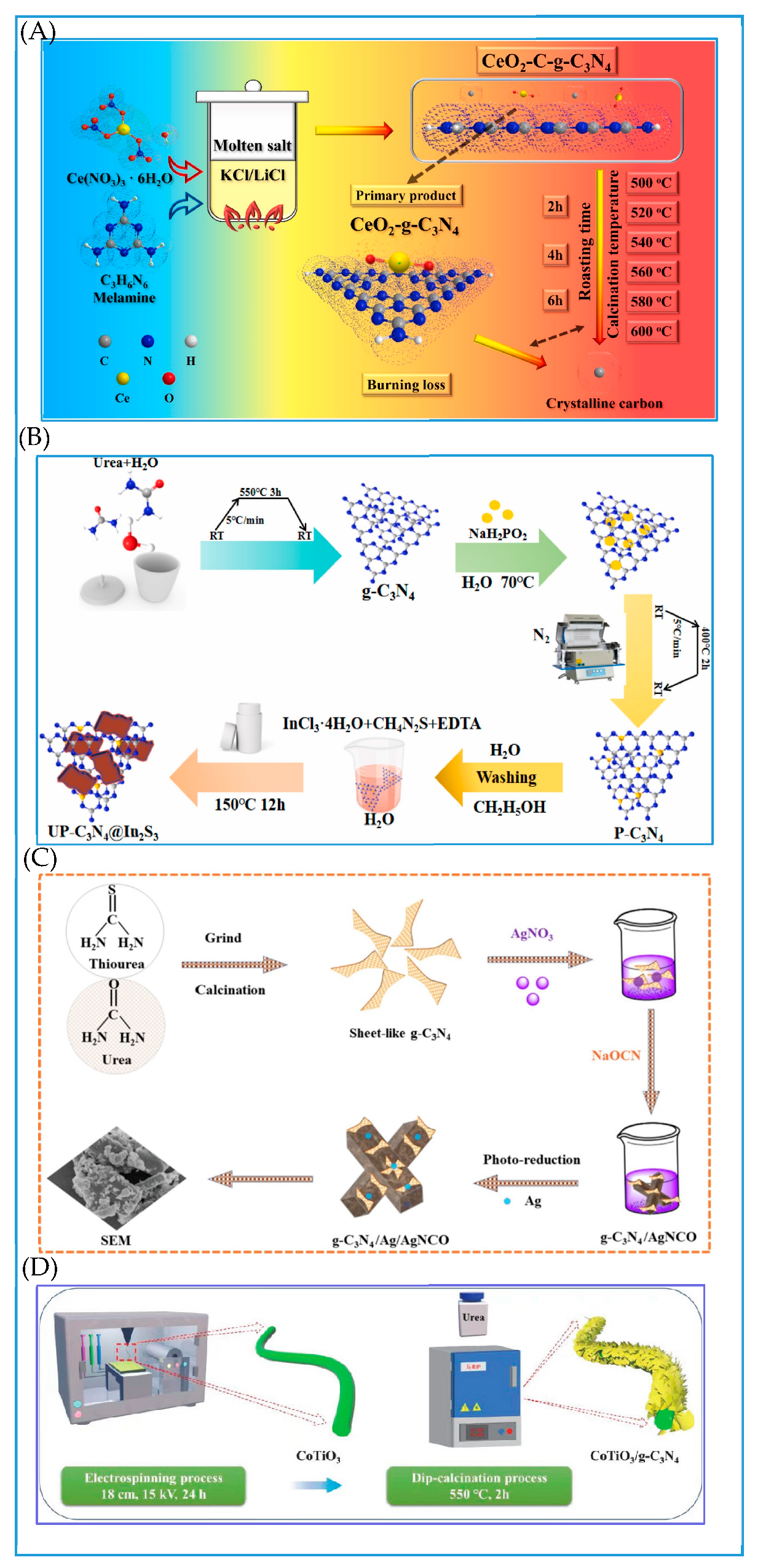
4.1. Hydrothermal Method
4.2. Calcination Method
4.3. Self-Assembly Method
4.4. In Situ Growth Method
4.5. Alternative Approaches
5. Photocatalytic Degradation of Different Types of Antibiotics
5.1. Quinolones
5.1.1. Photocatalytic Degradation of Ciprofloxacin
5.1.2. Norfloxacin
5.1.3. Ofloxacin
5.2. Tetracyclines
6. Summary
6.1. Semiconductor Materials for Constructing S-Scheme Heterojunctions with g-C3N4: From Single Metal Oxides/Sulfides to Multicomponent Metal Oxides/Sulfides
6.2. Preparation Methods: Evolving from Simple Tradition to Diverse Collaborative Innovation
6.3. Application Area: Focus on Quinolone and Tetracycline Antibiotics
7. Prospects
7.1. Innovations in Heterojunction Material: Composition, Structure, and Morphology
7.2. Innovations in Heterojunction Fabrication: Efficiency, Precision, and Sustainability
7.3. Innovations in Heterojunction Applications: Diversification, Versatility, and Mechanism Exploration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, D.; Peng, J.; Zhao, X.; Wu, H.; Zheng, G.; Zhao, Y.; Jiang, Y.; Sheng, X.; Guo, M.; Tan, Z. Quality and Safety Profiles of Chlamys Farreri Cultured in the Shandong Peninsula: Analysis of Nutritional Content, Flavor, and Hazards. J. Food Compos. Anal. 2023, 118, 105193. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic Carbon Nitride (g-C3N4)–Based Metal-Free Photocatalysts for Water Splitting: A Review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Al Mamari, S.; Kuvarega, A.T.; Selvaraj, R. Recent Advancements in the Development of Graphitic like Carbon Nitride (g-C3N4) Photocatalyst for Volatile Organic Compounds Removal: A Review. Desalin Water Treat. 2021, 235, 141–176. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Fang, L.; Ohfuji, H.; Shinmei, T.; Irifune, T. Experimental Study on the Stability of Graphitic C3N4 under High Pressure and High Temperature. Diam. Relat. Mater. 2011, 20, 819–825. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Idris, A.O.; Feleni, U.; Mamba, B.B.; Msagati, T.A.M. A Review on Water Treatment Technologies for the Management of Oxoanions: Prospects and Challenges. Environ. Sci. Pollut. Res. 2021, 28, 61979–61997. [Google Scholar] [CrossRef]
- Pretali, L.; Fasani, E.; Sturini, M. Current Advances on the Photocatalytic Degradation of Fluoroquinolones: Photoreaction Mechanism and Environmental Application. Photochem. Photobiol. Sci. 2022, 21, 899–912. [Google Scholar] [CrossRef]
- Yang, B.; Mao, X.; Pi, L.; Wu, Y.; Ding, H.; Zhang, W. Effect of pH on the Adsorption and Photocatalytic Degradation of Sulfadimidine in Vis/g-C3N4 Progress. Environ. Sci. Pollut. Res. 2017, 24, 8658–8670. [Google Scholar] [CrossRef]
- Kinsinger, N.M.; Dudchenko, A.; Wong, A.; Kisailus, D. Synergistic Effect of pH and Phase in a Nanocrystalline Titania Photocatalyst. ACS Appl. Mater. Interfaces 2013, 5, 6247–6254. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-Dimensional Porous g-C3N4 for Highly Efficient Photocatalytic Overall Water Splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Wang, Y.; Qiu, K.; Zhang, C.; Fang, J.; Sheng, X. Facile One-Step Synthesis of Hollow Mesoporous g-C3N4 Spheres with Ultrathin Nanosheets for Photoredox Water Splitting. Carbon 2018, 126, 247–256. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Cheng, K.; Zhang, Y.; Ran, J.; Wu, G. Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials 2021, 11, 1480. [Google Scholar] [CrossRef]
- Sakakibara, N.; Shizuno, M.; Kanazawa, T.; Kato, K.; Yamakata, A.; Nozawa, S.; Ito, T.; Terashima, K.; Maeda, K.; Tamaki, Y.; et al. Surface-Specific Modification of Graphitic Carbon Nitride by Plasma for Enhanced Durability and Selectivity of Photocatalytic CO2 Reduction with a Supramolecular Photocatalyst. ACS Appl. Mater. Interfaces 2023, 15, 13205–13218. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, J.; Pang, J.; Wang, A.; Wang, X.; Zhu, W. Fabrication of Pyrimidine/g-C3N4 Nanocomposites for Efficient Photocatalytic Activity under Visible-Light Illumination. Dye. Pigment. 2019, 163, 634–640. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, L.; Tang, Q.; Xiao, X.; Sun, S. Photocatalytic Degradation of Organic Pollutants by Carbon Quantum Dots Functionalized g-C3N4: A Review. Ecotoxicol. Environ. Saf. 2023, 262, 115133. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Xu, T.; Li, Y.; Wang, C. Size Effect of Pt Co-Catalyst on Photocatalytic Efficiency of g-C3N4 for Hydrogen Evolution. Appl. Surf. Sci. 2019, 464, 36–42. [Google Scholar] [CrossRef]
- Caux, M.; Menard, H.; AlSalik, Y.M.; Irvine, J.T.S.; Idriss, H. Photo-Catalytic Hydrogen Production over Au/g-C3N4: Effect of Gold Particle Dispersion and Morphology. Phys. Chem. Chem. Phys. 2019, 21, 15974–15987. [Google Scholar] [CrossRef]
- Fang, X.-X.; Ma, L.-B.; Liang, K.; Zhao, S.-J.; Jiang, Y.-F.; Ling, C.; Zhao, T.; Cheang, T.-Y.; Xu, A.-W. The Doping of Phosphorus Atoms into Graphitic Carbon Nitride for Highly Enhanced Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 11506–11512. [Google Scholar] [CrossRef]
- Qi, K.; Cui, N.; Zhang, M.; Ma, Y.; Wang, G.; Zhao, Z.; Khataee, A. Ionic Liquid-Assisted Synthesis of Porous Boron-Doped Graphitic Carbon Nitride for Photocatalytic Hydrogen Production. Chemosphere 2021, 272, 129953. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, W.; Tan, X.; Yu, T. Potassium Ions Intercalated into g-C3N4-Modified TiO2 Nanobelts for the Enhancement of Photocatalytic Hydrogen Evolution Activity under Visible-Light Irradiation. Nanotechnology 2018, 29, 215706. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Lou, J.; Huang, Y.; Peng, J.; Li, Y.; Liu, Y. Enhance ZnO Photocatalytic Performance via Radiation Modified G-C3N4. Molecules 2022, 27, 8476. [Google Scholar] [CrossRef]
- Li, Q.; Li, F. Recent Advances in Surface and Interface Design of Photocatalysts for the Degradation of Volatile Organic Compounds. Adv. Colloid Interface Sci. 2020, 284, 102275. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent Advances in Photodegradation of Antibiotic Residues in Water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Hasija, V.; Kumar, A.; Sudhaik, A.; Raizada, P.; Singh, P.; Van Le, Q.; Le, T.T.; Nguyen, V.-H. Step-Scheme Heterojunction Photocatalysts for Solar Energy, Water Splitting, CO2 Conversion, and Bacterial Inactivation: A Review. Environ. Chem. Lett. 2021, 19, 2941–2966. [Google Scholar] [CrossRef]
- Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-Scheme Heterojunction TiO2/CdS Nanocomposite Nanofiber as H2-Production Photocatalyst. ChemCatChem 2019, 11, 6301–6309. [Google Scholar] [CrossRef]
- Van Pham, V.; Mai, D.-Q.; Bui, D.-P.; Van Man, T.; Zhu, B.; Zhang, L.; Sangkaworn, J.; Tantirungrotechai, J.; Reutrakul, V.; Cao, T.M. Emerging 2D/0D g-C3N4/SnO2 S-Scheme Photocatalyst: New Generation Architectural Structure of Heterojunctions toward Visible-Light-Driven NO Degradation. Environ. Pollut. 2021, 286, 117510. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 Step-Scheme H2-Production Photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Hooshmand, S.E.; Asgari, E.; Sheikhmohammadi, A.; Rizehbandi, M. WO3 Nanoparticles on Nanosheets of Phosphorus-Doped g-C3N4 as S-Scheme Heterojunction Photocatalysts for the Degradation of Tetracycline. ACS Appl. Nano Mater. 2024, 7, 21659–21673. [Google Scholar] [CrossRef]
- Hassan, A.E.; Elsayed, M.H.; Hussien, M.S.A.; Mohamed, M.G.; Kuo, S.-W.; Chou, H.-H.; Yahia, I.S.; Mohamed, T.A.; Wen, Z. V2O5 Nanoribbons/N-Deficient g-C3N4 Heterostructure for Enhanced Visible-Light Photocatalytic Performance. Int. J. Hydrogen Energy 2023, 48, 9620–9635. [Google Scholar] [CrossRef]
- Yang, C.; Rong, Q.; Shi, F.; Cao, M.; Li, G.; Xin, Y.; Zhang, W.; Zhang, G. Rationally Designed S-Scheme Heterojunction of BiOCl/g-C3N4 for Photodegradation of Sulfamerazine: Mechanism Insights, Degradation Pathways and DFT Calculation. Chin. Chem. Lett. 2024, 35, 109767. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Liu, E.; Xu, J.; Sun, J.; Shi, H. Biochar Decorated Bi4O5Br2/g-C3N4 S-Scheme Heterojunction with Enhanced Photocatalytic Activity for Norfloxacin Degradation. J. Mater. Sci. Technol. 2024, 198, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.; Shi, S.; Jing, X.; Zheng, Z.; Yuan, W. Mechanism Insight into Double S-Scheme Heterojunctions and Atomic Vacancies with Tunable Band Structures for Notably Enhanced Light-Driven Enrofloxacin Decomposition. J. Colloid Interface Sci. 2024, 662, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Yun, S. Construction of 2D/2D g-C3N4@Bim+1Fem-3Ti3O3m+3 (m = 4, 5, 6) S-Scheme Heterojunctions for Efficient Photocatalytic Tetracycline Degradation. J. Mater. Res. 2023, 38, 2943–2957. [Google Scholar] [CrossRef]
- Das, K.K.; Mansingh, S.; Mohanty, R.; Sahoo, D.P.; Priyadarshini, N.; Parida, K. 0D–2D Fe2O3/Boron-Doped g-C3N4 S-Scheme Exciton Engineering for Photocatalytic H2O2 Production and Photo-Fenton Recalcitrant-Pollutant Detoxification: Kinetics, Influencing Factors, and Mechanism. J. Phys. Chem. C 2023, 127, 22–40. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, A.; Sharma, G.; Naushad, M.; Ubaidullah, M.; García-Peñas, A. Developing a g-C3N4/NiFe2O4 S-Scheme Hetero-Assembly for Efficient Photocatalytic Degradation of Cephalexin. Colloids Surf. Physicochem. Eng. Asp. 2022, 654, 129968. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.; Yan, X.; Gan, Y.; Xia, C.; Xu, Y.; Xie, M. Nitrogen-Defect-Modified g-C3N4/BaFe12O19 S-Scheme Heterojunction Photocatalyst with Enhanced Advanced Oxidation Technology Synergistic Photothermal Degradation Ability of Antibiotic: Insights into Performance, Electron Transfer Pathways and Toxicity. Appl. Catal. B Environ. 2024, 343, 123485. [Google Scholar] [CrossRef]
- Hemmati-Eslamlu, P.; Habibi-Yangjeh, A.; Khataee, A. S-Scheme g-C3N4/Ce2S3 Nanocomposites for Visible-Light Activation of Persulfate Ions: Photocatalytic Degradations of Antibiotics and Dyes. J. Photochem. Photobiol. Chem. 2024, 453, 115622. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Yao, B.; Wang, Y. Hollow Core-Shell B-g-C3N4-x@Bi2S3/In2S3 Dual S-Scheme Heterojunction Photothermal Nanoreactor: Boosting Photothermal Catalytic Activity in Confined Space. Chem. Eng. J. 2024, 484, 149399. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-Scheme Heterojunction g-C3N4/TiO2 for Efficient Photocatalytic Degradation of Tetracycline Hydrochloride under UV Light. Colloids Surf. Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Huang, X.; Wu, Y.; Li, L. S-Scheme g-C3N4/TiO2/CFs Heterojunction Composites with Multi-Dimensional through-Holes and Enhanced Visible-Light Photocatalytic Activity. Ceram. Int. 2022, 48, 8196–8208. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Bian, C.; Yang, N.; Qi, H. Designing Novel Step-Scheme Heterojunction g-C3N4/TMCs/GO with Effective Charge Transfer for Photocatalytic Degradation of Organic Pollutant under Visible Light. Sep. Purif. Technol. 2023, 306, 122736. [Google Scholar] [CrossRef]
- Madona, J.; Sridevi, C.; Indumathi, N.; Gokulavani, G.; Velraj, G. A Novel Carbon Doped CeO2/g-C3N4 Heterostructure for Disinfection of Microorganisms and Degradation of Malachite Green and Amoxicillin under Sunlight. Surf. Interfaces 2024, 44, 103803. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Zhang, C.; Chen, F.; Song, Y.; Tang, Y. Construction of a Novel Double S-Scheme Heterojunction CeO2/g-C3N4/Bi2O4 for Significantly Boosted Degradation of Tetracycline: Insight into the Dual Charge Transfer Mode. Chem. Eng. J. 2024, 479, 147333. [Google Scholar] [CrossRef]
- Long, Z.; Shi, H.; Chen, Y. Photothermal-Catalytic Activation Periodate over MnO2/g-C3N4 S-Scheme Heterojunction for Rapidly Tetracycline Removal: Intermediates, Toxicity Evaluation and Mechanism. J. Colloid Interface Sci. 2025, 678, 1169–1180. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Vinesh, V.; Neppolian, B. Construction of S-Scheme 1D/2D Rod-like g-C3N4/V2O5 Heterostructure with Enhanced Sonophotocatalytic Degradation for Tetracycline Antibiotics. Chemosphere 2022, 287, 132380. [Google Scholar] [CrossRef]
- Dai, B.; Li, Y.; Xu, J.; Sun, C.; Li, S.; Zhao, W. Photocatalytic Oxidation of Tetracycline, Reduction of Hexavalent Chromium and Hydrogen Evolution by Cu2O/g-C3N4 S-Scheme Photocatalyst: Performance and Mechanism Insight. Appl. Surf. Sci. 2022, 592, 153309. [Google Scholar] [CrossRef]
- Dou, X.; Li, Q.; Shi, H. Ag Nanoparticle-Decorated 2D/2D S-Scheme g-C3N4/Bi2 WO6 Heterostructures for an Efficient Photocatalytic Degradation of Tetracycline. CrystEngComm 2021, 23, 4638–4647. [Google Scholar] [CrossRef]
- Cao, D.; Su, N.; Wang, X.; Wang, X.; Xu, C.; Liu, Z.; Li, J.; Lu, C. Construction of Unique Floating Bi2WO6/g-C3N4 S-Scheme Heterojunction to Promote Photocatalytic Activity. J. Environ. Chem. Eng. 2024, 12, 112939. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Wang, C.; Wang, Y.; Yin, S.; Javed, M.S.; Han, W. S-Scheme Ti0.7Sn0.3O2/g-C3N4 Heterojunction Composite for Enhanced Photocatalytic Pollutants Degradation. J. Environ. Chem. Eng. 2022, 10, 107118. [Google Scholar] [CrossRef]
- Xu, F.; Chai, B.; Liu, Y.; Liu, Y.; Fan, G.; Song, G. Superior Photo-Fenton Activity toward Tetracycline Degradation by 2D α-Fe2O3 Anchored on 2D g-C3N4: S-Scheme Heterojunction Mechanism and Accelerated Fe3+/Fe2+ Cycle. Colloids Surf. Physicochem. Eng. Asp. 2022, 652, 129854. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Chen, Y.; Shi, H. Efficient Degradation of Tetracycline via Coupling of Photocatalysis and Photo-Fenton Processes over a 2D/2D α-Fe2O3/g-C3N4 S-Scheme Heterojunction Catalyst. Acta Phys. Chim. Sin. 2022, 38, 2201008. [Google Scholar] [CrossRef]
- Xiao, Y.; Yao, B.; Wang, Z.; Chen, T.; Xiao, X.; Wang, Y. Plasma Ag-Modified α-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances. Nanomaterials 2022, 12, 4212. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Zhang, J.; Wang, X. Highly Active BiFeO3–Fe2O3/g-C3N4 S-Scheme Heterojunction with Improved Antibiotic Degradation under Visible-Light: Revealing Mechanism. Res. Chem. Intermed. 2024, 50, 3505–3521. [Google Scholar] [CrossRef]
- Lu, C.; Wang, J.; Cao, D.; Guo, F.; Hao, X.; Li, D.; Shi, W. Synthesis of Magnetically Recyclable g-C3N4/NiFe2O4 S-Scheme Heterojunction Photocatalyst with Promoted Visible-Light-Response Photo-Fenton Degradation of Tetracycline. Mater. Res. Bull. 2023, 158, 112064. [Google Scholar] [CrossRef]
- Yan, J.; Chai, B.; Liu, Y.; Fan, G.; Song, G. Construction of 3D/2D ZnFe2O4/g-C3N4 S-Scheme Heterojunction for Efficient Photo-Fenton Degradation of Tetracycline Hydrochloride. Appl. Surf. Sci. 2023, 607, 155088. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Sonu; Nguyen, V.-H.; Van Le, Q.; Ahamad, T.; Thakur, S.; Kumar, N.; Hussain, C.M.; Singh, P.; et al. Graphene Oxide Modified K, P Co-Doped g-C3N4 and CoFe2O4 Composite for Photocatalytic Degradation of Antibiotics. J. Taiwan Inst. Chem. Eng. 2023, 150, 105077. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Xie, L.-J.; He, L.; Wu, P.-P.; Lu, K.-Q.; Yang, K.; Li, D.; Huang, W.-Y. Constructing Metal Sulfide-Based S-Scheme Heterojunctions for Efficient Photocatalytic Reaction: A Mini Review of Recent Advances. Curr. Opin. Chem. Eng. 2024, 44, 101021. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Li, L.; Cao, W.; Zhang, J.; Zhao, H.; Wang, L. A Novel F-BiVO4/g-C3N4/CdS Dual S-Scheme Heterojunction for High Efficient Photocatalytic Removal of Multiple Pollutants. Appl. Surf. Sci. 2024, 672, 160738. [Google Scholar] [CrossRef]
- Sun, H.; Qin, P.; Guo, J.; Jiang, Y.; Liang, Y.; Gong, X.; Ma, X.; Wu, Q.; Zhang, J.; Luo, L.; et al. Enhanced Electron Channel via the Interfacial Heterotropic Electric Field in Dual S-Scheme g-C3N4/WO3/ZnS Photocatalyst for Year-Round Antibiotic Degradation under Sunlight. Chem. Eng. J. 2023, 470, 144217. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Sun, W.; Guan, X.; Zheng, X.; Li, J. Fabrication of S-Scheme CdS-g-C3N4-Graphene Aerogel Heterojunction for Enhanced Visible Light Driven Photocatalysis. Environ. Res. 2021, 197, 111136. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Chi, Y.; Yin, P.; Wei, L.; Liu, W.; Wang, X.; Zhang, H.; Song, H. Construction of S-Scheme p-n Heterojunction between Protonated g-C3N4 and α-MnS Nanosphere for Photocatalytic H2O2 Production and in Situ Degradation of Oxytetracycline. J. Environ. Chem. Eng. 2023, 11, 109968. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, X.; Zhao, Y.; Zhang, K.; Yan, Y.; Qi, K. Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites. Nanomaterials 2023, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yao, B.; Cao, M.; Wang, Y. Super-Photothermal Effect-Mediated Fast Reaction Kinetic in S-Scheme Organic/Inorganic Heterojunction Hollow Spheres Toward Optimized Photocatalytic Performance. Small 2023, 19, 2207499. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Chu, G.; Wen, K.; Qiu, L.; Li, P.; Cheng, L.; Cao, B.; Tang, Y.; Chen, X.; et al. S-Scheme 2D/2D B-Doped N-Deficient g-C3N4/ZnIn2S4 Heterojunction for Efficient H2 Production Intergrated with Tertracycline Degradation under Visible-Light Illumination. Process Saf. Environ. Prot. 2024, 191, 883–896. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Jiang, X.; Feng, Y.; Ding, X.; Jiang, N.; Wang, J. An Internal Electric Field and Interfacial S–C Bonds Jointly Accelerate S-Scheme Charge Transfer Achieving Efficient Sunlight-Driven Photocatalysis. J. Mater. Chem. A 2022, 10, 25279–25294. [Google Scholar] [CrossRef]
- Yang, X.; Hesami, M.D.; Nazemipool, E.; Bahadoran, A.; Al-Bahrani, M.; Azizi, B. Fabrication of CuCo2S4 Yolk-Shell Spheres Embedded with S-Scheme V2O5-Deposited on Wrinkled g-C3N4 for Effective Promotion of Levofloxacin Photodegradation. Sep. Purif. Technol. 2022, 301, 122005. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, S.; Fang, L.; Yang, Q.; Wu, G.; Lv, H.; Guo, Y.; Song, H. A Flower-like g-C3N4/CDs/Bi2WO6 Hierarchical Structure for Enhanced Photocatalytic Degradation of Tetracycline. J. Mol. Struct. 2025, 1321, 140041. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Su, B.; Ma, J. Hydrothermal Construction of Flower-like g-C3N4/NiZnAl-LDH S-Scheme Heterojunction with Oxygen Vacancies for Enhanced Visible-Light Triggered Photocatalytic Performance. J. Alloys Compd. 2022, 922, 166098. [Google Scholar] [CrossRef]
- Lahootifar, Z.; Habibi-Yangjeh, A.; Salmanzadeh-Jamadi, Z.; Khataee, A. g-C3N4 Tubes Decorated with MnMoO4·H2O: Outstanding S-Scheme Photocatalyst for Detoxification of Water Pollutants upon Visible Light. FlatChem 2024, 48, 100738. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Xie, X.; Wang, H.; Wei, L.; Ma, M.; Yan, Q. Functionalized Carbon Nanotube Bridge Interface Drove Bi2O2CO3/g-C3N4 S-Scheme Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Sep. Purif. Technol. 2021, 274, 119032. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, L.; Jiang, J.; Li, H. Synthesis of S-Scheme 0D/2D Co2ZrO5/g-C3N4 Heterojunction Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for Tetracycline. Diam. Relat. Mater. 2024, 143, 110917. [Google Scholar] [CrossRef]
- Kuate, L.J.N.; Chen, Z.; Yan, Y.; Lu, J.; Guo, F.; Wen, H.; Shi, W. Construction of 2D/3D Black g-C3N4/BiOI S-Scheme Heterojunction for Boosted Photothermal-Assisted Photocatalytic Tetracycline Degradation in Seawater. Mater. Res. Bull. 2024, 175, 112776. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, J.; Wang, A.; Ji, Q.; Cheng, R.; Liang, H.; Chen, F.; Kannan, P.; Brouzgou, A.; Tsiakaras, P. Efficient Photocatalytic Production of H2O2 and Photodegradation of Tetracycline by CdS/Square Tubular g-C3N4 S-Scheme Heterojunction Photocatalyst. Chem. Eng. J. 2024, 479, 147150. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhang, Y.; Qiu, L.; Xing, Y. A 0D/2D Bi4V2O11/g-C3N4 S-Scheme Heterojunction with Rapid Interfacial Charges Migration for Photocatalytic Antibiotic Degradation. Acta Phys. Chim. Sin. 2022, 38, 2112027. [Google Scholar] [CrossRef]
- Hu, G.; Ren, X.; Meng, D.; Gao, D.; Guo, Q.; Hu, X.; Wang, L.; Song, J. Facile Fabrication of S-Scheme Bi2MoO6/g-C3N4/Sepiolite Ternary Photocatalyst for Efficient Tetracycline Degradation under Visible Light. Mater. Sci. Semicond. Process 2023, 166, 107712. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Azimi, H.; Ebadi, M.; Shirmardi, A.; Yousefi, R. Rapid Tetracycline Degradation by S-Scheme Se/g-C3N4 Heterostructure. J. Aust. Ceram. Soc. 2024. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Zhang, J.; Wei, H.; Dang, L. Integrating g-C3N4 Nanosheets with MOF-Derived Porous CoFe2O4 to Form an S-Scheme Heterojunction for Efficient Pollutant Degradation via the Synergy of Photocatalysis and Peroxymonosulfate Activation. Environ. Res. 2024, 241, 117653. [Google Scholar] [CrossRef]
- Shi, J.; Yang, T.; Zhao, T.; Pu, K.; Shi, J.; Zhou, A.; Li, H.; Wang, S.; Xue, J. Insights on the Efficiency and Contribution of Single Active Species in Photocatalytic Degradation of Tetracycline: Priority Attack Active Sites, Intermediate Products and Their Toxicity Evaluation. J. Environ. Manag. 2024, 367, 121970. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, D.; Zhang, R.; Si, P.; Zhang, H.; Wang, X.; Su, N.; Liu, Z.; Lu, C. Construction of Floating Photothermal-Assisted S-Scheme Heterojunction with Enhanced Photocatalytic Degradation of Tetracycline: Insights into Mechanisms, Degradation Pathways and Toxicity Assessment. J. Environ. Manag. 2024, 370, 122586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Zhang, Y.; Wang, Y.; Liang, Q.; Zhou, M.; Xu, S.; Li, Z. Bifunctional Synergistic S-Scheme Mn0.25Cd0.75S/Honeycomb-like g-C3N4 Heterojunction for Efficient Photocatalytic H2 Evolution Integrated with Amoxicillin Degradation. Chem. Eng. J. 2023, 476, 146494. [Google Scholar] [CrossRef]
- Wang, J.; Ren, P.; Du, Y.; Zhao, X.; Chen, Z.; Pei, L.; Jin, Y. Construction of Tubular g-C3N4/TiO2 S-Scheme Photocatalyst for High-Efficiency Degradation of Organic Pollutants under Visible Light. J. Alloys Compd. 2023, 947, 169659. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Z.; Yang, Y.; Kong, W.; Wu, C.; Peng, H.; Li, Z.; Xie, Y.; Zhou, W. Oxygen-Defective Bi2MoO6/g-C3N4 Hollow Tubulars S-Scheme Heterojunctions toward Optimized Photocatalytic Performance. J. Colloid Interface Sci. 2024, 653, 1566–1576. [Google Scholar] [CrossRef]
- Xu, C.-X.; Kong, Y.-L.; Zhang, W.-J.; Yang, M.-D.; Wang, K.; Chang, L.; Chen, W.; Huang, G.-B.; Zhang, J. S-Scheme 2D/2D FeTiO3/g-C3N4 Hybrid Architectures as Visible-Light-Driven Photo-Fenton Catalysts for Tetracycline Hydrochloride Degradation. Sep. Purif. Technol. 2022, 303, 122266. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Miao, C.; Xia, F.; Wang, Y.; Wang, Y.; Liu, C.; Che, G. g-C3N4/BiOI S-scheme Heterojunction: A 2D/2D Model Platform for Visible-Light-Driven Photocatalytic CO2 Reduction and Pollutant Degradation. J. Environ. Chem. Eng. 2022, 10, 108201. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, P.; Wang, Z.; Shen, J.; Han, X.; Zheng, X.; Wei, Y.; Li, C.; Song, K. Aerosol Self-Assembly Synthesis of g-C3N4/MXene/Ag3PO4 Heterostructure for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Colloids Surf. Physicochem. Eng. Asp. 2022, 648, 129392. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Che, G.; Zhu, E.; Guo, H.; Charpentier, P.A.; Xu, W.Z.; Liu, C. Enhanced Photocatalytic Properties of All-Organic IDT-COOH/O–CN S-Scheme Heterojunctions Through π–π Interaction and Internal Electric Field. ACS Appl. Mater. Interfaces 2024, 16, 6367–6381. [Google Scholar] [CrossRef]
- Jiang, S.; Jia, Y.; Yang, X.; Ou, Q.; Liu, Y.; Zhang, S.; Huang, D.; Wang, H. FeCo-LDH/g-C3N4 S-Scheme Photocatalyst with Visible Light Response and Enhanced Photocatalytic Performance. J. Photochem. Photobiol. Chem. 2024, 456, 115870. [Google Scholar] [CrossRef]
- Lee, D.-E.; Moru, S.; Reddy, K.P.; Jo, W.-K.; Tonda, S. 2D/2D BiOIO3/g-C3N4 S-Scheme Hybrid Heterojunction with Face-to-Face Interfacial Contact for Effective Photocatalytic H2 Production and Norfloxacin Degradation. J. Mater. Sci. Technol. 2023, 148, 19–30. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Qi, K.; Liu, S. CuInS2 Quantum-Dot-Modified g-C3N4 S-Scheme Heterojunction Photocatalyst for Hydrogen Production and Tetracycline Degradation. J. Mater. Sci. Technol. 2024, 172, 145–155. [Google Scholar] [CrossRef]
- Chu, Z.; Li, J.; Sohn, H.Y.; Chen, C.; Huang, X.; Lan, Y.; Murali, A.; Zhang, J. CeO2-g-C3N4 S-Scheme Heterojunctions for Enhanced Photocatalytic Performance: Effects of Surface C/N Ratio on Photocatalytic and Adsorption Properties. Compos. Part B Eng. 2023, 257, 110689. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Li, W.; Shao, Z.; Yu, Y.; Yan, H.; Jiao, S.; Lin, D.; Zhang, W.; Lv, C.; et al. P-Doped Ultrathin g-C3N4/In2S3 S-Scheme Heterojunction Enhances Photocatalytic Hydrogen Production and Degradation of Ofloxacin. Phys. B Condens. Matter 2024, 685, 416053. [Google Scholar] [CrossRef]
- Fang, H.; Han, Y.; Feng, X.; Ji, W.; Au, C.-T. S-Scheme Heterojunction g-C3N4/Ag/AgNCO for Efficient Tetracycline Removal in a Photo-Assisted Peroxymonosulfate System. Sep. Purif. Technol. 2022, 296, 121210. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Wang, X.; Li, L.; Chen, Z. Facile Electrospinning Synthesis of S-Scheme Heterojunction CoTiO3/g-C3N4 Nanofiber with Enhanced Visible Light Photocatalytic Activity. Chin. J. Catal. 2024, 59, 237–249. [Google Scholar] [CrossRef]
- Sun, F.; Xu, D.; Xie, Y.; Liu, F.; Wang, W.; Shao, H.; Ma, Q.; Yu, H.; Yu, W.; Dong, X. Tri-Functional Aerogel Photocatalyst with an S-Scheme Heterojunction for the Efficient Removal of Dyes and Antibiotic and Hydrogen Generation. J. Colloid Interface Sci. 2022, 628, 614–626. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic Polyacrylamide-Assisted Construction of Thin 2D-2D WO3/g-C3N4 Step-Scheme Heterojunction for Enhanced Tetracycline Degradation under Visible Light Irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef]
- Jalalat, Z.; Habibi-Yangjeh, A.; Hemmati-Eslamlu, P.; Akinay, Y. Anchoring Modified g-C3N4 with Bi5O7Br: S-Scheme Photocatalysts with Boosted Activities in Elimination of Inorganic and Organic Pollutants. Inorg. Chem. Commun. 2023, 158, 111565. [Google Scholar] [CrossRef]
- Arabian, S.; Gordanshekan, A.; Farhadian, M.; Solaimany Nazar, A.R.; Tangestaninejad, S.; Sabzyan, H. Adsorption/Photocatalytic Degradation of Cefixime by the Green Bi2WO6/g-C3N4/ZIF-67 Dual S-Scheme Heterojunction: Artificial Neural Network, Genetic Algorithm, Density Functional Theory, and Toxicity Assessments. Chem. Eng. J. 2024, 488, 150686. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Fan, J.; Hu, X.; Liu, E.; Yu, Q. Nanoarchitectonics of S-Scheme 0D/2D SbVO4/g-C3N4 Photocatalyst for Enhanced Pollution Degradation and H2 Generation. J. Alloys Compd. 2022, 919, 165752. [Google Scholar] [CrossRef]
- Xu, L.; Dai, R.; Yang, J.; Yan, J.; Zhang, Y.; Dai, Y.; Liao, C.; Zhang, Z.; Zhao, W.; Lei, X.; et al. A Novel S-Scheme g-C3N4/Mn(VO3)2 Heterojunction Photocatalyst for Its Superior Photocatalytic Degradation of Broad-Spectrum Antibiotics. J. Alloys Compd. 2023, 936, 168163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Jia, X.; Zhang, N.; Xia, R.; Zhang, X.; Wang, Z.; Yu, M. In Situ Fabrication of a Novel S-Scheme Heterojunction Photocatalyts Bi2O3/P-C3N4 to Enhance Levofloxacin Removal from Water. Sep. Purif. Technol. 2021, 268, 118691. [Google Scholar] [CrossRef]
- Li, R.; Chen, A.; Deng, Q.; Zhong, Y.; Kong, L.; Yang, R. Well-Designed MXene-Derived Carbon-Doped TiO2 Coupled Porous g-C3N4 to Enhance the Degradation of Ciprofloxacin Hydrochloride under Visible Light Irradiation. Sep. Purif. Technol. 2022, 295, 121254. [Google Scholar] [CrossRef]
- Pham, V.V.; Truong, T.K.; Hai, L.V.; La, H.P.P.; Nguyen, H.T.; Lam, V.Q.; Tong, H.D.; Nguyen, T.Q.; Sabbah, A.; Chen, K.-H.; et al. S-Scheme α-Fe2O3/g-C3N4 Nanocomposites as Heterojunction Photocatalysts for Antibiotic Degradation. ACS Appl. Nano Mater. 2022, 5, 4506–4514. [Google Scholar] [CrossRef]
- Sedaghati, N.; Habibi-Yangjeh, A.; Khataee, A. Fabrication of g-C3N4 Nanosheet/Bi5O7Br/NH2-MIL-88B (Fe) Nanocomposites: Double S-Scheme Photocatalysts with Impressive Performance for the Removal of Antibiotics under Visible Light. Int. J. Miner. Metall. Mater. 2023, 30, 1363–1374. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, M.; Xu, Z.; Xiong, W.; Yang, Z.; Cao, J.; Peng, H.; Xu, H.; Xiang, Y.; Jing, Y. Constructing 2D/2D N-ZnO/g-C3N4 S-Scheme Heterojunction: Efficient Photocatalytic Performance for Norfloxacin Degradation. Chem. Eng. J. 2022, 430, 132652. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, L.; Zhou, M.; Wang, Y.; Xu, S.; Chen, X.; Li, Z. Dual Functional S-Scheme ZnIn2S4/Crystalline Polymeric Carbon Nitride (ZIS/CPCN) Heterojunction for Efficient Photocatalytic Hydrogen Evolution and Degradation of Levofloxacin. Chem. Eng. J. 2024, 495, 153563. [Google Scholar] [CrossRef]
- Tu, B.; Che, R.; Wang, F.; Li, Y.; Li, J.; Qiu, J. Switching Heterojunction System from Type-II to S-Scheme for Efficient Photocatalytic Degradation of Ciprofloxacin. Sep. Purif. Technol. 2024, 345, 127323. [Google Scholar] [CrossRef]
- Chen, P.; Ou, X.; Xia, C.; Wang, K.; Zhang, M.; Wei, M.; Wang, Y. A Novel Dual S-Scheme Heterojunction Photocatalyst KBCN/t-BiVO4/m-BiVO4 Induced by Phase-Transformed Bismuth Vanadate for Highly Efficient Degradation of Ciprofloxacin in Full-Spectrum: Degradation Pathway, DFT Calculation and Mechanism Insight. Sep. Purif. Technol. 2024, 349, 127866. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, L.; Zhu, Y.; Zhang, Y.; Liu, J.; Wang, J.; Feng, C.; Qiao, L.; Chen, Y. Conjugated Polymers S-Scheme Homojunction with Large Internal Electric Field and Matching Interface for Efficient Visible Light Photocatalytic Degradation of Ciprofloxacin. J. Clean. Prod. 2023, 419, 138199. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Zhao, X.; Jiao, Y.; Yan, Y.; Jiang, J. Synergistic Enhancement of Ce–ZnO/g-C3N4 Photocatalytic Performance Using N,O-Bis-Vacancy Induction and S-Scheme Heterojunctions. J. Rare Earths 2024, 42, 817–826. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, L.; Ban, Y.; Li, H. Hydrothermal Synthesis of Step-Scheme Layer g-C3N4/Columnar ZnZrO3 Heterojunction Photocatalyst toward Efficient Norfloxacin Degradation under Solar Light. J. Solid State Chem. 2024, 337, 124815. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, N.; Li, Y.; Zhang, X.; Zhang, L. Step Scheme Fe2O3/S Doped g-C3N4 Heterojunction Photocatalysts for Photo-Fenton Norfloxacin and Tetracycline Degradation. Mater. Sci. Semicond. Process 2023, 160, 107423. [Google Scholar] [CrossRef]
- Lu, G.; Li, W.; Li, Z.; Gu, G.; Han, Q.; Liang, J.; Chen, Z. Enhanced Degradation of Norfloxacin Under Visible Light by S-Scheme Fe2O3/g-C3N4 Heterojunctions. Molecules 2024, 29, 5212. [Google Scholar] [CrossRef]
- Wei, H.; Meng, F.; Yu, W.; Li, J.; Zhang, H. Highly Efficient Photocatalytic Degradation of Levofloxacin by Novel S-Scheme Heterojunction Co3O4/Bi2MoO6@g-C3N4 Hollow Microspheres: Performance, Degradation Pathway and Mechanism. Sep. Purif. Technol. 2023, 318, 123940. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Khazaee, Z.; Mahjoub, A. Electron Flux at the Schottky Junction of Bi NPs and WO3-Supported g-C3N4: An Efficient Ternary S-Scheme Catalyst for Removal of Fluoroquinolone-Type Antibiotics from Water. Environ. Sci. Pollut. Res. 2022, 30, 18461–18479. [Google Scholar] [CrossRef]
- Kong, X.; Cao, L.; Shi, Y.; Chen, Z.; Shi, W.; Du, X. Construction of S-Scheme 2D/2D Crystalline Carbon Nitride/BiOIO3 van Der Waals Heterojunction for Boosted Photocatalytic Degradation of Antibiotics. Molecules 2023, 28, 5098. [Google Scholar] [CrossRef]
- Jia, X.; Hu, C.; Sun, H.; Cao, J.; Lin, H.; Li, X.; Chen, S. A Dual Defect Co-Modified S-Scheme Heterojunction for Boosting Photocatalytic CO2 Reduction Coupled with Tetracycline Oxidation. Appl. Catal. B Environ. 2023, 324, 122232. [Google Scholar] [CrossRef]
- Dou, K.; Peng, C.; Wang, R.; Cao, H.; Yao, C.; Qiu, J.; Liu, J.; Tsidaeva, N.; Wang, W. S-Scheme Tubular g-C3N4/BiOI Heterojunctions for Boosting Photodegradation of Tetracycline and Cr(VI): Mechanism Insight, Degradation Pathway and DFT Calculation. Chem. Eng. J. 2023, 455, 140813. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Tian, F.; Chen, X.; Wu, Z. Synthesis of BiVO4/g-C3N4 S-Scheme Heterojunction via a Rapid and Green Microwave Route for Efficient Removal of Glyphosate. Sep. Purif. Technol. 2022, 287, 120507. [Google Scholar] [CrossRef]
- Deng, X.; Wang, D.; Li, H.; Jiang, W.; Zhou, T.; Wen, Y.; Yu, B.; Che, G.; Wang, L. Boosting Interfacial Charge Separation and Photocatalytic Activity of 2D/2D g-C3N4/ZnIn2S4 S-Scheme Heterojunction under Visible Light Irradiation. J. Alloys Compd. 2022, 894, 162209. [Google Scholar] [CrossRef]
- Liu, S.; Zada, A.; Yu, X.; Liu, F.; Jin, G. NiFe2O4/g-C3N4 Heterostructure with an Enhanced Ability for Photocatalytic Degradation of Tetracycline Hydrochloride and Antibacterial Performance. Chemosphere 2022, 307, 135717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Zhang, K.; Zada, A.; Qi, K. Sonocatalytic Degradation of Tetracycline Hydrochloride with CoFe2O4/g-C3N4 Composite. Ultrason. Sonochem. 2023, 94, 106325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sudhaik, A.; Sonu; Raizada, P.; Nguyen, V.-H.; Van Le, Q.; Ahamad, T.; Thakur, S.; Hussain, C.M.; Singh, P. Integrating K and P Co-Doped g-C3N4 with ZnFe2O4 and Graphene Oxide for S-Scheme-Based Enhanced Adsorption Coupled Photocatalytic Real Wastewater Treatment. Chemosphere 2023, 337, 139267. [Google Scholar] [CrossRef]
- Hou, C.; Niu, M.; Hao, J.; Liu, Q.; Wang, X.; Zhang, M.; Wang, L. Construction of an S-Scheme g-C3N4/TiOF2 Heterostructures with Abundant O Vacancies: Enhanced Photocatalytic Activity and Mechanism Insight. J. Alloys Compd. 2023, 938, 168560. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Guo, H.; Liu, H.-Y.; Niu, C.-G.; Huang, D.-W.; Yang, Y.-Y.; Liang, C.; Li, L.; Li, J.-C. Construction of Dual S-Scheme Ag2CO3/Bi4O5I2/g-C3N4 Heterostructure Photocatalyst with Enhanced Visible-Light Photocatalytic Degradation for Tetracycline. Chem. Eng. J. 2022, 438, 135471. [Google Scholar] [CrossRef]
- Feyzi, L.; Rahemi, N.; Allahyari, S. Efficient Degradation of Tetracycline in Aqueous Solution Using a Coupled S-Scheme ZnO/g-C3N4/Zeolite P Supported Catalyst with Water Falling Film Plasma Reactor. Process Saf. Environ. Prot. 2022, 161, 827–847. [Google Scholar] [CrossRef]






| Classification | Photocatalyst | Performance Characteristics | Application | Reference |
|---|---|---|---|---|
| g-C3N4/metal oxide | P-CN/WO3 | Promotes charge separation and strong redox ability. | Remove pollutants from wastewater. | [31] |
| V2O5/N-deficient g-C3N4 | Rapid charge separation; enhanced visible-light absorption. | Remove organic pollutants (dyes and antibiotics). | [32] | |
| g-C3N4/multicomponent metal oxide | BiOCl/g-C3N4 | High removal rate; good stability; efficient charge transfer. | Remove the sulfonamide antibiotic sulfamerazine. | [33] |
| Bi4O5Br2/g-C3N4 | Effectively improve the separation of photogenerated carriers. | The degradation of contaminants like NOR in water. | [34] | |
| CeO2-x/C3-yN4/ Ce(CO3)(OH)-2 | High-efficiency photocatalytic performance; advantages of the unique heterojunction structure. | Photocatalytic degradation of enrofloxacin. | [35] | |
| C3N4@Bim + 1Fem-3Ti3O3m + 3 (m = 4, 5, 6) | High electrical conductivity; efficient charge separation ability. | The degradation of tetracycline under visible light. | [36] | |
| g-C3N4/magnetic oxide | Fe2O3 QD/B-g-C3N4 | High catalytic activity; high carrier separation efficiency. | The degradation of antibiotics such as amoxicillin. | [37] |
| g-C3N4/multicomponent magnetic oxide | g-C3N4/NiFe2O4 | High degradation efficiency; effective charge separation; good stability and easy recovery. | The degradation of cephalexin in water. | [38] |
| g-C3N4/BaFe12O19 | Accelerated electron migration; high degradation efficiency; low-toxicity degradation products. | The degradation of antibiotics, such as enrofloxacin. | [39] | |
| g-C3N4/metal sulfide | g-C3N4/Ce2S3 | Excellent performance under visible light; remarkable structural advantages; good cycle stability. | Activate persulfate ions and remove three antibiotics, including tetracycline, amoxicillin, and azithromycin. | [40] |
| g-C3N4/multicomponent metal sulfide | B-g-C3N4-x@Bi2S3/In2S3 | Generate heat; enhanced chemical reaction kinetics. | Tetracycline degradation and hydrogen evolution. | [41] |
| Antibiotic Degradation | Photocatalyst | Performance Characteristics | Synthesis Strategy | k (min−1) | Reference |
|---|---|---|---|---|---|
| Tetracycline | WO3/g-C3N4 | Significantly enhanced photocatalytic performance, fast charge transfer, high quantum efficiency. | Constructed with anionic polyacrylamide (APAM), where APAM acts as an auxiliary template and a carbon source. | 0.0378 | [98] |
| Amoxicillin Tetracycline | g-C3N4(M)/Bi5O7Br | Good degradation or conversion capabilities. Under visible light, it can generate more charges. | Prepared by a facile precipitation method. | — | [99] |
| Cefixime | Bi2WO6/g-C3N4/ZIF | Good photocatalytic adsorption, degradation, along with certain stability and reusability. | Hydrothermal synthesis method. | — | [100] |
| Ceftriaxone Sodium | SbVO4/g-C3N4 | The charge carriers with high redox activity enhance its activity. | A simple physical mixing strategy. | 0.0159 | [101] |
| Sulfamethoxazole | g-C3N4/Mn(VO3)2 | Demonstrating high-efficiency photocatalytic performance and stability. | Microwave hydrothermal method. | — | [102] |
| Levofloxacin | Bi2O3/P-C3N4 | Spatially separate the electrons and holes, and the BET specific surface area and hydrophilicity are improved. | In situ thermal polymerization. | 0.0276 | [103] |
| Ciprofloxacin Hydrochloride | g-C3N4/C-TiO2 | The larger specific surface area of the sample greatly improved the charge separation efficiency and photocatalytic performance. | One-step calcination method. | 0.0411 | [104] |
| Commercial Cefalexin and Amoxicillin | α-Fe2O3/g-C3N4 | It is easy to improve the recycling performance, and the performance of degrading antibiotics is excellent. | Synthesized by a simple method. | 0.0200 | [105] |
| Azithromycin, Metronidazole, and Cephalexin | GCN-NSh/Bi5O7Br/Fe—MOF | It has a double S-Scheme charge transfer mechanism and good cycling stability. | Synthesized via a facile solvothermal route. | — | [106] |
| Norfloxacin, Enrofloxacin, Levofloxacin, and Ciprofloxacin | 2D/2D N-ZnO/CN | Strong light capture capacity, effective migration and separation of carriers, and highly efficient photocatalytic performance. | Ultrasonic-assisted electrostatic self-assembly method. | 0.0340 | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Xu, K.; Zhao, Y.; Li, B.; Jiang, S.; Liu, Y.; Huang, S.; Yang, Q.; Gao, T.; Xie, S.; et al. Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation. Molecules 2025, 30, 1240. https://doi.org/10.3390/molecules30061240
Huang B, Xu K, Zhao Y, Li B, Jiang S, Liu Y, Huang S, Yang Q, Gao T, Xie S, et al. Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation. Molecules. 2025; 30(6):1240. https://doi.org/10.3390/molecules30061240
Chicago/Turabian StyleHuang, Bin, Kaidi Xu, Yu Zhao, Bohao Li, Siyuan Jiang, Yaxin Liu, Shengnan Huang, Qingyuan Yang, Tianxiang Gao, Simeng Xie, and et al. 2025. "Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation" Molecules 30, no. 6: 1240. https://doi.org/10.3390/molecules30061240
APA StyleHuang, B., Xu, K., Zhao, Y., Li, B., Jiang, S., Liu, Y., Huang, S., Yang, Q., Gao, T., Xie, S., Chen, H., & Li, Y. (2025). Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation. Molecules, 30(6), 1240. https://doi.org/10.3390/molecules30061240







