Influence of Malted Chickpea on the Composition of Volatiles in Hummus
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.1.1. Raw Material
3.1.2. Reagents and Standards
3.2. Methods
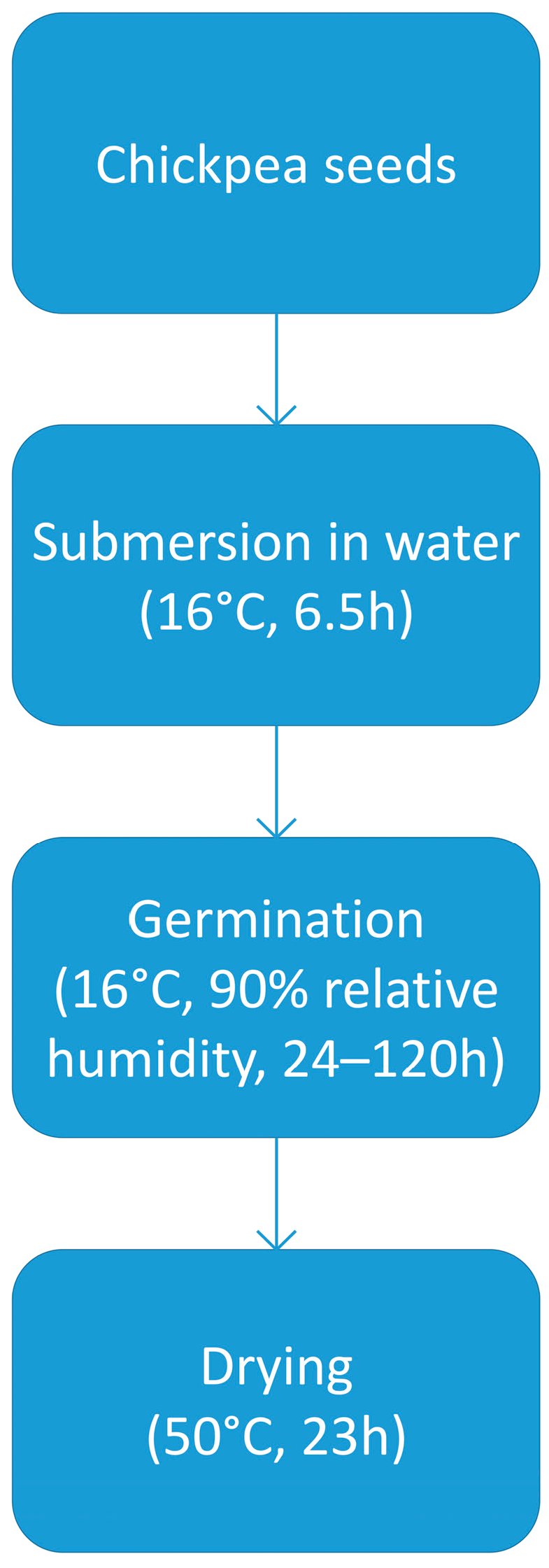
3.2.1. Chickpea Malt Production
3.2.2. Hummus Production
- Hummus produced from unmalted chickpea seeds (0D);
- Hummus produced from chickpea malt germinated for 24 h (1D);
- Hummus produced from chickpea malt germinated for 48 h (2D);
- Hummus produced from chickpea malt germinated for 72 h (3D);
- Hummus produced from chickpea malt germinated for 96 h (4D);
- Hummus produced from chickpea malt germinated for 120 h (5D).
3.2.3. Extraction and Analysis of Volatile Compounds in the Hummus
3.2.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nussbaum, H. Hummus: A Global History; Reaktion Books: London, UK, 2021. [Google Scholar]
- Andersen, N.R.; van Deurs Petersen, R.; Frøst, M.B. Consumer interest in hummus made from different pulses: Effects of information about origin and variety seeking tendency. Int. J. Gastron. Food Sci. 2022, 29, 100572. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing and Malting, 6th ed.; VLB Berlin: Berlin, Germany, 2019. [Google Scholar]
- Gasiński, A.; Kawa-Rygielska, J. Mashing quality and nutritional content of lentil and bean malts. LWT 2022, 169, 113927. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G. Changes in the raffinose family oligosaccharides content in the lentil and common bean seeds during malting and mashing processes. Sci. Rep. 2022, 12, 17911. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Henle, T. Maillard Reaction Products in Different Types of Brewing Malt. J. Agric. Food Chem. 2020, 68, 14274–14285. [Google Scholar] [CrossRef]
- Filipowska, W.; Jaskula-Goiris, B.; Ditrych, M.; Bustillo Trueba, P.; De Rouck, G.; Aerts, G.; Powell, C.; Cook, D.; De Cooman, L. On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: A review. J. Inst. Brew. 2021, 127, 107–126. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J. Malting—A method for modifying volatile composition of black, brown and green lentil seeds. PLoS ONE 2023, 18, e0290616. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J. Assessment of green lentil malt as a substrate for gluten-free beer brewing. Sci. Rep. 2024, 14, 504. [Google Scholar] [CrossRef]
- Jiménez, M.J.; Tárrega, A.; Fuentes, R.; Canet, W.; Álvarez, M.D. Consumer perceptions, descriptive profile, and mechanical properties of a novel product with chickpea flour: Effect of ingredients. Food Sci. Technol. Int. 2016, 22, 547–562. [Google Scholar] [CrossRef]
- Acevedo Martinez, K.A.; Yang, M.M.; Gonzalez de Mejia, E. Technological properties of chickpea (Cicer arietinum): Production of snacks and health benefits related to type-2 diabetes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3762–3787. [Google Scholar] [CrossRef]
- Losano Richard, P.; Steffolani, M.E.; Allende, M.J.; Carreras, J.; León, A.E. By-products of the classification of chickpea as an alternative in the production of hummus. Int. J. Food Sci. Technol. 2021, 56, 1759–1765. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Zafar, T.; Benyathiar, P.; Nasir, M. Production, processing, and nutritional profile of chickpeas and lentils. In Dry Beans and Pulses: Production, Processing, and Nutrition, 2nd ed.; Siddiq, M., Uebersax, M.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar]
- Martínez-Preciado, A.H.; Ponce-Simental, J.A.; Schorno, A.L.; Contreras-Pacheco, M.L.; Michel, C.R.; Rivera-Ortiz, K.G.; Soltero, J.F.A. Characterization of nutritional and functional properties of “Blanco Sinaloa” chickpea (Cicer arietinum L.) variety, and study of the rheological behavior of hummus pastes. J. Food Sci. Technol. 2020, 57, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Available online: https://www.thegoodscentscompany.com/ (accessed on 25 February 2025).
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Aroma Compounds. In Food Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 340–400. [Google Scholar]
- Schmidt, R.; Cain, W.S. Making scents: Dynamic olfactometry for threshold measurement. Chem. Senses 2010, 35, 109–120. [Google Scholar] [CrossRef]
- Yu, M.G.; Zheng, C.D.; Li, T.; Song, H.L.; Wang, L.J.; Zhang, S.H.; Xie, Q.G.; Jiang, S.L. Comparison of aroma properties of infant formulas: Differences in key aroma compounds and their possible origins in processing. J. Dairy Sci. 2023, 106, 5970–5987. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Inoue, T.; Shikata, H.; Sakakibara, K. Evaluation of the Odor Activity of Pyrazine Derivatives Using Structural and Electronic Parameters Derived from Conformational Study by Molecular Mechanics (MM3) and Ab Initio Calculations. J. Mol. Struct. 2005, 749, 169–176. [Google Scholar] [CrossRef]
- Forss, D.A. Odor and flavor compounds from lipids. Prog. Chem. Fats Other Lipids. 1972, 13, 177–258. [Google Scholar] [CrossRef]
- Tandon, K.; Baldwin, E.; Shewfelt, R. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Evans, C.D.; Moser, H.A.; List, G.R. Odor and flavor responses to additives in edible oils. J. Am. Oil Chem. Soc. 1971, 48, 495–498. [Google Scholar] [CrossRef]
- Chastrette, M.; Cretin, D.; Aïdi, E. Structure–Odor relationships: Using neural networks in the estimation of camphoraceous or fruity odors and olfactory thresholds of aliphatic alcohols. J. Chem. Inf. Comput. Sci. 1996, 36, 108–113. [Google Scholar] [CrossRef]
- Xue, J.; Guo, G.; Liu, P.; Chen, L.; Wang, W.; Zhang, J.; Yin, J.; Ni, D.; Englehardt, U.H.; Jiang, H. Identification of aroma-active compounds responsible for the floral and sweet odors of Congou black teas using gas chromatography–mass spectrometry/olfactometry, odor activity value, and chemometrics. J. Sci. Food Agric. 2022, 102, 5399–5410. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Huang, L.; Li, M.; Gao, J.; Liu, C.; Bao, X.; Huang, Z.; Yang, J.; Huang, H.; et al. Comparison and identification of aroma components in 21 kinds of frankincense with variety and region based on the odor intensity characteristic spectrum constructed by HS–SPME–GC–MS combined with E-nose. Food Res. Int. 2024, 195, 114942. [Google Scholar] [CrossRef]
- Tajima, K.; Tanaka, S.; Yamaguchi, T.; Fujita, M. Analysis of green and yellow yuzu peel oils (Citrus junos Tanaka). Novel aldehyde components with remarkably low odor thresholds. J. Agric. Food Chem. 1990, 38, 1544–1548. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- Ritter, S.W.; Gastl, M.I.; Becker, T.M. The modification of volatile and nonvolatile compounds in lupines and faba beans by substrate modulation and lactic acid fermentation to facilitate their use for legume-based beverages—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4018–4055. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, S.; Mariani, M.; Alberti, J.C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile flavor profile of Saskatchewan grown pulses as affected by different thermal processing treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef]
- Trindler, C.; Kopf-Bolanz, K.A.; Denkel, C. Aroma of peas, its constituents and reduction strategies–effects from breeding to processing. Food Chem. 2021, 376, 131892. [Google Scholar] [CrossRef]
- Reister, E.J.; Belote, L.N.; Leidy, H.J. The Benefits of Including Hummus and Hummus Ingredients into the American Diet to Promote Diet Quality and Health: A Comprehensive Review. Nutrients 2020, 12, 3678. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of Pyrazines in Maillard Model Systems: Effects of Structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Wainaina, I.; Kyomugasho, C.; Delbaere, S.; Wafula, E.; Van Loey, A.; Sila, D.; Hendrickx, M. (Bio) chemical reactions associated with ageing of red kidney beans (Phaseolus vulgaris) during storage probed by volatile profiling: The role of glass transition temperature. Food Res. Int. 2022, 162, 112102. [Google Scholar] [CrossRef]
- Huang, X.Q.; Li, R.; Fu, J.; Dudareva, N. A Peroxisomal Heterodimeric Enzyme Is Involved in Benzaldehyde Synthesis in Plants. Nat. Commun. 2022, 13, 1352. [Google Scholar] [CrossRef]
- Liang, X.; Qian, R.; Wang, D.; Liu, L.; Sun, C.; Lin, X. Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses. Biology 2022, 11, 1590. [Google Scholar] [CrossRef]
- Mao, H.; Yuan, S.; Li, Q.; Zhao, X.; Zhang, X.; Liu, H.; Ming, Y.; Wang, M. Influence of germination on the bioactivity, structural, functional and volatile characteristics of different chickpea flours. Food Chem. X 2024, 21, 101195. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Lan, Y.; Rao, J.; Chen, B. HS-SPME-GC-MS/olfactometry combined with chemometrics to assess the impact of germination on flavor attributes of chickpea, lentil, and yellow pea flours. Food Chem. 2019, 280, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Rajhi, I.; Ouertani, R.N.; Rajhi, F.; Mhadhbi, H.; Flamini, G. Fingerprinting of volatile profiles of sprouted and unsprouted seeds and flours of Phaseolus vulgaris using HS-SPME/GC-MS. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris). Molecules 2022, 27, 8204. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Liang, L.S.Y.; Balasubramanian, P. Volatile Compounds of dry beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 2007, 62, 177. [Google Scholar] [CrossRef]
- Rajhi, I.; Baccouri, B.; Rajhi, F.; Hammami, J.; Souibgui, M.; Amri, M.; Mhadhbi, H.; Flamini, G. Evaluation of germination effect on volatile compounds of different faba bean cultivars using HS-SPME/GC-MS. J. Food Compos. Anal. 2022, 112, 104692. [Google Scholar] [CrossRef]
- Lampi, A.M.; Yang, Z.; Mustonen, O.; Piironen, V. Potential of faba bean lipase and lipoxygenase to promote formation of volatile lipid oxidation products in food models. Food Chem. 2020, 311, 125982. [Google Scholar] [CrossRef]
- Yang, M.; Hou, L.; Dong, Y.; Wang, B.; Liu, H.; Wang, X. SAFE-GC-O-MS and descriptive sensory analysis were used to reveal the chemical sensory characteristics of sesame paste (tahini) at different storage stages. Food Chem. 2024, 454, 139809. [Google Scholar] [CrossRef]
- Adelina, N.M.; Wang, H.; Zhang, L.; Zhao, Y. Comparative Analysis of Volatile Profiles in Two Grafted Pine Nuts By Headspace-Spme/Gc-Ms and Electronic Nose As Responses To Different Roasting Conditions. Food Res. Int. 2021, 140, 110026. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.; Hamel, C.; Yang, C.; Matsubara, T.; Gan, Y.; Singh, A.K.; Kuwada, K.; Ishii, T. Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. Phytochemistry 2012, 78, 72–80. [Google Scholar] [CrossRef]
- Ma, S.; Ding, C.; Shi, H.; Zhang, H.; Bi, Y.; Xu, X. Impact of the Water Content in Peanut Kernels on the Generation of Benzeneacetaldehyde in Roasted Peanut Oils. ACS Food Sci. Technol. 2024, 4, 1756–1764. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, L.; Dong, S.; Li, Z.; Liu, Y.; Wu, Y.; Li, S.; Ye, L. Targeted metabolomics reveals the contribution of degradation and oxidation of lipids and proteins mediated by pH to the formation of characteristic volatiles in preserved egg yolk during pickling. Food Res. Int. 2024, 195, 114945. [Google Scholar] [CrossRef]
- Waldvogel, S.R. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sezer, E.N.Ş.; Yorgancılar, M.; Uysal, T. The Characterization of Volatile Compounds of Lupin Türkiye Genotype HS-SPME/GC-MS Method. Selcuk. J. Agric. Food Sci. 2023, 37, 515–523. [Google Scholar]
- Osei-Owusu, J.; Vuts, J.; Caulfield, J.C.; Woodcock, C.M.; Withall, D.M.; Hooper, A.M.; Osafo-Acquaah, S.; Birkett, M.A. Identification of Semiochemicals from cowpea, Vigna unguiculata, for low-input management of the legume pod borer, Maruca vitrata. J. Chem. Ecol. 2020, 46, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Vurro, F.; De Angelis, D.; Squeo, G.; Caponio, F.; Summo, C.; Pasqualone, A. Exploring Volatile Profiles and De-Flavoring Strategies for Enhanced Acceptance of Lentil-Based Foods: A Review. Foods 2024, 13, 2608. [Google Scholar] [CrossRef] [PubMed]
- Bozdemir, S.; Güneşer, O.; Yilmaz, E. Properties and Stability of Deep-Fat Fried Chickpea Products. Grasas Aceites 2015, 66, e065. [Google Scholar] [CrossRef]
- Viana, L.; English, M. The application of chromatography in the study of off-flavour compounds in pulses and pulse by-products. LWT—Food Sci. Technol. 2021, 150, 111981. [Google Scholar] [CrossRef]
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids–A Biofortification Approach. Front. Plant Sci. 2021, 12, 734980. [Google Scholar] [CrossRef]
- Noordraven, L.E.C.; Buvé, C.; Grauwet, T.; Van Loey, A.M. Effect of experimental flour preparation and thermal treatment on the volatile properties of aqueous chickpea flour suspensions. LWT 2022, 160, 113171. [Google Scholar] [CrossRef]
- Filipowska, W.; Bolat, I.; De Rouck, G.; Bauwens, J.; Cook, D.; De Cooman, L. Formation of staling aldehydes in different grain bed layers in an industrial scale maltings. J. Inst. Brew. 2023, 129, 276–306. [Google Scholar] [CrossRef]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. A Comprehensive Characterisation of Volatile and Fatty Acid Profiles of Legume Seeds. Foods 2019, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; He, L.; Xing, R.; Yu, N.; Chen, Y. New Insight into the Evolution of Volatile Profiles in Four Vegetable Oils with Different Saturations during Thermal Processing by Integrated Volatolomics and Lipidomics Analysis. Food Chem. 2023, 403, 134342. [Google Scholar] [CrossRef]

| Compound | KI Exp. | KI Lit. 1 | Similarity Search | Perceived Odor of the Compound 2 | Odor Threshold [ppb] 3 |
|---|---|---|---|---|---|
| 1-Hexanol | 849 | 863 | 95% | green; herbaceous; woody; sweet, beany; grassy | 2500 |
| 2-Heptanone | 874 | 889 | 95% | fruity; spicy; sweet; herbal; coconut; woody | 224 |
| Heptanal | 891 | 902 | 96% | fresh; aldehydic; fatty; green; herbal; cognac; ozone | 2.8 |
| Pyrazine, 2,5- dimethyl- | 905 | 911 | 92% | nutty; peanutty; musty; earthy; powdery; roasted; cocoa-like. | 7.9 |
| Benzaldehyde | 949 | 960 | 94% | almond; fruity; nutty. | 350 |
| 1-Heptanol | 962 | 966 | 92% | oily; nutty; fatty; green; aldehydic | 520 |
| 1-Octen-3-ol | 974 | 980 | 93% | beany, mushroom; fungal; earthy; floral. | 100 |
| 5-Hepten-2-one, 6-methyl- | 979 | 985 | 96% | fatty; green; citrusy. | 525 |
| Furan, 2-pentyl | 984 | 988 | 95% | fruity; green; earthy; beany | 4.8 |
| Pyrazine, 2-ethyl-3-methyl- | 999 | 1002 | 96% | nutty; peanut; musty; corn-like; earthy; bready. | 0.55 |
| Octanal | 1004 | 998 | 93% | aldehydic; waxy; citrusy; soapy, fatty, orange-like. | 3.4 |
| 1-Hexanol, 2-ethyl | 1008 | 1002 | 91% | rosey; citrusy; fresh; floral; oily; sweet. | 1493 |
| 3-Octen-2-one | 1028 | 1030 | 94% | earthy; spicy; herbal; sweet; mushroom; hay-like; blueberry. | 6.7 |
| Benzeneacetaldehyde | 1046 | 1042 | 92% | honey; sweet; floral; chocolate; cocoa | 4 |
| β-Ocimene | 1052 | 1050 | 91% | floral; herbal; flowery; sweet. | 34 |
| 1-Octanol | 1061 | 1068 | 94% | waxy; green; citrusy; aldehydic; oily, floral; sweet; fatty; coconutty. | 4.4 |
| Pyrazine, 3-ethyl-2,5-dimethyl- | 1079 | 1074 | 96% | potato-like; cocoa; roasted; nutty. | 0.00186 |
| Nonanal | 1108 | 1100 | 94% | waxy; aldehydic; citrusy; fresh; green; lemon peel; cucumber-like; fatty. | 2.8 |
| trans-2-Nonenal | 1159 | 1149 | 93% | beany; green; soapy; cucumber-like; melon; aldehydic; fatty. | 0.19 |
| Decanal | 1213 | 1201 | 94% | sweet; aldehydic; orange; waxy; citrus rind. | 9.3 |
| Undecanal | 1314 | 1306 | 92% | waxy; aldehydic; soapy; citrusy. | 5 |
| Dodecanal | 1419 | 1408 | 91% | soapy; waxy; citrus; orange; mandarin. | 0.53 |
| Compound | 0D 1 | 1D | 2D | 3D | 4D | 5D |
|---|---|---|---|---|---|---|
| 1-Hexanol | 136.45 ± 21.32 c | 61.37 ± 14.66 d | 66.82 ± 6.48 d | 71.55 ± 11.19 d | 335.48 ± 23.59 b | 434.21 ± 34.21 a |
| 2-Heptanone | 15.26 ± 4.57 b | 18.20 ± 3.42 b | 13.47 ± 3.26 b | 18.07 ± 2.56 b | 44.55 ± 19.07 a | 44.32 ± 18.19 a |
| Heptanal | 15.22 ± 2.78 b | 7.60 ± 1.69 c | 7.04 ± 1.67 c | 7.50 ± 1.63 c | 44.23 ± 13.78 a | 53.02 ± 9.56 a |
| Pyrazine, 2,5- dimethyl- | 15.03 ± 1.16 b | 17.27 ± 1.97 b | 16.24 ± 1.25 b | 16.69 ± 1.27 b | 23.18 ± 5.74 a | 23.73 ± 4.21 a |
| Benzaldehyde | 16.50 ± 3.26 bc | 10.76 ± 3.33 cd | 12.10 ± 1.39 c | 9.55 ± 0.82 d | 35.16 ± 7.44 a | 29.85 ± 8.42 a |
| 1-Heptanol | 10.87 ± 1.02 c | 16.16 ± 3.05 b | 6.41 ± 0.58 d | 4.70 ± 0.37 e | 23.19 ± 3.76 a | 22.22 ± 3.59 a |
| 1-Octen-3-ol | 12.37 ± 2.48 b | 6.18 ± 0.75 d | 7.32 ± 0.30 c | 6.41 ± 0.66 cd | 27.02 ± 9.86 a | 34.09 ± 11.05 a |
| 5-Hepten-2-one, 6-methyl- | 17.41 ± 3.45 bc | 11.88 ± 1.81 d | 9.74 ± 1.25 d | 8.99 ± 2.35 d | 37.63 ± 15.11 ab | 33.67 ± 10.81 ab |
| Furan, 2-pentyl | 21.89 ± 8.21 c | 8.96 ± 2.84 e | 12.97 ± 2.50 d | 14.96 ± 1.75 d | 62.98 ± 23.02 b | 114.80 ± 28.42 a |
| Pyrazine, 2-ethyl-3-methyl- | 9.99 ± 1.37 b | 6.69 ± 1.05 c | 9.33 ± 0.67 b | 7.33 ± 0.93 bc | 19.66 ± 6.19 a | 23.32 ± 5.34 a |
| Octanal | 15.57 ± 4.09 cd | 15.33 ± 6.43 cd | 6.28 ± 2.30 e | 11.32 ± 2.01 d | 47.55 ± 11.63 b | 82.34 ± 20.87 a |
| 1-Hexanol, 2-ethyl | 7.29 ± 0.33 a | 5.27 ± 0.39 b | 6.54 ± 1.11 ab | 6.63 ± 0.97 ab | 7.50 ± 1.15 a | 6.52 ± 1.62 ab |
| 3-Octen-2-one | 9.14 ± 1.85 b | 3.52 ± 0.80 c | 3.46 ± 0.88 c | 4.24 ± 0.18 c | 16.69 ± 8.70 ab | 19.00 ± 6.54 a |
| Benzeneacetaldehyde | 41.30 ± 8.89 b | 17.75 ± 4.12 c | 20.47 ± 1.94 c | 19.19 ± 2.02 c | 69.59 ± 12.01 a | 68.81 ± 14.17 a |
| β-Ocimene | 29.34 ± 12.31 a | 9.30 ± 2.02 b | 8.11 ± 3.20 b | 8.67 ± 1.65 b | 7.67 ± 1.43 a | 7.11 ± 1.07 a |
| 1-Octanol | 11.67 ± 1.96 b | 9.64 ± 2.09 bc | 5.10 ± 1.73 c | 7.70 ± 1.06 c | 21.96 ± 7.95 a | 29.65 ± 13.48 a |
| Pyrazine, 3-ethyl-2,5-dimethyl- | 5.56 ± 2.13 bc | 3.25 ± 0.86 cd | 2.89 ± 0.26 d | 3.82 ± 1.51 cd | 9.09 ± 4.19 ab | 7.32 ± 2.28 ab |
| Nonanal | 33.65 ± 8.91 c | 38.11 ± 5.81 c | 24.70 ± 4.14 d | 30.16 ± 6.91 c | 102.85 ± 21.17 b | 181.85 ± 43.39 a |
| trans-2-Nonenal | 3.03 ± 0.63 b | n.d. | 1.67 ± 0.38 c | 1.61 ± 0.37 c | 5.06 ± 2.16 ab | 8.38 ± 2.43 a |
| Decanal | 17.33 ± 5.91 d | 42.56 ± 20.61 bc | 9.91 ± 2.34 e | 26.24 ± 4.80 c | 69.96 ± 20.91 b | 169.55 ± 47.17 a |
| Undecanal | n.d. | 1.33 ± 0.79 bc | 0.73 ± 0.42 c | 0.90 ± 0.37 c | 2.97 ± 1.15 b | 6.50 ± 2.58 a |
| Dodecanal | 3.16 ± 0.44 a | 1.86 ± 0.34 c | 1.69 ± 0.52 cd | 1.20 ± 0.20 d | 2.41 ± 0.26 b | 1.34 ± 0.49 cd |
| Compound | LOD [ppb] | LOQ [ppb] |
|---|---|---|
| 1-Hexanol | 43.72 | 132.5 |
| 2-Heptanone | 1.57 | 4.77 |
| Heptanal | 1.95 | 5.91 |
| Pyrazine, 2,5- dimethyl- | 2.02 | 6.13 |
| Benzaldehyde | 7.4 | 22.44 |
| 1-Heptanol | 1.9 | 5.77 |
| 1-Octen-3-ol | 2.55 | 7.74 |
| 5-Hepten-2-one, 6-methyl- | 5.15 | 15.6 |
| Furan, 2-pentyl | 4.97 | 15.08 |
| Pyrazine, 2-ethyl-3-methyl- | 3.46 | 10.49 |
| Octanal | 5.73 | 17.35 |
| 1-Hexanol, 2-ethyl | 3.81 | 11.56 |
| 3-Octen-2-one | 0.53 | 1.61 |
| Benzeneacetaldehyde | 17.07 | 51.71 |
| β-Ocimene | 4.94 | 14.97 |
| 1-Octanol | 3.22 | 9.77 |
| Pyrazine, 3-ethyl-2,5-dimethyl- | 0.4 | 1.2 |
| Nonanal | 12.52 | 37.95 |
| trans-2-Nonenal | 0.24 | 0.73 |
| Decanal | 3.96 | 11.99 |
| Undecanal | 0.65 | 1.96 |
| Dodecanal | 0.27 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasiński, A.; Noguera-Artiaga, L.; Kawa-Rygielska, J. Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules 2025, 30, 1231. https://doi.org/10.3390/molecules30061231
Gasiński A, Noguera-Artiaga L, Kawa-Rygielska J. Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules. 2025; 30(6):1231. https://doi.org/10.3390/molecules30061231
Chicago/Turabian StyleGasiński, Alan, Luis Noguera-Artiaga, and Joanna Kawa-Rygielska. 2025. "Influence of Malted Chickpea on the Composition of Volatiles in Hummus" Molecules 30, no. 6: 1231. https://doi.org/10.3390/molecules30061231
APA StyleGasiński, A., Noguera-Artiaga, L., & Kawa-Rygielska, J. (2025). Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules, 30(6), 1231. https://doi.org/10.3390/molecules30061231







