Evaluation of NO Synthase Activity in Meat-Brining Solutions: Implications for Meat Curing and Color Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. pH and Color Analysis
2.2. Nitrite Content
2.3. Arginine and Citrulline Content
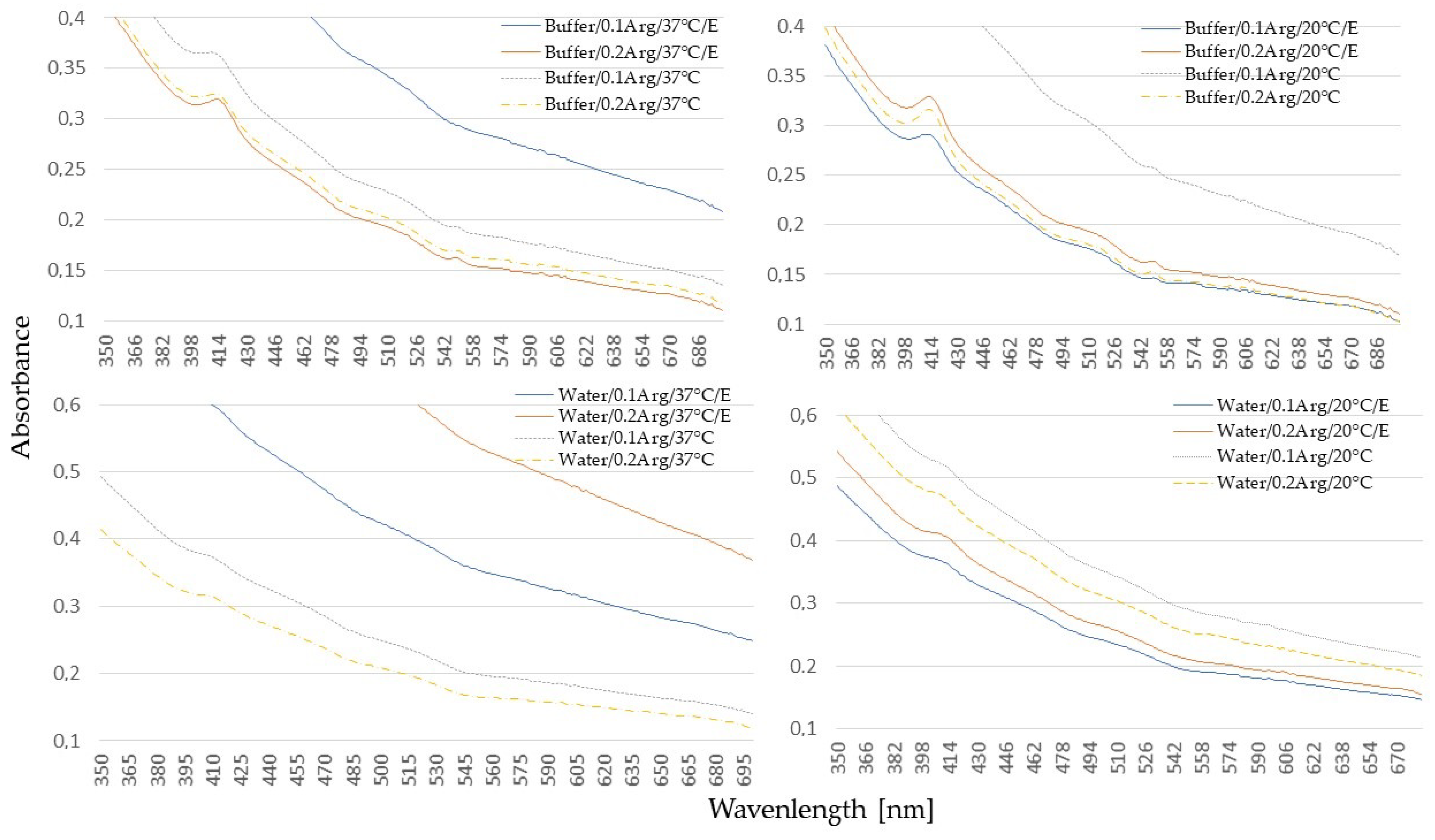
2.4. UV-Vis Analysis
2.5. Raman Spectra
3. Materials and Methods
3.1. Materials
3.2. Meat Sample Preparation
3.3. pH Measurement
3.4. CIELab Color Analysis
3.5. Nitrite Content Analysis
3.6. Arginine and Citrulline Content Analysis
3.6.1. Sample Preparation
3.6.2. HPLC Analysis
3.7. UV-Vis Spectra Analysis of Myoglobin Forms
3.8. Raman Data Acquisition and Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores, M.; Toldra, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products—Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- Melios, S.; Grasso, S.; Bolton, D.; Crofton, E. Sensory quality and consumer perception of reduced/free-from nitrates/nitrites cured meats. Curr. Opin. Food Sci. 2024, 58, 101183. [Google Scholar] [CrossRef]
- de Carvalho, T.B.; Oliveira, M.; Gomes, A.M.; Monteiro, M.J.; Pintado, M.; Komora, N.; Durães, T.; Nunes, F.M.; Cosme, F.; Patarata, L.; et al. Clean labelling sodium nitrite at pilot scale: In-situ reduction of nitrate from plant sources and its effects on the overall quality and safety of restructured cooked ham. Meat Sci. 2024, 216, 109572. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Chengli, H.; Wenting, W.; Zheng, L.; Li, X.; Liguo, W.; Jie, Z.; Dequan, Z. Effect of arginine-hemoglobin on color stability of ground lamb during storage. In Proceedings of the 63rd Internation Congress of Meat Science and Technology, Cork, Ireland, 13–18 August 2017. [Google Scholar]
- Bludau, S.E.; Modrow, K.M.; Osburn, W.N. Evaluation of an amino acid based alternative curing system on the physiochemical and shelf life attributes of beef frankfurters. Abstracts from the 2021 AMSA Reciprocal Meat Conference. Meat Muscle Biol. 2022, 6, 23–24. [Google Scholar]
- Zhou, C.; Ye, H.; Wang, H.; Qin, H.; Li, J. Coordination of L-arginine and iron cation improves stability of hemoglobin concentrates. Eur. Food Res. Technol. 2015, 240, 743–751. [Google Scholar] [CrossRef]
- Premi, L.; Rocchetti, G.; Lucini, L.; Morelli, L.; Rebecchi, A. Replacement of nitrates and nitrites in meat-derived foods through the utilization of coagulase-negative staphylococci: A review. Curr. Res. Food Sci. 2024, 8, 100731. [Google Scholar] [CrossRef]
- Gou, M.; Liu, X.; Qu, H. The role of nitric oxide in the mechanism of lactic acid bacteria substituting for nitrite. CyTA J. Food 2019, 17, 593–602. [Google Scholar] [CrossRef]
- Huang, P.; Luo, H.; Chen, C.; Li, P.; Xu, B. Bacterial nitric oxide synthase in colorizing meat products: Current development and future directions. Crit. Rev. Food Sci. Nutr. 2024, 64, 4362–4372. [Google Scholar] [CrossRef]
- Zajac, M.; Zajac, K.; Dybas, J. The effect of nitric oxide synthase and arginine on the color of cooked meat. Food Chem. 2022, 373. [Google Scholar] [CrossRef]
- Lorin, J.; Zeller, M.; Guilland, J.C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and nitric oxide synthase: Regulatory mechanisms and cardiovascular aspects. Mol. Nutr. Food Res. 2014, 58, 101–116. [Google Scholar] [CrossRef]
- Liu, R.; Warner, R.D.; Zhou, G.; Zhang, W. Contribution of nitric oxide and protein S-nitrosylation to variation in fresh meat quality. Meat Sci. 2018, 144, 135–148. [Google Scholar] [CrossRef]
- González Arbeláez, L.F.; Ciocci Pardo, A.; Burgos, J.I.; Vila Petroff, M.G.; Godoy Coto, J.; Ennis, I.L.; Mosca, S.M.; Fantinelli, J.C. New advances in the protective mechanisms of acidic pH after ischemia: Participation of NO. Arch. Biochem. Biophys. 2024, 758, 110059. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, A.; Suberville, S.; Philippe, C.; Bertrand, F.; Perez, J.; Fouqueray, B.; Cherqui, G.L.; Baud, L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages: Evidence for involvement of nuclear factor-κB activation. J. Biol. Chem. 1998, 273, 5086–5092. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.E.; Bohrer, B.M.; Mejia, S.M.V. Phosphate alternatives for meat processing and challenges for the industry: A critical review. Food Res. Int. 2023, 166, 112624. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.L.; Lorenzo, J.M.; Machado, J.M.; Manfio, M.; Cichoski, A.J.; Fries, L.L.M.; Morgano, M.A.; Campagnol, P.C.B. Application of arginine and histidine to improve the technological and sensory properties of low-fat and low-sodium bologna-type sausages produced with high levels of KCl. Meat Sci. 2020, 159, 107939. [Google Scholar] [CrossRef]
- Lei, Z.; Fu, Y.; Xu, P.; Zheng, Y.D.; Zhou, C.L. Effects of L-arginine on the physicochemical and gel properties of chicken actomyosin. Int. J. Biol. Macromol. 2016, 92, 1258–1265. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Li, S.; Ning, C.; Zhou, C. l–Arginine/l–lysine improves emulsion stability of chicken sausage by increasing electrostatic repulsion of emulsion droplet and decreasing the interfacial tension of soybean oil-water. Food Hydrocoll. 2019, 89, 492–502. [Google Scholar] [CrossRef]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors influencing internal color of cooked meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef]
- Ishibashi, M.; Tsumoto, K.; Tokunaga, M.; Ejima, D.; Kita, Y.; Arakawa, T. Is arginine a protein-denaturant? Protein Expr. Purif. 2005, 42, 1–6. [Google Scholar] [CrossRef]
- Ras, G.; Leroy, S.; Talon, R. Nitric oxide synthase: What is its potential role in the physiology of staphylococci in meat products? Int. J. Food Microbiol. 2018, 282, 28–34. [Google Scholar] [CrossRef]
- Foley, E.L.; Hvitved, A.N.; Eich, R.F.; Olson, J.S. Mechanisms of nitric oxide reactions with globins using mammalian myoglobin as a model system. J. Inorg. Biochem. 2022, 233, 111839. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, R.; Zhou, C. l-Arginine and l-lysine can weaken the intermolecular interactions of main myofibrillar proteins: The roles in improving the tenderness of pork Longissimus lumborum muscle. Int. J. Food Sci. Technol. 2023, 58, 3085–3096. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaiser, W.M. Production and Scavenging of Nitric Oxide by Barley Root Mitochondria. Plant Cell Physiol. 2010, 51, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bianco Junior, A.; Daguer, H.; Kindlein, L. Baseline sodium nitrate and nitrite concentrations in fresh and processed meats. J. Food Compos. Anal. 2022, 105, 104227. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj/eng (accessed on 7 January 2025).
- Wang, X.; Yu, Q.; He, L.; Zhang, Q.; Ma, J. Effects of nitrite concentrations on the quality and protein oxidation of salted meat. J. Food Sci. 2022, 87, 3978–3994. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Xu, B.; Zhou, C. Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT 2020, 129, 109524. [Google Scholar] [CrossRef]
- Kato, T.; Kikugawa, K. Proteins and amino acids as scavengers of nitrite: Inhibitory effect on the formation of nitrosodimethylamine and diazoquinone. Food Chem. Toxicol. 1992, 30, 617–626. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M.; Aristoy, M.C. Enzyme generation of free amino acids and its nutritional significance in processed pork meats. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1303–1322. [Google Scholar]
- Bahelka, I.; Bučko, O.; Hozáková, K.; Stupka, R.; Čítek, J.; Hanusová, E.; Gondeková, M. Amino acid, fatty acid and chemical composition of meat and fat from entire males, castrates and gilts. Acta Fytotechn Zootech. 2020, 23, 167–173. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, C.; Zheng, J.; Ma, L.; Ma, X.; Zhong, Y.; Zhao, X.; Li, F.; Guo, Q.; Yin, Y. Profiles of muscular amino acids, fatty acids, and metabolites in Shaziling pigs of different ages and relation to meat quality. Sci. China Life Sci. 2023, 66, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, D.; Wang, R.; Zhao, C.; Ma, Q.; Wu, H.; Xie, X. Reconstructing a recycling and nonauxotroph biosynthetic pathway in Escherichia coli toward highly efficient production of L-citrulline. Metab. Eng. 2021, 68, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Long, Y.; Zhang, Y.F.; Zhang, Z.Y.; Yang, B.; Chen, C.Y.; Huang, L.S.; Su, Y. Phenotypic and genetic correlations of pork myoglobin content with meat colour and other traits in an eight breed-crossed heterogeneous population. Animal 2021, 15, 100364. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Ragireddy, V.; Dent, M.R.; Tejero, J. Engineering neuroglobin nitrite reductase activity based on myoglobin models. Biochem. Biophys. Rep. 2023, 36, 101560. [Google Scholar] [CrossRef]
- Millar, S.J.; Moss, B.W.; Stevenson, M.H. Some observations on the absorption spectra of various myoglobin derivatives found in meat. Meat Sci. 1996, 42, 277–288. [Google Scholar] [CrossRef]
- Ning, C.; Bao, P.; Zhang, D.; Li, L.; Chen, L.; Fang, H.; Tang, Y.; Zhou, C. Reduction and coordination properties of L-Lysine/L-arginine/L-cysteine for the improvement of the color of cured sausage. Food Chem. 2020, 312, 126122. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, B.; Xiong, Y.L. Production of cured meat color in nitrite-free Harbin red sausage by Lactobacillus fermentum fermentation. Meat Sci. 2007, 77, 593–598. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Wackerbarth, H.; Kuhlmann, U.; Tintchev, F.; Heinz, V.; Hildebrandt, P. Structural changes of myoglobin in pressure-treated pork meat probed by resonance Raman spectroscopy. Food Chem. 2009, 115, 1194–1198. [Google Scholar] [CrossRef]
- Dybas, J.; Bokamper, M.J.; Marzec, K.M.; Mak, P.J. Probing the structure-function relationship of hemoglobin in living human red blood cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118530. [Google Scholar] [CrossRef]
- Ma, G.; Chen, H.; Zhang, Q.; Ma, J.; Yu, Q.; Han, L.; Chen, C.; Song, R. Protective characterization of low dose sodium nitrite on yak meat myoglobin in a hydroxy radical oxidation environment: Fourier Transform Infrared spectroscopy and laser Micro-Raman spectroscopy. LWT 2019, 116, 108556. [Google Scholar] [CrossRef]
- Ma, P.; Yin, J.; Sun, Y.; Wu, D.; Zhang, Y.; Feng, Y.; Liu, G. New insights into NO bonding in Tan sheep myoglobin for meat pigmentation: Spectroscopic and density functional theory investigations. J. Mol. Struct. 2024, 1309, 138227. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Z.; Yu, Q.; Han, L.; Chen, C.; Guo, Z. Effects of low-dose sodium nitrite on the structure of yak meat myoglobin during wet curing. Food Chem. X 2022, 15, 100434. [Google Scholar] [CrossRef]
- Marzec, K.M.; Dybas, J.; Chlopicki, S.; Baranska, M. Resonance Raman in Vitro Detection and Differentiation of the Nitrite-Induced Hemoglobin Adducts in Functional Human Red Blood Cells. J. Phys. Chem. B 2016, 120, 12249–12260. [Google Scholar] [CrossRef]
- Xi, W.; Haes, A.J. Elucidation of HEPES Affinity to and Structure on Gold Nanostars. J. Am. Chem. Soc. 2019, 141, 4034–4042. [Google Scholar] [CrossRef] [PubMed]
- Kostrewa, S.; Emgenbroich, M.; Klockow, D.; Wulff, G. Surface-Enhanced Raman Scattering on Molecularly Imprinted Polymers in Water. Macromol. Chem. Phys. 2003, 204, 481–487. [Google Scholar] [CrossRef]
- Zajac, M.; Duda, I.; Skoczylas, L.; Tabaszewska, M. Potential Use of Hyssopus officinalis and Borago officinalis as Curing Ingredients in Pork Meat Formulations. Animals 2020, 10, 2327. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Borawska-Dziadkiewicz, J.; Kulawik, P.; Duda, I.; Morawska, M.; Mickowska, B. The effects of hydrolysis condition on the antioxidant activity of protein hydrolysate from Cyprinus carpio skin gelatin. Lwt-Food Sci. Technol. 2020, 117, 108616. [Google Scholar] [CrossRef]

| (a) | ||||
| L-Arginine Concentration (%) | Incubation Temperature (°C) | Buffer/Water | Enzyme/No Enzyme | pH |
| 0% | 37 °C | Buffer | Yes | |
| No | 7.2 bcd ± 0.0 | |||
| Water | Yes | |||
| No | 5.9 g ± 0.1 | |||
| 20 °C | Buffer | Yes | ||
| No | 7.2 bcd ± 0.0 | |||
| Water | Yes | |||
| No | 6.0 g ± 0.1 | |||
| 0.1% | 37 °C | Buffer | Yes | 7.4 bc ± 0.1 |
| No | 7.2 bcd ± 0.0 | |||
| Water | Yes | 7.1 cde ± 0.0 | ||
| No | 6.9 ef ± 0.1 | |||
| 20 °C | Buffer | Yes | 7.3 bc ± 0.1 | |
| No | 7.3 bcd ± 0.0 | |||
| Water | Yes | 7.0 def ± 0.1 | ||
| No | 6.8 f ± 0.1 | |||
| 0.2% | 37 °C | Buffer | Yes | 7.3 bcd ± 0.0 |
| No | 7.7 a ± 0.2 | |||
| Water | Yes | 7.0 def ± 0.1 | ||
| No | 7.5 b ± 0.1 | |||
| 20 °C | Buffer | Yes | 7.3 bcd ± 0.0 | |
| No | 7.8 a ± 0.2 | |||
| Water | Yes | 7.0 def ± 0.1 | ||
| No | 7.8 a ± 0.2 | |||
| (b) | ||||
| L-Arginine Concentration (%) | Incubation Temperature (°C) | Buffer/Water | Meat/No Meat | pH |
| 0.1% | 37 °C | Buffer | Yes | 7.4 abc ± 0.1 |
| No | 7.4 abc ± 0.1 | |||
| Water | Yes | 7.1 d ± 0.0 | ||
| No | 7.5 a ± 0.0 | |||
| 20 °C | Buffer | Yes | 7.3 abc ± 0.1 | |
| No | 7.5 a ± 0.1 | |||
| Water | Yes | 7.0 d ± 0.1 | ||
| No | 7.4 abc ± 0.0 | |||
| 0.2% | 37 °C | Buffer | Yes | 7.3 c ± 0.0 |
| No | 7.3 bc ± 0.0 | |||
| Water | Yes | 7.0 d ± 0.1 | ||
| No | 7.3 bc ± 0.0 | |||
| 20°C | Buffer | Yes | 7.3 bc ± 0.0 | |
| No | 7.3 bc ± 0.0 | |||
| Water | Yes | 7.0 d ± 0.1 | ||
| No | 7.3 c ± 0.1 | |||
| L-Arginine Concentration | Incubation Temperature (°C) | Buffer/Water | Enzyme/No Enzyme | L* (D65) | a* (D65) | b* (D65) |
|---|---|---|---|---|---|---|
| 0% | 37 °C | Buffer | Yes | |||
| No | 59.34 ghi ± 0.25 | 1.35 abc ± 0.06 | 12.61 abc ± 0.18 | |||
| Water | Yes | |||||
| No | 64.04 cd ± 0.21 | 1.11 bcde ± 0.05 | 12.88 ab ± 0.12 | |||
| 20 °C | Buffer | Yes | ||||
| No | 59.04 i ± 0.23 | 1.18 abcd ± 0.13 | 12.02 cdef ± 0.26 | |||
| Water | Yes | |||||
| No | 63.40 d ± 0.15 | 1.41 ab ± 0.03 | 12.83a b ± 0.09 | |||
| 0.1% | 37 °C | Buffer | Yes | 59.30 hi ± 0.20 | 0.65 f ± 0.16 | 11.49 fg ± 0.18 |
| No | 60.02 fghi ± 0.42 | 1.35 abc ± 0.06 | 12.38 abcd ± 0.20 | |||
| Water | Yes | 64.18 cd ± 0.33 | 1.15 bcde ± 0.07 | 12.77 ab ± 0.15 | ||
| No | 65.08 bc ± 0.38 | 1.13 bcde ± 0.06 | 12.72 ab ± 0.15 | |||
| 20 °C | Buffer | Yes | 59.13 hi ± 0.22 | 0.66 f ± 0.10 | 11.30 g ± 0.18 | |
| No | 60.34 efgh ± 0.49 | 1.51 a ± 0.08 | 12.58 abc ± 0.15 | |||
| Water | Yes | 64.40 bcd ± 0.27 | 1.02 cde ± 0.10 | 12.95 a ± 0.13 | ||
| No | 64.63 bcd ± 0.38 | 1.26 abc ± 0.07 | 12.62 abc ± 0.14 | |||
| 0.2% | 37 °C | Buffer | Yes | 61.06 ef ± 0.50 | 0.90 def ± 0.18 | 12.60 abc ± 0.36 |
| No | 59.36 ghi ± 0.59 | 0.64 f ± 0.15 | 11.98 cdef ± 0.30 | |||
| Water | Yes | 65.60 ab ± 0.45 | 0.82 ef ± 0.13 | 12.22 bcde ± 0.24 | ||
| No | 66.33 a ± 0.40 | 0.63 f ± 0.08 | 11.89 defg ± 0.15 | |||
| 20 °C | Buffer | Yes | 61.50 e ± 0.55 | 0.91 def ± 0.12 | 12.50 abcd ± 0.30 | |
| No | 60.57 efg ± 0.54 | 0.59 f ± 0.17 | 11.87 cdef ± 0.34 | |||
| Water | Yes | 66.39 a ± 0.60 | 0.68 f ± 0.16 | 12.19 bcde ± 0.24 | ||
| No | 66.59 a ± 0.44 | 0.57 f ± 0.08 | 11.70 efg ± 0.13 |
| L-Arginine Concentration (%) | Incubation Temperature (°C) | Buffer/Water | Enzyme/No Enzyme | NO2− (µg/mL) |
|---|---|---|---|---|
| 0% | 37 °C | Buffer | No | 0.48 abcde ± 0.06 |
| Water | No | 0.51 abcde ± 0.20 | ||
| 20 °C | Buffer | No | 0.55 abc ± 0.06 | |
| Water | No | 0.52 abcd ± 0.10 | ||
| 0.1% | 37 °C | Buffer | Yes | 0.61 ab ± 0.06 |
| Water | Yes | 0.24 efg ± 0.05 | ||
| 20 °C | Buffer | Yes | 0.68 a ± 0.08 | |
| Water | Yes | 0.40 abcdef ± 0.08 | ||
| 37 °C | Buffer | No | 0.27 defg ± 0.03 | |
| Water | No | 0.07 g ± 0.02 | ||
| 20 °C | Buffer | No | 0.35 bcdef ± 0.02 | |
| Water | No | 0.13 fg ± 0.02 | ||
| 0.2% | 37 °C | Buffer | Yes | 0.48 abcde ± 0.06 |
| Water | Yes | 0.29 cdefg ± 0.05 | ||
| 20 °C | Buffer | Yes | 0.48 acde ± 0.07 | |
| Water | Yes | 0.41 abcde ± 0.08 | ||
| 37 °C | Buffer | No | 0.35 bcdef ± 0.10 | |
| Water | No | 0.03 g ± 0.02 | ||
| 20 °C | Buffer | No | 0.41 abcde ± 0.12 | |
| Water | No | 0.35 bcdef ± 0.15 |
| Incubation Temperature (°C) | Buffer/Water | Meat/No Meat | Enzyme/No Enzyme | L-Arginine mg/100 mL | Citrulline |
|---|---|---|---|---|---|
| 37 °C | Buffer | No | yes | 68.73 ef ± 4.62 | nd |
| Yes | yes | 88.55 cd ± 3.33 | 9.85 a ± 0.54 | ||
| No | 95.67 bc ± 5.81 | 9.89 a ± 0.81 | |||
| Water | No | yes | 55.85 f ± 4.47 | nd | |
| Yes | yes | 84.76 cd ± 6.00 | 7.67 ab ± 1.03 | ||
| no | 109.73 ab ± 1.49 | 8.71 ab ± 0.61 | |||
| 20 °C | Buffer | No | yes | 59.99 f ± 4.33 | nd |
| Yes | yes | 79.26 de ± 6.62 | 8.48 ab ± 0.60 | ||
| no | 94.75 bcd ± 4.81 | 7.15 b ± 0.65 | |||
| Water | No | yes | 66.77 ef ± 6.21 | nd | |
| Yes | yes | 84.67 cd ± 4.59 | 8.86 ab ± 1.06 | ||
| no | 123.28 a ± 3.01 | 9.59 ab ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, M.; Szram, R. Evaluation of NO Synthase Activity in Meat-Brining Solutions: Implications for Meat Curing and Color Stability. Molecules 2025, 30, 1215. https://doi.org/10.3390/molecules30061215
Zając M, Szram R. Evaluation of NO Synthase Activity in Meat-Brining Solutions: Implications for Meat Curing and Color Stability. Molecules. 2025; 30(6):1215. https://doi.org/10.3390/molecules30061215
Chicago/Turabian StyleZając, Marzena, and Rafał Szram. 2025. "Evaluation of NO Synthase Activity in Meat-Brining Solutions: Implications for Meat Curing and Color Stability" Molecules 30, no. 6: 1215. https://doi.org/10.3390/molecules30061215
APA StyleZając, M., & Szram, R. (2025). Evaluation of NO Synthase Activity in Meat-Brining Solutions: Implications for Meat Curing and Color Stability. Molecules, 30(6), 1215. https://doi.org/10.3390/molecules30061215








