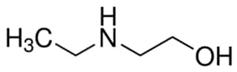
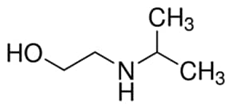
Abstract
One of the most used methods for capturing acidic gases from the atmosphere is the use of amines that react with the acids and can later be recovered. The choice of amines that are most efficient in capturing has been the subject of several studies; however, the energy effort for their regeneration is also important. While the polarity of the solvent plays a critical role in determining which amines efficiently capture CO2, the heat capacity of the solvent is also a significant factor in the regeneration process. In this work, we present values for Reichardt’s and Kamlet−Taft parameters, such as π* (dipolarity/polarizability), α (acidity), and β (basicity), for solutions of two alkanolamines and two alkoxyamines dissolved in propane-1,3-diol, at 298.15 K, a solvent with a lower heat capacity than water. In addition to the polarity characterization of the amines in that solvent, the aim of this study is to analyze the differences observed in the solvatochromic parameters when water is replaced by alcohol. The impact of this change on the values of those parameters for the binary amine + solvent solutions was assessed by calculating the transfer values, . Defined as, , these transfer values represent the difference in the parameters when the amines are transferred from water to alcohol. While the water medium is more favourable in terms of π* for CO2 capture, the alcohol medium appears to hold more promise in terms of β.
1. Introduction
Although the chemical-based proposals for the CO2 absorption mechanism by the amine-based CO2 capture method are based on the formation of carbamate and bicarbonate ions [1,2,3], its mechanism is not yet fully understood [4]. In fact, CO2 is a non-polar molecule with low solubility in polar mediums, such as aqueous amine solutions, and the absorption/adsorption process begins at the gas/liquid interface where intermediate short-living species formed are experimentally difficult to characterize.
The choice of different types of amines that have been revealed to be more efficient in the capture process has been the subject of many studies in recent years [5,6,7], showing that certain sterically hindered amines would be more efficient in this process [8]. However, considering the overall cost of the process and bearing in mind that throughout the process we will consider CO2 as the solute and the amine solution as the solvent, the subsequent regeneration of the amine is also an important step with high associated costs (between 60 and 70% of the total cost). One of the important characteristics of the solvents encompassing this last step of the process is its heat capacity; the lower it is, the lower energy costs are associated with it [6,9,10].
Recent studies combining experimental results on the absorption of CO2 by the well-studied 30% aqueous solution of 2-aminoethanol (MEA) with results from classical molecular dynamics (MD) showed the possibility of improving the CO2 capture process by modifying the polarity of the solvent to obtain more stable intermediate compounds in the interface of gas/liquid with better solubility in the solution of the amine, thus reducing the overall cost of the process [11].
The polarity of a solvent, which reflects its solvation capability, is influenced by the combined effects of all possible specific and non-specific solute–solvent interactions [12]. As a result, it serves as a crucial factor in the CO2 capture process. This concept has been extensively explored in numerous studies [12,13,14,15,16,17], including several outstanding reviews.
On the other hand, it was also recently found that there is a good correlation between the CO2 absorption capacity of a given amine and the Kamlet–Taft parameters β and π* of mixtures of a given organic solvent with water [3], being that the CO2 uptake is mainly governed by both the solvent basicity and dipolarity/polarizability. Based on these findings, the purpose of the present work is to characterize, in terms of the solvent polarity, four non-aqueous binary mixtures containing a dialcohol and one of two different alkanolamines or one of two different alkoxyamines.
Considering the characterization of the solvents regarding their ability to better adsorb the CO2 in the interface of gas/liquid and its release after capture, we aim to open other hints on suggesting the possible advantage of substituting water with a non-aqueous solvent, showing that the different solvatochromic parameters can be tuned out.
In this work, we determine the solvatochromic parameters, namely, Reichardt’s normalized parameter, ; dipolarity/polarizability, π*; acidity, α; and basicity, β, in two secondary alkanolamines (one of them hindered) and two linear alkoxyamines in an organic solvent, propane-1,3-diol (1,3-PD), with a heat capacity of 2.31 kJ kg−1 K−1 [18] (nearly half that of the water 4.15 kJ kg−1 K−1), across the entire concentration range, and at 298.15 K. The differences observed in the amine-solvent parameters, when the amines are transferred from aqueous to the non-aqueous solvent, were also determined and analyzed.
2. Results and Discussion
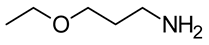
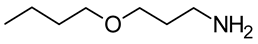
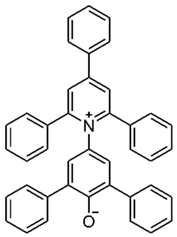
Using the three molecular probes, 2,6-diphenyl-4-(2,4,6-triphenylpyridinium-1-yl) phenolate, Reichardt’s betaine, RB(30); 4-amino-nitrobenzene, 4-NA; and 4-(dimethylamino)-nitrobenzene, NN-4-NA, experimental wavelengths across the whole composition range of the binary liquid mixtures: {propane-1,3-diol + 2-(ethylamino)ethanol (EEA), or 2-(isopropylamino)ethanol (IPAE), or 3-ethoxypropan-1-amine (EPA), or 3-butoxypropan-1-amine (BPA)}, were obtained at 298.15 K. Values are presented in the Supplementary Information (SI), Table S1.
The solvent parameter was calculated using Equation (1) [12,19,20]:
where NA is the Avogadro number, h is the Planck constant, c is the speed of light in the vacuum, and is the wavenumber of the RB probe. was normalized to give a value of 0 for tetramethylsilane (TMS) and 1 for water according to Equation (2) [12,19,20].
The dipolarity/polarizability parameter, , was calculated from Equation (3) to give a value of 0 for cyclohexane and 1 for dimethylsulfoxide [12,21]. is the wavenumber of the NN-4-NA probe.
The acidity parameter, α, was calculated using Equation (4), giving a value of 1 for methanol [12,21].
The basicity parameter, β, was estimated to give a value of 1 for hexamethylphosphoramide and is given by Equation (5) [12,21]. is the wavenumber of the 4-NA probe.

Table 1.
Solvatochromic parameters for {1,3-PD (1) + EEA, or IPAE, or EPA, or BPA (2)} binary mixtures at T/K = 298.15 and P/MPa = 0.1 a.
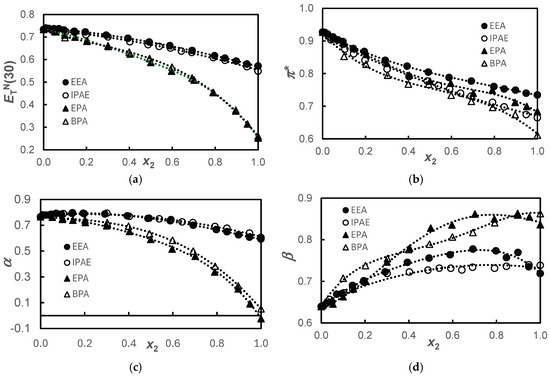
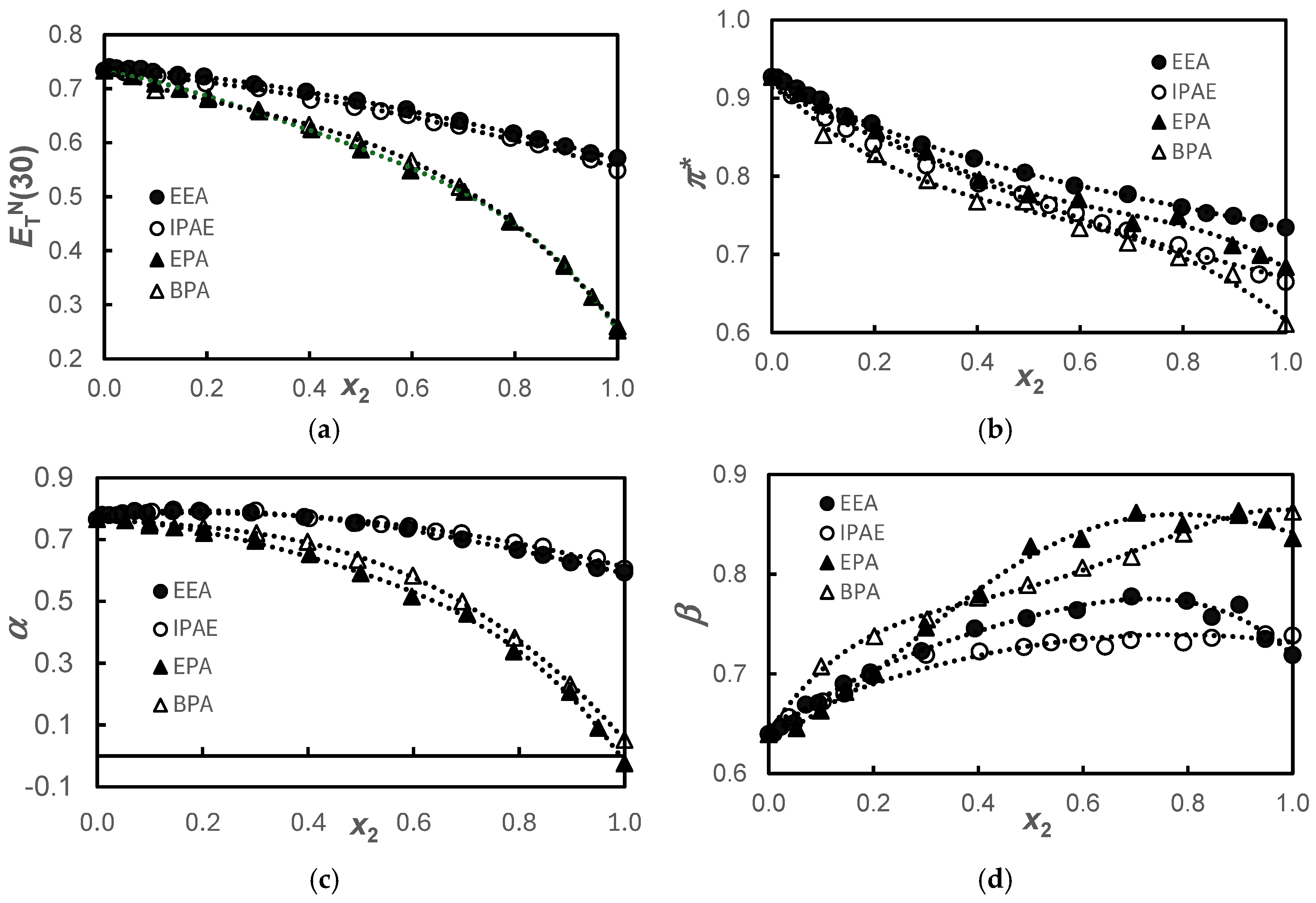
Figure 1.
Solvatochromic parameters, ; π* (b); α (c); and β (d), for the {propane-1,3-diol+amine} systems (●), EEA; (ο), IPAE; (▲), EPA; and (Δ), BPA at T = 298.15 K. The dotted lines were drawn to guide the eyes adjusting polynomial functions.
Since the four solvatochromic parameters of the pure amphiphiles were previously published [22,23], in Table 2, we report their values for comparison. The displayed uncertainty values show a good agreement within the mutual uncertainty for all the parameters.

Table 2.
Solvatochromic parameters for the pure amines, EEA, IPAE, EPA, or BPA, at T/K = 298.15 and P/MPa = 0.1 a.
Figure 1 shows similar behaviors of and α for the alkanolamine and alkoxyamine pairs. Knowing that the parameter measures both the dipolarity/polarizability, π*, and acidity, α, of the solvent, it can be observed that in these systems, the parameter predominantly reflects the acidity of the solvent since the behavior of π* does not show that same pairing. This dichotomy of α and π* behavior is explained because, regarding acidity, we have two very different types of compounds, with pure basic alkoxyamines having acidity very close to zero and alkanolamines being rather more acidic. On the other hand, regarding the dipolarity/polarizability, we found that the size of the amine molecules is the factor that governs the observed behavior.
Regarding basicity, as previously mentioned, alkoxyamines are inherently more basic than alkanolamines. In the same class of compounds, the fact that the pure compound with the longer chain is more basic than the pure compound with the shorter chain could be justified by the inductive effect that would exist in molecules with longer carbon chains, displacing the negative charge towards the more electronegative atoms, -O- in the alkoxyamines and -NH- in the alkanolamines. Also, synergistic effects are observed for the systems of the smallest molecules in the concentrated composition ranges 0.4 < x2 < 0.9 and 0.7 < x2 < 0.9, for EEA and EPA, respectively. This peculiar behavior had already been observed and interpreted by us in the systems H2O + EEA [22] and H2O + EPA [23] as being the result of the formation of the stable entities EPA·H2O or EEA·H2O as water is added starting from the pure amphiphile. It is believed that, in the same way, the stable entities EEA·1,3-PD or EPA·1,3-PD would be formed. This phenomenon has been previously observed in earlier studies [14,16,17], where the role of hydrogen bonding in enhancing polarity compared to the pure components was thoroughly discussed. In the present study, however, this effect is only evident in basicity.
As stated in the introduction, recent studies, with the aim of outlining alternatives to the use of aqueous solvents to optimize the cost-benefit of the carbon capture and storage process (CCS), indicate that non-aqueous solvents may present advantages. In this work, we chose a dialcohol, namely, 1,3-PD, with the aim of forming non-aqueous solutions with two secondary amines, one of which is hindered, and two primary amines and evaluating the change in the characteristics of the solvent in terms of its solvatochromic parameters when water is replaced by this alcohol. A convenient way to achieve this goal is to calculate the transfer values of those parameters when the amines are transferred from water to alcohol. The transfer function, Δtransf(Fi,xi), is defined by Equation (6) and the results are presented in Table S2 and Figure 2:
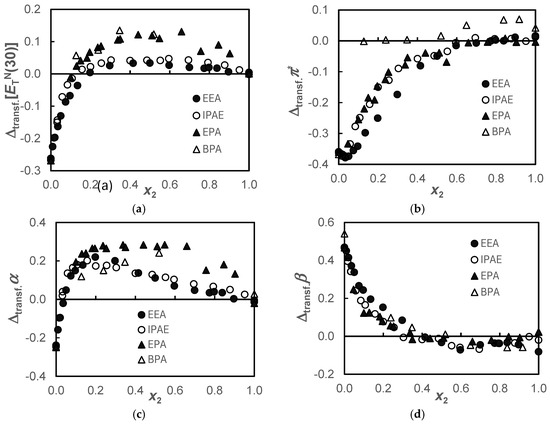
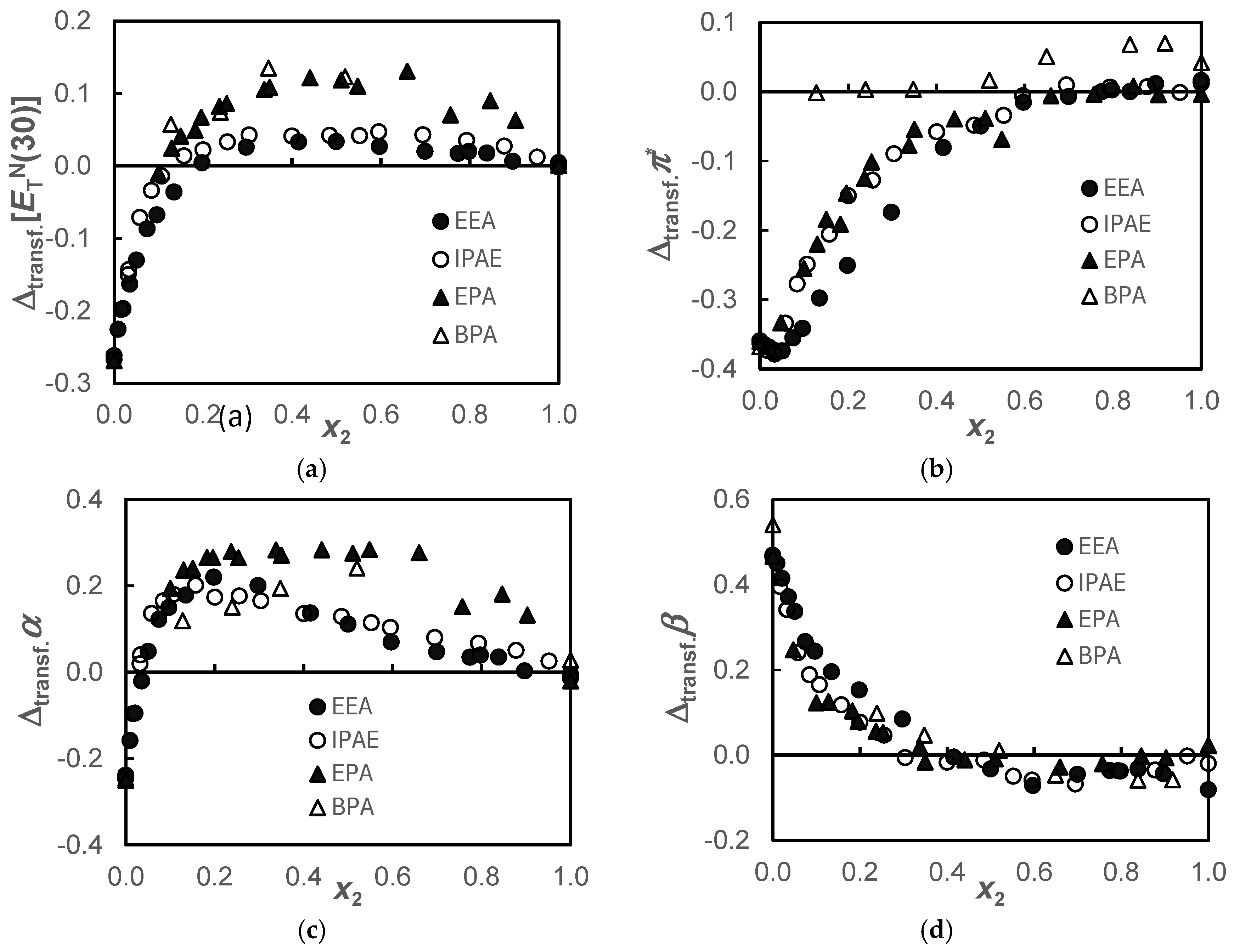
Figure 2.
Transfer values, , of the solvatochromic parameters, (a); π* (b); α (c); and β (d), for the {propane-1,3-diol+amine} systems when the amines are transferred from water to the alcohol, at T = 298.15 K.
and are the values of each solvatochromic parameter, , π*, α, or β, at the same composition, xi, of the amine in 1,3-PD and in water, respectively.
The pairing of the behavior observed in Figure 1a for the secondary amines EEA and IPAE and the primary amines EPA and BPA is also verified in the transfer function, , depicted in Figure 2a. This transfer function presents negative values up to x2 ≈ 0.2, becoming positive from this molar fraction onward, but more positive ones in primary amines. Since is a mixed parameter, simultaneously measuring dipolarity/polarizability, π*, and acidity, α, these parameters will be analyzed below. In Figure 2c, the same pairing described for the function is observed for α, showing that this last function predominantly reflects the acidity of the solvent.
Regarding the , Figure 2b, the marked negative π* difference between alcohol and water attenuates as the amine is added, becoming practically null from x2 ≈ 0.6 for the EEA, IPAE, and EPA systems and from x2 ≈ 0.1 for the BPA system. However, a minimum is observed in the case of secondary amines for x2 ≈ 0.03. In the BPA system, the values are slightly positive, although they are within mutual uncertainty.
Concerning the function in all systems, there is a zone of greater basicity of the amine in alcohol compared to that of the amine in water for a range of mole fractions 0 ≤ x2 ≤ 0.4. This fact indicates that the 1,3-PD + amine solvent would be more promising in capturing CO2 in the range of molar fractions indicated above. Figure 2c shows that despite water being much more acidic than 1,3-PD, giving negative values for the transfer property, as amine is added, they rapidly became positive beyond x2 > 0.03. Curiously, between this composition and x2 = 0.4, there is also a higher basicity (Figure 2d) of all amines in alcohol than in water. This is probably due to the coexisting hydrogen bond acceptor and hydrogen bond donor capabilities of the solvent in the alcohol. This effect is more pronounced in the alkoxyamine systems.
3. Experimental
3.1. Materials
All the amines and the propane-1,3-diol were reagent grade and were used as received without further purification. Table 3 presents the characterization of the chemicals used.

Table 3.
Characteristics of the chemicals used.
3.2. Methods
All solutions were prepared by mass using a Kern AEJ balance (Kern & Sohn, Balingen, Germany) with a resolution of 0.00001 g. Airtight designed flasks provided with Teflon stoppers were used to avoid evaporation and air contamination. Different total volumes of solution were used, attending to the minimization of the vapor phase. Buoyancy corrections were applied. Standard combined uncertainties in the calculated mole fractions were found to be less than 0.0005.
UV−vis Spectroscopy: Wavelengths of the molecular probes in the solutions prepared were performed with a double-beam UV-1800 Shimazu spectrometer (Shimazu, Kyoto, Japan) with a resolution of 0.1 nm. The path length of the cell used was 1 cm. The temperature was kept constant at (298.15 ± 0.10) K. The amount of each dye, added directly to the cell, was evaluated to obtain absorbance values ranging from 0.5 to 1 to avoid the formation of aggregates. The spectra were recorded 3 to 5 times and the wavelength corresponding to the peak of maximum absorbance, λmax, was obtained from the average. The uncertainty of the λmax was found to be 1 nm. The wavenumbers of maximum absorbance of each dye, , expressed in kilo Kaiser (1 kK = 1000 cm−1), were used to calculate the solvatochromic parameters.
4. Conclusions
In this study, the polarity of four binary mixtures was characterized at 298.15 K. These mixtures consisted of a dialcohol, propane-1,3-diol, combined with either two secondary amines—2-(ethylamino)ethanol, EEA, or the sterically hindered 2-(isopropylamino)ethanol, IPAE—or two primary amines—3-ethoxy-1-propylamine, EPA, or 3-butoxy-1-propylamine, BPA. Polarity measurements were conducted using three molecular probes: 2,6-diphenyl-4-(2,4,6-triphenylpyridinium-1-yl) phenolate (Reichardt’s betaine, RB(30)); 4-amino-nitrobenzene (4-NA); and 4-(dimethylamino)-nitrobenzene (NN-4-NA). This study determined values for Reichardt’s as well as Kamlet–Taft parameters, including π* (dipolarity/polarizability), α (acidity), and β (basicity).
Similar trends for and α in alkanolamine and alkoxyamine pairs were observed. Since reflects both dipolarity/polarizability (π*) and acidity (α), the acidity contribution is the dominant factor as π* does not follow the same pattern.
Regarding basicity within each class, longer-chain compounds are more basic due to the inductive effect, which shifts the negative charge toward more electronegative atoms (-O- in alkoxyamines and -NH- in alkanolamines). Additionally, synergistic effects were observed in concentrated mixtures of the small EEA and EPA molecules, likely due to the formation of stable entities, such as EEA·1,3-PD or EPA·1,3-PD, similar to previously observed EPA·H2O and EEA·H2O formations, mainly due to H-bonding.
This study investigates the potential benefits of using non-aqueous solvents, specifically 1,3-propanediol (1,3-PD), as an alternative to water-based solvents for carbon capture. By analyzing solvatochromic parameters, the research evaluates how replacing water with 1,3-PD influences solvent properties. The transfer values of solvent polarity parameters, when amines are transferred from water to alcohol, suggest that in the presence of amines, 1,3-PD medium exhibits greater basicity than water medium, particularly within the mole fraction range of 0 ≤ x2 ≤ 0.40, making it a more promising candidate for CO2 capture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30061213/s1, Table S1: Wavelengths of maximum absorbances, , of the molecular probes: Reichardt’s Betaine, RB(30), 4-amino-nitrobenzene, 4-NA, and 4-(dimethylamino)-nitrobenzene, NN-4-NA, in the binary liquid mixtures: {propane-1,3-diol + 2-(ethylamino)ethanol (EEA) or 2-(isopropylamino)ethanol (IPAE) or 3-ethoxypropan-1-amine (EPA) or 3-butoxypropan-1-amine (BPA)}, at T/K = 298.15 and P/MPa = 0.1. Table S2: Transfer functions, of the amines-solvent parameters ; dipolarity/polarizability, π*; acidity, α and basicity, β, when the amines are transferred from the aqueous to the non-aqueous solvent, at T/K =298.15 and P/MPa = 0.1.
Author Contributions
Conceptualization, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Validation, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Formal analysis, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Investigation, M.-L.C.J.M., Â.F.S.S. and M.A.B.S.S.C.; Data curation, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Writing—original draft, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Writing—review & editing, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Visualization, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Supervision, M.-L.C.J.M., Â.F.S.S. and I.M.S.L.; Funding acquisition, Â.F.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from Fundação para a Ciência e a Tecnologia, Portugal, under projects UID/QUIM/00100/2020, UIDB/QUI/00100/2020 and LA/P/0056/2020 is greatly appreciated.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jamal, A.; Meisen, A.; Lim, C.J. Kinetics of carbon dioxide absorption and desorption in aqueous alkanolamine solutions using a novel hemispherical contactor—I. Experimental apparatus and mathematical modelling. Chem. Eng. Sci. 2006, 61, 6571–6589. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Q.; Zhou, Q.; Yang, J.; Ding, J.; Wen, J. Absorption of Carbon Dioxide from Flue Gas using Blended Amine Solutions. Chem. Eng. Technol. 2014, 37, 635–642. [Google Scholar] [CrossRef]
- Bui, T.Q.; Khokarale, S.G.; Shukla, S.K.; Mikkola, J.-P. Switchable aqueous Pentaethylenehexamine system for CO2 capture: An alternative technology with industrial potential. ACS Sustain. Chem. Eng. 2018, 6, 10395–10407. [Google Scholar] [CrossRef]
- Said, R.B.; Kolle, J.M.; Essalah, K.; Tangour, B.; Sayari, A. A unified approach to CO2-Amine reaction mechanisms. ACS Omega 2020, 5, 26125–26133. [Google Scholar] [CrossRef]
- Padurean, A.; Cormos, C.C.; Cormos, A.M.; Agachi, P.S. Multicriterial analysis of post-combustion carbon dioxide capture using alkanolamines. Int. J. Greenh. Gas Control 2011, 5, 676–685. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Reversible carbon dioxide capture by aqueous and non-aqueous amine-based absorbents: A comparative analysis carried out by 13C NMR spectroscopy. Appl. Energy 2018, 220, 208–219. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Yamada, H.; Onoda, M.; Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 2011, 4, 201–208. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents—A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193. [Google Scholar] [CrossRef]
- Schulze-Hulbe, A.; Shaahmadi, F.; Burger, A.J.; Cripwell, J.T. Toward nonaqueous alkanolamine-based carbon capture systems: Parameterizing amines, secondary alcohols, and carbon dioxide-containing systems in s-SAFT-γ Mie. Ind. Eng. Chem. Res. 2023, 62, 14061–14083. [Google Scholar] [CrossRef]
- Gladich, I.; Abotaleb, A.; Sinopoli, A. Tuning CO2 capture at the Gas/amine solution interface by changing the solvent polarity. J. Phys. Chem. B 2020, 124, 10245–10256. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Langhals, H. Polarity of Binary Liquid Mixtures. Angew. Chem. Int. Ed. Engl. 1982, 21, 724–733. [Google Scholar] [CrossRef]
- Rosés, M.; Ràfols, C.; Ortega, J.; Bosch, E. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 1. A comparison of several preferential solvation models for describing ET(30) polarity of dipolar hydrogen bond acceptor-cosolvent mixtures. J. Chem. Soc. Perkin Trans. 2 1995, 8, 1607–1615. [Google Scholar] [CrossRef]
- Tada, E.B.; Novaki, L.P.; El Seoud, O.A. Solvatochromism in pure and binary solvent mixtures: Effects of the molecular structure of the zwitterionic probe. J. Phys. Org. Chem. 2000, 13, 679–687. [Google Scholar] [CrossRef]
- Testoni, F.M.; Ribeiro, E.A.; Giusti, L.A.; Machado, V.G. Merocyanine solvatochromic dyes in the study of synergistic effects in mixtures of chloroform with hydrogen-bond accepting solvents. Spectrochim. Acta Part A 2009, 71, 1704–1711. [Google Scholar] [CrossRef]
- Langhals, H. How the concept of solvent polarity investigated with solvatochromic probes helps studying intermolecular interactions. Liquids 2023, 3, 481–511. [Google Scholar] [CrossRef]
- Góralsk, P.; Tkaczyk, M. Heat Capacities of Some Liquid α,ω-Alkanediols within the Temperature Range between (293.15 and 353.15) K. J. Chem. Eng. Data 2008, 53, 1932–1934. [Google Scholar] [CrossRef]
- Moita, M.-L.C.J.; Santos, A.F.S.; Silva, J.F.C.C.; Lampreia, I.M.S. Polarity of Some [NR1R2R3R4]+[Tf2N]− Ionic Liquids in Ethanol: Preferential Solvation versus Solvent−Solvent Interactions. J. Chem. Eng. Data 2012, 57, 2702–2709. [Google Scholar] [CrossRef]
- Machado, V.G.; Stock, R.I.; Reichardt, C. Pyridinium N-phenolate betaine dyes. Chem. Rev. 2014, 114, 10429–10475. [Google Scholar] [CrossRef]
- Lagalante, A.F.; Spadi, M.; Bruno, T.J. Kamlet-Taft Solvatochromic parameters of eight alkanolamines. J. Chem. Eng. Data 2000, 45, 382–385. [Google Scholar] [CrossRef]
- Moita, M.-L.C.J.; Fialho, B.G.; Santos, A.F.S.; Lampreia, I.M.S. Exploring molecular interactions in two aqueous alkanolamines mixtures using refractive indices and molecular probes. J. Mol. Liq. 2023, 388, 122760. [Google Scholar] [CrossRef]
- Moita, M.-L.C.J.; Santos, Â.F.S.; Alves, M.A.S.; Nobre, L.C.S.; Lampreia, I.M.S. New insights on molecular interactions in the aqueous systems (3-ethoxy or 3-butoxy)propan-1-amine mixtures using refractive indices and molecular probes. J. Chem. Eng. Data 2024, 69, 2756–2763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).