Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency
Abstract
1. Introduction
2. Results and Discussion
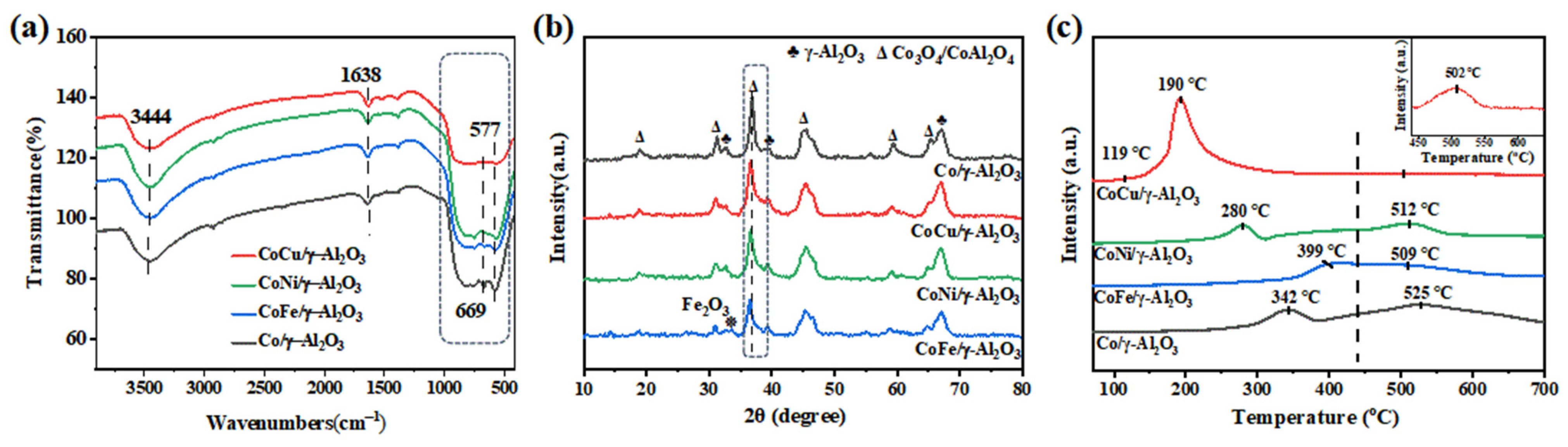
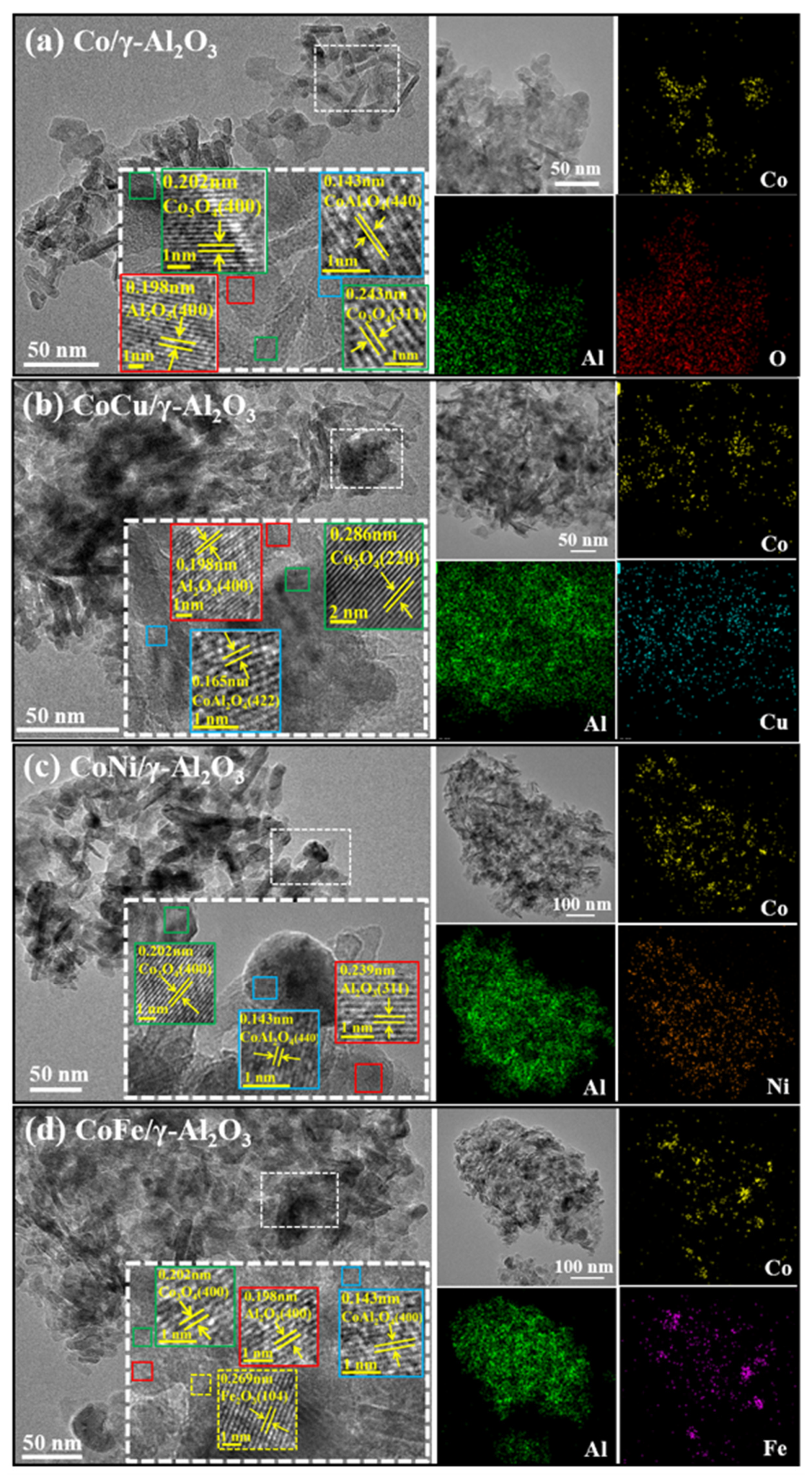
2.1. Synergistic Effects
2.2. The Effect of the Additional Metal on the Acidity of the Catalyst
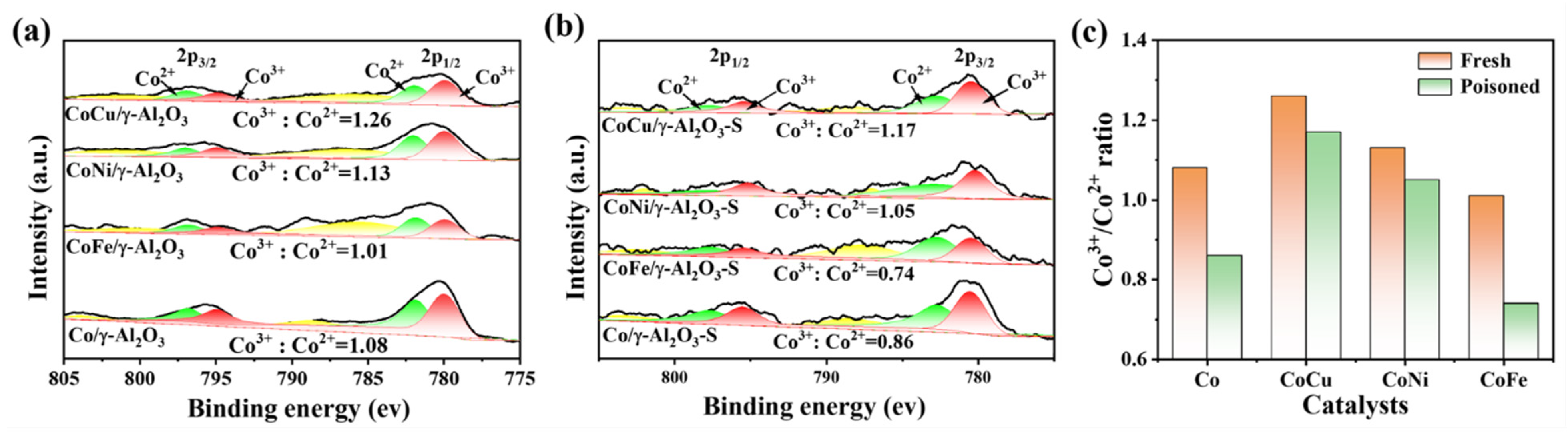
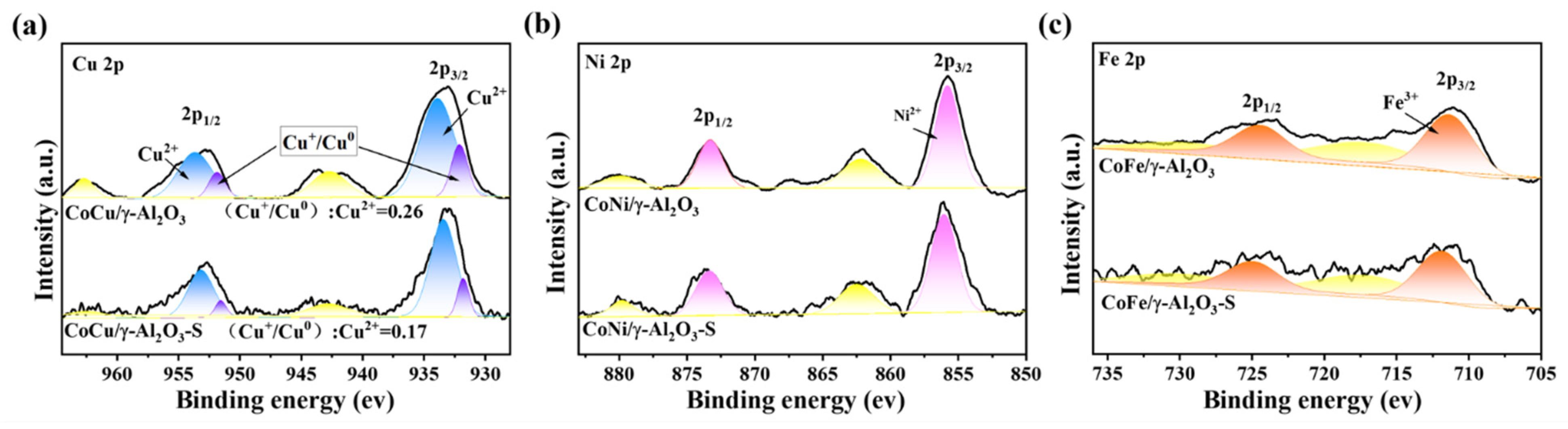
2.3. Evaluation of Catalytic Activity and Sulfur Tolerance
3. Experimental Methods
3.1. Catalyst Preparation
3.2. Catalytic Performance Evaluation
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murindababisha, D.; Yusuf, A.; Sun, Y.; Wang, C.; Ren, Y.; Lv, J.; Xiao, H.; Chen, G.Z.; He, J. Current Progress on Catalytic Oxidation of Toluene: A Review. Environ. Sci. Pollut. Res. 2021, 28, 62030–62060. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattison, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Mao, M.; Shi, J. A Review of the Treatment Techniques of VOC. Appl. Math. Nonlinear Sci. 2023, 8, 2063–2074. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N. Atomic dispersion of bulk/nano metals to atomic-sites catalysts and their application in thermal catalysis. Nano Res. 2023, 16, 6380–6401. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent Advances in VOC Elimination by Catalytic Oxidation Technology onto Various Nanoparticles Catalysts: A Critical Review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Chen, X.; Li, J.; Schwank, J.W. Sodium-Promoted Ag/CeO2 Nanospheres for Catalytic Oxidation of Formaldehyde. Chem. Eng. J. 2018, 350, 419–428. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, H.; Pan, W.; Mu, X.; Qiu, K.; Ma, J.; Zhao, Y.; Song, J.; Zhang, Z. Research Progress of a Composite Metal Oxide Catalyst for VOC Degradation. Environ. Sci. Technol. 2022, 56, 9220–9236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Im, M.; Cho, E.; Jang, H.; Jang, S.Y.; Kim, D.W.; Kim, K.W.; Heo, I.; Kim, Y.J.; Lee, J.H. Effects of Thermal Aging on the Electronic and Structural Properties of Pt-Pd and Toluene Oxidation Activity. Sci. Total Environ. 2022, 847, 157482. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt-Pd Bimetallic Nanoparticles Anchored on Uniform Mesoporous MnO2 Sphere as an Advanced Nanocatalyst for Highly Efficient Toluene Oxidation. Green Energy Environ. 2022, 7, 1349–1360. [Google Scholar] [CrossRef]
- Tu, L.; Liu, R.; Zhao, D.; Ding, H.; Cui, J.; Liang, B. PtPd/TiO2 Catalysts for Low-Temperature Toluene Oxidation. Catal. Surv. Asia 2021, 25, 389–398. [Google Scholar] [CrossRef]
- Ran, Y.; Xu, C.; Ji, D.; Zhao, H.; Li, L.; Lei, Y. Research Progress of Transition Metal Compounds as Bifunctional Catalysts for Zinc-Air Batteries. Nano Res. Energy 2024, 3, e9120092. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; van Hoof, A.J.F.; Hensen, E.J.M. On the Surface-Dependent Oxidation of Cu2O During CO Oxidation: Cu2+ is More Active Than Cu+. Appl. Catal. A Gen. 2020, 602, 117712. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Zhang, L.; Wang, H.; Yang, Y.; Hung, S.-F.; Hsu, Y.-Y.; Liu, B. Tuning Chemical Bonding of MnO2 Through Transition-Metal Doping for Enhanced CO Oxidation. J. Catal. 2016, 341, 82–90. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Xie, K. Activating Lattice Oxygen at the Twisted Surface in a Mesoporous CeO2 Single Crystal for Efficient and Durable Catalytic CO Oxidation. Angew. Chem. Int. Ed. 2021, 60, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, F.; Li, J.; You, Z. Dispersion–Precipitation Synthesis of Highly Active Nanosized Co3O4 for Catalytic Oxidation of Carbon Monoxide and Propane. Appl. Surf. Sci. 2017, 411, 136–143. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Wang, G.; Du, R.; Zhao, H.; Lu, H.; Yang, S.; Tang, S.; Guo, Z.; Yang, J.; et al. Constructing core@shell structured photothermal nanosphere with thin carbon layer confined Co-Mn bimetals for pollutant degradation and solar interfacial water evaporation. Rare Met. 2024, 43, 1686–1701. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Ding, J.; Wu, X.; Si, Z.; Weng, D. The Controlled Preparation and Performance of Fe, Co-Modified Porous Ceria Nanorods for the Total Oxidation of Propane. Mol. Catal. 2020, 480, 110663. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Wang, Y.; Gong, X.; Lu, G. A Highly Effective Ni-Modified MnOx Catalyst for Total Oxidation of Propane: The Promotional Role of Nickel Oxide. RSC Adv. 2016, 6, 50228–50237. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.; Zheng, J.; Zuo, S. Studies of Sulfur Poisoning Process via Ammonium Sulfate on MnO2/γ-Al2O3 Catalyst for Catalytic Combustion of Toluene. Appl. Catal. B Environ. 2021, 298, 120595. [Google Scholar] [CrossRef]
- Niu, K.; Lin, D.; Lan, W.; Feng, X.; Gong, W.; Zhang, Z.; Huang, B.; Luo, Y.; Qian, Q.; Chen, Q. Waste Eggshell Membrane-Templated Three-Dimensional CoOx Catalyst for Efficient Low-Temperature Toluene Oxidation with Strong Thermal Stability. J. Environ. Chem. Eng. 2022, 10, 108710. [Google Scholar] [CrossRef]
- Jiang, B.; Xie, K.; Wang, Z.; Ning, H.; Zuo, S.; Li, J. Study on the Mechanism of Sulfur Poisoning in Toluene Catalyzed by Co3O4/γ-Al2O3 Sulfur Tolerant Catalyst Containing Spinel Structure. J. Environ. Chem. Eng. 2023, 11, 110518. [Google Scholar] [CrossRef]
- Han, J.-K.; Jia, L.-T.; Hou, B.; Li, D.-B.; Liu, Y.; Liu, Y.-C. Catalytic Properties of CoAl2O4/Al2O3 Supported Cobalt Catalysts for Fischer-Tropsch Synthesis. J. Fuel Chem. Technol. 2015, 43, 846–851. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, Z.; Ren, Y.; Dong, C. Enhancement of Toluene Oxidation Performance Over Cu–Mn Composite Oxides by Regulating Oxygen Vacancy. Appl. Surf. Sci. 2021, 560, 149983. [Google Scholar] [CrossRef]
- Barama, A.; Ouaguenouni, M.H.S.; Barama, S. Textural Properties and Catalytic Activity of Ni–Mn Mixed Oxides in the Combustion of Toluene at Low-Temperatures. Arab. J. Sci. Eng. 2023, 48, 8679–8692. [Google Scholar] [CrossRef]
- Soltan, W.B.; Peng, J.; Cao, Z.; Fu, Z.; Liu, H. Bimetallic Fe-Mn Loaded H-ZSM-5 Zeolites for Excellent VOCs Catalytic Oxidation at Low-Temperatures: Synergistic Effects and Catalytic Mechanisms. Chem. Eng. J. 2023, 475, 146251. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Sun, Y.; Ma, Y.; Zhu, L.; Zhu, Y.; Zhang, J.; Qiao, S.; Gao, J.; Ji, W.; et al. Synergistic Double Doping of Co and Cu Constructs Multiple Active Sites to Achieve Catalyzed Degradation of Toluene at High Humidity. Fuel 2023, 342, 127875. [Google Scholar] [CrossRef]
- Li, Z.; Öztuna, E.; Skorupska, K.; Vinogradova, O.V.; Jamshaid, A.; Steigert, A.; Rohner, C.; Dimitrakopoulou, M.; Prieto, M.J.; Kunkel, C.; et al. Rationally Designed Laterally-Condensed-Catalysts Deliver Robust Activity and Selectivity for Ethylene Production in Acetylene Hydrogenation. Nat. Commun. 2024, 15, 10660. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Jing, Y.; Qian, Y.; Chen, D.; Maeda, N.; Murayama, T.; Toyao, T.; Shimizu, K. In situ/operando spectroscopic evidence on associative redox mechanism for periodic unsteady-state water–gas shift reaction on Au/CeO2 catalyst. J. Catal. 2024, 433, 115500. [Google Scholar] [CrossRef]
- Zhang, N.; Miyazaki, S.; Qian, Y.; Jing, Y.; Toyao, T.; Shimizu, K. Mechanism of the periodic unsteady-state water–gas shift reaction on highly dispersed Cu-loaded CeO2 catalysts. ACS Catal. 2023, 13, 8503–8515. [Google Scholar] [CrossRef]
- Martra, G.; Coluccia, S.; Marchese, L.; Augugliaro, V.; Loddo, V.; Palmisano, L.; Schiavello, M. The Role of H2O in the Photocatalytic Oxidation of Toluene in Vapour Phase on Anatase TiO2 Catalyst: A FTIR Study. Catal. Today 1999, 53, 695–702. [Google Scholar] [CrossRef]
- Stuart, N.M.; Sohlberg, K. The Microstructure of γ-Alumina. Energies 2022, 14, 6472. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Li, H.; An, X.; Wang, A. Cobalt Blue Hybrid Pigment Doped with Magnesium Derived from Sepiolite. Appl. Clay Sci. 2018, 157, 111–120. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Dai, Y.; Quan, Y.; Zhao, J.; Ren, J. Highly Active Urchin-Like MCo2O4 (M = Co, Cu, Ni or Zn) Spinel for Toluene Catalytic Combustion. Fuel 2022, 318, 123648. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Ma, Y.; Xu, C.; Zhou, S. Synthesis of Mesoporous Silica-Supported NiCo Bimetallic Nanocatalysts and Their Enhanced Catalytic Hydrogenation Performance. ACS Omega 2023, 8, 12339–12347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Du, J.; Luo, D.; Shi, H.; Wang, J.; Zhang, J.; Liu, X.; Liu, M.; Mei, K.; Liu, D.; et al. Structurally Ordered FeCo@FeCoOx@NC Dual-Shell Nanoparticles Synthesized Under Micro-Oxygen Conditions: An Efficient Cocatalyst for BiVO4 Photoelectrochemical Water Oxidation. Appl. Catal. B Environ. 2025, 363, 124779. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Wang, L.; Wu, T. Magnetic Properties of α-Fe2O3 Nanopallets. Rare Met. 2019, 38, 14–19. [Google Scholar] [CrossRef]
- Shi, X.; Yu, Y.; Xue, L.; He, H. Effect of Sulfur Poisoning on Co3O4/CeO2 Composite Oxide Catalyst for Soot Combustion. Chin. J. Catal. 2014, 35, 1504–1510. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, J.; Ma, Y.; Liu, B.; Zhang, X.; Wang, L.; Yuan, Y.; Liu, J.; Wang, M.; Du, Q.; et al. Increasing Sensing Sensitivity of the Fe-α-Fe2O3 (104) Surface by Hydrogenation and the Sensing Reaction Molecule Mechanism. Sens. Actuator B Chem. 2019, 281, 366–374. [Google Scholar] [CrossRef]
- Torres-Ochoa, J.A.; Cabrera-German, D.; Cortazar-Martinez, O.; Bravo-Sanchez, M.; Gomez-Sosa, G.; Herrera-Gomez, A. Peak-Fitting of Cu 2p Photoemission Spectra in Cu0, Cu1+, and Cu2+ Oxides: A method for Discriminating Cu0 from Cu1+. Appl. Surf. Sci. 2023, 622, 156960. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New Interpretations of XPS Spectra of Nickel Metal and Oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, X.; Liu, M.; Zhao, H.; Wang, Z.; Ni, R.; Wang, Y.; Yang, J.; Gao, F.; Li, Y.; et al. Composition-engineered FeCo nanoalloys with lattice expansion and optimized electron structure boosting electrocatalytic nitrate reduction. Appl. Catal. B Environ. Energy 2024, 355, 124205. [Google Scholar] [CrossRef]
- Stehr, J.E.; Hofmann, D.M.; Schörmann, J.; Becker, M.; Chen, W.M.; Buyanova, I.A. Electron Paramagnetic Resonance Signatures of Co2+ and Cu2+ in β-Ga2O3. Appl. Phys. Lett. 2019, 115, 242101. [Google Scholar] [CrossRef]
- Lv, X.; Cai, S.; Chen, J.; Yan, D.; Jiang, M.; Chen, J. Tuning the Degradation Activity and Pathways of Chlorinated Organic Pollutants over CeO2 Catalyst with Acid Sites: Synergistic Effect of Lewis and Brønsted Acid Sites. Catal. Sci. Technol. 2021, 11, 4581–4595. [Google Scholar] [CrossRef]
- Ye, L.; Lu, P.; Yan, X.H.; Huang, H. Boosting Simultaneous Catalytic Removal of NOx and Toluene via Cooperation of Lewis Acid and Oxygen Vacancies. Appl. Catal. B 2023, 331, 122696. [Google Scholar] [CrossRef]
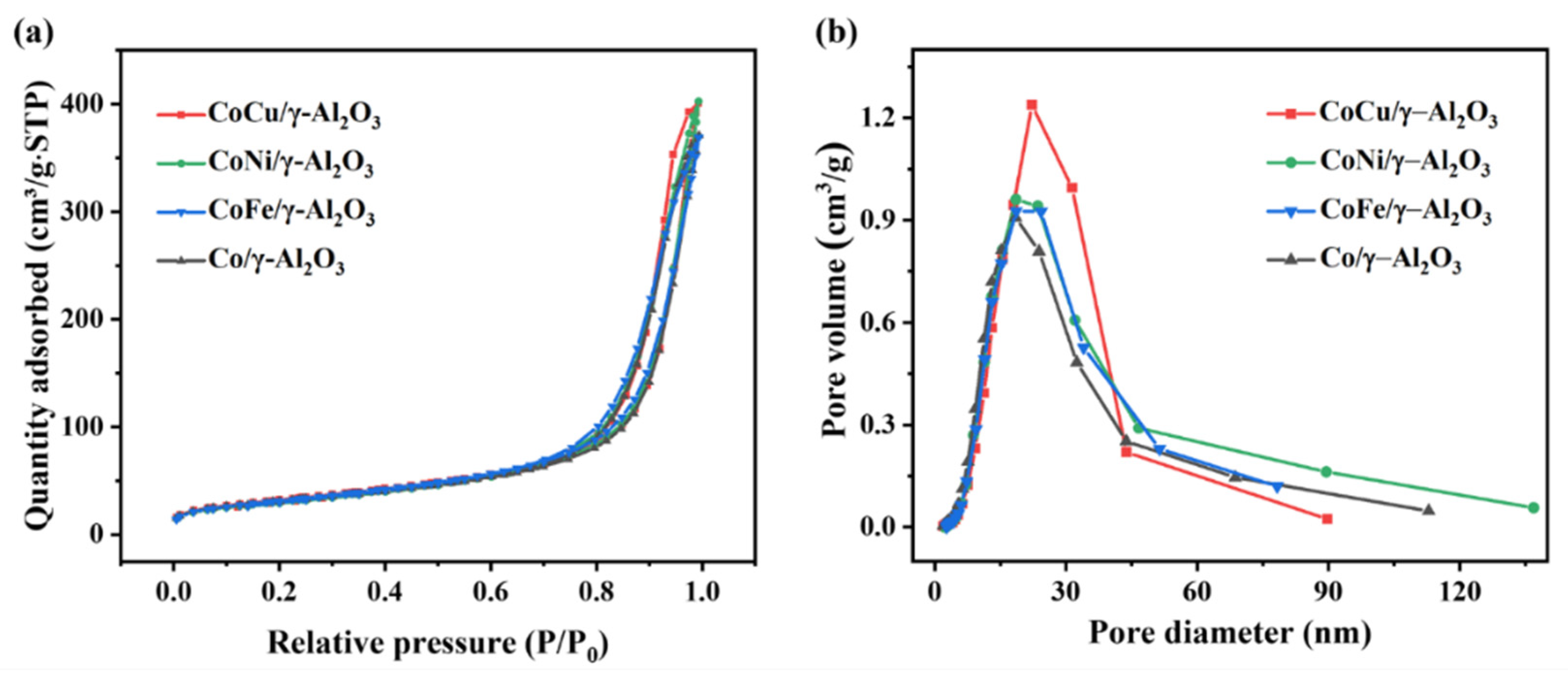
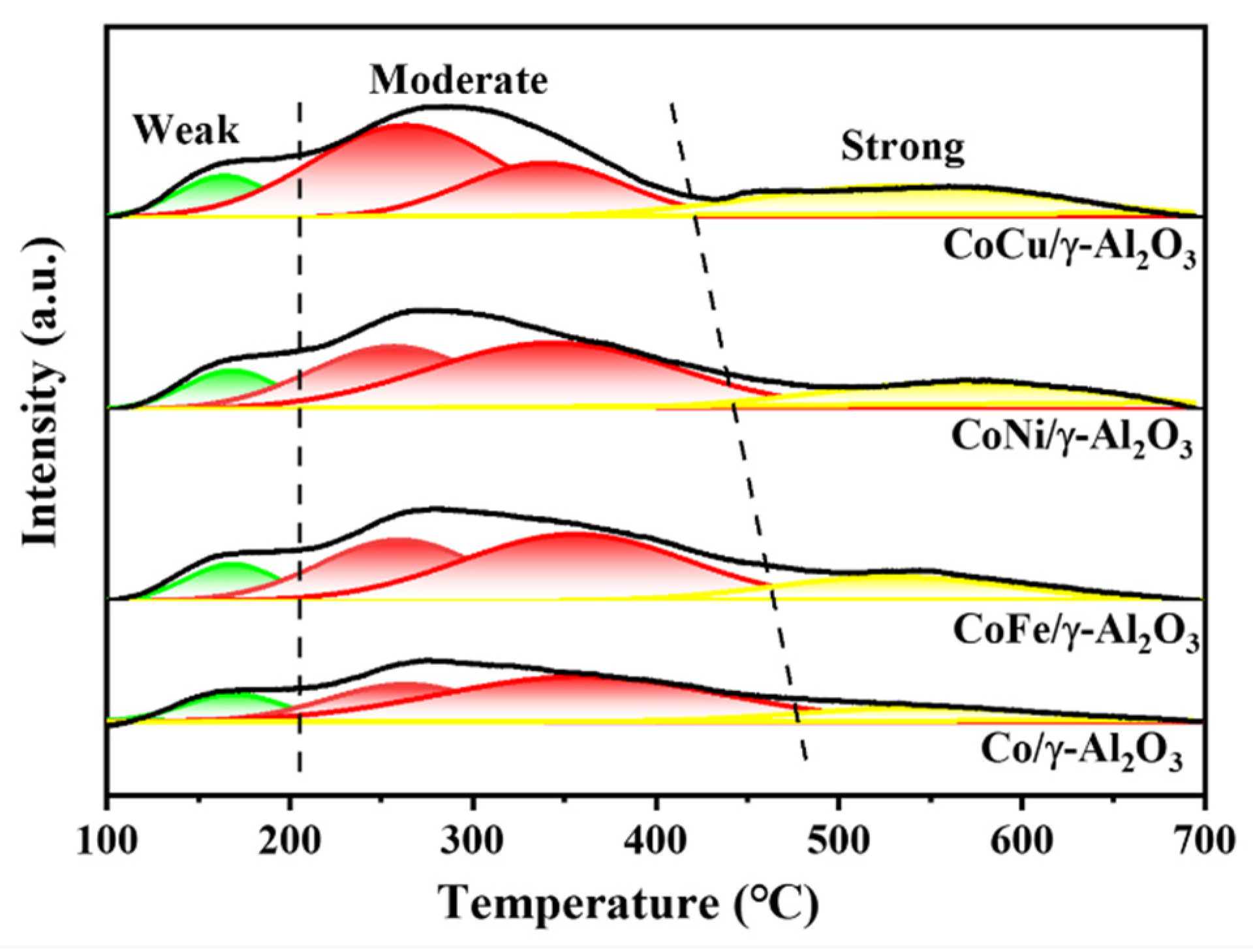
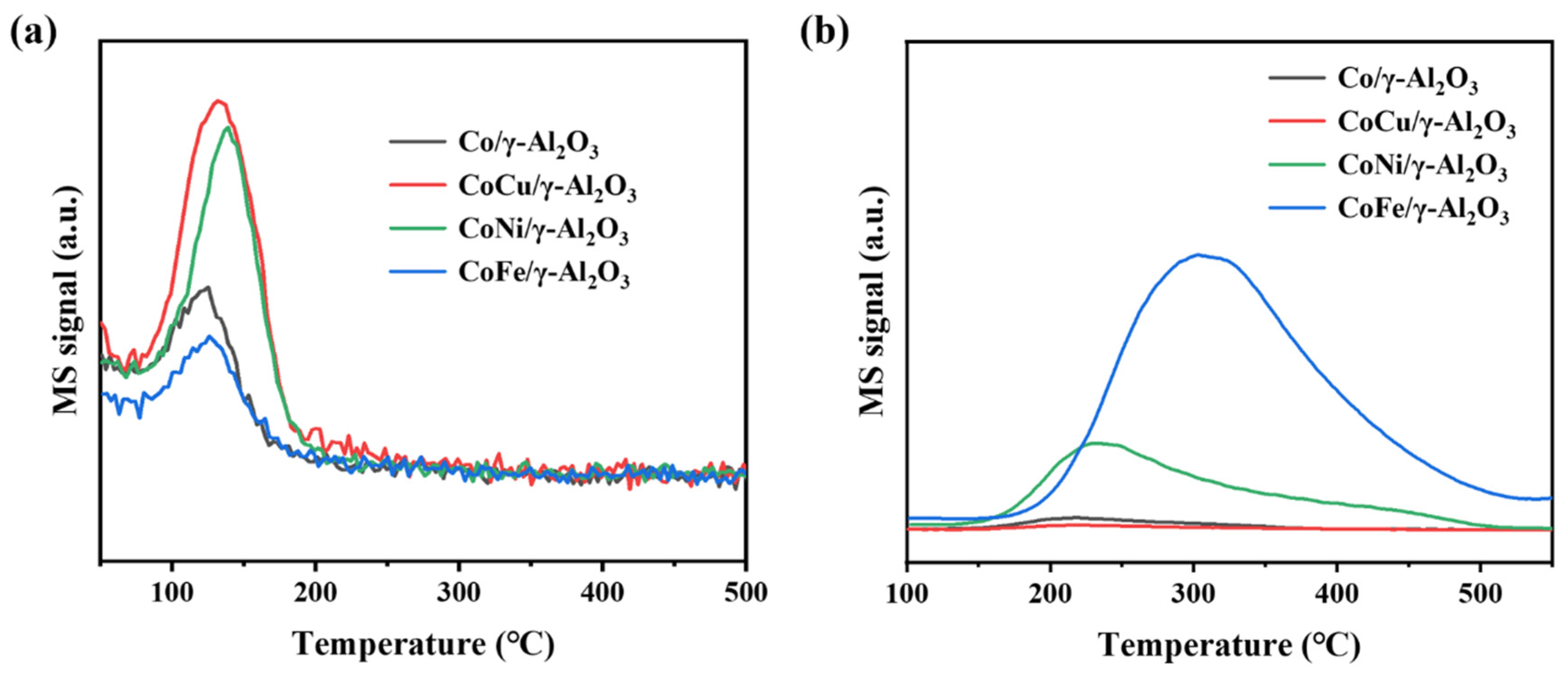
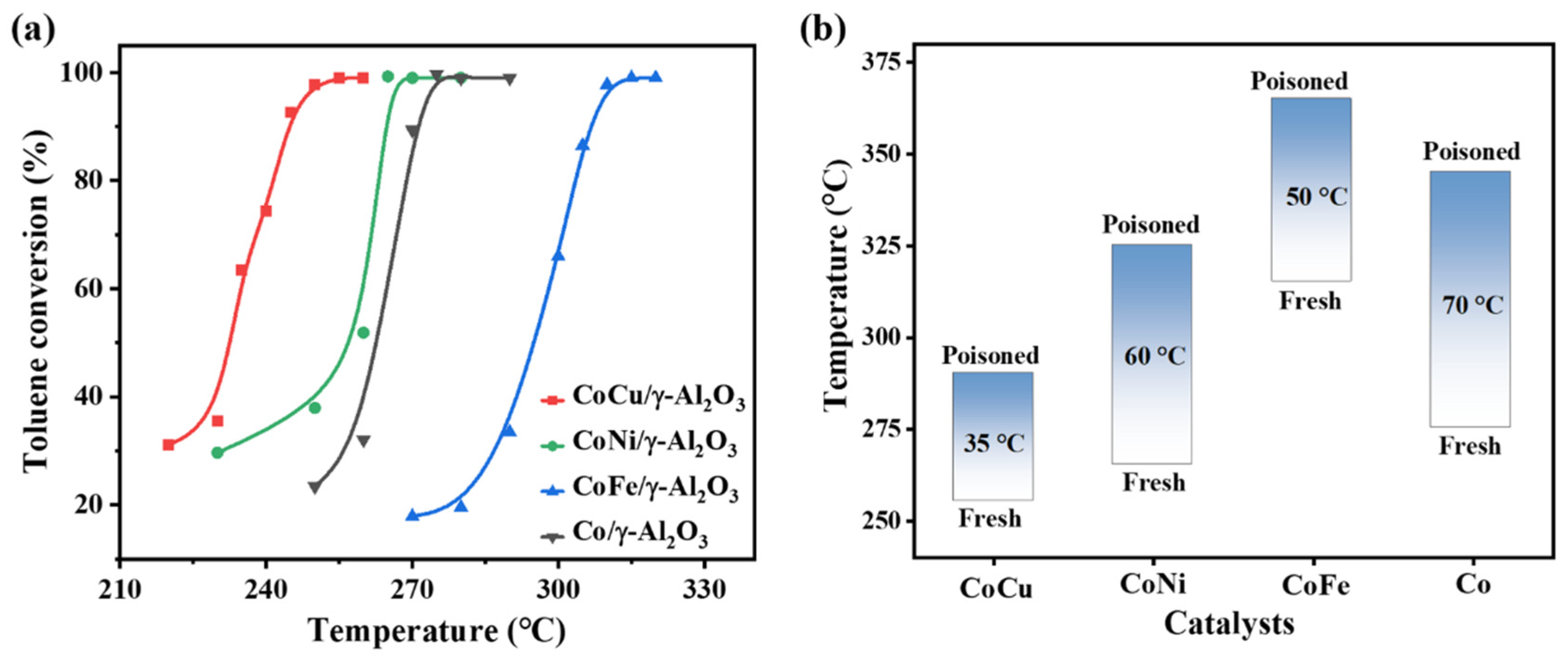
| Sample | Co/γ-Al2O3 | CoCu/γ-Al2O3 | CoNi/γ-Al2O3 | CoFe/γ-Al2O3 | ||||
|---|---|---|---|---|---|---|---|---|
| Fresh | Poisoned | Fresh | Poisoned | Fresh | Poisoned | Fresh | Poisoned | |
| SBET a (m2/g) | 112.4 | 100.6 | 117.6 | 121.0 | 111.9 | 114.2 | 110.8 | 102.7 |
| Vp b (m3/g) | 0.5740 | 0.5614 | 0.6205 | 0.6591 | 0.6200 | 0.5872 | 0.5706 | 0.5339 |
| Sample | Weak (mmol/gcat.) | Moderate (mmol/gcat.) | Strong (mmol/gcat.) |
|---|---|---|---|
| CoCu/γ-Al2O3 | 0.04248 | 0.28546 | 0.10390 |
| CoNi/γ-Al2O3 | 0.03998 | 0.29940 | 0.08208 |
| CoFe/γ-Al2O3 | 0.03992 | 0.29330 | 0.06744 |
| Co/γ-Al2O3 | 0.03986 | 0.27139 | 0.04998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Yang, X.; Zhang, Q.; Lv, D.; Zuo, S.; Li, J. Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency. Molecules 2025, 30, 1188. https://doi.org/10.3390/molecules30051188
Tang Y, Yang X, Zhang Q, Lv D, Zuo S, Li J. Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency. Molecules. 2025; 30(5):1188. https://doi.org/10.3390/molecules30051188
Chicago/Turabian StyleTang, Yuwei, Xu Yang, Qinglong Zhang, Dongmei Lv, Shufeng Zuo, and Jing Li. 2025. "Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency" Molecules 30, no. 5: 1188. https://doi.org/10.3390/molecules30051188
APA StyleTang, Y., Yang, X., Zhang, Q., Lv, D., Zuo, S., & Li, J. (2025). Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency. Molecules, 30(5), 1188. https://doi.org/10.3390/molecules30051188






