Preparation of CL-20 with Controllable Particle Size Using Microfluidic Technology
Abstract
1. Introduction
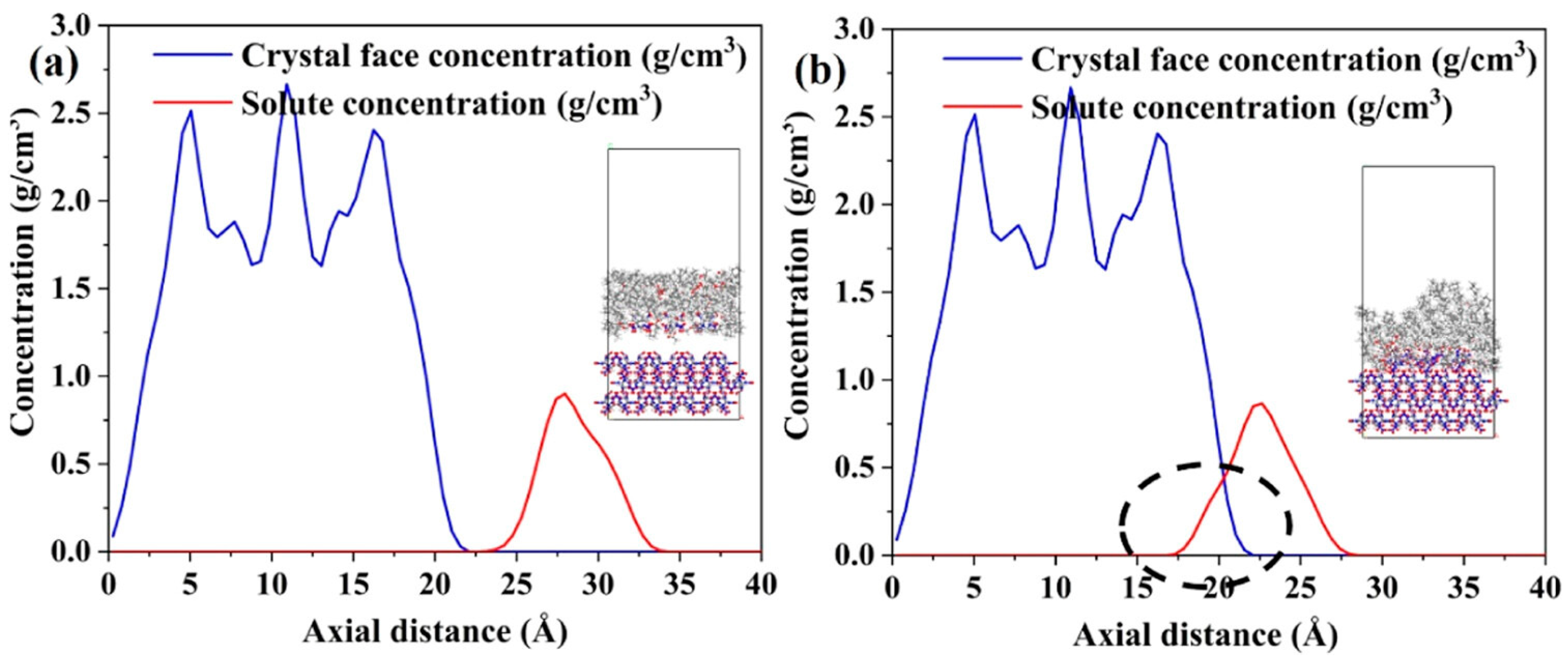
2. Results and Discussion
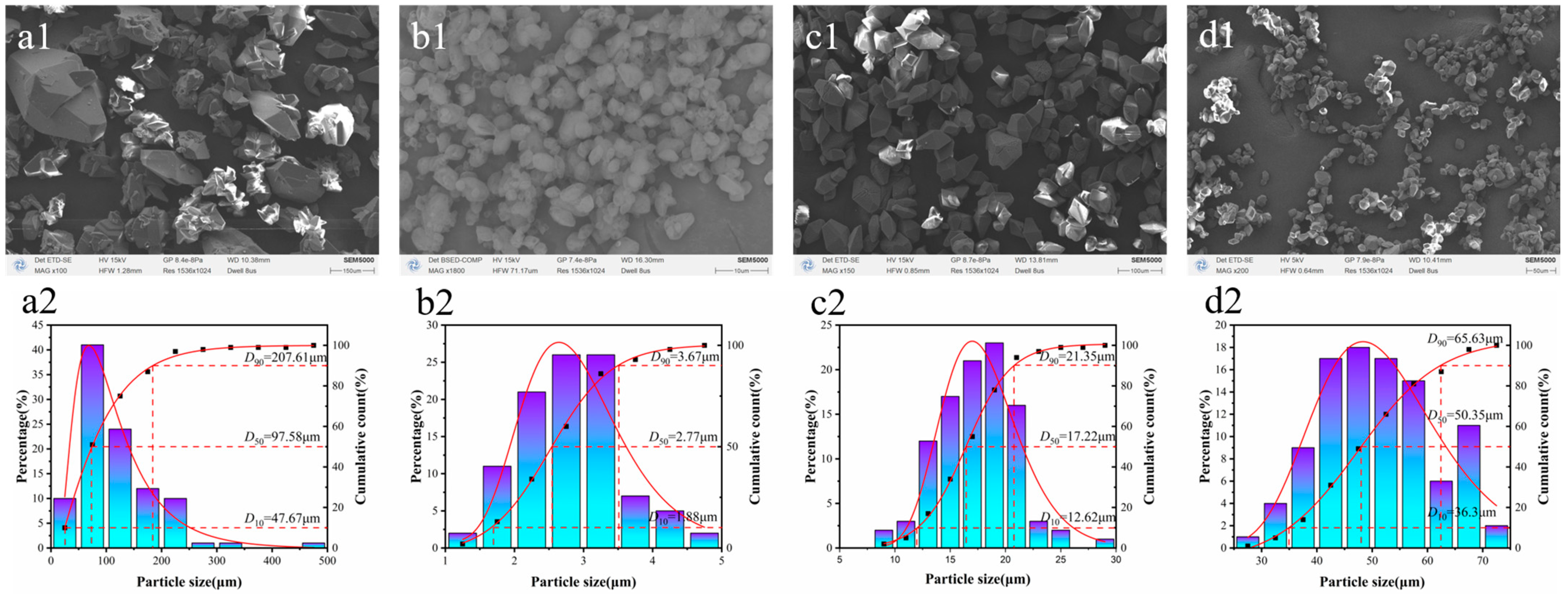
2.1. Morphology Analysis
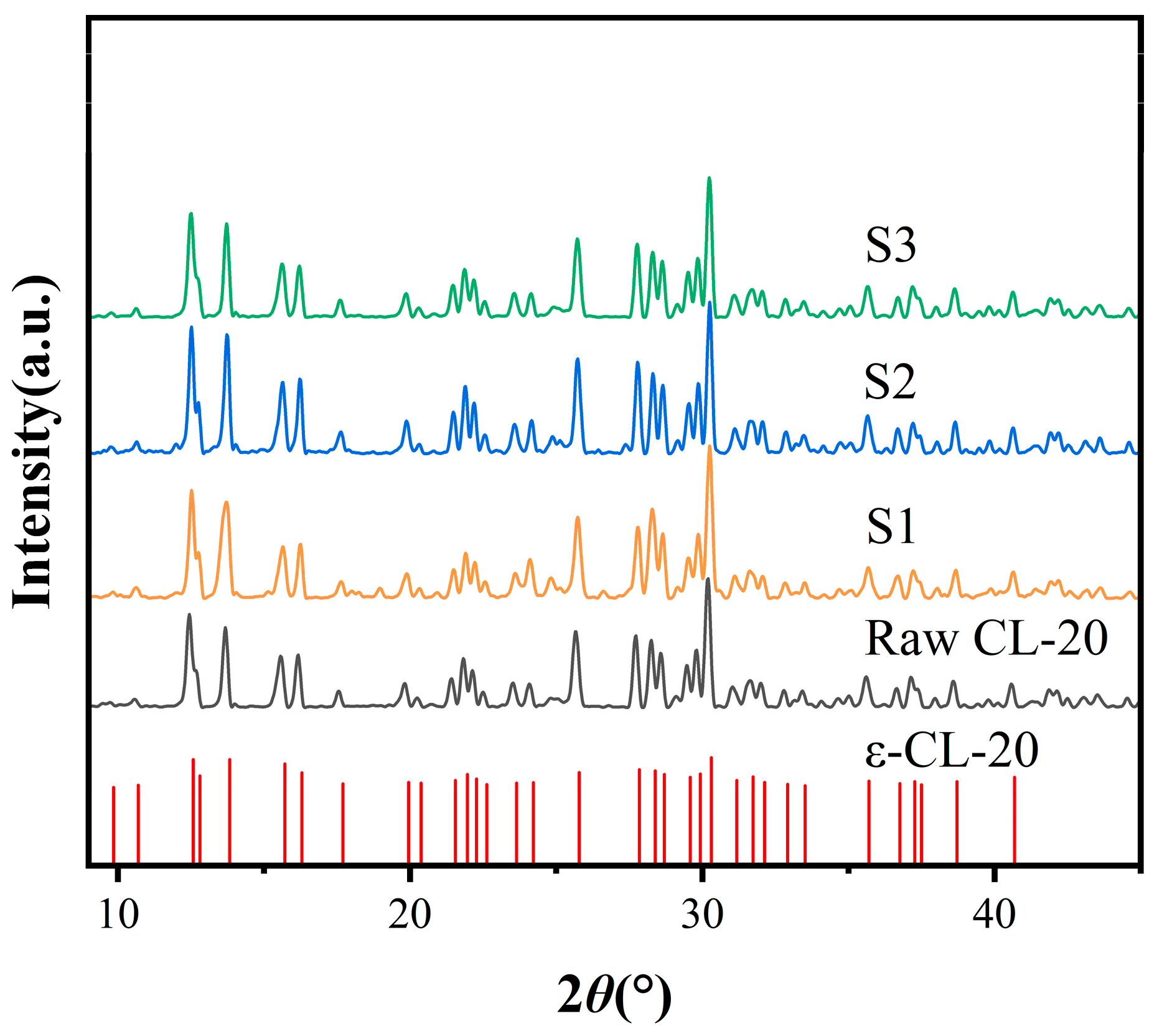
2.2. XRD Analysis
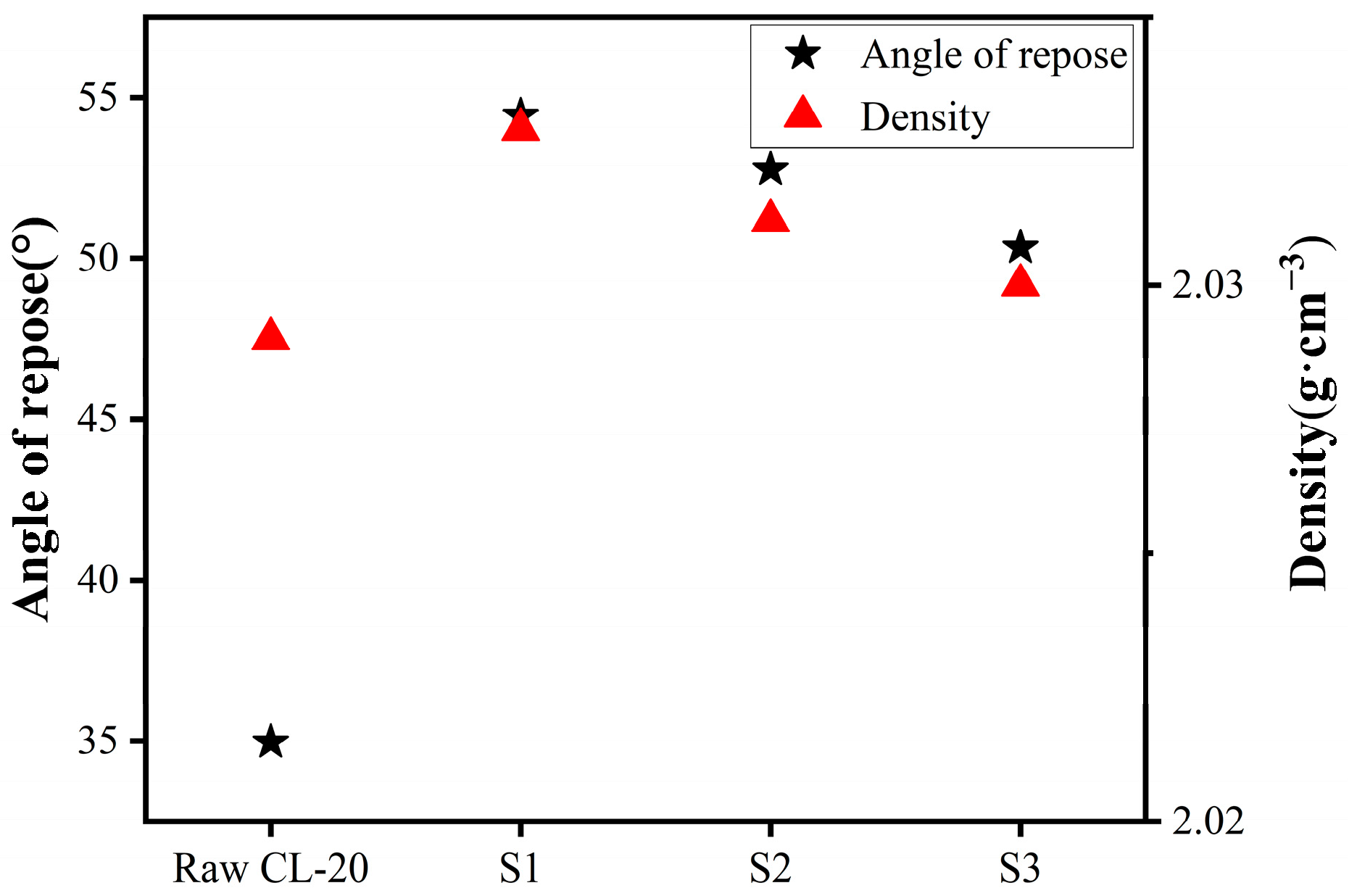
2.3. Dispersion Performance and True Density Analysis
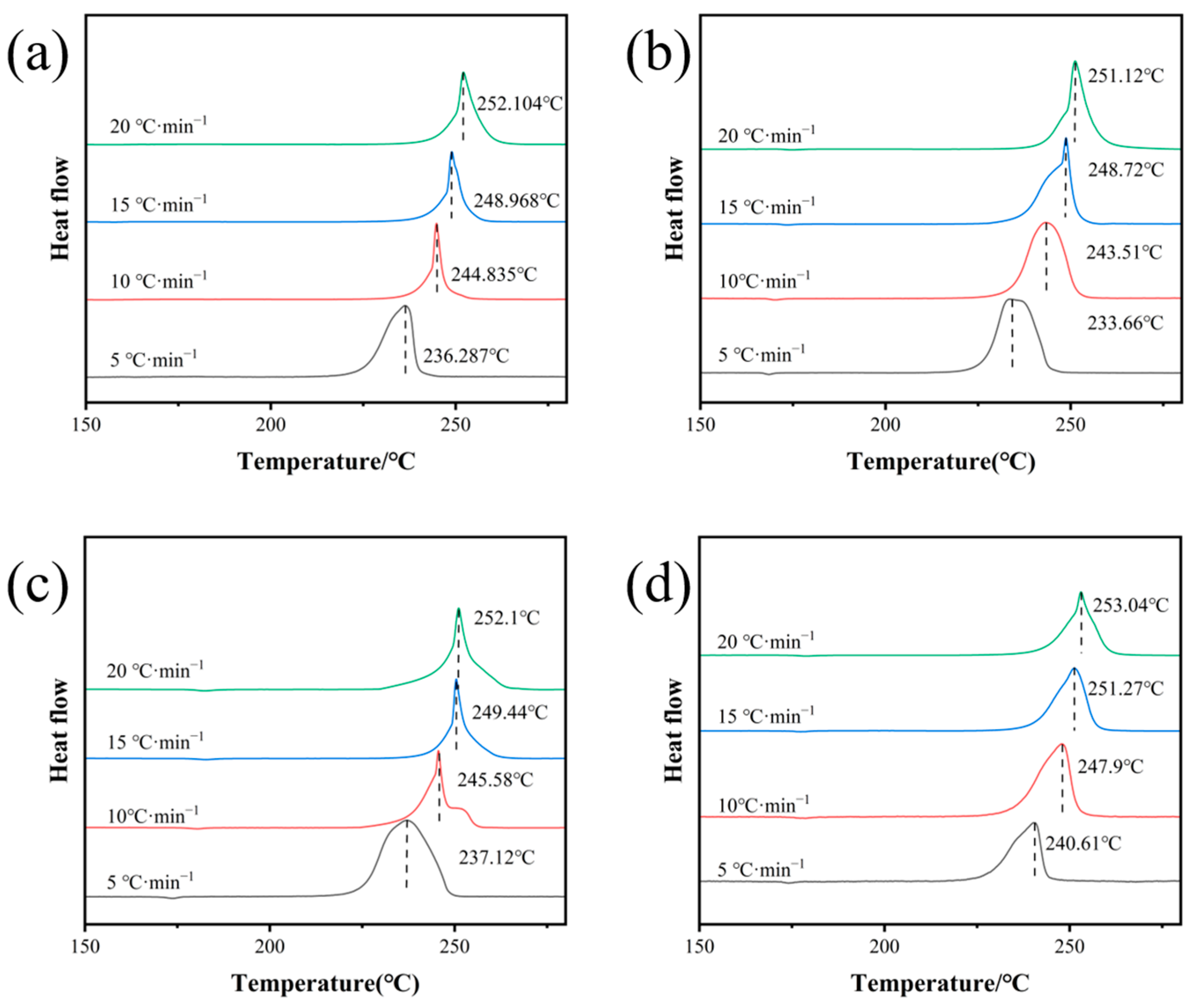
2.4. Thermal Performance Analysis
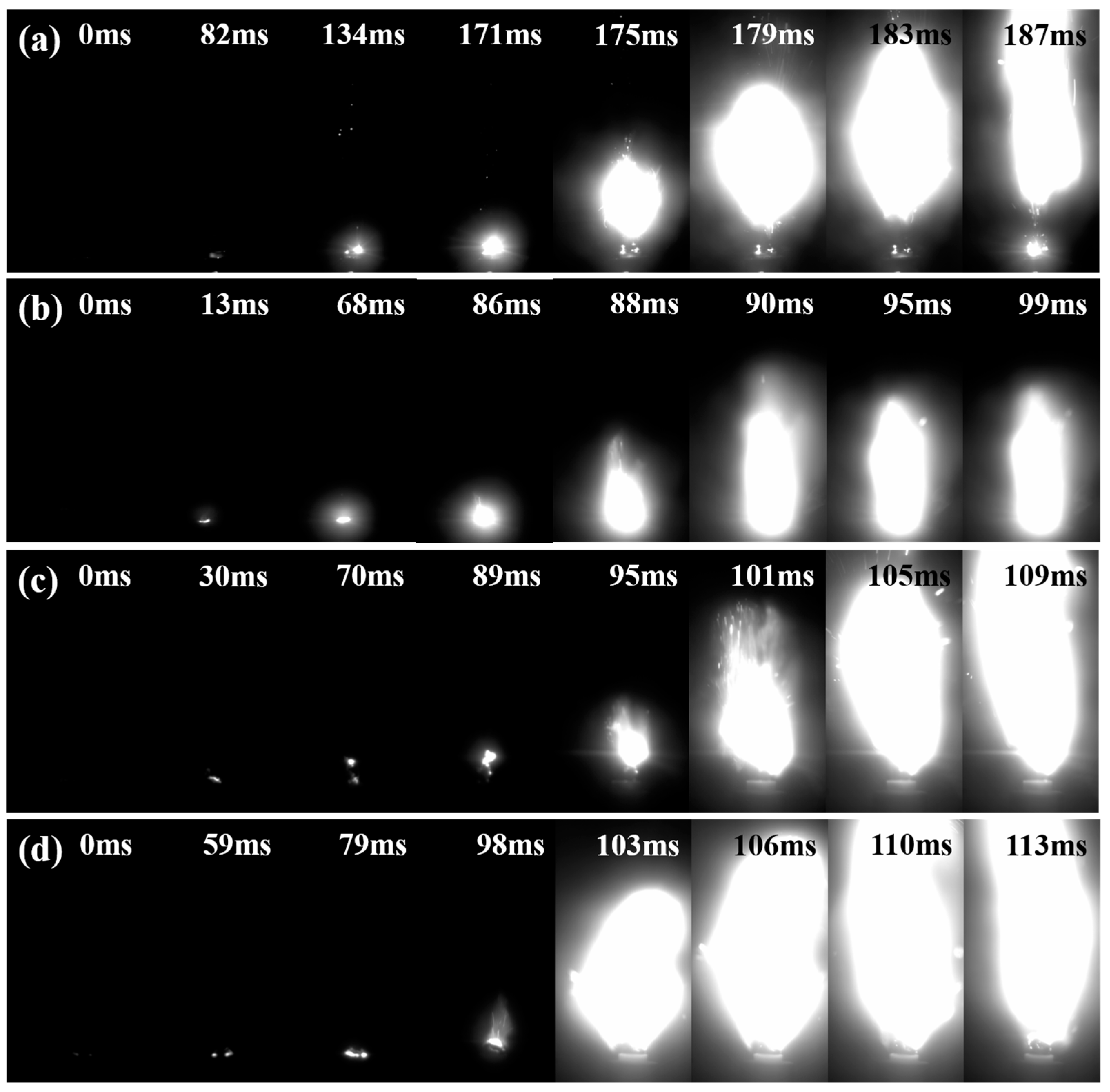
2.5. Analysis of Combustion Behavior
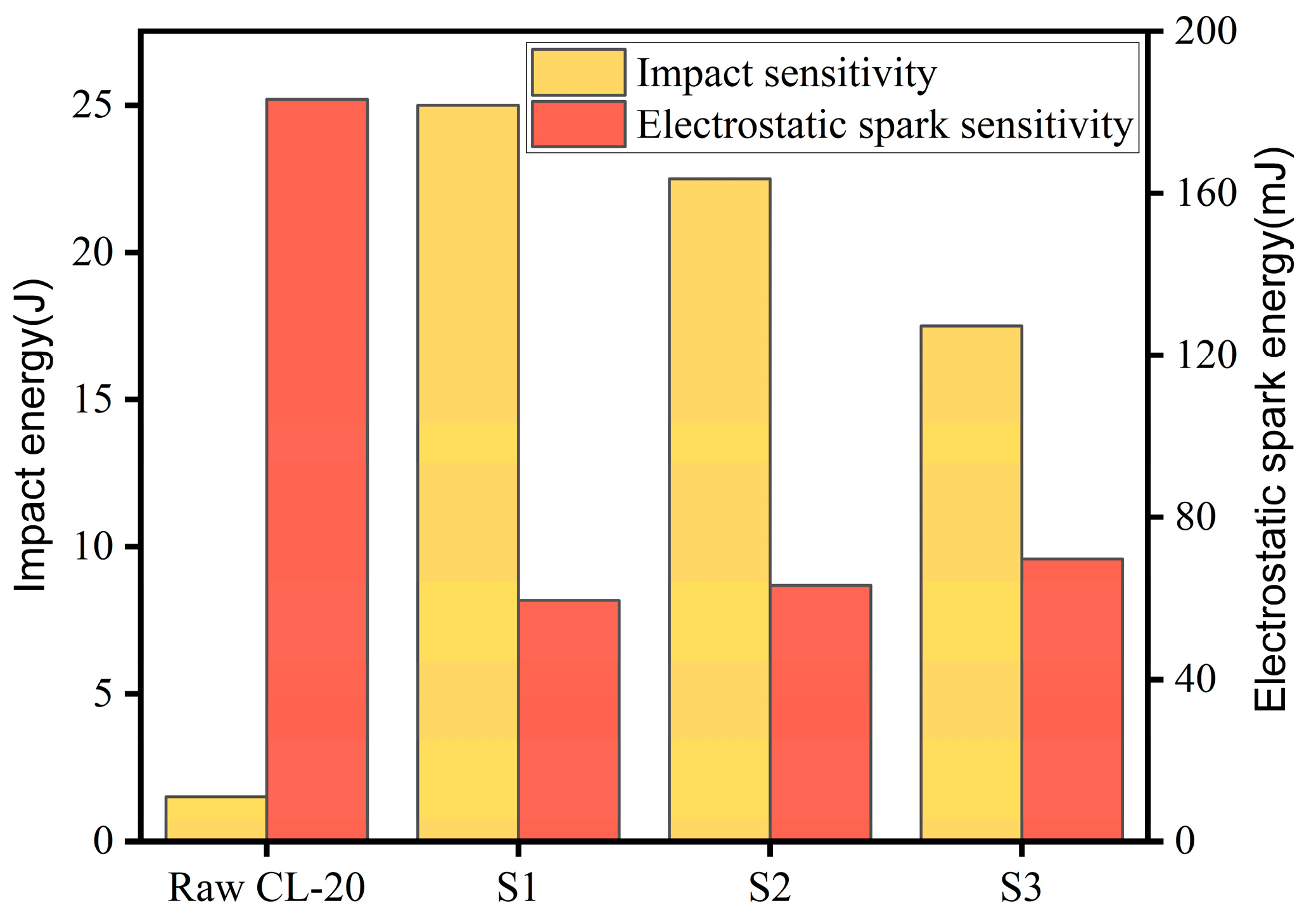
2.6. Sensitivity Analysis
3. Materials and Methods
3.1. Experimental Materials and Facilities
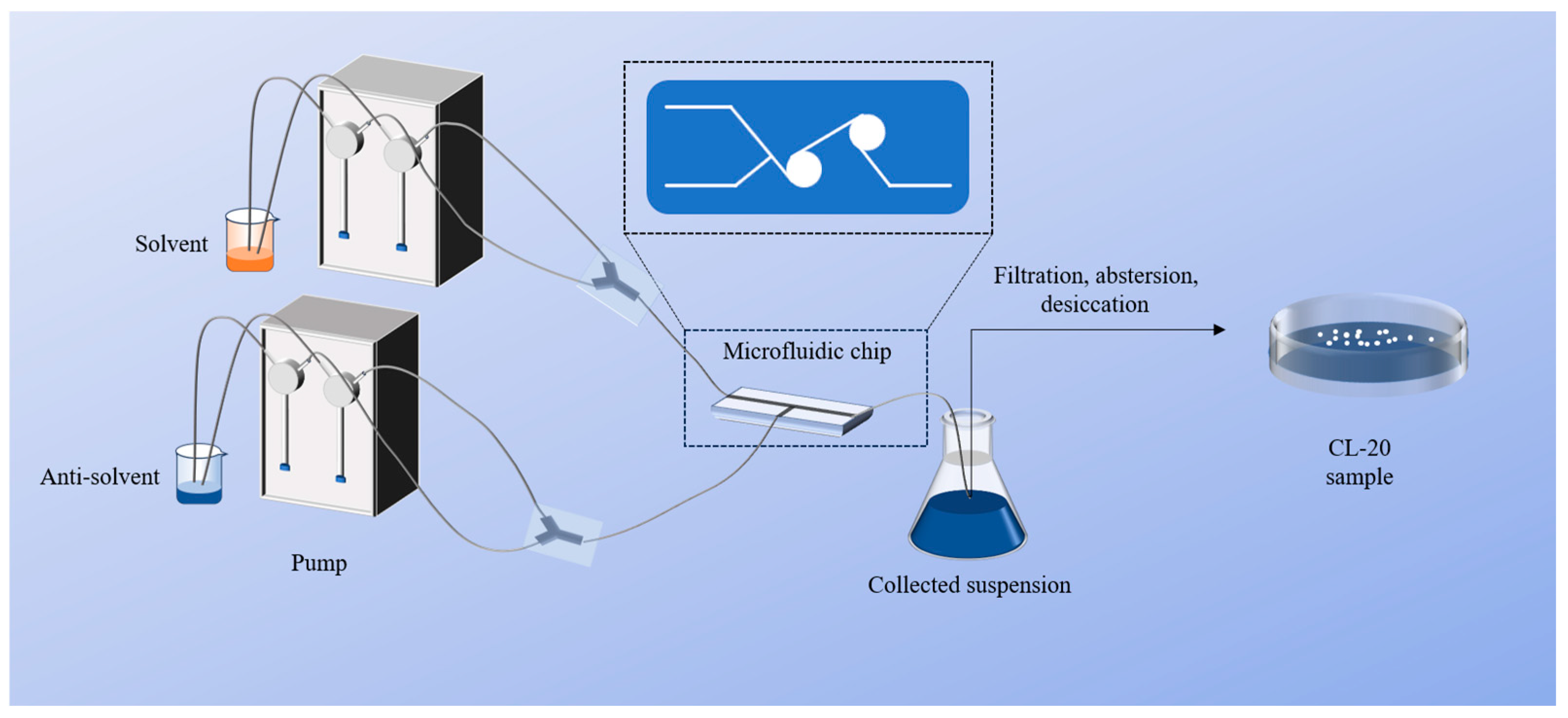
3.2. Preparation of CL-20 with Multiple Particle Sizes
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, A.T.; Chafin, A.P.; Christian, S.L.; Moore, D.W.; Nadler, M.P.; Nissan, R.A.; Vanderah, D.J.; Gilardi, R.D.; George, C.F.; Flippen-Anderson, J.L. Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 1998, 54, 11793–11812. [Google Scholar] [CrossRef]
- Bayat, Y.; Zarandi, M.; Zarei, M.A.; Soleyman, R.; Zeynali, V. A novel approach for preparation of CL-20 nanoparticles by microemulsion method. J. Mol. Liq. 2014, 193, 83–86. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, L.; Wu, P.; Liu, Y.; Nie, F.; Huang, L. Fabrication of RDX, HMX and CL-20 based microcapsules via in situ polymerization of melamine–formaldehyde resins with reduced sensitivity. Chem. Eng. J. 2015, 268, 60–66. [Google Scholar] [CrossRef]
- Feng, C.; Ye, B.; Ma, Y.; Cheng, W.; Shi, S.; Zhao, F.; An, C.; Wang, J. Electrospray fabrication of CL-20 composite microspheres for high-energy EFIs: Microstructure modulation and performance optimization. Powder Technol. 2024, 437, 119563. [Google Scholar] [CrossRef]
- Bao, P.; Chen, Y.; Li, Y.; Ma, S.; Xiao, W.; Wang, B. Devising a composite membrane with a creeper sucker structure to enhance the interfacial performance and desensitization of CL-20. Mater. Today Commun. 2024, 41, 110436. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Zhu, R.; Zhu, R.; Zhou, J.; Lin, Z.; Han, K.; An, C.; Wang, J.; Wu, B. Crystal phase control and ignition properties of HNS/CL-20 composite microspheres prepared by microfluidics combined with emulsification techniques. Particuology 2024, 85, 241–251. [Google Scholar] [CrossRef]
- Gao, F.; Jing, J.; Cheng, W.; Song, H.; Li, S.; Zhang, Z.; Wang, J.; An, C. Molecular dynamics simulation of bilayer core-shell structure of CL-20 surface-modified by polydopamine coated with polymer binder. Mater. Today Commun. 2023, 37, 107099. [Google Scholar] [CrossRef]
- Wang, D.; Gao, B.; Yang, G.; Nie, F.; Huang, H. Preparation of CL-20 Explosive Nanoparticles and Their Thermal Decomposition Property. J. Nanomater. 2016, 2016, 5462097. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X.; Zhang, J.; Wang, Z.; Li, S. Preparation of Spherical Ultrafine CL-20 by Mechanical grinding. Chin. J. Energetic Mater. 2013, 21, 738–742. [Google Scholar] [CrossRef]
- Li, Y.; Ni, B.; Li, K.; Nan, H.; Niu, Y.; Jiang, F.; Li, X. Preparation of Ultrafine CL-20 by Wet Grinding Method and the Study on Its Properties. Propellants Explos. Pyrotech. 2020, 45, 1720–1728. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, F.; Xie, X.; Jiang, Y.; Jiang, Z.; Ran, R.; Zhang, M.; Li, H.; Qu, W. Effects of particle size of CL-20 on its thermal decomposition and combustion characteristics. Fuel 2024, 369, 131555. [Google Scholar] [CrossRef]
- Joseph, K.; Jonathan, E. Influence of Particle Size on the Combustion of CL-20/HTPB Propellants. Propellants Explos. Pyrotech. 2017, 42, 1261–1267. [Google Scholar] [CrossRef]
- Wu, B.; Shi, J.; Wei, W.; Zhu, R.; Zhou, J.; An, C.; Wang, J. Process and performance of DAAF microspheres prepared by continuous integration from synthesis to spherical coating based on microfluidic system. Def. Technol. 2024, 32, 629–643. [Google Scholar] [CrossRef]
- Yu, J.; Xu, S.; Jiang, H.; Zhao, F. Application and development trend of microfluidic technology in the preparation of energetic materials. Chin. J. Explos. Propellants 2022, 4, 439–451. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, J.; Zhu, P.; Xia, H.; Chen, C.; Shen, R. Microfluidic platform for preparation and screening of narrow size-distributed nanoscale explosives and supermixed composite explosives. Ind. Eng. Chem. Res. 2018, 57, 13191–13204. [Google Scholar] [CrossRef]
- Pu, C.; Luan, Y.; Wang, Y.; Xiao, Z. Thermal kinetics and flash DSC experimental validation of conventional nitrocellulose and ladder-structured nitrocellulose under non-isothermal condition. Combust. Flame. 2024, 260, 113216. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Yu, J.; Zhou, W.; Zhao, S.; Xu, S.; Zhao, F. Size, Morphology and Crystallinity Control Strategy of Ultrafine HMX by Microfluidic Platform. Nanomaterials 2023, 13, 464. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Guo, Y.; Xue, Z.; Han, K.; Liu, S.; An, C.; Ma, Z.; Wu, B. Regulating hollow structure of CL-20 microspheres using microjet droplet technology to enhance safety and combustion performance. Fuel 2024, 361, 130748. [Google Scholar] [CrossRef]
- Yang, L.; Shi, X.; Li, C.; Wu, B.; Pei, Z. Microfluidic assisted 90% loading CL-20 spherical particles: Enhancing self-sustaining combustion performance. Def. Technol. 2023, 22, 176–184. [Google Scholar] [CrossRef]
- Yang, B.; Lv, J.; Zhen, Z.; Wang, J.; Shen, Y.; Zhang, Y.; Wang, Y. Crystallization kinetics of stearic acid and stearic acid/MXene composite phase change materials. Energetic Storage Sci. Technol. 2022, 12, 3836–3844. [Google Scholar] [CrossRef]
- Shi, J.; Fei, Y.; Xia, H.; Zhou, X.; Yu, Q.; Zhu, P.; Shen, R. Microfluidic Avenue to Manipulate Polycrystalline Materials: A Case Study of 2, 4, 6, 8, 10, 12-Hexanitro-2, 4, 6, 8, 10, 12-Hexaazaisowurtzitane. Cryst. Growth Des. 2024, 24, 7755–7773. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Q.; Guo, X.; Wei, H. Production of the Large Smooth ε-CL-20 Particle by an Alternative Method of Solvent and Anti-solvent. Chin. J. Energetic Mater. 2017, 25, 221–225. [Google Scholar] [CrossRef]
- Pan, B.; Wei, H.; Jiang, J.; Zong, S.; Lv, P.; Dang, L. Solution-mediated polymorphic transformation of CL-20: An approach to prepare purified form ε particles. J. Mol. Liq. 2018, 265, 216–225. [Google Scholar] [CrossRef]
- Pan, B.; Dang, L.; Wang, Z.; Jiang, J.; Wei, H. Preparation, crystal structure and solution-mediated phase transformation of a novel solid-state form of CL-20. CrystEngComm 2018, 20, 1553–1563. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, F.; Gao, H.; Zhang, J.; Zhang, H.; Zhang, H. Estimation of critical temperature of thermal explosion for nitrosubstituted azetidines using non-isothermal DSC. Chin. J. Chem. 2009, 27, 2145–2154. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Y.; Huang, H.; Shang, F.; Song, X. An electrospun preparation of the NC/GAP/Nano-LLM-105 nanofiber and its properties. Nanomaterials 2019, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, S.; Shi, S.; Cheng, X.; An, C.; Wang, J. Preparation of microstructure controllable CL-20/FOX-7 composite microspheres by microchannel recrystallization technology to enhance safety and combustion performance. Chem. Eng. J. 2024, 499, 155869. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Zhou, J.; Ren, D.; Zhang, D.; An, C.; Wang, J. One-step rapid preparation of CL-20/TNT co-crystal assembly and spheroidized coating based on droplet microfluidic technology. Def. Technol. 2023, 27, 251–262. [Google Scholar] [CrossRef]
- QJ 20019-2011; Test Method for Safety Performance of Composite Solid Propellants. State Administration of Science, Technology and Industry for National Defence: Beijing, China, 2011.

| Reagent | Ethyl Acetate | Petroleum Ether | Normal Octane |
|---|---|---|---|
| Boiling point/K | 349.65 | 333.15 | 399.55 |
| Sample | Kissinger Method | Ozawa Method | Tp0/°C | Tb/°C | ||
|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | |||
| Raw CL-20 | 176.38 | 0.9913 | 185.06 | 0.9973 | 219.91 | 222.12 |
| S1 | 162.2 | 0.9933 | 152.33 | 0.9891 | 217.34 | 219.81 |
| S2 | 194.14 | 0.9939 | 185.93 | 0.9926 | 220.66 | 222.74 |
| S3 | 226.02 | 0.9869 | 232.26 | 0.9888 | 227.08 | 228.96 |
| Sample | Solvent | Concentration/g·mL−1 | Anti-Solvent | Flow Ratio | Post-Processing Method and Time |
|---|---|---|---|---|---|
| S1 | Ethyl acetate | 0.3 | Normal octane | 1:20 | Treatment at 50 °C for 15 min |
| S2 | Ethyl acetate | 0.3 | Petroleum ether | 1:15 | Stir for 1 h and stand for 4 h |
| S3 | Ethyl acetate | 0.3 | Petroleum ether | 1:10 | Stir for 1 h and stand for 6 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yu, J.; Wen, Y.; Jiang, H.; Xu, S.; Shao, Y.; Yao, E.; Li, H.; Zhao, F. Preparation of CL-20 with Controllable Particle Size Using Microfluidic Technology. Molecules 2025, 30, 1176. https://doi.org/10.3390/molecules30051176
Zhang Z, Yu J, Wen Y, Jiang H, Xu S, Shao Y, Yao E, Li H, Zhao F. Preparation of CL-20 with Controllable Particle Size Using Microfluidic Technology. Molecules. 2025; 30(5):1176. https://doi.org/10.3390/molecules30051176
Chicago/Turabian StyleZhang, Zihao, Jin Yu, Yujia Wen, Hanyu Jiang, Siyu Xu, Yubao Shao, Ergang Yao, Heng Li, and Fengqi Zhao. 2025. "Preparation of CL-20 with Controllable Particle Size Using Microfluidic Technology" Molecules 30, no. 5: 1176. https://doi.org/10.3390/molecules30051176
APA StyleZhang, Z., Yu, J., Wen, Y., Jiang, H., Xu, S., Shao, Y., Yao, E., Li, H., & Zhao, F. (2025). Preparation of CL-20 with Controllable Particle Size Using Microfluidic Technology. Molecules, 30(5), 1176. https://doi.org/10.3390/molecules30051176






