Triple Design Strategy for Quinoxaline-Based Hole Transport Materials in Flexible Perovskite Solar Cells
Abstract
1. Introduction
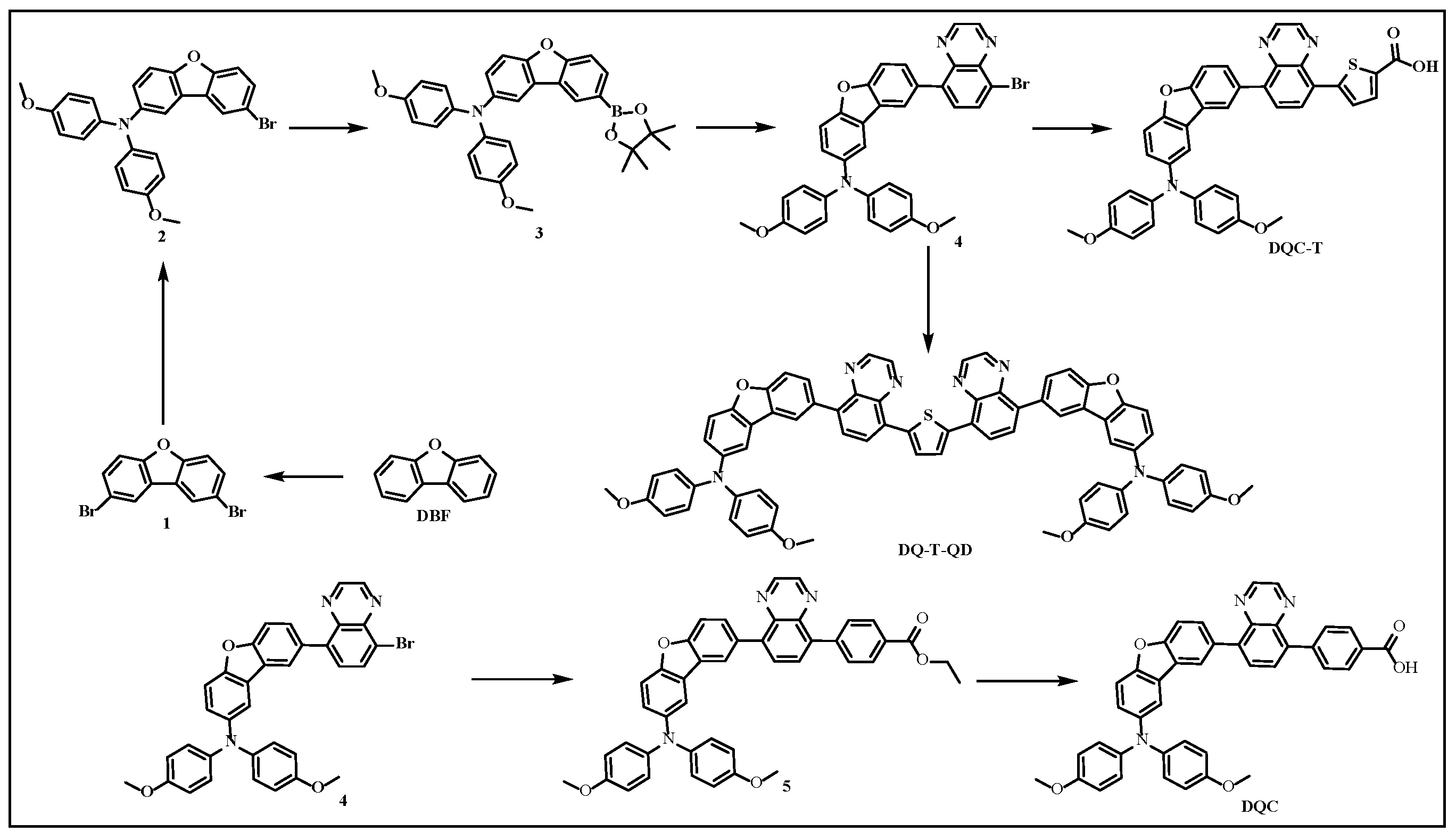
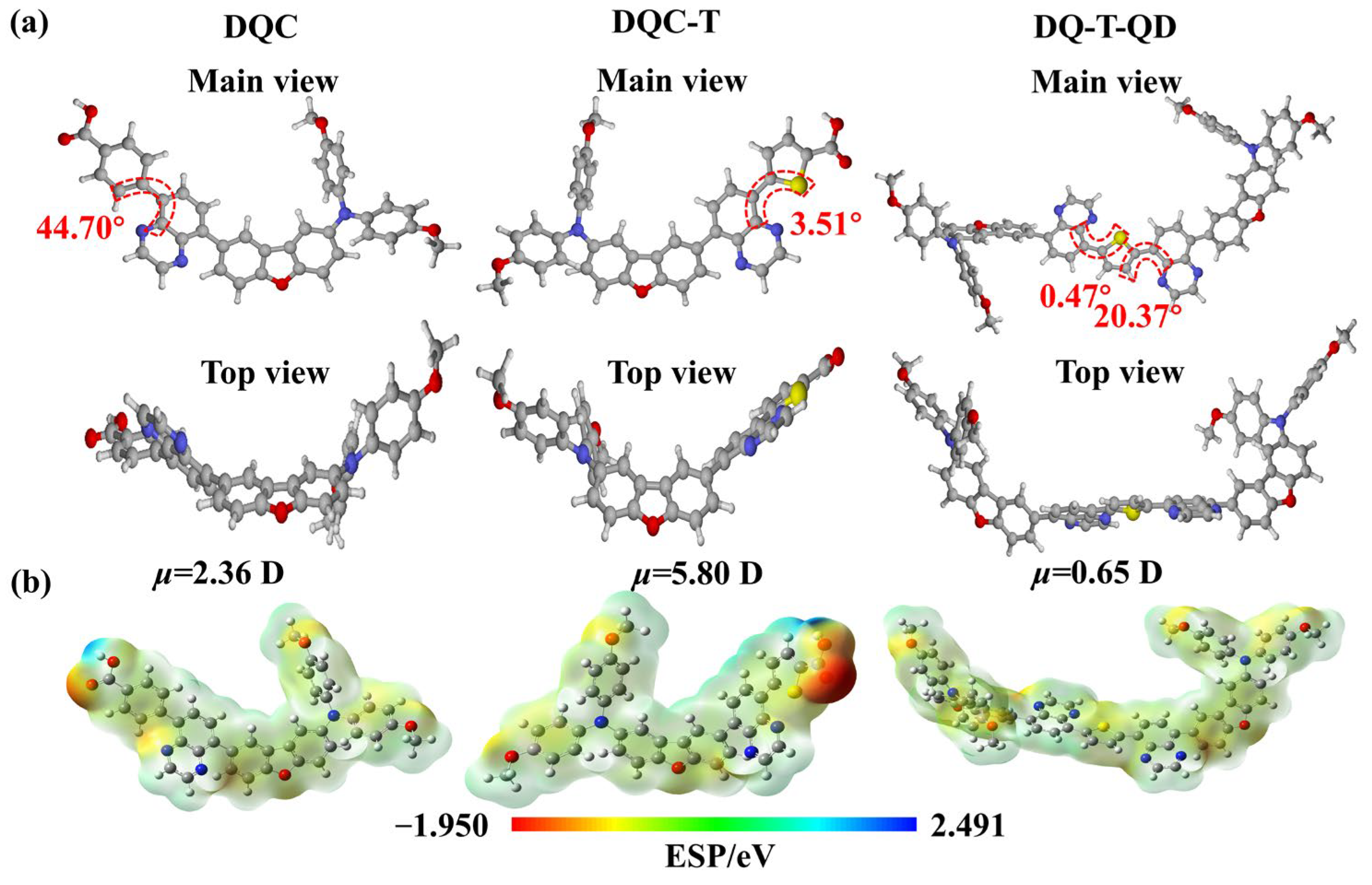
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of DQC-T and DQ-T-QD
3.2. Device Fabrication
3.3. Measurement and Characterization
3.4. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Cheng, C.; Zhang, C.; Hu, H.; Wang, K.; De Wolf, S. Organic Hole-Transport Layers for Efficient, Stable, and Scalable Inverted Perovskite Solar Cells. Adv. Mater. 2022, 34, 2203794. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Du, S.; Li, J.; Liu, C.; Pu, Z.; Tong, X.; Liu, J.; Wang, Y.; Meng, Y.; Yang, M.; et al. Molecular Dipole Engineering-assisted Strain Release for Mechanically Robust Flexible Perovskite Solar Cells. Energy Environ. Sci. 2023, 16, 5423–5433. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Liu, M.; Matta, S.K.; Tian, J.; Deng, Z.; Russo, S.P.; Vivo, P.; Zhou, Z.; Zhang, H. Synergistic Fluorine⋅Sulfur Intra- and Intermolecular Interactions on Dopant-Free Hole Transport Material for Efficient and Stable Inverted Perovskite Solar Cells. Sol. RRL 2023, 7, 2300031. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced Photovoltage for Inverted Planar Heterojunction Perovskite Solar Cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef]
- Huang, S.-M.; Xu, S.-Y.; Belopolski, I.; Lee, C.-C.; Chang, G.; Wang, B.; Alidoust, N.; Bian, G.; Neupane, M.; Zhang, C.; et al. A Weyl Fermion Semimetal with Surface Fermi Arcs in the Transition Metal Monopnictide TaAs Class. Nat. Commun. 2015, 6, 7373. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Marčinskas, M.; Kasparavičius, E.; Malinauskas, T.; Palmstrom, A.; Getautis, V.; Albrecht, S.; McGehee, M.D.; Magomedov, A. Wettability Improvement of a Carbazole-Based Hole-Selective Monolayer for Reproducible Perovskite Solar Cells. ACS Energy Lett. 2023, 8, 898–900. [Google Scholar] [CrossRef]
- Hossain, K.; Kulkarni, A.; Bothra, U.; Klingebiel, B.; Kirchartz, T.; Saliba, M.; Kabra, D. Resolving the Hydrophobicity of the Me-4PACz Hole Transport Layer for Inverted Perovskite Solar Cells with Efficiency >20%. ACS Energy Lett. 2023, 8, 3860–3867. [Google Scholar] [CrossRef]
- Kulkarni, A.; Sarkar, R.; Akel, S.; Häser, M.; Klingebiel, B.; Wuttig, M.; Wiegand, S.; Chakraborty, S.; Saliba, M.; Kirchartz, T. A Universal Strategy of Perovskite Ink—Substrate Interaction to Overcome the Poor Wettability of a Self-Assembled Monolayer for Reproducible Perovskite Solar Cells. Adv. Funct. Mater. 2023, 33, 2305812. [Google Scholar] [CrossRef]
- Bertoluzzi, L.; Boyd, C.C.; Rolston, N.; Xu, J.; Prasanna, R.; O’Regan, B.C.; McGehee, M.D. Mobile Ion Concentration Measurement and Open-Access Band Diagram Simulation Platform for Halide Perovskite Solar Cells. Joule 2020, 4, 109–127. [Google Scholar] [CrossRef]
- Niu, B.; Liu, H.; Huang, Y.; Gu, E.; Yan, M.; Shen, Z.; Yan, K.; Yan, B.; Yao, J.; Fang, Y.; et al. Multifunctional Hybrid Interfacial Layers for High-Performance Inverted Perovskite Solar Cells. Adv. Mater. 2023, 35, 2212258. [Google Scholar] [CrossRef]
- Yang, K.; Liao, Q.; Huang, J.; Zhang, Z.; Su, M.; Chen, Z.; Wu, Z.; Wang, D.; Lai, Z.; Woo, H.Y.; et al. Intramolecular Noncovalent Interaction-Enabled Dopant-Free Hole-Transporting Materials for High-Performance Inverted Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202113749. [Google Scholar]
- Wang, H.; Zhang, W.; Wang, B.; Yan, Z.; Chen, C.; Hua, Y.; Wu, T.; Wang, L.; Xu, H.; Cheng, M. Modulating Buried Interface with Multi-fluorine Containing Organic Molecule toward Efficient NiOx-based Inverted Perovskite Solar Cell. Nano Energy 2023, 111, 108363. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Duan, Y.; Sun, R.; Li, Y.; Xie, Z.; Xu, D.; Wu, M.; Wang, Y.; Li, H.; et al. Glycol Monomethyl Ether-Substituted Carbazolyl Hole-Transporting Material for Stable Inverted Perovskite Solar Cells with Efficiency of 25.52%. Angew. Chem. Int. Ed. 2024, 63, e202403068. [Google Scholar]
- Niu, T.; Zhu, W.; Zhang, Y.; Xue, Q.; Jiao, X.; Wang, Z.; Xie, Y.-M.; Li, P.; Chen, R.; Huang, F.; et al. D-A-π-A-D-type Dopant-free Hole Transport Material for Low-Cost, Efficient, and Stable Perovskite Solar Cells. Joule 2021, 5, 249–269. [Google Scholar] [CrossRef]
- Guo, Y.; He, L.; Guo, J.; Guo, Y.; Zhang, F.; Wang, L.; Yang, H.; Xiao, C.; Liu, Y.; Chen, Y.; et al. A Phenanthrocarbazole-Based Dopant-Free Hole-Transport Polymer with Noncovalent Conformational Locking for Efficient Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202114341. [Google Scholar]
- Ji, X.; Zhou, T.; Fu, Q.; Wang, W.; Wu, Z.; Zhang, M.; Guo, X.; Liu, D.; Woo, H.Y.; Liu, Y. Dopant-Free Two-Dimensional Hole Transport Small Molecules Enable Efficient Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2203756. [Google Scholar] [CrossRef]
- Xie, G.; Wang, J.; Yin, S.; Liang, A.; Wang, W.; Chen, Z.; Feng, C.; Yu, J.; Liao, X.; Fu, Y.; et al. Dual-Strategy Tailoring Molecular Structures of Dopant-Free Hole Transport Materials for Efficient and Stable Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202403083. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, Q.; Ding, Y.; Wang, Z.; Jiang, N.; Zhou, E. Constructing D-π-A Type Polymers as Dopant-Free Hole Transport Materials for High-Performance CsPbI2Br Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 9784–9791. [Google Scholar] [CrossRef]
- Duan, C.; Tang, A.; Guo, Q.; Zhang, W.; Yang, L.; Ding, Y.; Dai, Z.; Zhou, E. DTBDT-Based Polymer Hole Transport Materials for Low Voltage Loss CsPbI2Br Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2313462. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Shen, C.; Zhang, D.; Liu, S.; Wu, Y.; Zhu, W.-H. A Coplanar π-Extended Quinoxaline Based Hole-Transporting Material Enabling over 21% Efficiency for Dopant-Free Perovskite Solar Cells. Angew. Chem. Int. Ed. 2021, 60, 2674–2679. [Google Scholar] [CrossRef]
- Afraj, S.N.; Kuan, C.-H.; Lin, J.-S.; Ni, J.-S.; Velusamy, A.; Chen, M.-C.; Diau, E.W.-G. Quinoxaline-Based X-Shaped Sensitizers as Self-Assembled Monolayer for Tin Perovskite Solar cells. Adv. Funct. Mater. 2023, 33, 2213939. [Google Scholar] [CrossRef]
- Cheng, Y.; Qi, Y.; Tang, Y.; Zheng, C.; Wan, Y.; Huang, W.; Chen, R. Controlling Intramolecular Conformation through Nonbonding Interaction for Soft-Conjugated Materials: Molecular Design and Optoelectronic Properties. J. Phys. Chem. Lett. 2016, 7, 3609–3615. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Facchetti, A.; Marks, T.J. Organic and Polymeric Semiconductors Enhanced by Noncovalent Conformational Locks. Chem. Rev. 2017, 117, 10291–10318. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, Q.; Li, Y.; Gao, P.; Xu, B.; Lin, H.; Li, X.; Miyasaka, T. Artemisinin-passivated Mixed-cation Perovskite Films for Durable Flexible Perovskite Solar Cells with over 21% Efficiency. J. Mater. Chem. A 2021, 9, 1574–1582. [Google Scholar] [CrossRef]
- Lv, H.; Sun, Q.J.; Zhao, J.; Zhang, C.; Hao, Y.; Qin, W. Collaboration of SnO2:PAN NWs and BABr Enable High-performance Flexible Perovskite Solar Cells. Chem. Eng. J. 2024, 496, 153945. [Google Scholar] [CrossRef]
- Tu, S.; Chen, W.; Gang, Y.; Xiong, Q.; Li, X. Engineering a Thermally Robust Hole-Selective Layer for Stable Flexible Perovskite Solar Cells. Chem. Eng. J. 2025, 503, 158389. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, Y.; Ding, F.; Chen, Z.; Xie, Q.; Xia, X.; Zhu, P.; Lu, X.; Zhu, H.; Liao, X.; et al. Terpolymerization and Regioisomerization Strategy to Construct Efficient Terpolymer Donors Enabling High-Performance Organic Solar Cells. Adv. Mater. 2023, 35, 2300820. [Google Scholar] [CrossRef]
- Aktas, E.; Phung, N.; Köbler, H.; González, D.A.; Méndez, M.; Kafedjiska, I.; Turren-Cruz, S.-H.; Wenisch, R.; Lauermann, I.; Abate, A.; et al. Understanding the Perovskite/Self-assembled Selective Contact Interface for Ultra-stable and Highly Efficient p–i–n Perovskite Solar Cells. Energy & Environ. Sci. 2021, 14, 3976–3985. [Google Scholar]
- Liu, X.; Ding, B.; Han, M.; Yang, Z.; Chen, J.; Shi, P.; Xue, X.; Ghadari, R.; Zhang, X.; Wang, R.; et al. Extending the π-Conjugated System in Spiro-Type Hole Transport Material Enhances the Efficiency and Stability of Perovskite Solar Modules. Angew. Chem. Int. Ed. 2023, 62, e202304350. [Google Scholar]
- Giron, D. Thermal Analysis and Calorimetric Methods in the Characterisation of Polymorphs and Solvates. Thermochim. Acta 1995, 248, 1–59. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, K.; Fo, Y.; Li, Y.; Guo, B.; Zhang, X.; Zhou, X. Rational Design of Small Molecule Hole-Transporting Materials with a Linear π-bridge for Highly Efficient Perovskite Solar Cells. Phys. Chem. Chem. Phys. 2022, 24, 18793–18804. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, R.; Mu, C.; Wang, Y.; Han, L.; Wu, Y.; Zhu, W.-H. Conjugated Self-Assembled Monolayer as Stable Hole-Selective Contact for Inverted Perovskite Solar Cells. ACS Mater. Lett. 2022, 4, 1976–1983. [Google Scholar] [CrossRef]
- Budiawan, W.; Lai, K.-W.; Karuppuswamy, P.; Jadhav, T.S.; Lu, Y.-A.; Ho, K.-C.; Wang, P.-C.; Chang, C.-C.; Chu, C.-W. Asymmetric Benzotrithiophene-Based Hole Transporting Materials Provide High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 29143–29152. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Correa-Baena, J.-P.; Saracco, G.; Gratzel, M.; Hagfeldt, A.; Turri, S.; Gerbaldi, C. Improving Efficiency and Stability of Perovskite Solar Cells with Photocurable Fluoropolymers. Science 2016, 354, 203–206. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Ji, X.; He, J.; Guo, H.; Wang, S.; Wu, W.; Zhu, W.-H.; Wu, Y. Formamidinium Lead Iodide-Based Inverted Perovskite Solar Cells with Efficiency over 25% Enabled by An Amphiphilic Molecular Hole-Transporter. Angew. Chem. Int. Ed. 2024, 63, e202401260. [Google Scholar]
- Zhu, H.; Xu, Z.; Zhang, Z.; Lian, S.; Wu, Y.; Zhang, D.; Zhan, H.; Wang, L.; Han, L.; Qin, C. Improved Hole-Selective Contact Enables Highly Efficient and Stable FAPbBr3 Perovskite Solar Cells and Semitransparent Modules. Adv. Mater. 2024, 36, 2406872. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Ma, Z.; Kang, J.; Wang, Z.; Zong, X.; Xue, S. Boosting Interfacial Contact for the NiOx-based Inverted Perovskite Solar Cells via D-A Type Semiconductor. Chem. Eng. J. 2024, 496, 154011. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, L.; Wang, C.; Huang, J.; Liu, S.; Liu, X.; Zhang, J.; Hu, Z.; Zhu, Y. Efficient Inverted Perovskite Solar Cells with a Low-Temperature Processed NiOx/SAM Hole Transport Layer. J. Mater. Chem. C 2024, 12, 1507–1515. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Lu, J.; Lin, X.; Lu, Y.; Li, X.; Tu, S. Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells. Molecules 2024, 29, 1208. [Google Scholar] [CrossRef]
- Ma, X.; Luo, H.; Jiang, S.; Zheng, L.; Xue, H.; Li, X. Phase-Engineering of Layered Nickel Hydroxide for Synthesizing High-Quality NiOx Nanocrystals for Efficient Inverted Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 38444–38453. [Google Scholar] [CrossRef]



| HTMs | Experiment Data | Calculation Data e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λmax a (nm) | λem a (nm) | Eg b (eV) | Td (℃) | Tg (℃) | HOMO c (eV) | LUMO d (eV) | HOMO (eV) | LUMO (eV) | |
| DQC-T | 303 | 488 | 2.77 | 243 | - | −5.29 | −2.52 | −4.87 | −2.72 |
| DQ-T-QD | 304 | 462 | 2.98 | 408 | - | −5.31 | −2.33 | −4.76 | −2.55 |
| HTMs | Voc (V) | Jsc (mA cm−2) | FF (%) | PCEmax (%) | Jsc-in (mA cm−2) |
|---|---|---|---|---|---|
| NiOx | 1.05 | 22.36 | 65.96 | 15.52 | 21.10 |
| NiOx/DQC | 1.08 | 21.99 | 70.82 | 16.75 | 21.41 |
| NiOx/DQC-T | 1.09 | 22.28 | 74.44 | 18.12 | 22.31 |
| NiOx/DQ-T-QD | 1.04 | 23.25 | 69.10 | 16.67 | 22.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Gao, Z.; Zhong, X.; Lu, Y.; Tu, S.; Li, X. Triple Design Strategy for Quinoxaline-Based Hole Transport Materials in Flexible Perovskite Solar Cells. Molecules 2025, 30, 1129. https://doi.org/10.3390/molecules30051129
Lin Y, Gao Z, Zhong X, Lu Y, Tu S, Li X. Triple Design Strategy for Quinoxaline-Based Hole Transport Materials in Flexible Perovskite Solar Cells. Molecules. 2025; 30(5):1129. https://doi.org/10.3390/molecules30051129
Chicago/Turabian StyleLin, Yuanqiong, Zeyuan Gao, Xiaoshang Zhong, Yinghua Lu, Song Tu, and Xin Li. 2025. "Triple Design Strategy for Quinoxaline-Based Hole Transport Materials in Flexible Perovskite Solar Cells" Molecules 30, no. 5: 1129. https://doi.org/10.3390/molecules30051129
APA StyleLin, Y., Gao, Z., Zhong, X., Lu, Y., Tu, S., & Li, X. (2025). Triple Design Strategy for Quinoxaline-Based Hole Transport Materials in Flexible Perovskite Solar Cells. Molecules, 30(5), 1129. https://doi.org/10.3390/molecules30051129






