Whitening and Anti-Inflammatory Activities of Exosomes Derived from Leuconostoc mesenteroides subsp. DB-21 Strain Isolated from Camellia japonica Flower
Abstract
1. Introduction
2. Results and Discussion
2.1. Exosome Analysis
2.2. Cytotoxicity Assessment
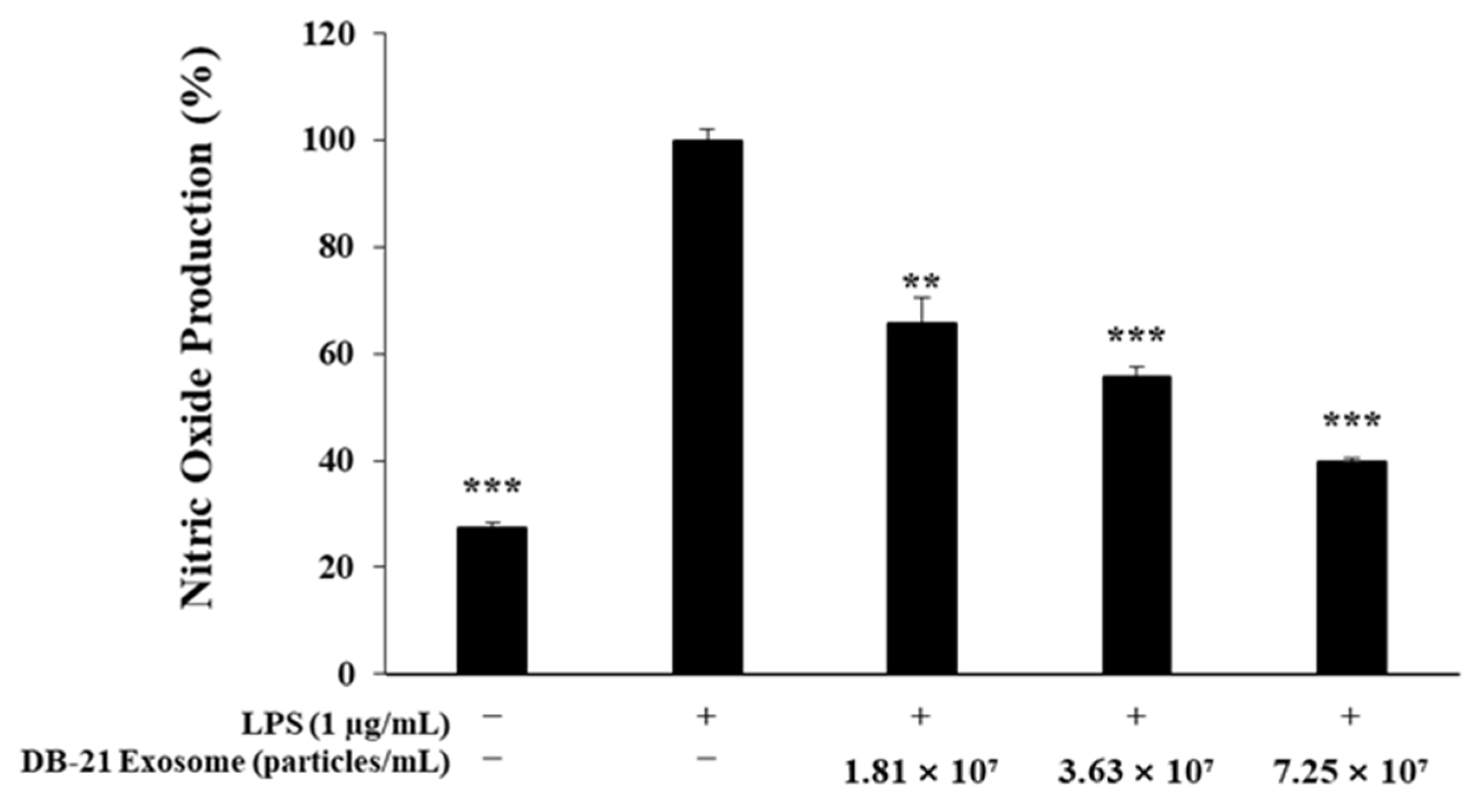
2.3. Nitric Oxide Production Inhibitory Activity Measurement
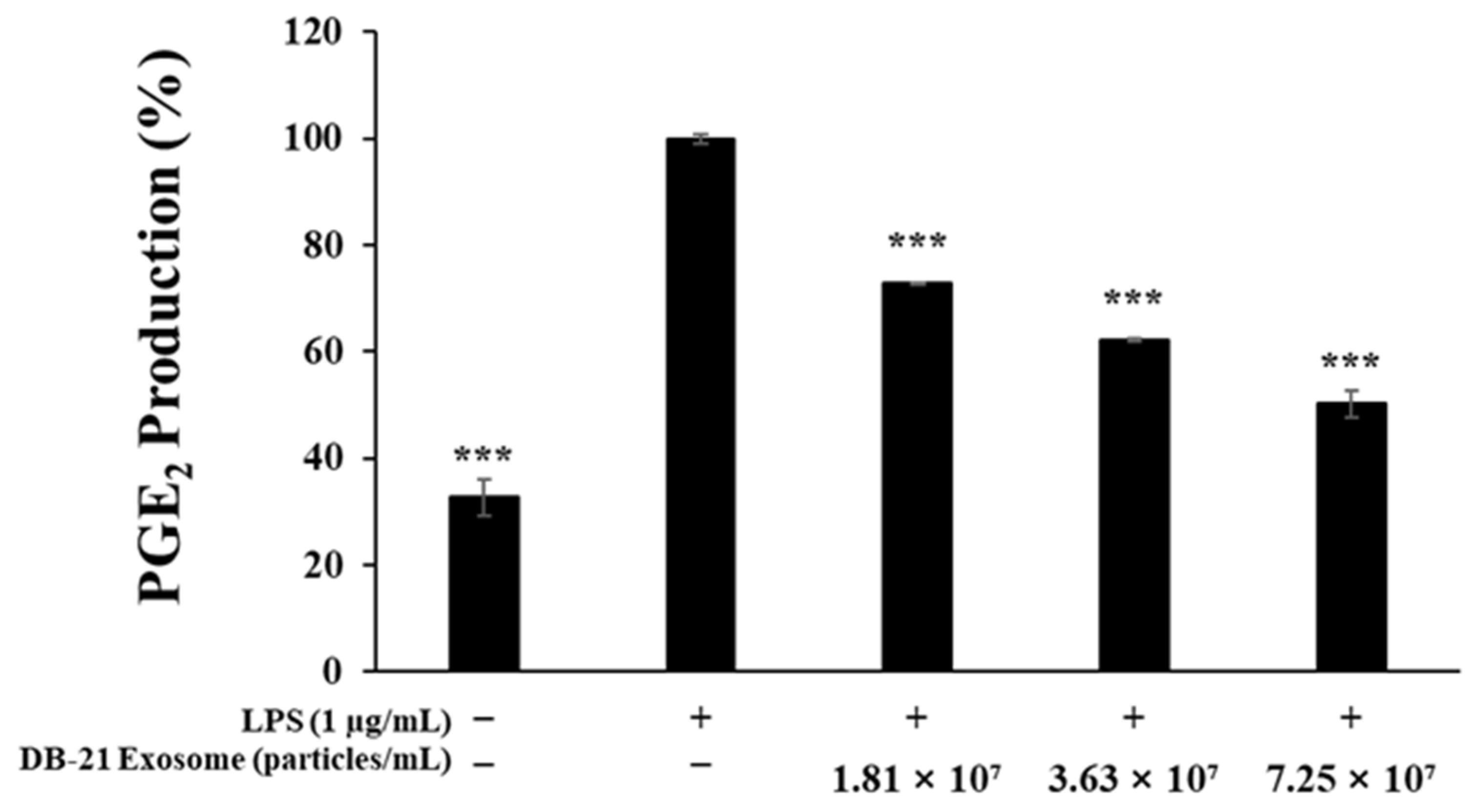
2.4. Prostaglandin E2 Production Inhibitory Activity Measurement
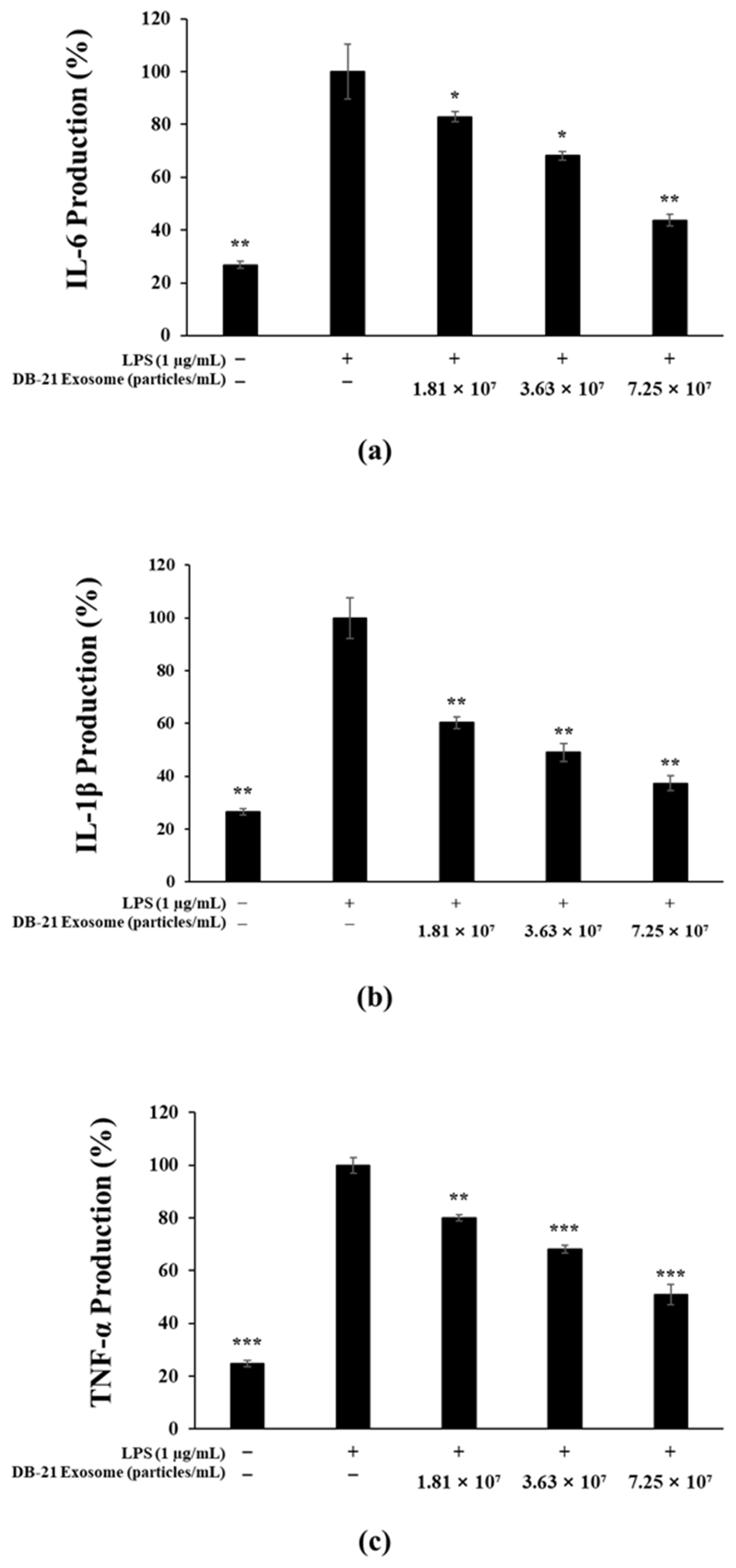
2.5. Pro-Inflammatory Cytokine (IL-6, IL-1β, TNF-α) Production Inhibitory Activity Measurement
2.6. Melanin Production Inhibition Activity Assessment
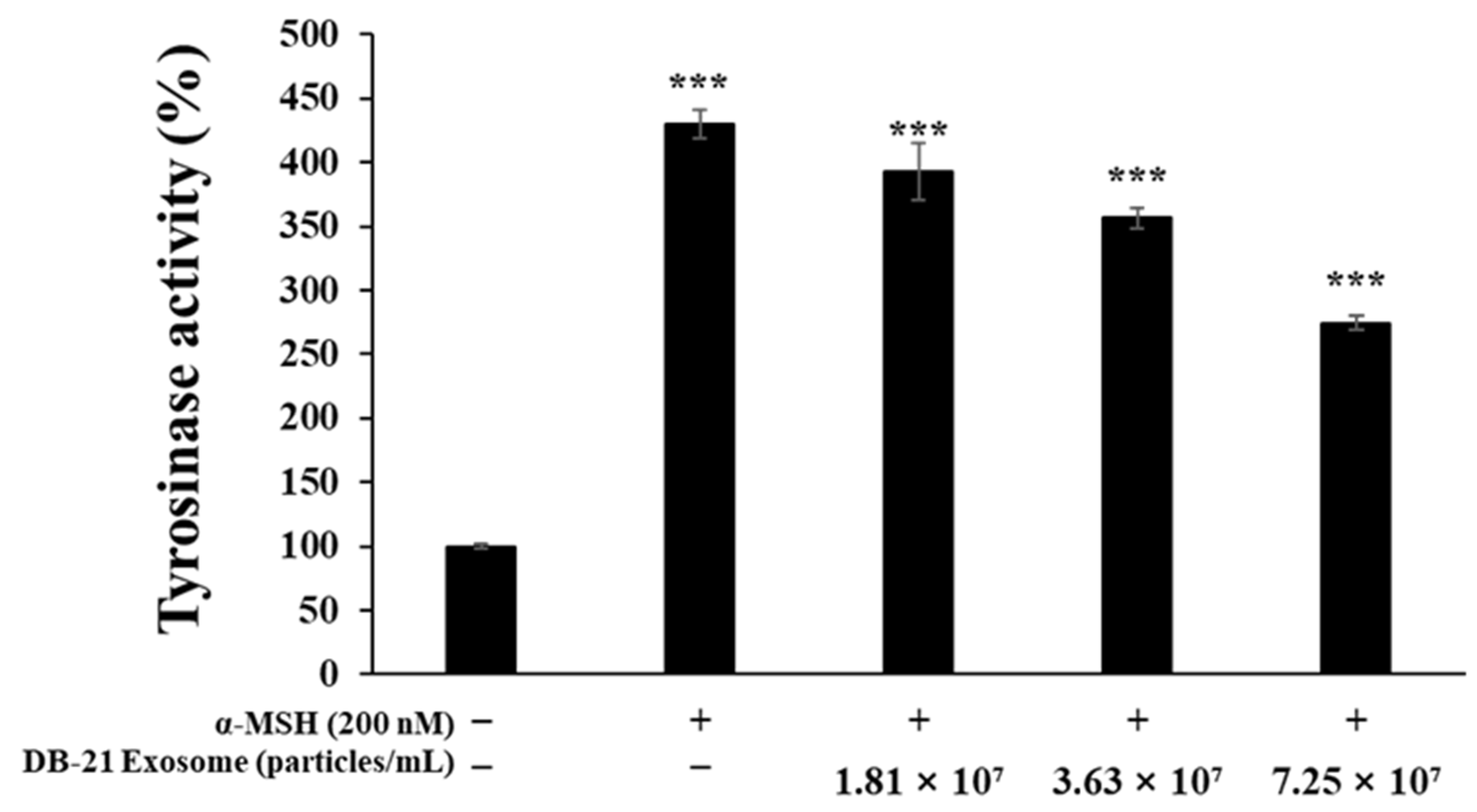
2.7. Tyrosinase Production Inhibition Activity Assessment
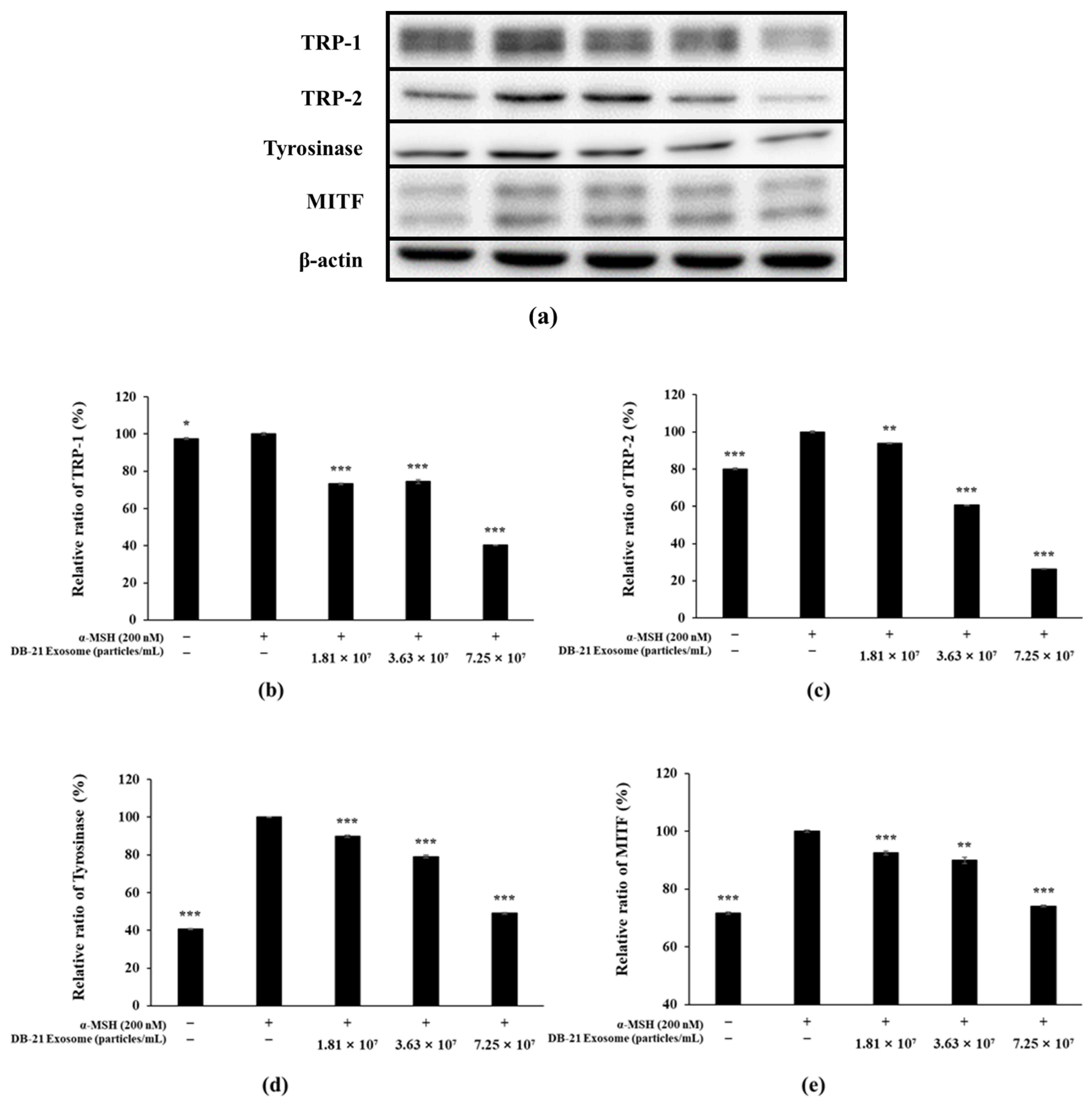
2.8. Western Blot Analysis
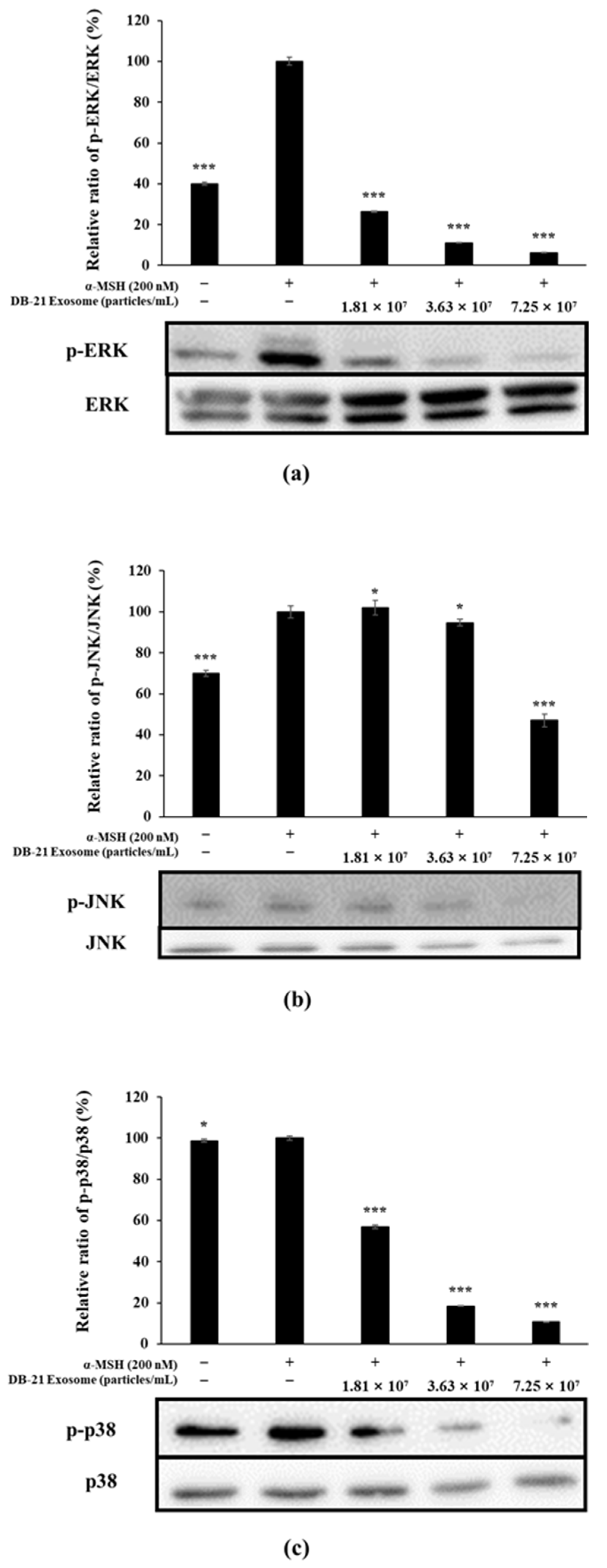
2.9. MAP Kinase Phosphorylation Inhibition in DB-21 Exosomes
2.10. Akt and GSK-3β Phosphorylation Inhibition by DB-21 Exosomes
3. Materials and Methods
3.1. Incubation of Leuconostoc mesenteroides subsp. DB-21
3.2. Exosome Isolation
3.3. Characterization of L. mesenteroides subsp. DB-21 Derived Exosomes
3.4. Materials and Cell Culture
3.5. Cytotoxicity Measurement
3.6. Nitric Oxide Inhibition Activity Measurement
3.7. Prostaglandin E2 Production Inhibition Activity Measurement
3.8. Pro-Inflammatory Cytokine (TNF-α, IL-6, IL-1β) Production Inhibitory Activity
3.9. Melanin Content Measurement
3.10. Tyrosinase Activity Measurements
3.11. Western Blotting
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The inflammation theory of disease. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef]
- Chung, K.F.; Adcock, I.M. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008, 31, 1334–1356. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K. Melanogenesis connection with innate immunity and toll-like receptors. Int. J. Mol. Sci. 2020, 21, 9769. [Google Scholar] [CrossRef]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Halaban, R.; Pomerants, S.H.; Marshall, S.; Lambert, D.T.; Lerner, A.B. Regulation of tyrosinase in human melanocytes grown in culture. J. Cell Biol. 1983, 97, 480–488. [Google Scholar] [CrossRef]
- Park, J.; Jung, H.; Kim, K.; Lim, K.M.; Kim, J.Y.; Jho, E.K.; Oh, E.S. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment. Cell Melanome Res. 2018, 31, 374–383. [Google Scholar] [CrossRef]
- Omata, Z.; Tomita, H.; Nakajima, T.; Natsume, B. Design of new melanin biosynthesis inhibitors. Pestic. Sci. 1989, 26, 271–281. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Becker, S.W.; Lerner, A.B.; Montgomery, H. Tyrosinase in human skin: Demonstration of its presence and of its role in human melanin formation. Science 1950, 112, 223–225. [Google Scholar] [CrossRef]
- Iozume, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef]
- Ferguson, C.A.; Kidson, S.H. The regulation of tyrosinase gene transcription. Pigment. Cell Res. 2006, 10, 127–138. [Google Scholar] [CrossRef]
- Fang, D.; Tsuji, Y.; Setaluri, V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002, 30, 3096–3106. [Google Scholar] [CrossRef]
- Thody, A.J.; Craham, A. Does α-MSH have a role in regulating skin pigmentation if humans? Pigment. Cell Res. 2006, 11, 265–274. [Google Scholar] [CrossRef]
- Fuller, B.B.; Lunsford, J.B.; Iman, D.S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in cloudman S-91 mouse melanoma cell cultures. J. Biol. Chem. 1987, 262, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Ploper, D.; Robertis, E.M.D. The MITF family of transcription factors: Role in endolysosomal biogenesis, Wnt signaling, and oncogenesis. Pharmacol. Res. 2015, 99, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 46. [Google Scholar]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Allmang, C.; Kufel, J.; Chanfreau, G.; Mitchell, P.; Petfalski, E.; Tollervey, D. Functions of the exosomes in rRNA, snoRNA, and snRNA synthesis. EMBO J. 1999, 18, 5399–5410. [Google Scholar] [CrossRef]
- Kalluri, R.; Lebleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef]
- Wang, H.; Lee, K.S.; Kang, Y.W. Skin barrier improvement effect of exosomal nanovesicles derived from lactic acid bacteria. J. Soc. Cosmet. Sci. Korea 2021, 47, 171–178. [Google Scholar]
- Lee, B.H.; Chen, Y.Z.; Shen, T.L.; Pan, T.M.; Hsu, W.H. Proteomic characterization of extracellular vesicles derived from lactic acid bacteria. Food Chem. 2023, 427, 136685. [Google Scholar] [CrossRef]
- Liu, R. A promising area of research in medicine: Recent advances in properties and applications of Lactobacillus-dereived exosomes. Front. Microbiol. 2024, 15, 1266510. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.H.; Hong, D.K.; Mo, S.J.; Jeon, S.; Park, S.D.; Shim, J.J.; Lee, J.L.; Lee, J.H. Targeting inflammation and skin aging via the gut-skin axis: The role of Lactiplantibacillus plantarum HY7714-derived extracellular vesilces. Microorganisms 2024, 12, 2466. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Yoom, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hwang, E.J.; Kim, G.H.; Choi, Y.B.; Lim, C.Y.; Kim, S.M. Antifungal and antioxidant activites extracts from leaves and flowers of Camellia japonica L. Korean J. Med. Crop Sci. 2005, 13, 93–100. [Google Scholar]
- Lee, H.S.; Choi, J.H.; Cui, L.; Li, Y.; Yang, J.M.; Yun, J.J.; Jung, J.E.; Choi, W.; Yoon, K.C. Anti-inflammatory and antioxidative effects of Camellia japonica on human corneal epithelial cells and experimental dry eye: In vivo and in vitro study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1196–1207. [Google Scholar] [CrossRef]
- Azuma, C.M.; dos Santos, F.C.S.; Lago, J.H.G. Flavonoids and fatty acids of Camellia japonica leaves extracts. Rev. Bras. Farmacogn. 2011, 21, 1159–1162. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Kim, H.R.; Ko, J.; Kong, C.S. Camellioside A, isolated from Cemellia japonica flowers, attenuates UVA-induced production of MMP-1 in HaCaT keratinocytes via suppression of MAPK activation. Exp. Ther. Med. 2020, 21, 16. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Cemellia japonica essential oil inhibits α-MSH-induced melanin production and tyrosinase activity in B16F10 melanoma cells. Evid-Based Compl. Alt. 2021, 2021, 6328767. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Park, D.; Byun, S.; Jung, E. Protective effects of Camellia japonica flower extract against urban air pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 6, pdb-prot095505. [Google Scholar] [CrossRef]
- Sakata, D.; Yao, C.; Narumiya, S. Prostaglandin E2, an immunoactivator. J. Pharmacol. Sci. 2010, 112, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signaling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. Roles and functions of ROS and RNS in cellular physiology and pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chem-Eur. J. 2017, 24, 47–55. [Google Scholar] [CrossRef]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Mackenzie, A.; Wadsworth, R.M. Extracellular L-arginine is required for optimal NO synthesis by eNOs and iNOs in the rat mesenteric artery wall. Br. J. Pharmacol. 2009, 139, 1487–1497. [Google Scholar] [CrossRef]
- Slominski, A.; Moellmann, G.; Kiklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of melanogenic pathway, L-tyrosine and L-dopa. J. Cell Sci. 1988, 89, 287–296. [Google Scholar] [CrossRef]
- Garcia-Carmona, F.; Garcia-Cánovas, F.; Iborra, J.L.; Lozano, J.A. Kinetic study of the pathway of melanizationn between L-dopa and dopachrome. BBA-Gen. Subj. 1982, 717, 124–131. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signaling to CREB. Pigment. Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Arozarena, I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment. Cell Res. 2015, 28, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, L.; Su, Q.; Fan, X.; Wang, Y.; Gao, S.; Wang, H.; Chen, H.; Chan, C.B.; Liu, Z. Phosphorylation of MITF by AKT effects its downstream targets and causes TP53-dependent cell senescence. Int. J. Biochem. Cell Biol. 2016, 80, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef]
- Kim, S.W.; Shin, D.H.; Gal, S.W.; Bang, K.H.; Kim, D.S.; Chi, W.J. Isolation and Characterization of Lactic acid bacteria Leuconostoc mesenteroides DB3 from Cemellia japonica Flower. J. Life Sci. 2023, 33, 915–922. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-M.; Lee, G.; Hong, H.; Park, C.-M.; Yeom, A.; Chi, W.-J.; Kim, S.-Y. Whitening and Anti-Inflammatory Activities of Exosomes Derived from Leuconostoc mesenteroides subsp. DB-21 Strain Isolated from Camellia japonica Flower. Molecules 2025, 30, 1124. https://doi.org/10.3390/molecules30051124
Choi B-M, Lee G, Hong H, Park C-M, Yeom A, Chi W-J, Kim S-Y. Whitening and Anti-Inflammatory Activities of Exosomes Derived from Leuconostoc mesenteroides subsp. DB-21 Strain Isolated from Camellia japonica Flower. Molecules. 2025; 30(5):1124. https://doi.org/10.3390/molecules30051124
Chicago/Turabian StyleChoi, Byeong-Min, Gibok Lee, Hyehyun Hong, Chang-Min Park, Areum Yeom, Won-Jae Chi, and Seung-Young Kim. 2025. "Whitening and Anti-Inflammatory Activities of Exosomes Derived from Leuconostoc mesenteroides subsp. DB-21 Strain Isolated from Camellia japonica Flower" Molecules 30, no. 5: 1124. https://doi.org/10.3390/molecules30051124
APA StyleChoi, B.-M., Lee, G., Hong, H., Park, C.-M., Yeom, A., Chi, W.-J., & Kim, S.-Y. (2025). Whitening and Anti-Inflammatory Activities of Exosomes Derived from Leuconostoc mesenteroides subsp. DB-21 Strain Isolated from Camellia japonica Flower. Molecules, 30(5), 1124. https://doi.org/10.3390/molecules30051124






