Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study
Abstract
1. Introduction
2. Results and Discussion
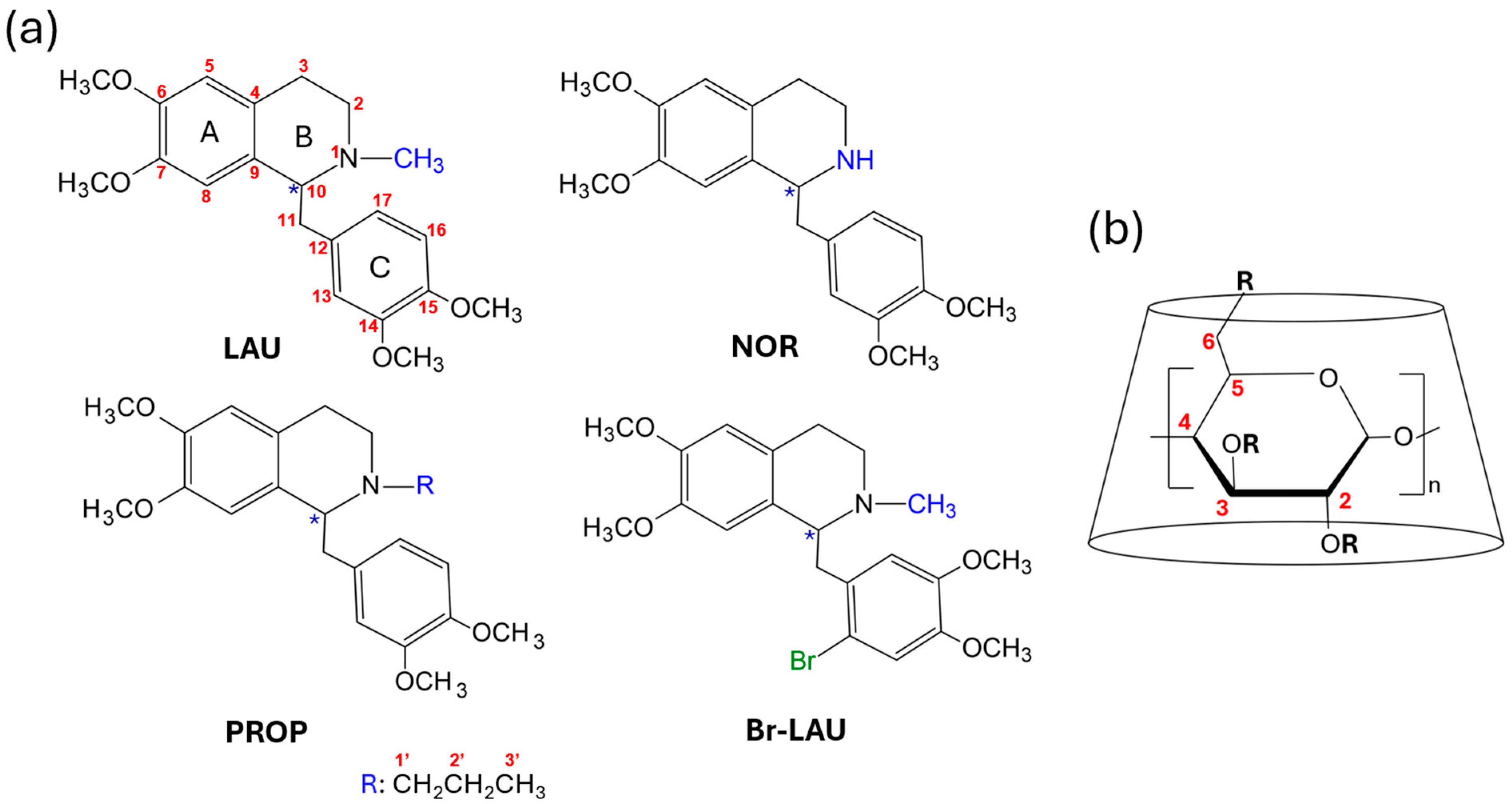
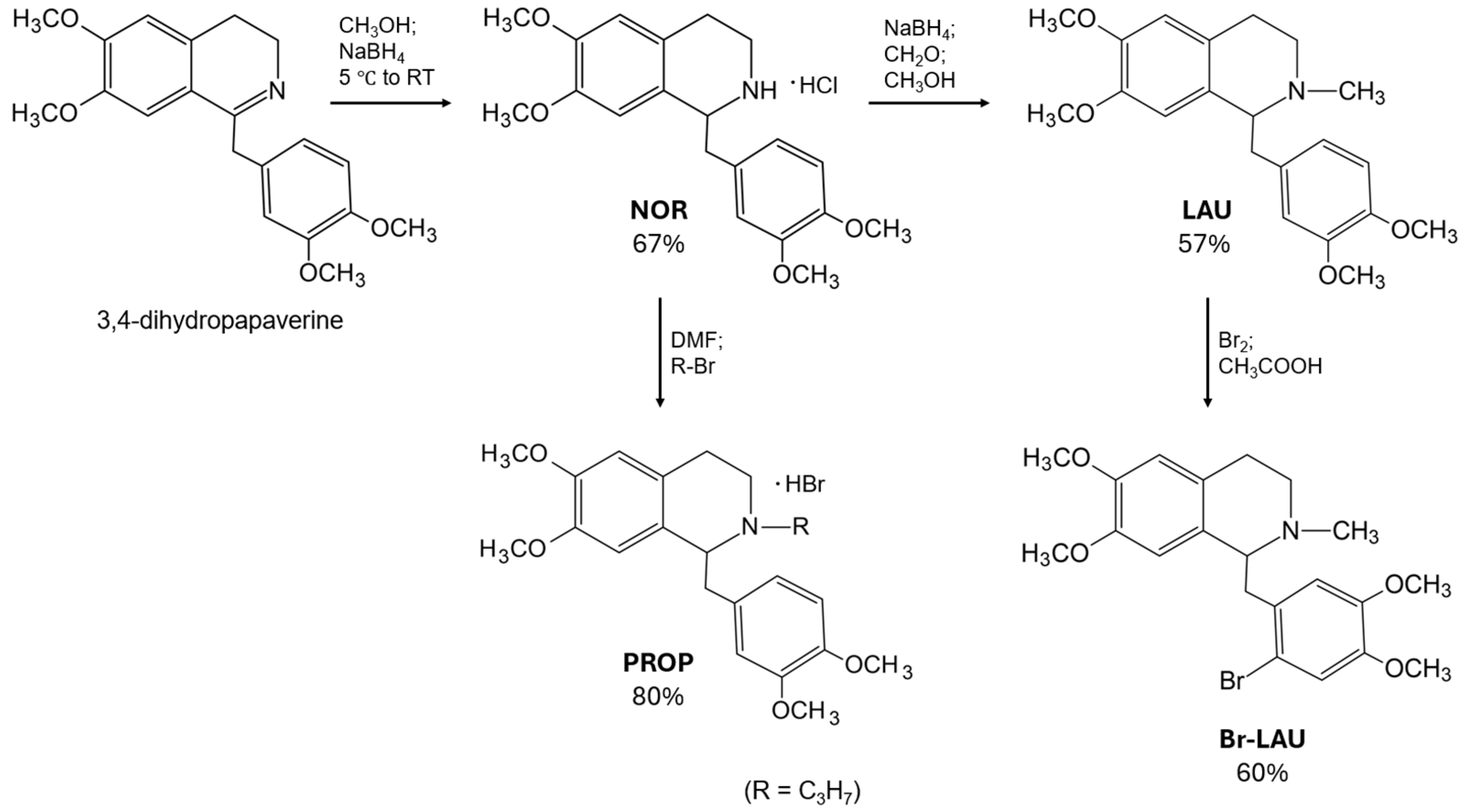
2.1. Synthetic Procedures
2.2. Analytical and Semi-Preparative Chiral HPLC Studies
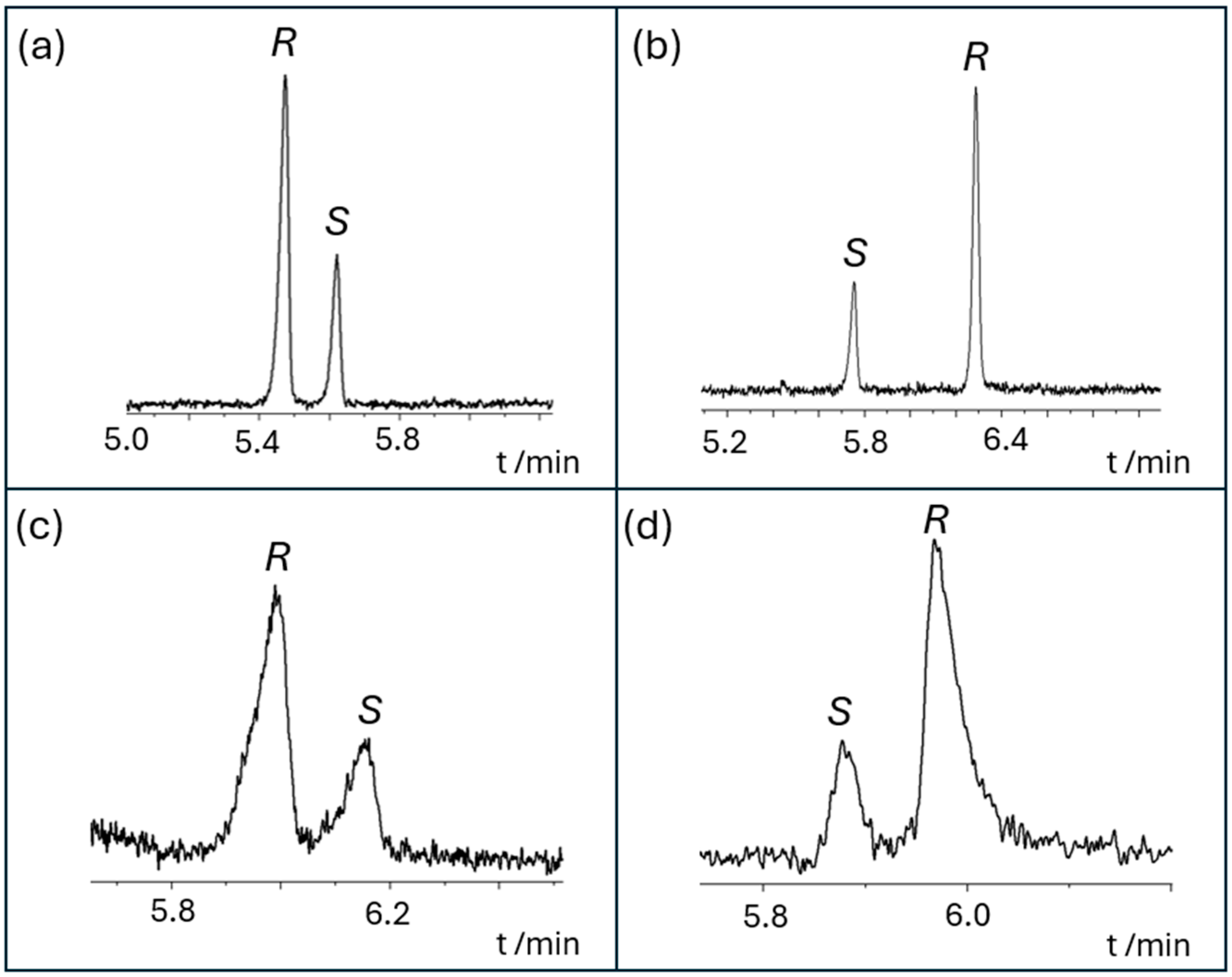
2.3. Enantioseparation of the Benzyltetrahydroisoquinoline Alkaloids by CE
2.4. Enantiomer Migration Order Reversal Determination by CE
2.5. Characterization of the Inclusion Complexes by NMR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Syntheses of Benzyltetrahydroisoquinoline Alkaloids
3.2.1. Synthesis of Racemic NOR
3.2.2. Synthesis of Racemic LAU
3.2.3. Synthesis of Racemic PROP
3.2.4. Synthesis of Racemic Br-LAU
3.3. Chiral HPLC
3.4. CD Spectroscopy
3.5. Capillary Electrophoresis
3.6. NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powder-George, Y.L. Alkaloids. In Pharmacognosy: Fundamentals, Applications, and Strategies, 2nd ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 167–209. [Google Scholar] [CrossRef]
- Bai, R.; Yao, C.; Zhong, Z.; Ge, J.; Bai, Z.; Ye, X.; Xie, T.; Xie, Y. Discovery of natural anti-inflammatory alkaloids: Potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 2021, 213, 113165. [Google Scholar] [CrossRef]
- Brochmann-Hanssen, E.; Fu, C.-C.; Leung, A.; Zanati, G. Opium alkaloids X: Biosynthesis of 1-benzylisoquinolines. J. Pharm. Sci. 1971, 60, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, Ç.; Sarıyar, G.; Akarsu, B.G.; Çevikbaş, A. Antimicrobial activity and phytochemical studies on turkish samples of Papaver macrostomum. Pharm. Biol. 2007, 45, 626–630. [Google Scholar] [CrossRef]
- Fodale, V.; Santamaria, L.B. Laudanosine, an atracurium and cisatracurium metabolite. Eur. J. Anaesthesiol. 2002, 19, 466–473. [Google Scholar] [CrossRef]
- Biological Test Results. 9.1 BioAssay Results. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/278172#section=Biological-Test-Results (accessed on 13 November 2024).
- qHTS Assay for MDR1-Selective Chemotherapeutics: Primary Screen Using The Drug-Selected MDR Subline Cells KB-V1. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1347076 (accessed on 13 November 2024).
- Reversal of VCR-Resistance in Human Eca-109 Cells Assessed as Vincristine IC50 by Measuring Cell Viability Incubated for 48 hrs by CCK-8 Method (Rvb = 6830.0+/−537.0 nM). Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1948023 (accessed on 13 November 2024).
- Zeng, R.; Yang, X.-M.; Li, H.-W.; Li, X.; Guan, Y.; Yu, T.; Yan, P.; Yuan, W.; Niu, S.-L.; Gu, J.; et al. Simplified Derivatives of Tetrandrine as Potent and Specific P-gp Inhibitors to Reverse Multidrug Resistance in Cancer Chemotherapy. J. Med. Chem. 2023, 66, 4086–4105. [Google Scholar] [CrossRef]
- Leaney, A.E.; Heath, J.; Midforth, E.; Beck, P.; Brown, P.; Mawson, D.H. Presence of higenamine in beetroot containing ‘foodstuffs’ and the implication for WADA-relevant anti-doping testing. Drug Test. Anal. 2023, 15, 173–180. [Google Scholar] [CrossRef]
- Kozhuharov, V.R.; Ivanov, K.; Ivanova, S. Dietary Supplements as Source of Unintentional Doping. BioMed Res. Int. 2022, 2022, 8387271. [Google Scholar] [CrossRef] [PubMed]
- Garris, P.; Budygin, E.; Phillips, P.; Venton, B.; Robinson, D.; Bergstrom, B.; Rebec, G.; Wightman, R. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience 2003, 118, 819–829. [Google Scholar] [CrossRef]
- Luethi, D.; Hoener, M.C.; Liechti, M.E. Effects of the new psychoactive substances diclofensine, diphenidine, and methoxphenidine on monoaminergic systems. Eur. J. Pharmacol. 2017, 819, 242–247. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Native and substituted cyclodextrins as chiral selectors for capillary electrophoresis enantioseparations: Structures, features, application, and molecular modeling. Electrophoresis 2021, 42, 1676–1708. [Google Scholar] [CrossRef]
- Ujj, D.; Kalydi, E.; Malanga, M.; Varga, E.; Sohajda, T.; Béni, S.; Benkovics, G. Sugammadex analogue cyclodextrins as chiral selectors for enantioseparation of cathinone derivatives by capillary electrophoresis. J. Chromatogr. A 2022, 1683, 463506. [Google Scholar] [CrossRef] [PubMed]
- Dobó, M.; Ádám, M.; Fiser, B.; Papp, L.A.; Dombi, G.; Sekkoum, K.; Szabó, Z.-I.; Tóth, G. Enantioseparation and molecular docking study of selected chiral pharmaceuticals on a commercialized phenylcarbamate-β-cyclodextrin column using polar organic mode. Sci. Rep. 2023, 13, 14778. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Benkovics, G.; Darcsi, A.; Várnai, B.; Sohajda, T.; Malanga, M.; Béni, S. Comparative analysis of the full set of methylated β-cyclodextrins as chiral selectors in capillary electrophoresis. Electrophoresis 2019, 40, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Kalydi, E.; Malanga, M.; Nielsen, T.T.; Wimmer, R.; Béni, S. Solving the puzzle of 2-hydroxypropyl β-cyclodextrin: Detailed assignment of the substituent distribution by NMR spectroscopy. Carbohydr. Polym. 2024, 338, 122167. [Google Scholar] [CrossRef]
- Ma, Q.; Cong, W.; Liu, Y.; Geng, Z.; Lin, Y.; Wang, Z. Experimental and computational study on the enantioseparation of four chiral fluoroquinolones by capillary electrophoresis with sulfated-β-cyclodextrin as chiral selector. Chirality 2021, 33, 549–557. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Zhu, F.; Chen, B. Evaluation of the enantioselectivity of glycogen-based dual chiral selector systems towards basic drugs in capillary electrophoresis. J. Chromatogr. A 2010, 1217, 7158–7163. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Zhu, F.; Chen, B.; Zhang, Q.; Du, S.; Li, P. Study of the enantioseparation capability of chiral dual system based on chondroitin sulfate C in CE. Electrophoresis 2015, 36, 607–614. [Google Scholar] [CrossRef]
- Wang, J.; Warner, I.M. Chiral Separations Using Micellar Electrokinetic Capillary Chromatography and a Polymerized Chiral Micelle. Anal. Chem. 1994, 66, 3773–3776. [Google Scholar] [CrossRef]
- Billiot, F.H.; Billiot, E.J.; Ng, Y.K.; Warner, I.M. Chiral separation of norlaudanosoline, laudanosoline, laudanosine, chlorthalidone, and three benzoin derivatives using amino acid based molecular micelles. J. Chromatogr. Sci. 2006, 44, 64–69. [Google Scholar] [CrossRef][Green Version]
- Zuo, M.; Gao, J.; Zhang, X.; Cui, Y.; Fan, Z.; Ding, M. Capillary electrophoresis with electrochemiluminescence detection for the simultaneous determination of cisatracurium besylate and its degradation products in pharmaceutical preparations. J. Sep. Sci. 2015, 38, 2332–2339. [Google Scholar] [CrossRef]
- Agnew-Heard, K.A.; Peña, M.S.; Shamsi, S.A.; Warner, I.M. Studies of Polymerized SodiumN-Undecylenyl-L-valinate in Chiral Micellar Electrokinetic Capillary Chromatography of Neutral, Acidic, and Basic Compounds. Anal. Chem. 1997, 69, 958–964. [Google Scholar] [CrossRef]
- Ruiz-Olalla, A.; Würdemann, M.A.; Wanner, M.J.; Ingemann, S.; van Maarseveen, J.H.; Hiemstra, H. Organocatalytic Enantioselective Pictet–Spengler Approach to Biologically Relevant 1-Benzyl-1,2,3,4-Tetrahydroisoquinoline Alkaloids. J. Org. Chem. 2015, 80, 5125–5132. [Google Scholar] [CrossRef]
- Dohárszky, A.; Kalydi, E.; Völgyi, G.; Béni, S.; Fejős, I. Cyclodextrin-Enabled Enantioselective Complexation Study of Cathinone Analogs. Molecules 2024, 29, 876. [Google Scholar] [CrossRef] [PubMed]
- Danel, C.; Azaroual, N.; Foulon, C.; Goossens, J.-F.; Vermeersch, G.; Bonte, J.-P.; Vaccher, C. NMR studies of chiral recognition mechanisms: Interaction of enantiomers of N-imidazole derivatives with cyclodextrin hosts. Correlation with the CD-EKC studies. Tetrahedron Asymmetry 2006, 17, 975–983. [Google Scholar] [CrossRef]
- Garibyan, A.; Delyagina, E.; Agafonov, M.; Khodov, I.; Terekhova, I. Effect of pH, temperature and native cyclodextrins on aqueous solubility of baricitinib. J. Mol. Liq. 2022, 360, 119548. [Google Scholar] [CrossRef]
- Huang, M.; Quan, Z.; Liu, Y. Computational modeling of inclusion complexes of β-cyclodextrin with enantiomers of salsolinol, N-methyl-salsolinol, and 1-benzyl-tetrahydroisoquinoline. Int. J. Quantum Chem. 2009, 109, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Louafi, F.; Hurvois, J.-P.; Chibani, A.; Roisnel, T. Synthesis of tetrahydroisoquinoline alkaloids via anodic cyanation as the key step. J. Org. Chem. 2010, 75, 5721–5724. [Google Scholar] [CrossRef]
- Li, X.; Leonori, D.; Sheikh, N.S.; Coldham, I. Synthesis of 1-substituted tetrahydroisoquinolines by lithiation and electrophilic quenching guided by in situ IR and NMR spectroscopy and application to the synthesis of salsolidine, carnegine and laudanosine. Chem. A Eur. J. 2013, 19, 7724–7730. [Google Scholar] [CrossRef]
- Elavarasan, S.; Preety, J.; Abinaya, R.; Saravanan, T.; Balasubramanian, K.K.; Venkatramaiah, N.; Baskar, B. Visible Light Driven Metal-Free Photoredox Catalyzed α-benzylation and α-oxygenation of N-substituted Tetrahydroisoquinolines: Applications to Synthesis of Natural Products. Chem. Asian J. 2022, 17, e202200878. [Google Scholar] [CrossRef]
- Iturriaga-Vásquez, P.; Zapata-Torres, G.; Rezende, M.C.; Cassels, B.K. 1-Benzyl-1,2,3,4-tetrahydroisoquinolines: ¹H NMR conformational studies and rotational barriers. J. Chil. Chem. Soc. 2004, 49, 17–23. [Google Scholar] [CrossRef]
- Orejarena Pacheco, J.C.; Lahm, G.; Opatz, T. Synthesis of alkaloids by stevens rearrangement of nitrile-stabilized ammonium ylides: (±)-laudanosine, (±)-laudanidine, (±)-armepavine, (±)-7-methoxycryptopleurine, and (±)-xylopinine. J. Org. Chem. 2013, 78, 4985–4992. [Google Scholar] [CrossRef] [PubMed]
- Kametani, T.; Sugi, H.; Shibuya, S. The absolute configuration of cryptostyline.III. Studies on the syntheses of heterocyclic compounds. CCCXCVII. Tetrahedron 1971, 27, 2409–2414. [Google Scholar] [CrossRef]
- Mujahidin, D.; Doye, S. Enantioselective synthesis of (+)-(S)-Laudanosine and (-)-(S)-xylopinine. Eur. J. Org. Chem. 2005, 2005, 2689–2693. [Google Scholar] [CrossRef]
- Uprety, H.; Bhakuni, D.S.; Kapil, R.S. Biosynthesis of papaverine. Phytochemistry 1975, 14, 1535–1537. [Google Scholar] [CrossRef]
- Reimann, E.; Ettmayr, C. A convenient sythesis of 1-benzyl-1,2,3,4-tetrahydroisoquinolines by combined Strecker/Bruylants reaction. Monats. Chem. 2004, 135, 1289–129540. [Google Scholar] [CrossRef]
- Anastasia, L.; Cighetti, G.; Allevi, P. Simple and selective one-pot replacement of the N-methyl group of tertiary amines by quaternization and demethylation with sodium sulfide or potassium thioacetate: An application to the synthesis of pergolide. J. Chem. Soc. Perkin 2001, 1, 2398–2403. [Google Scholar] [CrossRef]
- Blank, N.; Opatz, T. Enantioselective synthesis of tetrahydroprotoberberines and bisbenzylisoquinoline alkaloids from a deprotonated α-aminonitrile. J. Org. Chem. 2011, 76, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Spath, E.; Quietensky, H. Die Aufspaltung der Methylendioxygruppe. Berichte der Dtsch. Chem. Ges. (A B Series) 1921, 54, 3064. [Google Scholar] [CrossRef]
- Ferencz, E.; Szabó, Z.-I.; Zöldhegyi, A.; Dombi, G.; Molnár, G.; Dobó, M.; Varga, E.; Molnár, I.; Tóth, G. Possibilities and limitations of computer assisted chiral HPLC method development for ozanimod on polysaccharide based chiral stationary phases. Sci. Rep. 2024, 14, 26757. [Google Scholar] [CrossRef]
- Benmekhbi, L.; Louafi, F.; Roisnel, T.; Hurvois, J.-P. Synthesis of Tetrahydroisoquinoline Alkaloids and Related Compounds through the Alkylation of Anodically Prepared α-Amino Nitriles. J. Org. Chem. 2016, 81, 6721–6739. [Google Scholar] [CrossRef]
- ChemAxon. Calculator Playground. Available online: https://playground.calculators.cxn.io/ (accessed on 13 November 2024).
- Dohárszky, A.; Vági, E.M.; Könczöl, Á.; Simon, A.; Várnagy, E.; Muratov, M.; Steiger, K.I.; Várnai, B.; Béni, S.; Riethmüller, E.; et al. Kratom Alkaloids: A Blood–Brain Barrier Specific Membrane Permeability Assay-Guided Isolation and Cyclodextrin Complexation Study. Molecules 2024, 29, 5302. [Google Scholar] [CrossRef] [PubMed]
- Sohajda, T.; Varga, E.; Iványi, R.; Fejős, I.; Szente, L.; Noszál, B.; Béni, S. Separation of vinca alkaloid enantiomers by capillary electrophoresis applying cyclodextrin derivatives and characterization of cyclodextrin complexes by nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 2010, 53, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Modroiu, A.; Stroia, D.G.; Uilăcan, A. Analyzing the Chiral Purity of Pharmaceuticals: The Application of Cyclodextrin-Based Chiral Selectors in Capillary Electrophoresis. Symmetry 2024, 16, 1354. [Google Scholar] [CrossRef]
- Fejős, I.; Tóth, G.; Várnai, B.; Szabó, Z.I.; Köteles, I.; Malanga, M.; Béni, S. Enantioseparation of solriamfetol and its major impurity phenylalaninol by capillary electrophoresis using sulfated gamma cyclodextrin. Electrophoresis 2021, 42, 1818–1825. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis 2019, 40, 2420–2437. [Google Scholar] [CrossRef]
- Russell Falck, J.; Miller, L.L.; Stermitz, F.R. Electrooxidative synthesis of morphinandienones from 1-benzyltetrahydroisoquinolines. Tetrahedron 1974, 30, 931–934. [Google Scholar] [CrossRef]
- Dubský, P.; Ördögová, M.; Malý, M.; Riesová, M. CEval: All-in-one software for data processing and statistical evaluations in affinity capillary electrophoresis. J. Chromatogr. A 2016, 1445, 158–165. [Google Scholar] [CrossRef]
- Østergaard, J.; Jensen, H.; Holm, R. Affinity capillary electrophoresis method for investigation of bile salts complexation with sulfobutyl ether-β-cyclodextrin. J. Sep. Sci. 2012, 35, 2764–2772. [Google Scholar] [CrossRef]
- Beneš, M.; Zusková, I.; Svobodová, J.; Gaš, B. Determination of stability constants of complexes of neutral analytes with charged cyclodextrins by affinity capillary electrophoresis. Electrophoresis 2012, 33, 1032–1039. [Google Scholar] [CrossRef]




| Column | Mobile Phase | LAU | NOR | Br-LAU | PROP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | Rs | EEO | t1 | Rs | EEO | t1 | Rs | EEO | t1 | Rs | EEO | ||
| Chiral CD-Ph | ACN | 5.39 | - | - | 5.76 | - | - | 9.21 | - | - | 5.79 | - | - |
| MeOH | 11.85 | - | - | 12.17 | 0.8 | S,R | 9.53 | 8.16 | - | - | |||
| Cyclobond I | ACN | 7.79 | - | - | 8.48 | - | - | 6.34 | - | - | 5.04 | - | - |
| MeOH | 5.11 | - | - | 5.10 | - | - | 4.97 | - | - | 4.79 | - | - | |
| Nucleodex β-CD | ACN | 2.68 | - | - | 2.69 | - | - | 2.70 | - | - | 2.68 | - | - |
| MeOH | 2.87 | - | - | 3.21 | - | - | 2.88 | - | - | 2.88 | - | - | |
| Chiralcel OD | ACN | 5.44 | 2.7 | S,R | 7.33 | 2.9 | S,R | 5.68 | 2.4 | S,R | 5.17 | 0.8 | S,R |
| MeOH | 7.00 | 3.2 | S,R | 7.74 | 1.0 | S,R | 7.17 | 2.8 | S,R | 6.89 | 1.8 | S,R | |
| Chiralpak AD | ACN | 4.87 | - | - | 5.91 | 3.7 | S,R | 5.00 | - | - | 4.80 | - | - |
| MeOH | 5.92 | 0.6 | S,R | 6.98 | 4.7 | S,R | 5.87 | 1.9 | S,R | 6.50 | 1.9 | R,S | |
| Chiralpak IA | ACN | 5.10 | 0.5 | S,R | 6.37 | 3.6 | S,R | 5.28 | - | - | 5.03 | - | - |
| MeOH | 6.37 | - | - | 6.72 | 3.0 | S,R | 6.45 | - | - | 6.39 | 0.9 | R,S | |
| CyD | LAU | NOR | Br-LAU | PROP | |||
|---|---|---|---|---|---|---|---|
| Neutral CyDs | Randomly substituted | HP-β-CyD | EMO | - | - | R,S | - |
| Rs | - | - | 0.8 (30 mM) | - | |||
| HP-γ-CyD | EMO | S,R | - | S,R | S,R | ||
| Rs | 0.4 (30 mM) | - | 0.4 (30 mM) | 1.3 (30 mM) | |||
| Anionic CyDs | S-α-CyD | EMO | R,S | R,S | R,S | S,R | |
| Rs | 2.6 (10 mM) | 2.1 (10 mM) | 0.9 (10 mM) | 0.4 (10 mM) | |||
| S-β-CyD | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 3.4 (10 mM) | 7.5 (10 mM) | 0.7 (10 mM) | 5.4 (10 mM) | |||
| S-γ-CyD | EMO | S,R | R,S | S,R | - | ||
| Rs | 10.5 (10 mM) | 5.4 (10 mM) | 7.3 (10 mM) | - | |||
| SP-α-CyD | EMO | - | - | - | S,R | ||
| Rs | - | - | - | 0.7 (10 mM) | |||
| SP-β-CyD | EMO | - | - | - | S,R | ||
| Rs | - | - | - | 0.7 (10 mM) | |||
| SP-γ-CyD | EMO | S,R | - | S,R | S,R | ||
| Rs | 1.2 (10 mM) | - | 1.5 (10 mM) | 2.1 (10 mM) | |||
| SBE-α-CyD | EMO | - | - | S,R | - | ||
| Rs | - | - | 0.5 (10 mM) | - | |||
| SBE-β-CyD | EMO | R,S | R,S | ||||
| Rs | 1.0 (10 mM) | - | 2.3 (10 mM) | - | |||
| SBE-γ-CyD | EMO | - | - | S,R | S,R | ||
| Rs | - | - | 1.1 (10 mM) | 1.3 (10 mM) | |||
| CM-α-CyD | EMO | S,R | S,R | - | S,R | ||
| Rs | 0.6 (10 mM) | 1.0 (10 mM) | - | 4.8 (10 mM) | |||
| CM-β-CyD | EMO | S,R | - | - | S,R | ||
| Rs | 0.7 (10 mM) | - | - | 1.0 (10 mM) | |||
| CM-γ-CyD | EMO | S,R | S,R | S,R | S,R | ||
| Rs | 3.2 (7.5 mM) | 1.0 (10 mM) | 3.3 (7.5 mM) | 2.7 (10 mM) | |||
| SBX | EMO | S,R | S,R | R,S | - | ||
| Single isomer | Rs | 2.6 (7.5 mM) | 3.3 (7.5 mM) | 2.9 (7.5 mM) | - | ||
| SGX | EMO | S,R | S,R | S,R | S,R | ||
| Rs | 6.7 (5 mM) | 5.7 (5 mM) | 2.2 (2.5 mM) | 7.2 (2.5 mM) | |||
| HS-β-CyD | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 1.5 (4 mM) | 0.6 (5 mM) | 1.8 (4 mM) | 2.1 (5 mM) | |||
| HDAS | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 8.3 (2 mM) | 3.2 (2 mM) | 5.0 (2 mM) | 4.7 (2 mM) | |||
| HxDMS | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 2.2 (5 mM) | 1.0 (5 mM) | 1.9 (5 mM) | 0.5 (5 mM) | |||
| HDMS | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 7.0 (3 mM) | 6.1 (2 mM) | 5.6 (3 mM) | 8.3 (3 mM) | |||
| ODMS | EMO | R,S | R,S | R,S | R,S | ||
| Rs | 2.8 (4 mM) | 7.5 (2 mM) | 6.3 (2 mM) | 1.8 (2 mM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várnagy, E.; Tóth, G.; Hosztafi, S.; Dobó, M.; Fejős, I.; Béni, S. Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study. Molecules 2025, 30, 1125. https://doi.org/10.3390/molecules30051125
Várnagy E, Tóth G, Hosztafi S, Dobó M, Fejős I, Béni S. Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study. Molecules. 2025; 30(5):1125. https://doi.org/10.3390/molecules30051125
Chicago/Turabian StyleVárnagy, Erzsébet, Gergő Tóth, Sándor Hosztafi, Máté Dobó, Ida Fejős, and Szabolcs Béni. 2025. "Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study" Molecules 30, no. 5: 1125. https://doi.org/10.3390/molecules30051125
APA StyleVárnagy, E., Tóth, G., Hosztafi, S., Dobó, M., Fejős, I., & Béni, S. (2025). Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study. Molecules, 30(5), 1125. https://doi.org/10.3390/molecules30051125








