Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Metal-Loaded Vermiculite
2.2. Zn/Ni Desorption by Washing with 0.1 M Hydrochloric Acid Solution
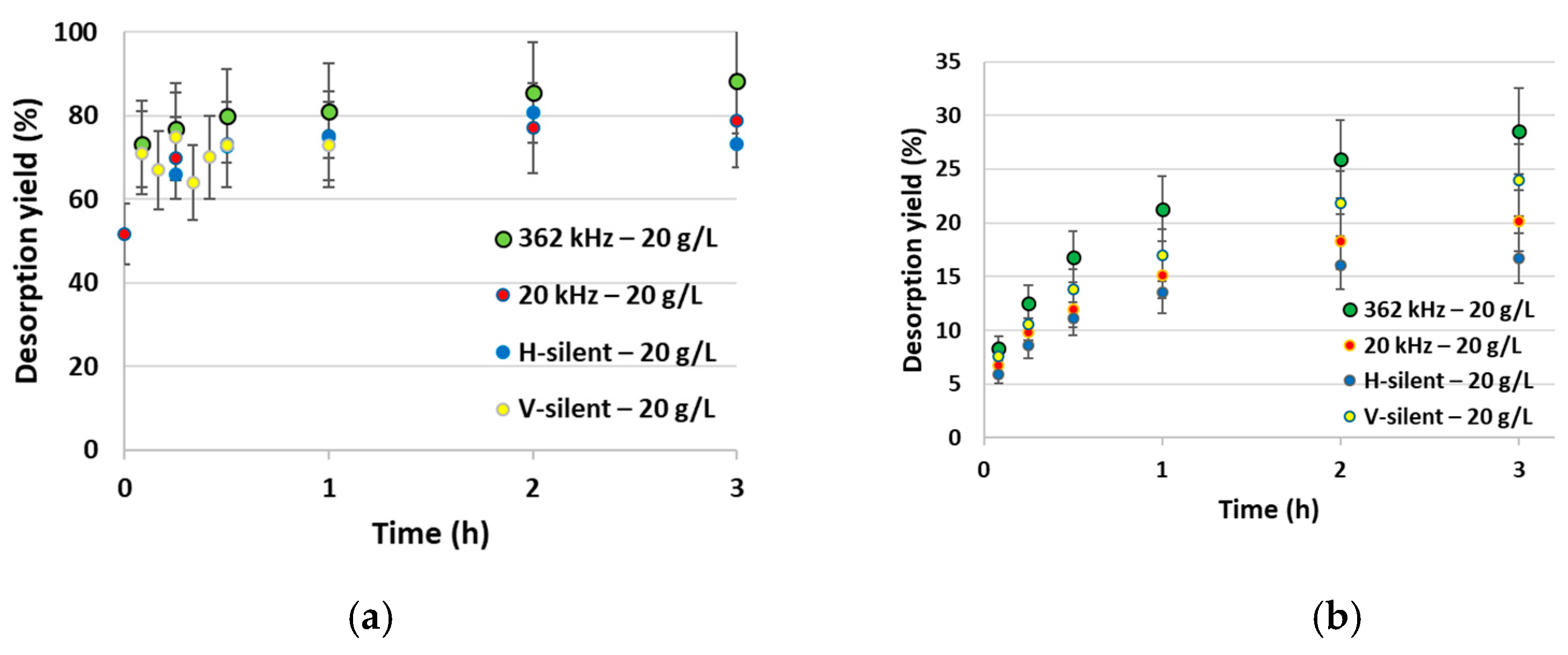
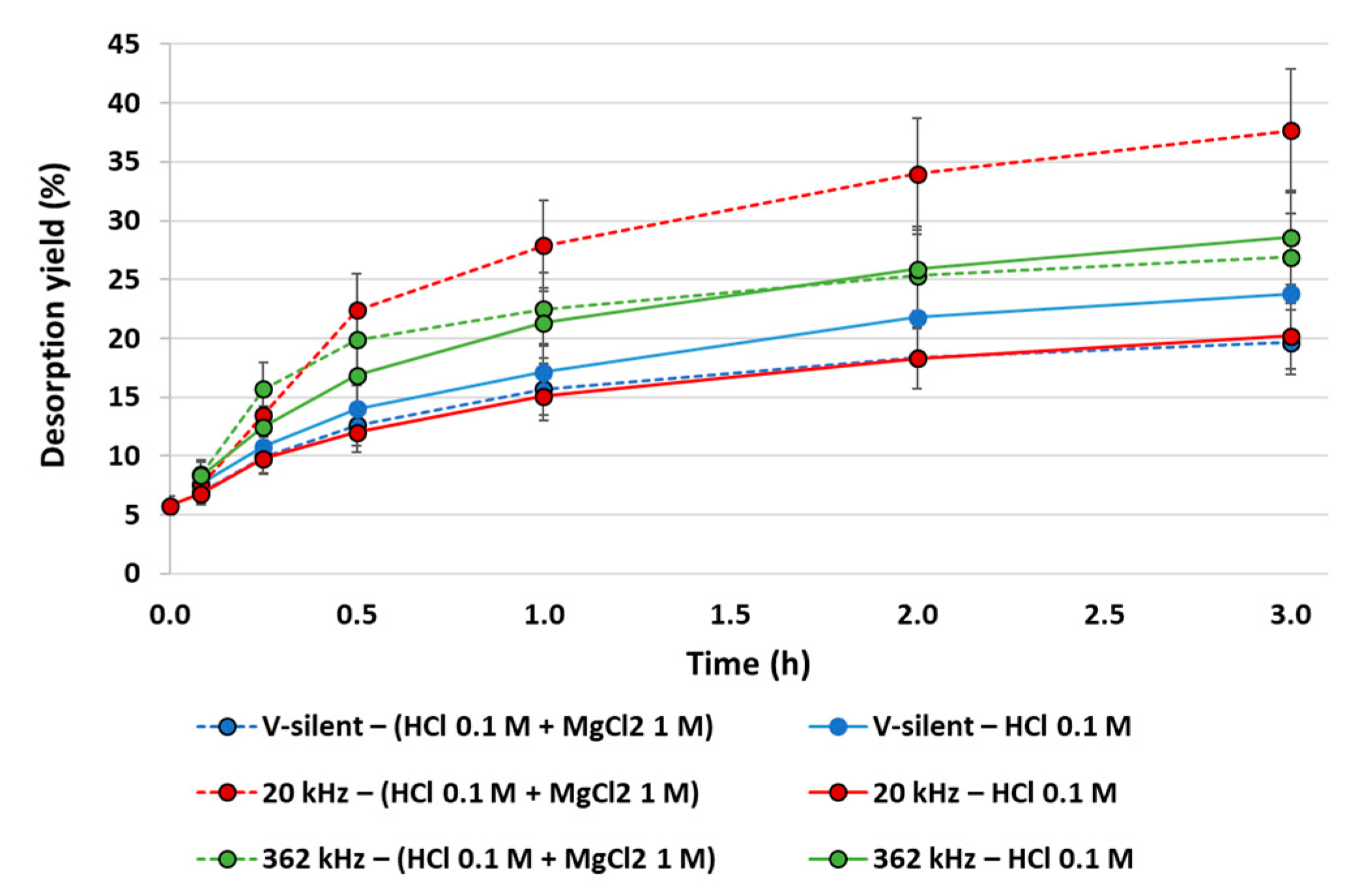
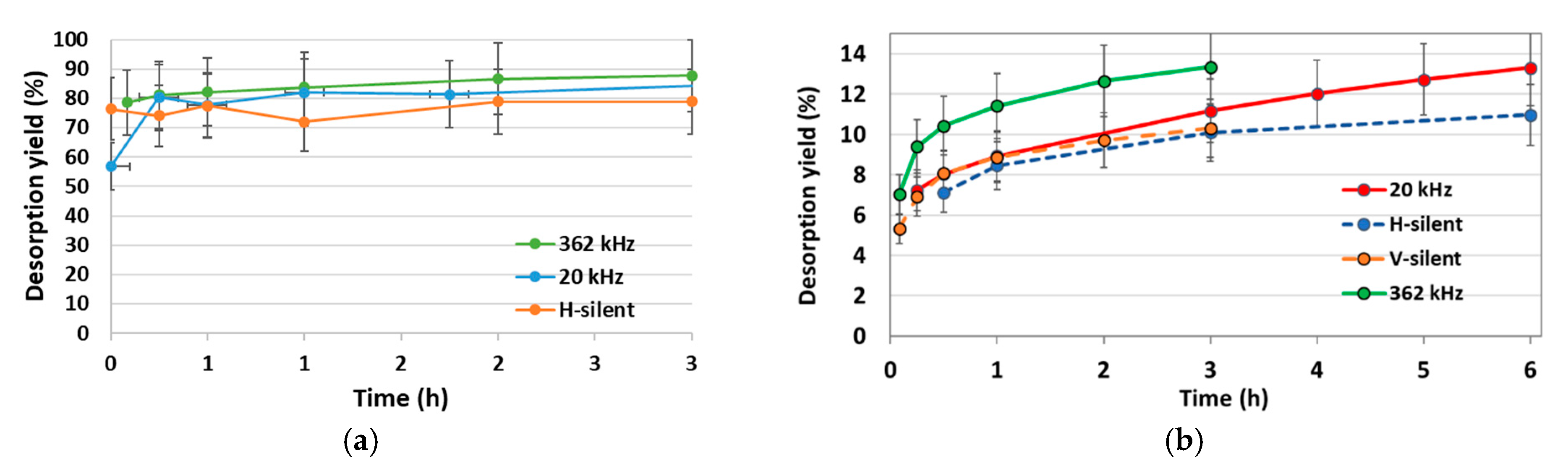
2.2.1. Desorption Kinetics
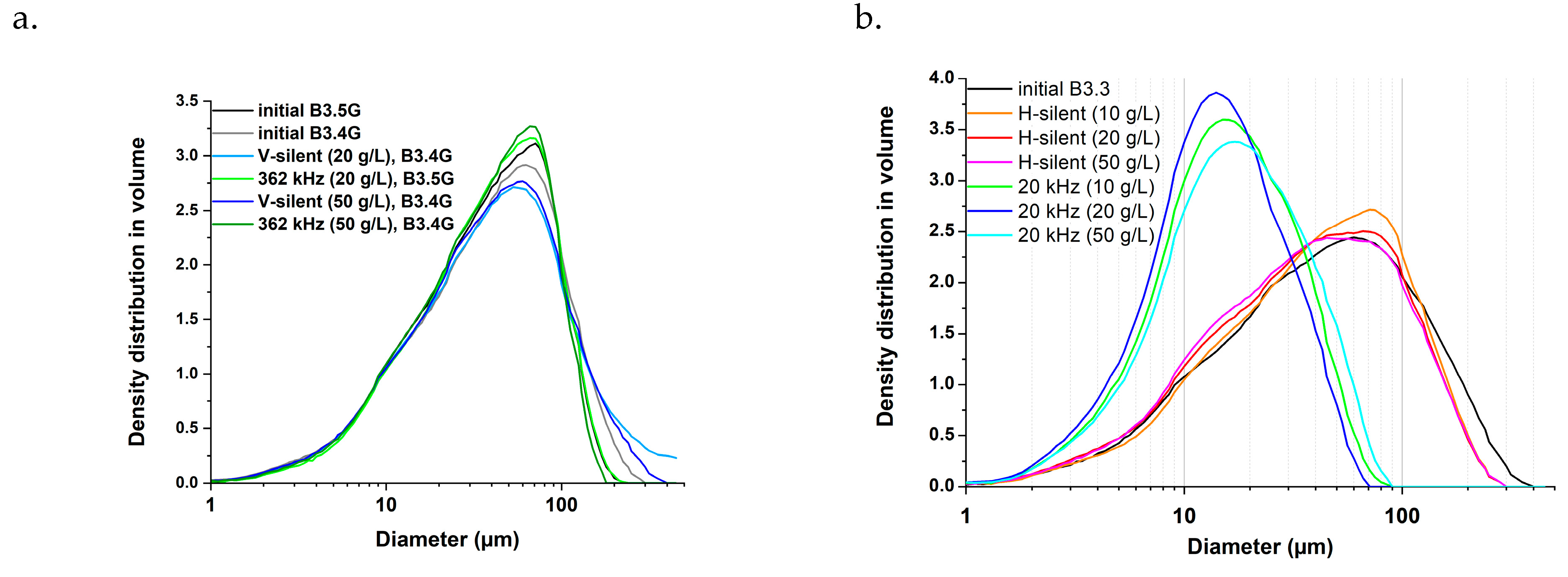
2.2.2. Particle Size Distributions
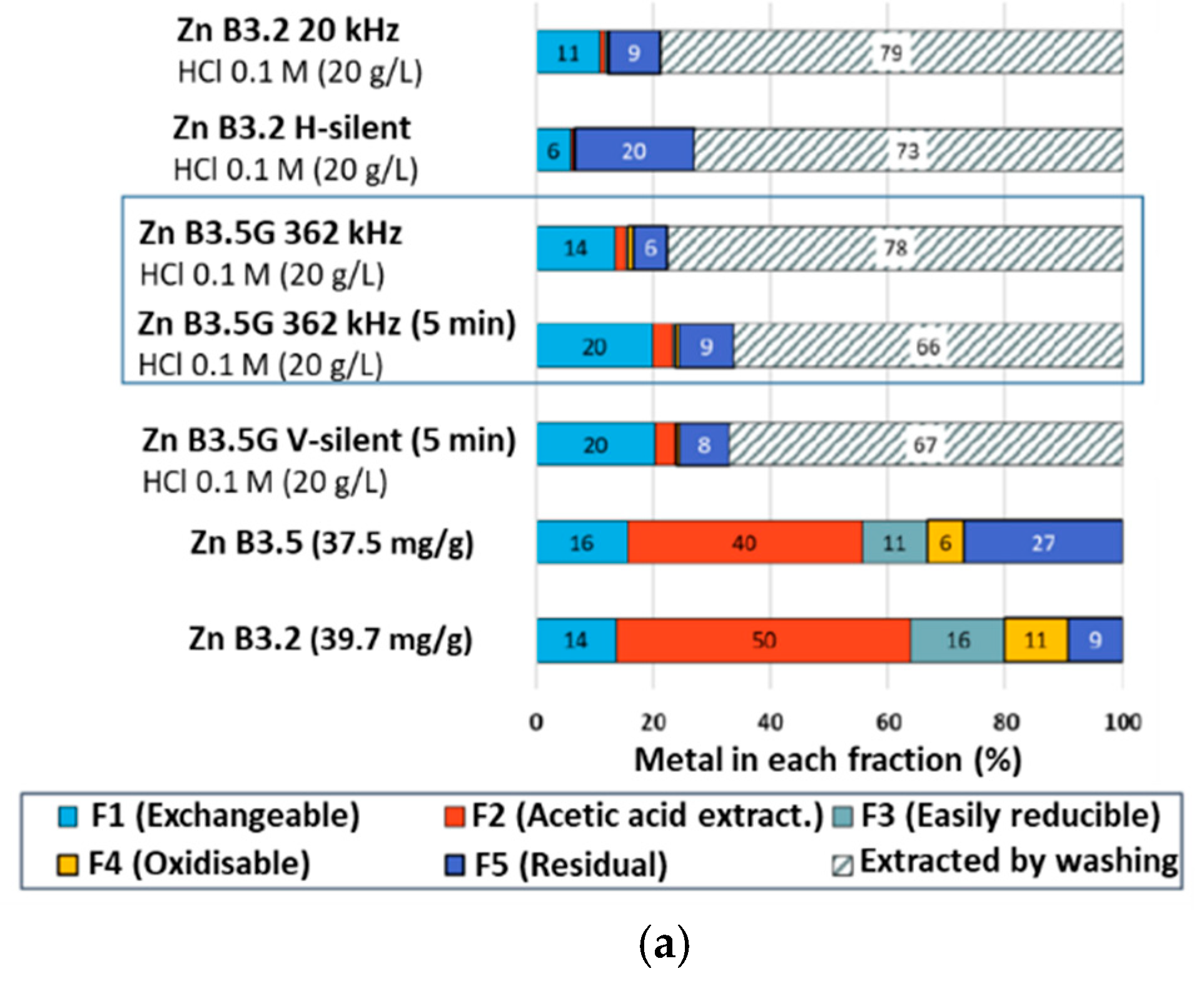
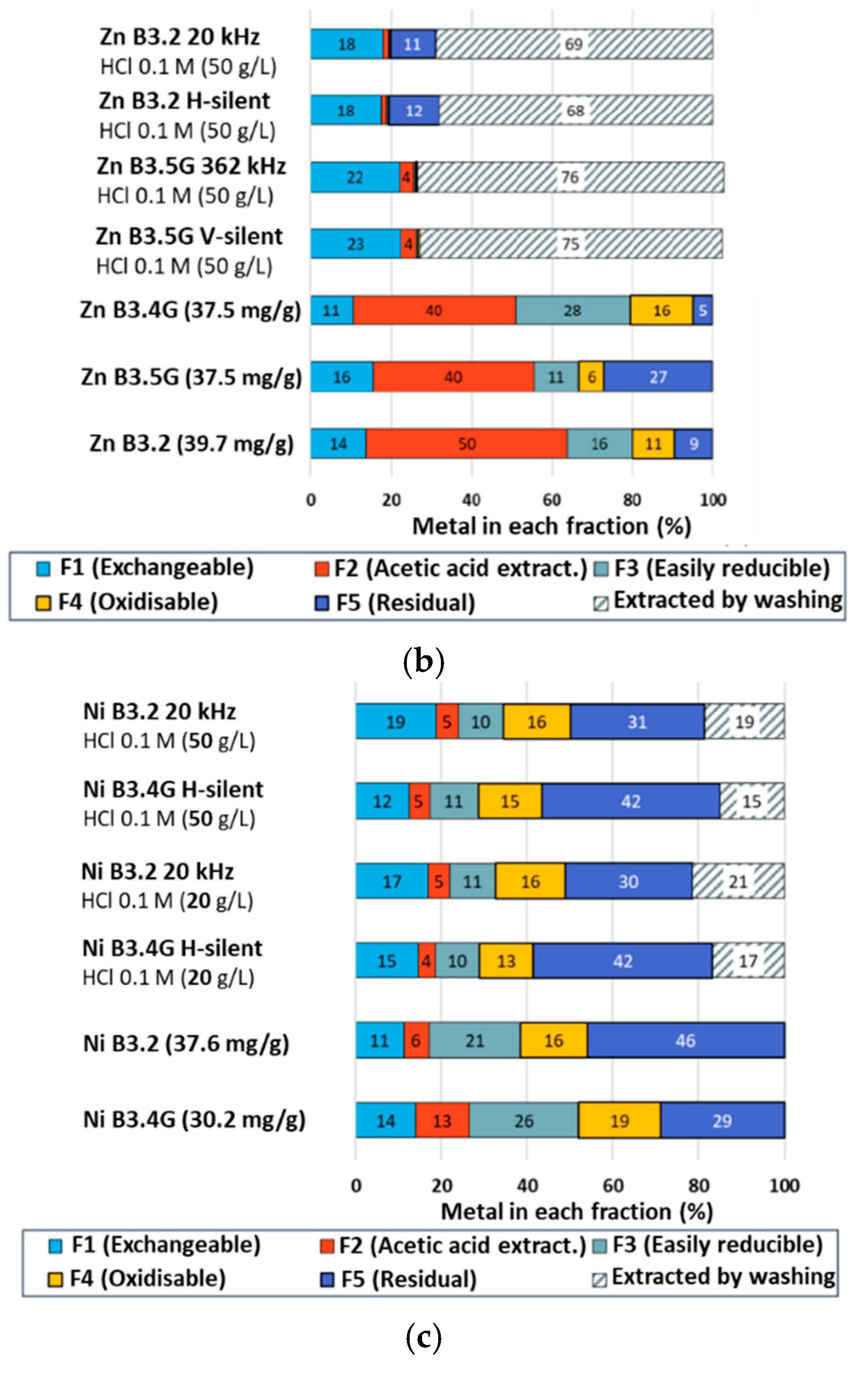
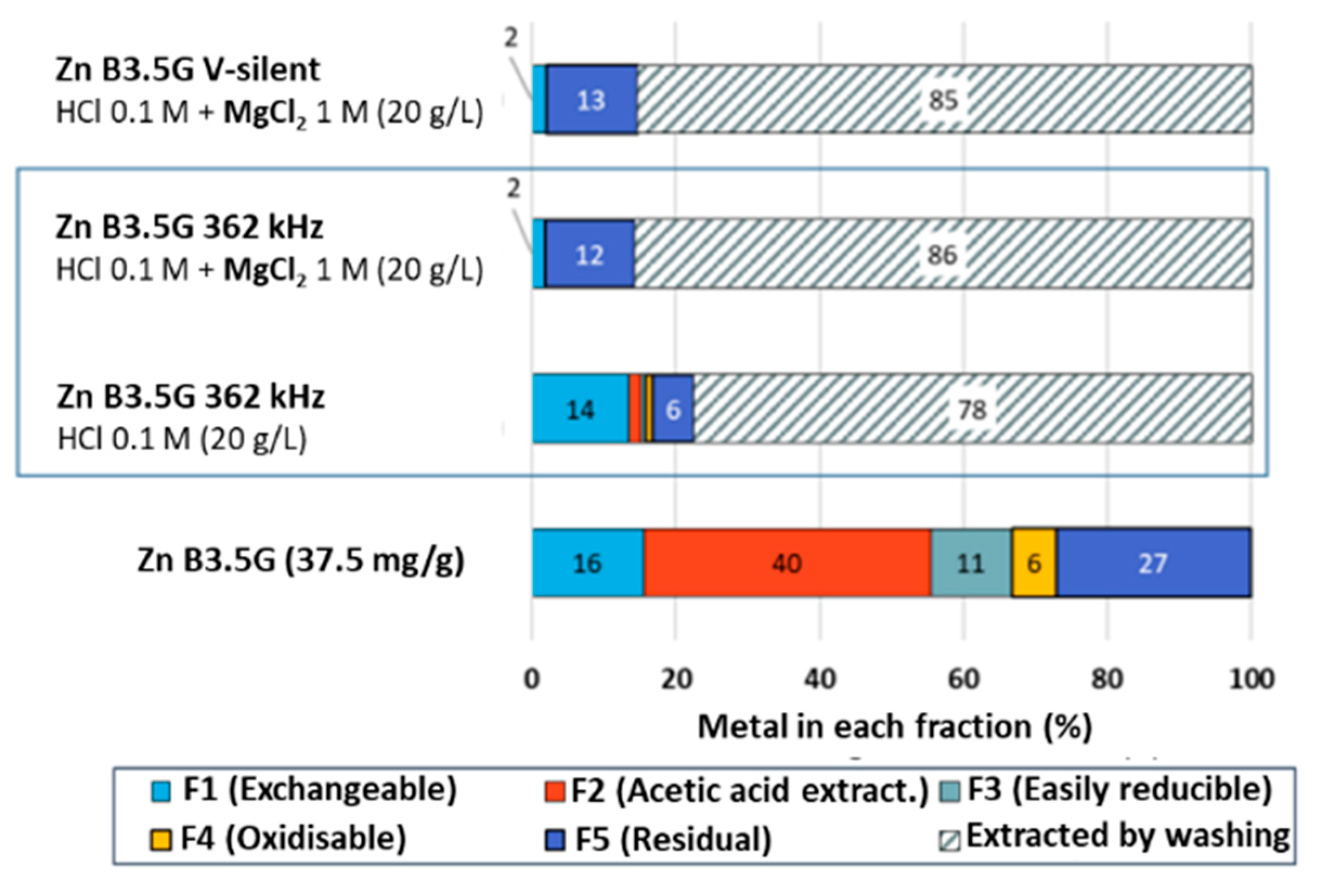
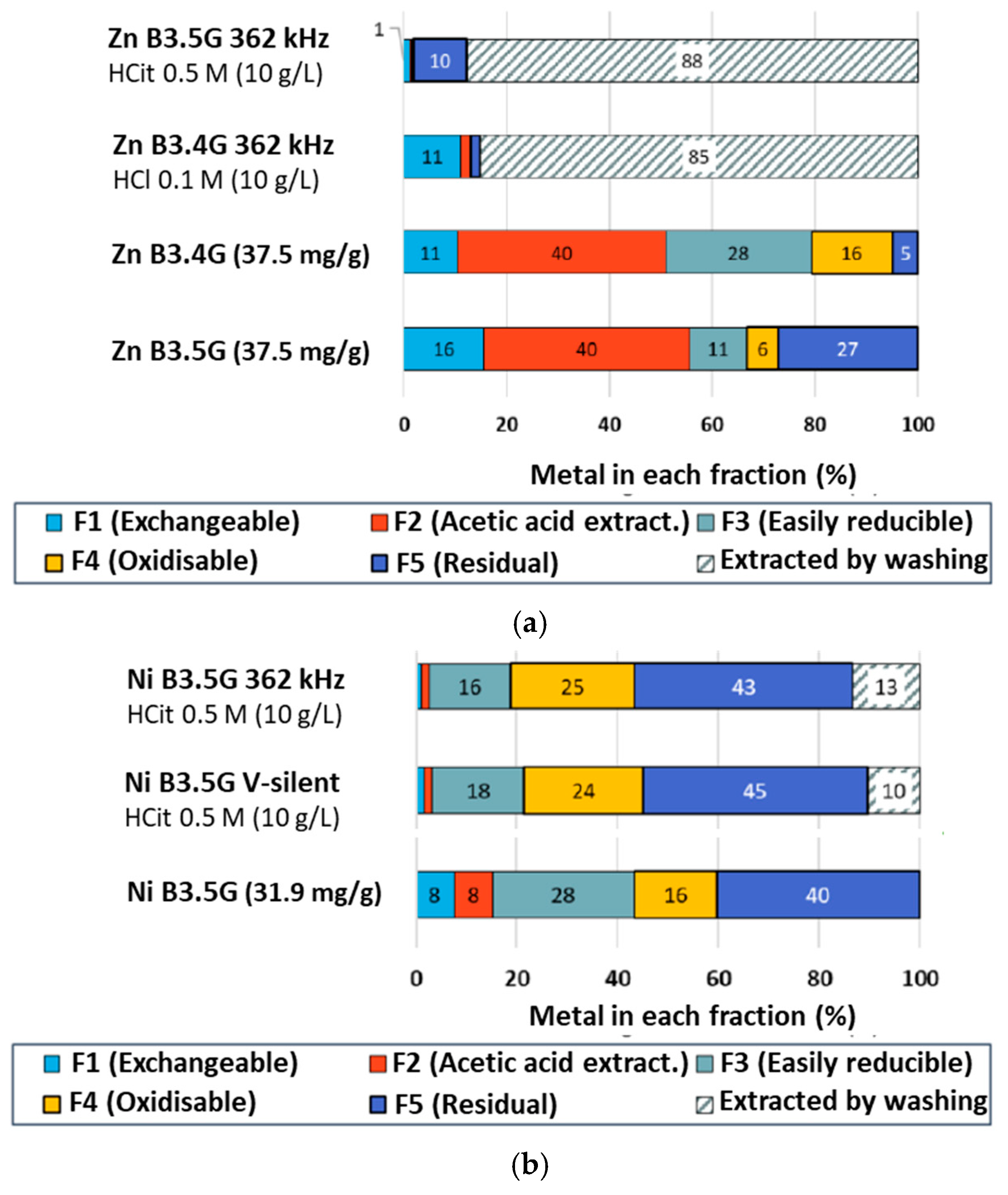
2.2.3. Tessier Sequential Extractions
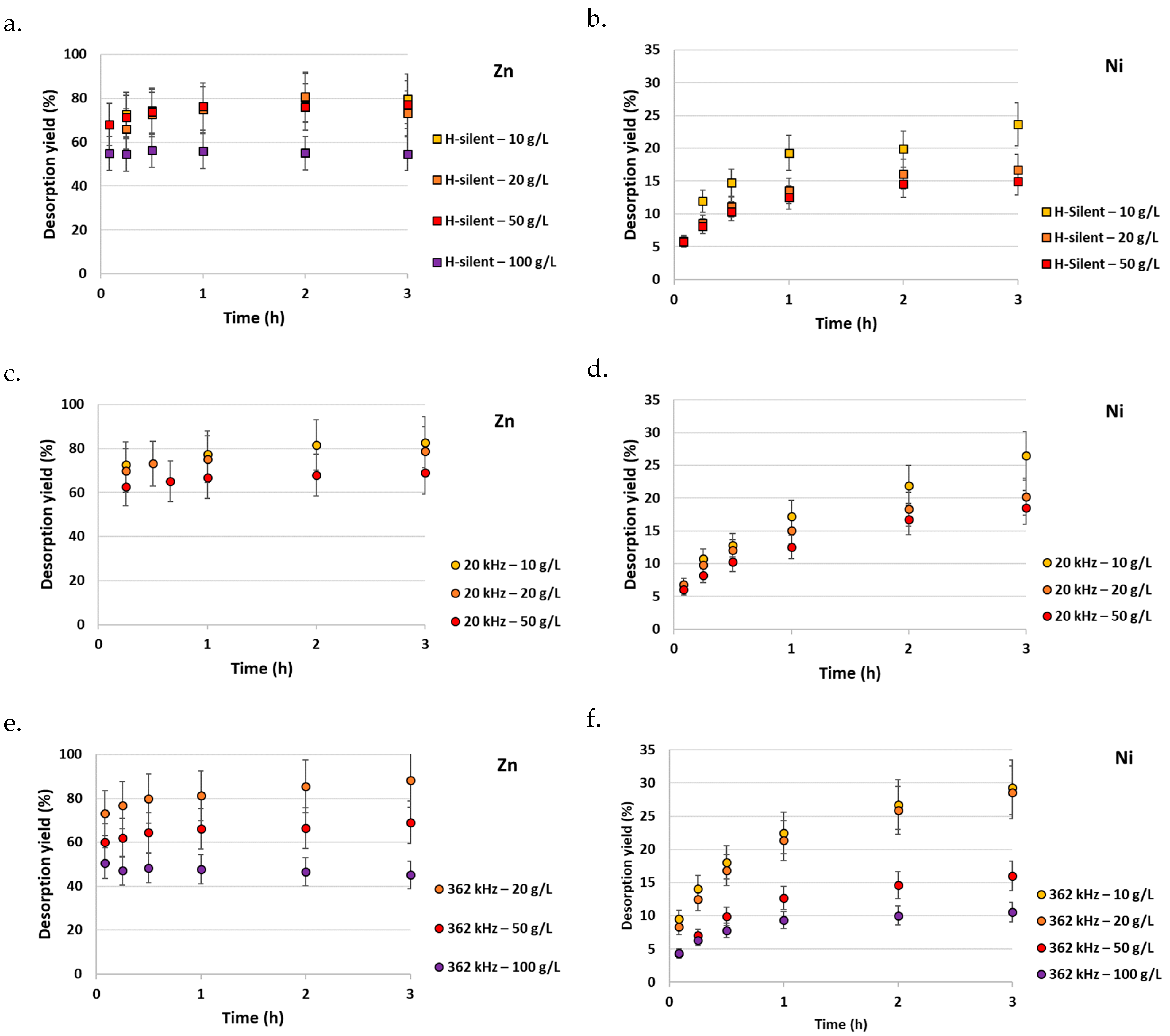
2.3. Improving Zn/Ni Desorption by Coupling HCl Acidic Washing with Ion Exchange
2.4. Metal Desorption Using Citric Acid
2.4.1. Kinetics
2.4.2. Tessier Sequential Extractions
3. Materials and Methods
3.1. Materials
3.2. Sample Analysis and Characterization
3.3. Adsorption Isotherms and Kinetics
3.4. Preparation of Metal-Loaded Vermiculite
3.5. Washing Experiments
3.6. Tessier Sequential Extraction Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T.; et al. Accelerating Circular Economy Solutions to Achieve the 2030 Agenda for Sustainable Development Goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global Soil Pollution by Toxic Elements: Current Status and Future Perspectives on the Risk Assessment and Remediation Strategies—A Review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Kaur, H.; Srivastava, S.; Goyal, N.; Walia, S. Behavior of Zinc in Soils and Recent Advances on Strategies for Ameliorating Zinc Phyto-Toxicity. Environ. Exp. Bot. 2024, 220, 105676. [Google Scholar] [CrossRef]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in Soil and Water: Sources, Biogeochemistry, and Remediation Using Biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metalloids Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy Metal Stabilization Remediation in Polluted Soils with Stabilizing Materials: A Review. Environ. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Khan, E.; Wick, A.F. Thermal Remediation Alters Soil Properties—A Review. J. Environ. Manag. 2018, 206, 826–835. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of Contaminated Soils by Heavy Metals and PAHs. A Brief Review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Liu, Q.; Li, J.; Qiao, Z.; Sun, P.; Yang, Y. A Critical Review on Soil Washing during Soil Remediation for Heavy Metals and Organic Pollutants. Int. J. Environ. Sci. Technol. 2022, 19, 601–624. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ure, A.M. Single Extraction Schemes for Soil Analysis and Related Applications. Sci. Total Environ. 1996, 178, 3–10. [Google Scholar] [CrossRef]
- Ure, A.M. Trace Element Speciation in Soils, Soil Extracts and Solutions. Mikrochim. Acta 1991, 104, 49–57. [Google Scholar] [CrossRef]
- Bacon, J.R.; Davidson, C.M. Is There a Future for Sequential Chemical Extraction? Analyst 2008, 133, 25–46. [Google Scholar] [CrossRef]
- Ko, I.; Lee, C.; Lee, K.; Lee, S.; Kim, K. Remediation of Soil Contaminated with Arsenic, Zinc, and Nickel by Pilot-scale Soil Washing. Environ. Prog. 2006, 25, 39–48. [Google Scholar] [CrossRef]
- Ko, I.; Chang, Y.; Lee, C.; Kim, K. Assessment of Pilot-Scale Acid Washing of Soil Contaminated with As, Zn and Ni Using the BCR Three-Step Sequential Extraction. J. Hazard. Mater. 2005, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Bossart, K.; Mueller, R.; Ritschel, J.; Hauser, L.; Schulin, R.; Nowack, B. Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents. Environ. Sci. Technol. 2004, 38, 937–944. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Lepkova, K.; Kubal, M. Comparison of Electrodialytic Removal of Cu from Spiked Kaolinite, Spiked Soil and Industrially Polluted Soil. J. Hazard. Mater. 2006, 137, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Vanthuyne, M.; Maes, A.; Cauwenberg, P. The Use of Flotation Techniques in the Remediation of Heavy Metal Contaminated Sediments and Soils: An Overview of Controlling Factors. Miner. Eng. 2003, 16, 1131–1141. [Google Scholar] [CrossRef]
- Moutsatsou, A.; Gregou, M.; Matsas, D.; Protonotarios, V. Washing as a Remediation Technology Applicable in Soils Heavily Polluted by Mining–Metallurgical Activities. Chemosphere 2006, 63, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Tejowulan, R.S.; Hendershot, W.H. Removal of Trace Metals from Contaminated Soils Using EDTA Incorporating Resin Trapping Techniques. Environ. Pollut. 1998, 103, 135–142. [Google Scholar] [CrossRef]
- Tampouris, S.; Papassiopi, N.; Paspaliaris, I. Removal of Contaminant Metals from Fine Grained Soils, Using Agglomeration, Chloride Solutions and Pile Leaching Techniques. J. Hazard. Mater. 2001, 84, 297–319. [Google Scholar] [CrossRef]
- Papassiopi, N.; Tambouris, S.; Kontopoulos, A. Removal of Heavy Metals from Calcareous Contaminated Soils by EDTA Leaching. Water. Air. Soil Pollut. 1999, 109, 1–15. [Google Scholar] [CrossRef]
- Elliott, H.A.; Shastri, N.L. Extractive Decontamination of Metal-Polluted Soils Using Oxalate. Water. Air. Soil Pollut. 1999, 110, 335–346. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The Removal of Cu, Ni, and Zn in Industrial Soil by Washing with EDTA-Organic Acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Lu, T.; Qi, W.; Zhu, Y.; Lu, M.; Qi, Z.; Chen, W. Enhanced Transport of Heavy Metal Ions by Low-Molecular-Weight Organic Acids in Saturated Porous Media: Link Complex Stability Constants to Heavy Metal Mobility. Chemosphere 2022, 290, 133339. [Google Scholar] [CrossRef]
- Kim, M.-S.; Koo, N.; Kim, J.-G.; Lee, S.-H. Effects of Washing Solution, Washing Time, and Solid-Solution Rate on the Maximum Heavy Metals Removal Efficiency. Appl. Sci. 2021, 11, 6398. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Calcagnoli, G. Assisted Washing for Heavy Metal and Metalloid Removal from Contaminated Dredged Materials. Water. Air. Soil Pollut. 2009, 196, 183–198. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.; Son, Y. Ultrasound-Assisted Soil Washing Processes for the Remediation of Heavy Metals Contaminated Soils: The Mechanism of the Ultrasonic Desorption. Ultrason. Sonochem. 2021, 74, 105574. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Son, Y. Ultrasonic and Mechanical Soil Washing Processes for the Removal of Heavy Metals from Soils. Ultrason. Sonochem. 2017, 35, 640–645. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, X.; Cao, J.; Shen, L. Ultrasound-Assisted Soil Washing for Metals-Contaminated Soil Using Various Washing Solutions. CLEAN–Soil Air Water 2022, 50, 2100419. [Google Scholar] [CrossRef]
- Son, Y.; Lee, D.; Lee, W.; Park, J.; Hyoung Lee, W.; Ashokkumar, M. Cavitational Activity in Heterogeneous Systems Containing Fine Particles. Ultrason. Sonochem. 2019, 58, 104599. [Google Scholar] [CrossRef]
- Chen, S.; Fei, X.; Zhang, C.; Chen, Y.; Ge, Q. Release Behavior of Heavy Metals from Soil in Ultrasound-Assisted EDTA Washing. J. Soils Sediments 2021, 21, 3825–3833. [Google Scholar] [CrossRef]
- Manickam, S.; Camilla Boffito, D.; Flores, E.M.M.; Leveque, J.-M.; Pflieger, R.; Pollet, B.G.; Ashokkumar, M. Ultrasonics and Sonochemistry: Editors’ Perspective. Ultrason. Sonochem. 2023, 99, 106540. [Google Scholar] [CrossRef] [PubMed]
- Egashira, K.; Osaka, K.; Nakashima, S. Technical Classification of the Clay Mineralogical Composition of Paddy Soils Using Multivariate Analysis in Reference to Rice Production. Soil Sci. Plant Nutr. 1992, 38, 431–442. [Google Scholar] [CrossRef]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Competitive Sorption and Desorption of Heavy Metals by Individual Soil Components. J. Hazard. Mater. 2007, 140, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A Review on Zinc and Nickel Adsorption on Natural and Modified Zeolite, Bentonite and Vermiculite: Examination of Process Parameters, Kinetics and Isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Giacomino, A.; Aceto, M.; Mentasti, E. Adsorption of Heavy Metals on Vermiculite: Influence of pH and Organic Ligands. J. Colloid Interface Sci. 2006, 299, 537–546. [Google Scholar] [CrossRef]
- Kuo, S.; Lai, M.S.; Lin, C.W. Influence of Solution Acidity and CaCl2 Concentration on the Removal of Heavy Metals from Metal-Contaminated Rice Soils. Environ. Pollut. 2006, 144, 918–925. [Google Scholar] [CrossRef]
- Pflieger, R.; Chave, T.; Vite, G.; Jouve, L.; Nikitenko, S.I. Effect of Operational Conditions on Sonoluminescence and Kinetics of H2O2 Formation during the Sonolysis of Water in the Presence of Ar/O2 Gas Mixture. Ultrason. Sonochem. 2015, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Syu, S.-Y.; Chen, C.-W.; Chen, C.-F.; Dong, C.-D. Assessment of Ex-Situ Chemical Washing of Heavy Metals from Estuarine Sediments around an Industrial Harbor in Southern Taiwan. J. Soils Sediments 2019, 19, 3108–3122. [Google Scholar] [CrossRef]
- Kim, K.; Choi, Y. Effect of Hydrochloric Acid Concentration on Removal Efficiency and Chemical Forms of Heavy Metals During Dredged Sediment Acid Washing. J. Soil Groundw. Environ. 2020, 25, 74–83. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Sun, Y.; Yang, W. Leaching Remediation of Dredged Marine Sediments Contaminated with Heavy Metals. J. Mar. Sci. Eng. 2022, 10, 636. [Google Scholar] [CrossRef]
- Parat, C.; Lévêque, J.; Dousset, S.; Chaussod, R.; Andreux, F. Comparison of Three Sequential Extraction Procedures Used to Study Trace Metal Distribution in an Acidic Sandy Soil. Anal. Bioanal. Chem. 2003, 376, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, C. Dissolution of Hydroxide Minerals in the 1 M Sodium Acetate, pH 5, Extracting Solution in Sequential Extraction Schemes. Environ. Geol. 2004, 45, 864–868. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, M.; Gao, Y.; Wang, J.; Li, D.; Li, T. Removal of Toxic Metals from Vanadium-Contaminated Soils Using a Washing Method: Reagent Selection and Parameter Optimization. Chemosphere 2017, 180, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, R.; Lejeune, M.; Draye, M. Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar. Molecules 2022, 27, 6050. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Reinert, L.; Levêque, J.-M.; Duclaux, L.; Muller, F.; Saeed, S.; Shah, S.S. Effect of Sonication Conditions: Solvent, Time, Temperature and Reactor Type on the Preparation of Micron Sized Vermiculite Particles. Ultrason. Sonochem. 2014, 21, 1002–1009. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Reinert, L.; Lévêque, J.-M.; Beziat, A.; Dehaudt, P.; Juliaa, J.-F.; Duclaux, L. Preparation and Characterization of Micron and Submicron-Sized Vermiculite Powders by Ultrasonic Irradiation. Appl. Clay Sci. 2013, 72, 9–17. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Shaikh, Q.; Jatoi, W.B.; Feng, X.; Sun, G.; Saleh, T.A. A Review of Sequential Extraction Methods for Fractionation Analysis of Toxic Metals in Solid Environmental Matrices. TrAC Trends Anal. Chem. 2024, 173, 117639. [Google Scholar] [CrossRef]
- Poulsen, I.F.; Hansen, H. Chr. B. Soil Sorption of Nickel in Presence of Citrate or Arginine. Water. Air. Soil Pollut. 2000, 120, 249–259. [Google Scholar] [CrossRef]




| Chemical Name | CAS N° | Source | Chemical Formula/Structure | Concentration or Purity (wt%) |
|---|---|---|---|---|
| Acetic acid | 64-19-7 | Aldrich | CH3COOH | 99.7 |
| Hydrochloric acid | 7647-01-0 | Carlo Erba (Milan, Italy) | HCl | 37 |
| Citric acid | 77-92-9 | Aldrich |  | 99.5 |
| Nitric acid | 7697-37-2 | Aldrich | HNO3 | 70 |
| Ammonium acetate | 631-61-8 | Thermo Fisher Scientific | CH3COONH4 | 99.99 |
| Magnesium chloride | 7786-30-3 | Aldrich | MgCl2 | 99.99 |
| Nickel (II) nitrate hexahydrate | 13478-00-7 | Aldrich | Ni(NO3)2·6H2O | 99.999 |
| Sodium acetate | 127-09-3 | Merck | CH3COONa | 99.99 |
| Sodium nitrate | 7631-99-4 | Aldrich | NaNO3 | 99.995 |
| Zinc (II) nitrate hexahydrate | 7779-88-6 | Aldrich | Zn(NO3)2·6H2O | 99.999 |
| Hydrogen peroxide | 7722-84-1 | Aldrich | H2O2 | 30 |
| Hydroxylamine | 7803-49-8 | Aldrich | NH2OH | 50 |
| Reactants and Conditions | Fraction | |
|---|---|---|
| F1 | 8 mL MgCl2 1 M, pH 7, 2 h, 25 °C | Exchangeable cations |
| F2 | 8 mL CH3COONa 1 M, pH fixed to 5.0 with CH3COOH, 5 h, 25 °C | Acetic acid extractable fraction |
| F3 | 20 mL NH2OH.HCl 0.04 M in CH3COOH 25% v/v, pH fixed to 2 with HNO3, 6 h, 96 °C (in an autoclave) | Easily reducible |
| F4 | (i) 5 mL H2O2 30%, pH 2 + 3 mL HNO3 0.02 M, 2 h, 85 °C (in a autoclave) (ii) then add 3 mL H2O2 30%, 3 h, 85 °C (in a autoclave) (iii) after cooling, add 5 mL CH3COONH4 3.2 M in HNO3 20 vol% and complete to 10 mL with H2O, 30 min, 20 °C | Oxidisable |
| F5 | Not measured but calculated by mass balance | Residual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leybros, A.; Herr, S.; Salameh, R.; Pflieger, R. Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite. Molecules 2025, 30, 1110. https://doi.org/10.3390/molecules30051110
Leybros A, Herr S, Salameh R, Pflieger R. Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite. Molecules. 2025; 30(5):1110. https://doi.org/10.3390/molecules30051110
Chicago/Turabian StyleLeybros, Antoine, Sophie Herr, Rita Salameh, and Rachel Pflieger. 2025. "Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite" Molecules 30, no. 5: 1110. https://doi.org/10.3390/molecules30051110
APA StyleLeybros, A., Herr, S., Salameh, R., & Pflieger, R. (2025). Benefit of an Ultrasonic Irradiation on the Depollution by Washing of Nickel- or Zinc-Contaminated Vermiculite. Molecules, 30(5), 1110. https://doi.org/10.3390/molecules30051110








