A Selective and Fast Approach for Volatile Metalorganics Assaying in Wastewater
Abstract
1. Introduction
2. Results
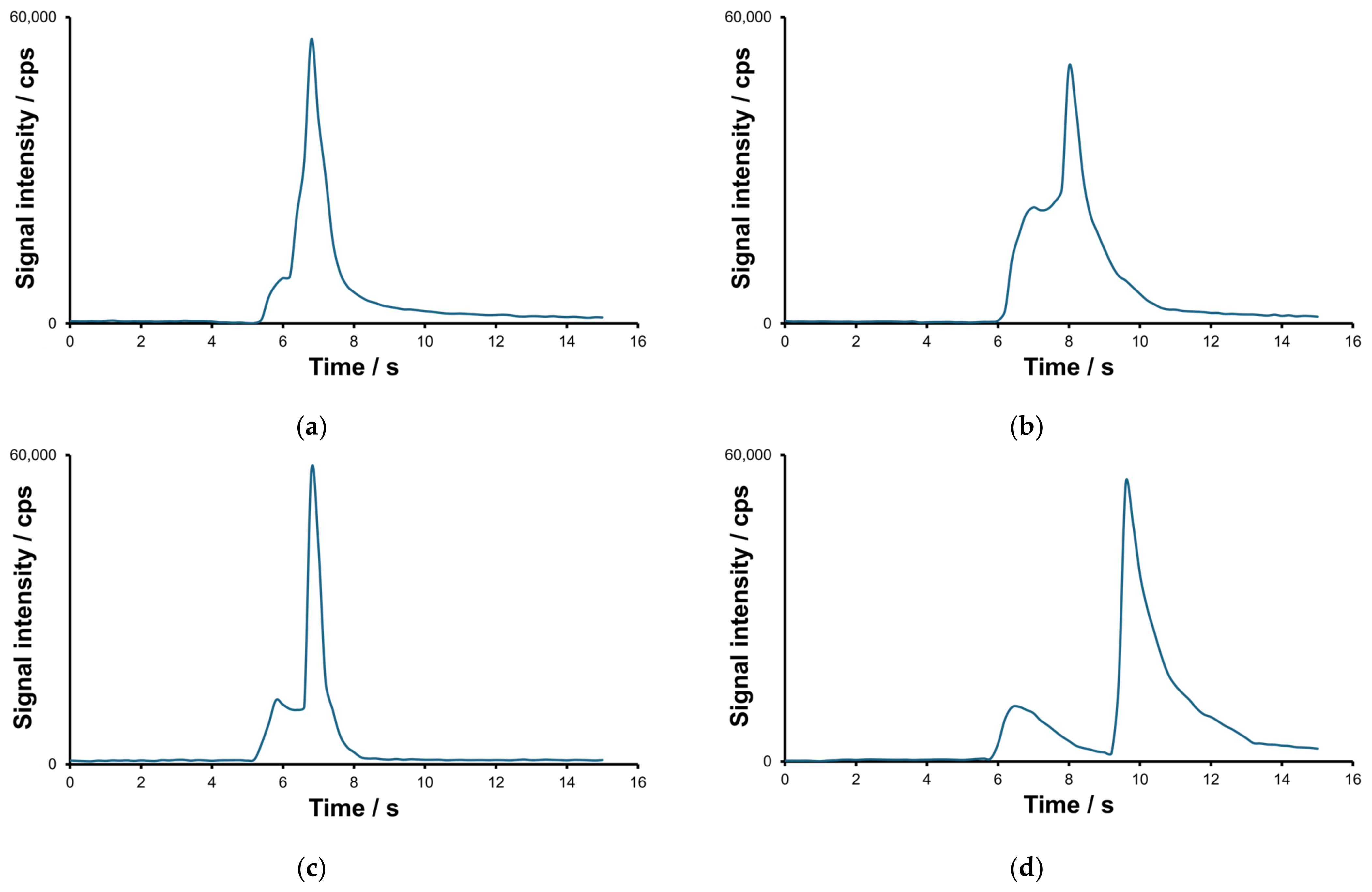
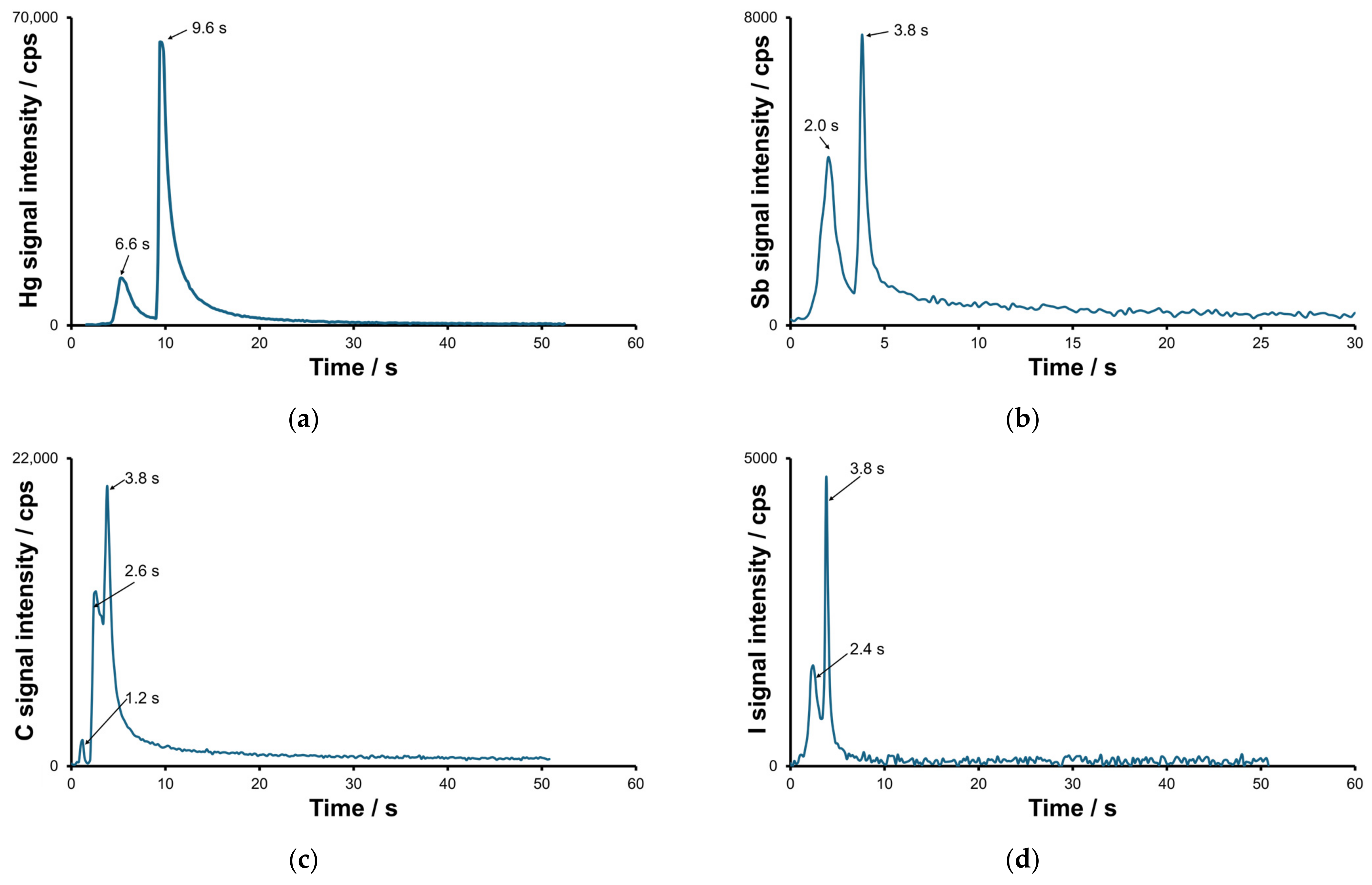
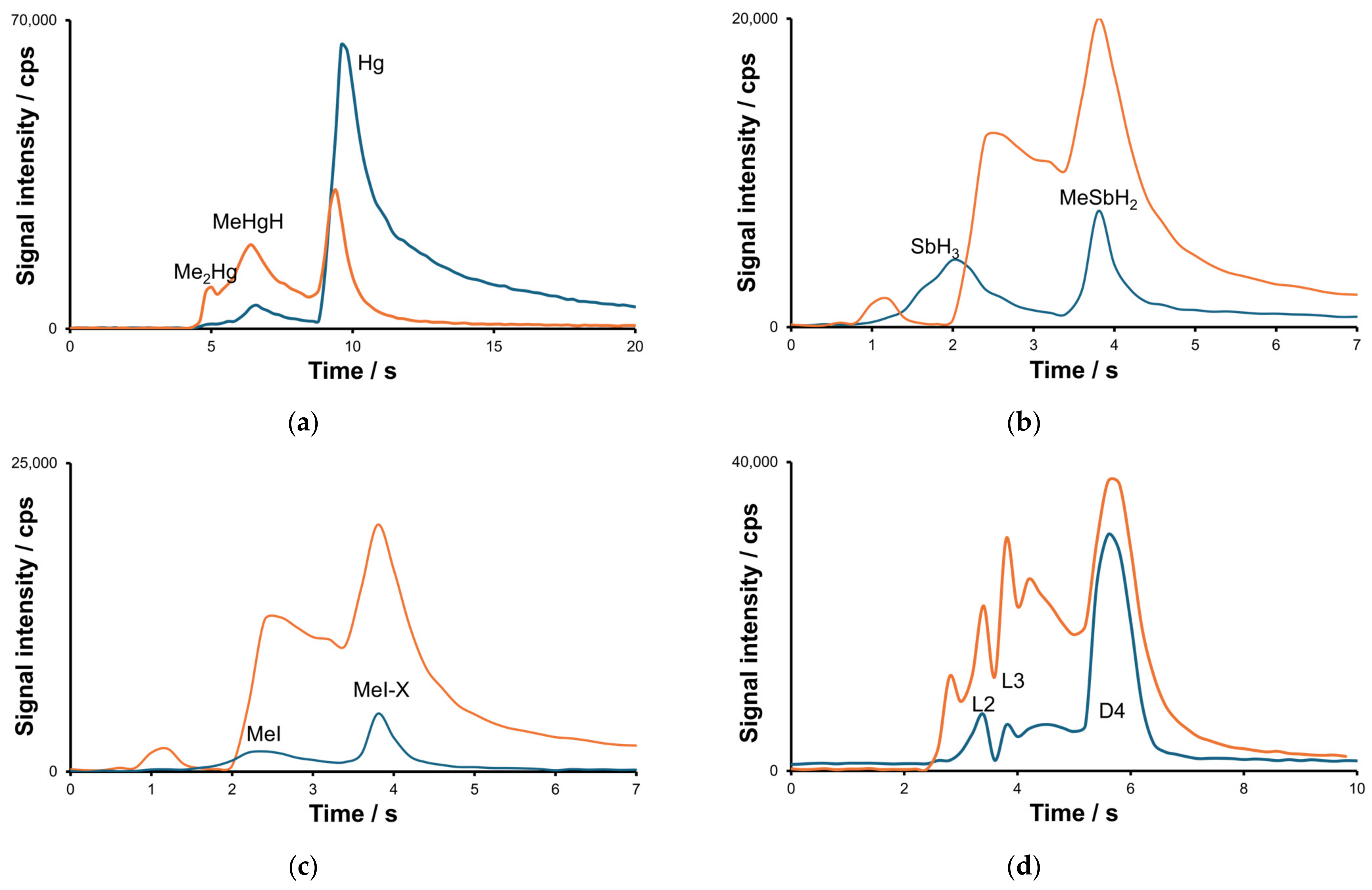
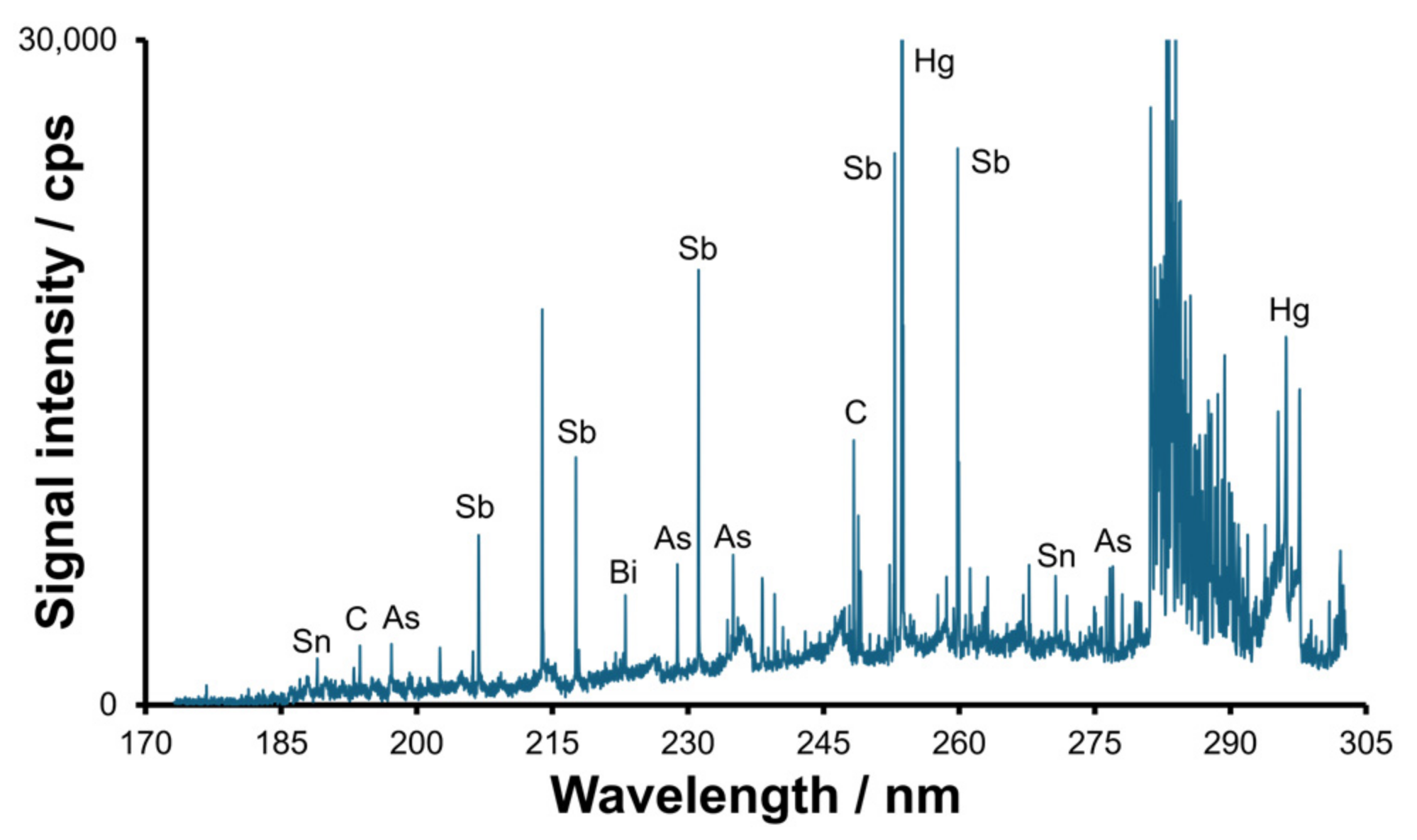
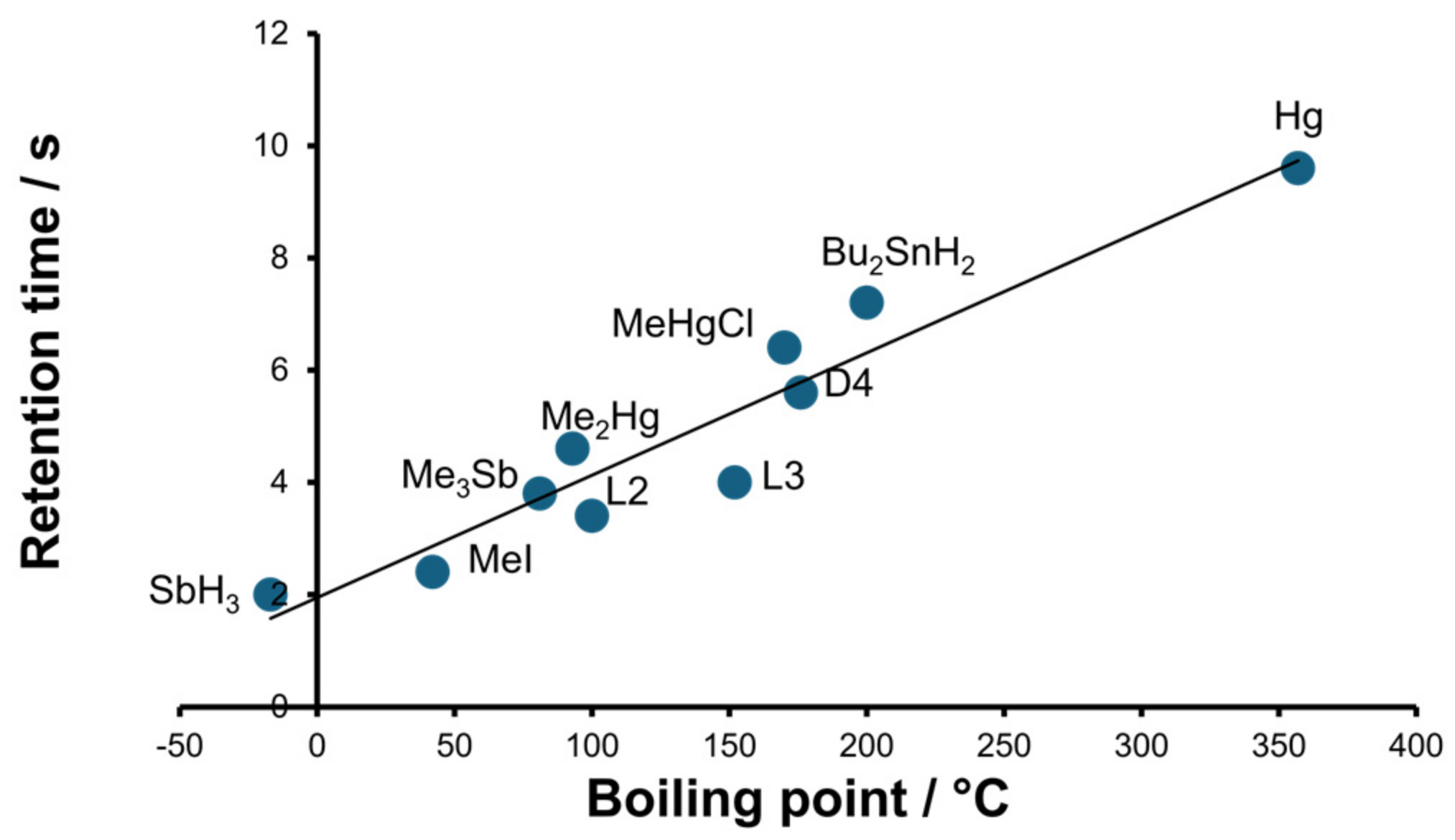
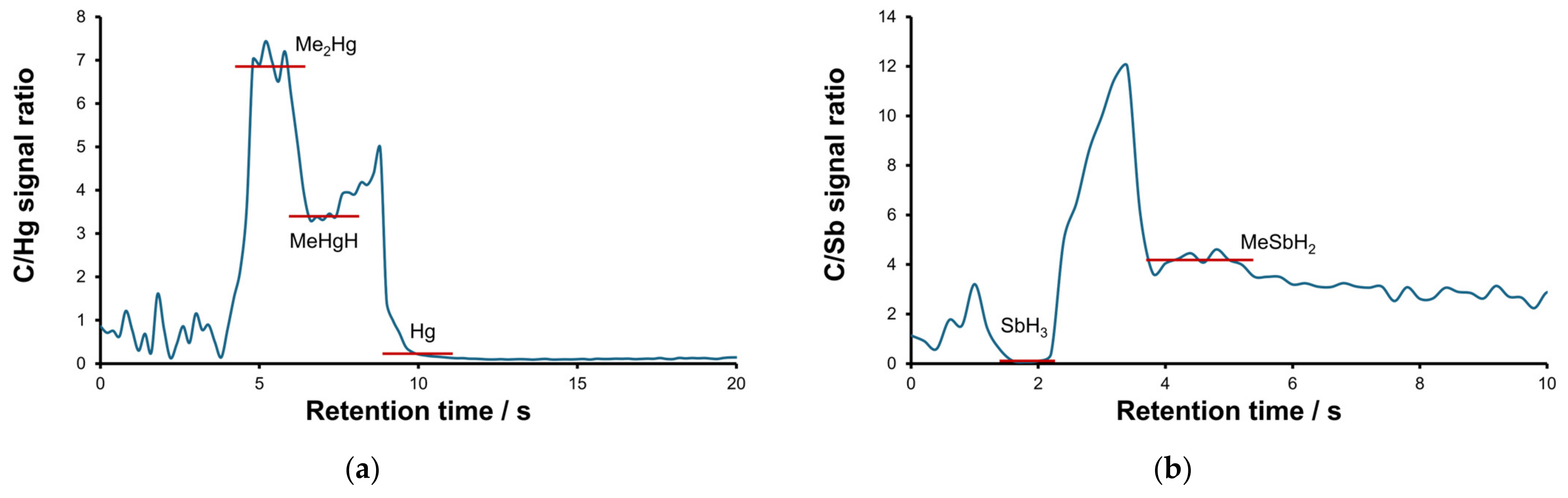
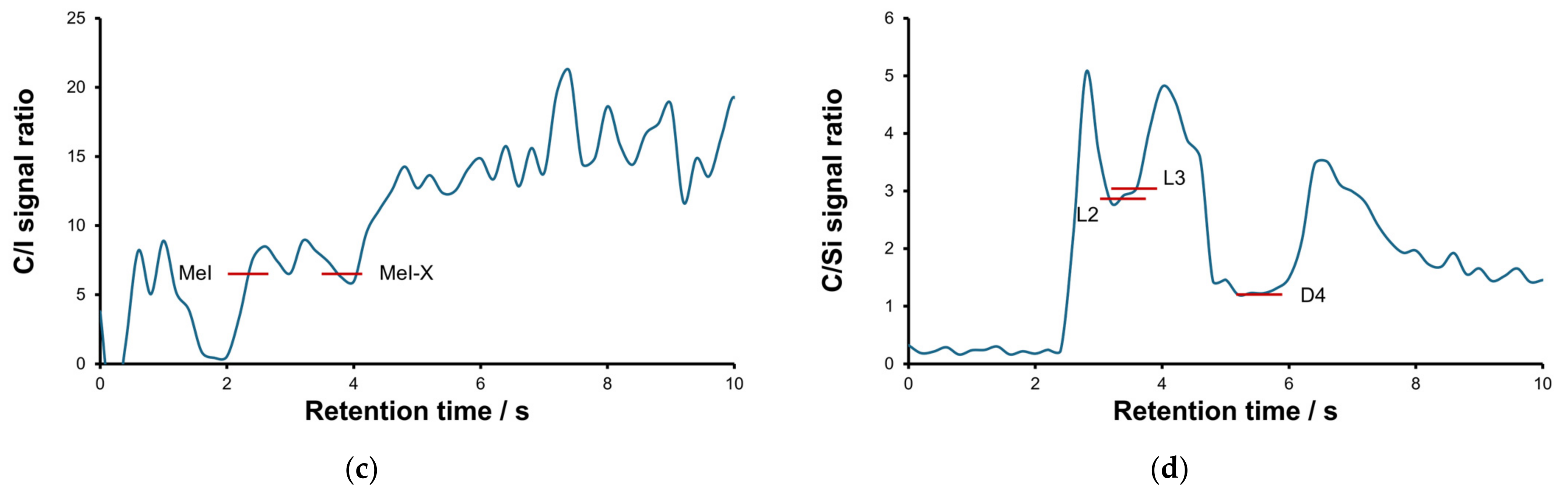
2.1. Optimization of the Separation and Detection Conditions
2.2. Analytical Performance
2.3. Analysis of Samples from WWTP
3. Materials and Methods
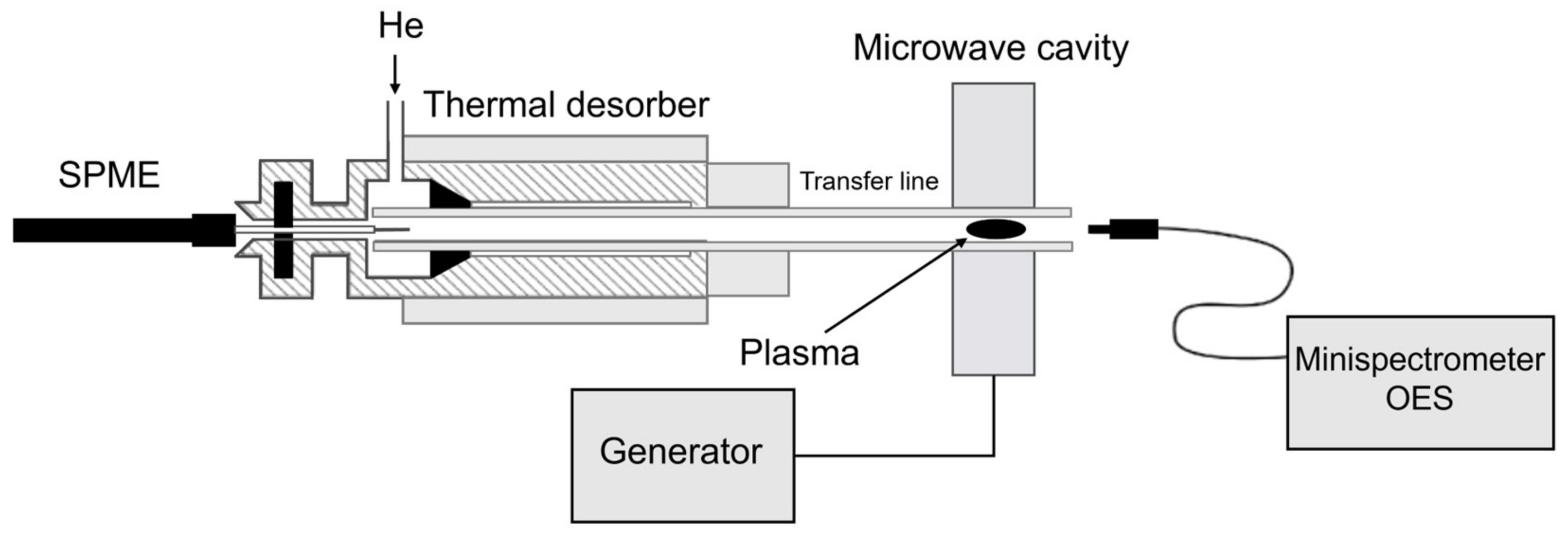
3.1. Solid-Phase Microextraction Device
3.2. TD-MIP-OES Instrument
3.3. Reagents and Reference Materials
3.4. Procedures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkmann, T.; Giner-Santonja, G.; Yükseler, H.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector; EUR 28112 EN; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Villaescusa, I. Chemical equilibria in wastewaters during toxic metal ion removal by agricultural biomass. Coord. Chem. Rev. 2010, 254, 2181–2192. [Google Scholar] [CrossRef]
- Craig, P.J. Organometallic Compounds in the Environment, 2nd ed.; John Wiley: Chichester, UK, 2003. [Google Scholar]
- Hirner, A.V.; Emons, H. Organic Metal and Metalloid Species in the Environment, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Feldmann, J.; Hirner, A.V. Occurrence of volatile metal and metalloid species in landfill and sewage gases. Int. J. Environ. Anal. Chem. 1995, 60, 339–359. [Google Scholar] [CrossRef]
- Michalke, K.; Wickenheiser, E.B.; Mehring, M.; Hirner, A.V.; Hensel, R. Production of volatile derivatives of metal (loid) s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 2000, 66, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Du, J.; Pei, W.; Liu, Y.; Guo, M. Modeling and monitoring cyclic and linear volatile methylsiloxanes in a wastewater treatment plant using constant water level sequencing batch reactors. Sci. Total Environ. 2015, 512–513, 472–479. [Google Scholar] [CrossRef] [PubMed]
- EN. Directive 2013/39/EU of the European Parliament and of the Council amending Directives 2000/60/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, L 226, 17. [Google Scholar]
- Smallwood, T.J.; Magnuson, J.K.; Thompson, J.T.; Lin, A.M.; Townsend, T.G. Insights on volatile metals in landfill gas as determined from advanced treatment media. J. Hazard. Mater. 2024, 462, 132777. [Google Scholar] [CrossRef] [PubMed]
- Capela, D.; Ratola, N.; Alves, A.; Homem, V. Volatile methylsiloxanes through wastewater treatment plants—A review of levels and implications. Environ Int. 2017, 102, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, J.; Grümping, R.; Hirner, A.V. Determination of volatile metal and metalloid compounds in gases from domestic waste deposits with GC/ICP-MS. Fresenius J. Anal. Chem. 1994, 350, 228–234. [Google Scholar] [CrossRef]
- Mitra, S.K.; Jiang, K.; Haas, K.; Feldmann, J. Municipal landfills exhale newly formed organotins. J. Environ. Monit. 2005, 7, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Bone, R.A.; Hitzke, M. Multi-element organometal(loid) speciation by hydride generation-GC-ICP-MS: Overcoming the problem of species-specific optima by using a pH-gradient during derivatization. J. Anal. At. Spectrom. 2008, 23, 861–870. [Google Scholar] [CrossRef]
- Serra, H.; Nogueira, J.M.F. Organotin speciation in environmental matrices by automated on-line hydride generation-programmed temperature vaporization-capillary gas chromatography-mass spectrometry detection. J. Chromatogr. A 2005, 1094, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, P.; Goenaga Infante, H.; Adams, F.C. Simultaneous multi-elemental speciation analysis of organometallic compounds by solid-phase microextraction and multicapillary gas chromatography hyphenated to inductively coupled plasma-time-of-flight-mass spectrometry. J. Anal. At. Spectrom. 2004, 19, 867–875. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, K.; Hou, X.; Jiang, X. Compact integration of gas chromatographer and atomic fluorescence spectrometer for speciation analysis of trace alkyl metals/semimetals. Microchem. J. 2014, 114, 16–21. [Google Scholar] [CrossRef]
- Botana, J.; Rodríguez, R.; Díaz, A.; Ferreira, R.; Torrijos, R.; Pereiro, I. Fast and simultaneous determination of tin and mercury species using SPME, multicapillary gas chromatography and MIP-AES detection. J. Anal. At. Spectrom. 2002, 17, 904–907. [Google Scholar] [CrossRef]
- Giersz, J.; Jankowski, K.; Truskolaska, M. Rapid separation of elemental species by fast multicapillary gas chromatography with multichannel optical spectrometry detection following headspace solid phase microextraction. Chromatography 2015, 2, 239–252. [Google Scholar] [CrossRef]
- Borowska, M.; Jankowski, K. Photochemical vapor generation combined with headspace solid phase microextraction for determining mercury species by microwave-induced plasma optical emission spectrometry. Microchem. J. 2022, 172, 106905. [Google Scholar] [CrossRef]
- Tyburska, A.; Jankowski, K.; Rodzik, A. Determination of arsenic and selenium by hydride generation and headspace solid phase microextraction coupled with optical emission spectrometry. Spectrochim. Acta Part B 2011, 66, 517–521. [Google Scholar] [CrossRef]
- Haberhauer-Troyer, C.; Rosenberg, E.; Grasserbauer, M. Evaluation of solid-phase microextraction for sampling of volatile organic sulfur compounds in air for subsequent gas chromatographic analysis with atomic emission detection. J. Chromatogr. A 1999, 848, 305–315. [Google Scholar] [CrossRef]
- Carmona, M.; Llanos, W.; Higueras, P.; Kocman, D. Mercury emissions in equilibrium: A novel approach for the quantification of mercury emissions from contaminated soils. Anal. Methods 2013, 5, 2793–2801. [Google Scholar] [CrossRef]
- Anderson, J.T. Some unique properties of gas chromatography coupled with atomic emission detection. Anal. Bioanal. Chem. 2002, 373, 344–355. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Sn as Bu3SnCl | Determination of Sb as SbH3 | Hg as MeHgCl | Hg as Hg0 |
|---|---|---|---|---|
| Linear dynamic range [ng L−1] | 50–20,000 | 75–10,000 | 20–20,000 | 20–50,000 |
| Limit of detection [ng L−1] | 16 | 25 | 6.7 | 5.2 |
| Relative standard deviation % | 3.5 | 3.8 | 3.1 | 3.3 |
| Sample | Hgtot Found Value [mg kg−1] | Hgtot Certified Value [mg kg−1] | MeHg Found Value [mg kg−1] | MeHg Certified Value [mg kg−1] | Recovery [%] |
|---|---|---|---|---|---|
| Estuarine sediment ERM CC580 | 128.7 ± 2.1 | 132 ± 3 | 0.081 ± 0.008 | 0.075 ± 0.004 | 97.5; 108 |
| Wastewater sediment | 22.5 ± 0.2 | 23.2 ± 0.3 | 0.161 ± 0.011 | n.a. | 97; - |
| Astot found value [ng mL−1] | Astot certified value [mg kg−1] | Setot found value [mg kg−1] | Setot certified value [mg kg−1] | ||
| Hard drinking water ERM-CA 011a | 10.5 ± 0.9 | 10.1 ± 0.6 | 10.4 ± 0.7 | 10.7 ± 0.7 | 104; 97 |
| 1 Influent | 2 Wastewater After Neutralization | 4 Concentrate Residue | 5 Effluent | 6 Sewage Sediment | |
|---|---|---|---|---|---|
| Analyte | Concentration/ppm | Content/mg kg−1 | |||
| Bi | 15 | n.d. | n.d. | n.d. | n.d. |
| Pb | 13.9 | 6.8 | 11.1 | 0.1 | 0.5 |
| Sb | 28.8 | 21.2 | 32.8 | 0.6 | 24 |
| Sn | 4.4 | 0.5 | 1.8 | 0.15 | 3 |
| Hg | 24.6 | 17.2 | 4.5 | 1.1 | 36 |
| Te | 0.25 | 0.1 | n.d. | 0.15 | n.d. |
| Zn | 220 | 50 | 65.5 | 1.3 | 63 |
| Cd | 30 | 29.7 | 25.8 | 1.1 | 117 |
| I | 25 | 22 | 1.5 | 0.1 | 62 |
| As | 9.4 | 2.1 | 3.1 | 0.2 | - |
| Se | 1.7 | 0.7 | 0.55 | 0.2 | - |
| Fe | 79 | 0.3 | 0.3 | n.d. | 70 |
| Mn | 106 | 38 | 36 | 0.4 | - |
| Cu | 36 | 35 | 34 | 27 | 78 |
| Cr | 45 | 28 | 40 | 0.2 | 42.5 |
| Ni | 57 | 25 | 32 | 0.3 | 34 |
| Sample | Total Metal(loid) Concentration/mg L−1 | Total VMOCs Concentration/ ng m−3 | Identified Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Volatile Hydrides Concentration/mg L−1 | |||||||||||
| Hg | Sb | Sn | As | I | Hg | Sb | Sn | As | I | ||
| Influent | 24.6 | 28.8 | 4.4 | 29.4 | 25.0 | 7200 | n.d. | n.d. | n.d. | n.d. | Me2Hg, MeHgCl, Hg |
| 23.8 | 5.6 | 2.1 | 7.4 | n.d. | Me2Hg, Hg, MeHgH, SbH3, MeSbH2, AsH3, SnH4 | ||||||
| After neutralization | 17.2 | 21.2 | 0.5 | 2.1 | 22.1 | 850 | n.d. | n.d. | n.d. | n.d. | MeHgCl, Hg |
| 15.1 | 0.10 | 0.06 | n.d. | n.d. | Me2Hg, Hg, MeHgH, SbH3, MeSbH2, SnH4 | ||||||
| Concentrated residue | 4.5 | 32.8 | 1.8 | 3.1 | 1.5 | 4700 | 60 | n.d. | n.d. | n.d. | Me2Hg, MeSbH2, MeHgCl, Hg |
| 3.8 | 7.5 | 0.2 | 0.6 | n.d. | Me2Hg, Hg, MeHgH, SbH3, MeSbH2, AsH3, SnH4 | ||||||
| Sewage sludge 1 | 29 * | 11 * | 2.8 * | 0.3 * | 54 * | 750 | n.d. | n.d. | n.d. | 250 | Me2Hg, Hg, MeHgCl, MeI |
| 1.4 * | 0.5 * | n.d. | n.d. | n.d. | MeHgCl, Hg, MeSbH2 | ||||||
| Sewage sludge 2 | 36 * | 24 * | 3 * | n.d. | 62 * | 480 | 50 | 100 | n.d. | 850 | Me2Hg, Hg, MeHgCl, MeI |
| 0.82 * | 0.91 * | 0.36 * | n.d. | 1.4 * | Me2Hg, Hg, MeHgH, MeSbH2, AsH3, | ||||||
| Effluent | 1.1 | 0.60 | 0.15 | 0.20 | 0.11 | 10 | n.d. | n.d. | n.d. | n.d. | Hg |
| 0.32 | n.d. | n.d. | n.d. | n.d. | Hg | ||||||
| Parameter | Value |
|---|---|
| Frequency | 2.45 GHz |
| Applied power | 50–150 W |
| Plasma configuration | Beenakker cavity with coaxial coupling |
| Fiber coating | Carboxen |
| Preconcentration time | 20 min |
| Desorption temperature | 150–250 °C |
| Carrier gas flow rate | 150–300 mL min−1 |
| Parameter | Value |
|---|---|
| Applied power | 1200 W |
| Height above coil | 4 mm |
| Nebulizer gas flow rate | 0.5 L min−1 |
| Plasma gas flow rate | 0.5 L min−1 |
| Cooling gas flow rate | 12 L min−1 |
| Wavelength | Hg 253.6 nm Sb 259.8 nm Sn 180 nm As 234.9 nm I 206.1 nm Si 251.6 nm |
| Wavelength | Bi 223.1 nm Cd 226.5 nm Cr 205.6 nm Cu 324.7 nm Fe 238.2 nm Mn 259.4 nm Ni 232.0 nm Pb 220.3 nm Te 214.3 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowski, K.; Truskolaska, M.; Borowska, M.; Giersz, J.; Reszke, E. A Selective and Fast Approach for Volatile Metalorganics Assaying in Wastewater. Molecules 2025, 30, 1111. https://doi.org/10.3390/molecules30051111
Jankowski K, Truskolaska M, Borowska M, Giersz J, Reszke E. A Selective and Fast Approach for Volatile Metalorganics Assaying in Wastewater. Molecules. 2025; 30(5):1111. https://doi.org/10.3390/molecules30051111
Chicago/Turabian StyleJankowski, Krzysztof, Monika Truskolaska, Magdalena Borowska, Jacek Giersz, and Edward Reszke. 2025. "A Selective and Fast Approach for Volatile Metalorganics Assaying in Wastewater" Molecules 30, no. 5: 1111. https://doi.org/10.3390/molecules30051111
APA StyleJankowski, K., Truskolaska, M., Borowska, M., Giersz, J., & Reszke, E. (2025). A Selective and Fast Approach for Volatile Metalorganics Assaying in Wastewater. Molecules, 30(5), 1111. https://doi.org/10.3390/molecules30051111






