Abstract
This review provides an overview of the synthesis, characterization and application of coordination polymers based on N,O-donor Schiff base ligands. The coordination polymers (CPs) represent a novel class of inorganic–organic hybrid materials with tunable compositions and fascinating structures. They are composed of metal ions and organic ligands. Therefore, the nature of the metal ion and type of organic ligand is the most significant factor in constructing targeted coordination polymers with the desired properties. Due to the versatile coordination modes, N,O-donor Schiff base ligands are also used to construct various CPs.
1. Introduction
In recent decades, the design and construction of coordination polymers (CPs) has undergone significant development in the domain of crystal engineering. This advancement is not only attributed to their intriguing structural topologies but also to their potential applications in areas such as catalysis [1,2,3,4,5,6], luminescence [1,7], magnetism [1,4,8,9,10], gas adsorption and separation [4,11,12], biological activity [1,4,13,14,15,16] optics [4,17,18], sensors [4,19,20,21], nanomaterials [22], photoconduction [13,23], and photoelectrochemical materials [24,25]. CPs are composed of metal ions or cluster nodes, which are linked by organic ligands. Nonetheless, the synthesis of CPs with predictable structures and specific properties remains a significant challenge. This is due to the numerous subtle factors related to the crystallization process, including type of metal ions, type of organic ligands, type on solvents pH value, temperature, reaction time, and so on. The key to constructing a CP is to design suitable organic ligands, which could be, for example, Schiff bases, due to their versatile coordination modes. The aforementioned ligands are characterized by the possession of multiple coordination sites, a property that engenders high selectivity. This facilitates their capacity to react with a variety of metal ions, predominantly transition metals (e.g., vanadium, cadmium, zinc, lead, nickel, copper, manganese, mercury etc.), and lanthanides, thereby forming mononuclear, dinuclear, multinuclear, 1D chain, and 2D and 3D network structures [18,26,27,28,29,30,31,32]. A comprehensive review of the extant literature and the CSD database [33] revealed that N,O-donor Schiff bases predominantly form discrete complexes. Much less information is available on coordination polymers. In the coordination chemistry, vanadium mainly in the high valence state is attractive due to the use of numerous vanadium complexes as potential catalytic reagents in oxidation reactions of alcohols, olefins, benzene/alkyl aromatic compounds and sulphides [34,35,36,37,38,39,40,41,42,43,44,45]. This element can interconvert among various oxidation states and readily access higher oxidation states. Additionally, vanadium displays a range of coordination numbers, possesses a strong affinity for oxygen, and can act as a Lewis acid. These characteristics facilitate its application in redox and Lewis acid-catalyzed or -promoted reactions. Vanadium complexes, when combined with oxidants such as H2O2, serve as effective oxidation catalysts [46,47,48]. They readily generate intermediate species that feature an electron-rich peroxido group, which is capable of transferring oxygen to the organic substrate. Coordination polymers of vanadium are typically insoluble in common solvents. They possess the advantage over monomer analogues of easy separation from the catalytic reaction mixture, allowing for operational flexibility and recyclability. Vanadium complexes are also valuable models for understanding the biological functions of vanadium [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. Scientists pay particular attention to the lower toxicity and harmless side effects of vanadium chelating complexes. In animals, vanadate inhibits various important metabolic enzyme systems in the liver or muscle, to utilize or accumulate glucose in the cells, while also blocking the effects of hormones that counteract insulin action [65]. Vanadium complexes with N,O-donor ligands of the Schiff base type mainly form discrete structures, while coordination polymers are much less common [33]. Furthermore, in the synthesis of vanadium coordination polymers, additional metal ions are frequently utilized, including Zn2+, Cd2+, and Na+, among others. This results in the formation of heteronuclear polymers. In contrast, ions of other metals, such as Mn2+, Ag+, Pb2+, Cd2+, and Cu2+, exhibit a greater propensity to yield homonuclear coordination polymers [33]. This review presents the synthesis, crystal structures, and potential applications of vanadium coordination polymers as well as examples of coordination polymers formed by other metal ions.
2. Synthesis of Coordination Polymers
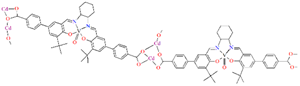
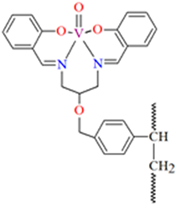
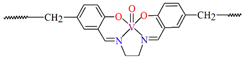
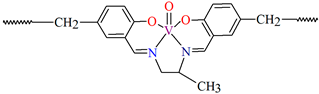
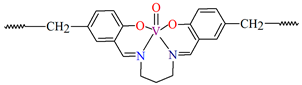
Schiff bases, characterized by structural versatility, are of great interest to researchers due to their extensive complexation range and high stability under various oxidizing and reducing conditions. Among these, the most popular are probably the salen-type N2O2, N2O4 donor ligands prepared by 1:2 condensation of the respective diamine and salicylaldehyde or salicylaldehyde derivatives. The use of N-substituted diamines led to the obtainment of tridentate N2O donor Schiff bases. It is possible to reduce these ligands to form more flexible “reduced Schiff bases”. These ligands can be used in the synthesis of coordination polymers, which have interesting applications in materials science. Unfortunately, in most cases the reaction of such ligands with metal ions does not lead to the formation of coordination polymers, but only to the formation of discrete mono-, di- or polynuclear units. Therefore, additional atoms or donor groups capable of coordinating metal ions are often introduced into such structures, examples being the ligands L8, L9, and L14 (Figure 1). For these salen-type ligands, substituents pyridine (L8), a carboxyl group (L9) and benzoic acid (L14) have been introduced into the aromatic ring, which can coordinate additional metal ions (Table 1). Another way to obtain coordination polymers of symmetric Schiff bases is the synthesis of polymeric ligands such as L15, L16, L19, L20, or L21. To obtain coordination polymers, in addition to modifying the Schiff base, additional bridging ligands, such as N3−, O2−, SCN−, CH3O−, C2H5O−, N(CN)2−, or carboxylate ions are introduced (Table 1 and Table 2). In the case of asymmetric ligands: L1, L2, L5, L6, L7, L13, L17, L22, L23, L24, L25, L27, L28, L29, L30, L31, L32, L33, L34, L35, L36, L40 (Figure 1 and Figure 2), additional bridging ligands (e.g., complexes 1, 2, 6, 16, 25–28, etc., Table 1 and Table 2) or additional donor atoms in the Schiff base structure (e.g., 29, 33, 34, 35, 37, 38, 39, etc., Table 2) are often introduced.
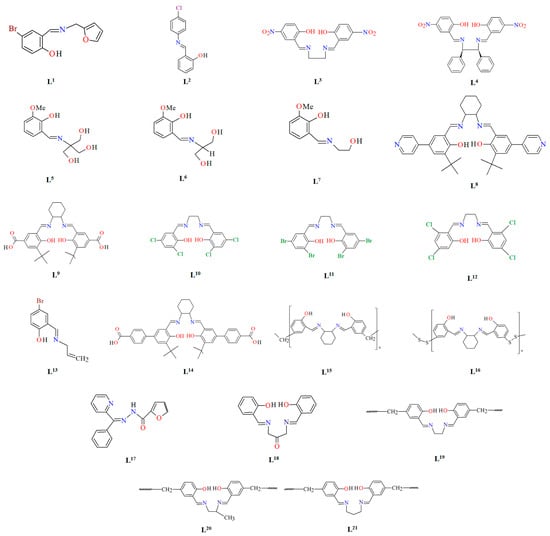
Figure 1.
Scheme of N,O-donor Schiff base ligands using for coordination of vanadium ions L1 [66], L2 [67], L3 [68], L4 [68], L5 [69], L6 [69], L7 [69], L8 [70,71,72], L9 [71,72], L10 [73], L11 [73], L12 [73], L13 [74], L14 [75], L15 [76], L16 [76], L17 [77], L18 [78], L19 [79], L20 [79], L21 [79].

Table 1.
Vanadium coordination polymers—scheme and conditions of their synthesis.

Table 2.
Coordination polymers of selected metal ions—scheme and conditions of their synthesis.
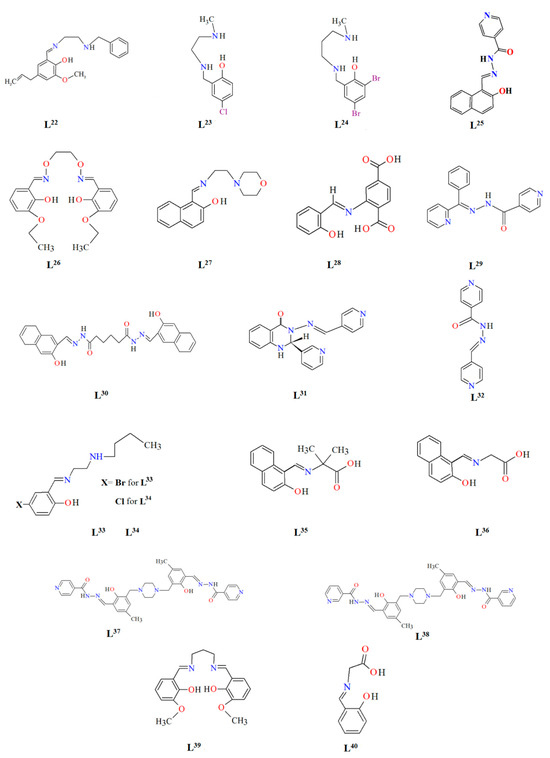
Figure 2.
Scheme of N,O-donor Schiff base ligands using for coordination of selected metal ions L22 [80], L23 [81], L24 [81], L25 [1], L26 [82], L27 [83], L28 [84], L29 [85], L30 [86], L31 [87], L32 [88], L33 [4], L34 [4], L35 [89], L36 [89], L37 [90], L38 [90], L39 [91], L40 [92].
Metal coordination polymers based on Schiff bases are mainly obtained by indirect reaction of metal ions with previously prepared N,O-donor ligands (e.g., 1, 3, 4, 13, 25, 28, 30) or in a one-pot reaction (6, 7, 5, 16, 44, 45). Compounds are obtained in different solvents, e.g., methanol, DMF, ethanol, or their mixtures (Table 1 and Table 2).
3. Crystal Structure and Properties of Vanadium Coordination Polymers
1D coordination polymer [VOL12]n (1) was obtained during reaction of N,O-bidentate Schiff base (L1 = 5-bromo-2-hydroxybenzyl-2-furyl(methyl)imine)) and VO2+ in methanol solution. 1 crystallizes in the monoclinic system. The complex is characterized by the presence of two symmetry-independent vanadium centres, which are located on a two-fold axis and have a distorted octahedral N2O4 coordination sphere. The oxygen atoms placed in the apical positions (the basal plane is formed by two nitrogen atoms and two oxygen atoms from two Schiff bases) link the metal centres to form an oxovanadium(IV) coordination polymer (Figure 3) [66].
The polynuclear oxovanadium(IV) Schiff base complex [VOL2]n 1, with TBHP as oxidant may be used as catalyst for epoxidation of cyclooctene [66].
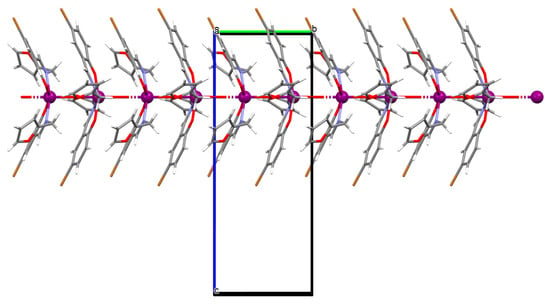
The oxygen-bridging coordination polymer {[(VO)2(L2)4]}n (2) was obtained during reaction of VO2+ and N-(4-chlorophenyl)-salicylaldimine (L2) in the presence of Na[O2CMe] (ethanol as solvent was used). 2 crystalizes in the triclinic system, in the form of the needles (the monomeric compound crystalizes in the prism form). In crystal 2, the monomeric units are connected through oxygen atoms V=O···V=O forming a chain along the c-axis (Figure 4). The magnetic measurements of the oxygen bridging indicated an occurrence of ferromagnetic interaction between metal centres. Conversely, no such interaction was observed in the monomeric compound [67].
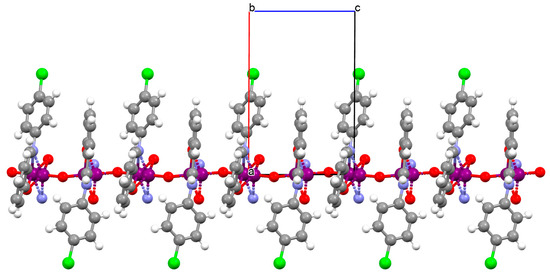
Figure 4.
Fragment of crystal structure showing formation of a 1D chain of 2 (Table 1) viewed along the b-axis [33,67,93].
[VO(L3)] 2H2O (3) and [VO(L4)] 0.5H2O (4) were obtained during reaction of tetradentate N,N,O,O-Schiff bases N,N′-di-5-nitrosalicylidene-1, 2-ethanediamine (H2(5-NO2salen = L3) or N,N′-di-5-nitrosalicylidene-(R,S)(S,R)-1, 2-diphenyl-1,2-ethanediamine (H2(5-NO2sal-meso-stien = L4) and VO2+ in methanol solution. 4 crystalizes in the monoclinic system. Complex 4 forms a polymeric structure with a distorted octahedral coordination geometry around the metal centres. Two azomethine nitrogens and the phenoxide oxygen of the Schiff base ligand and two oxo ions surround each vanadium(IV) ion. Oxo ions assemble VIV-Schiff base units into 1D chains running along to the b-axis (Figure 5). The results of the spectroscopic and magnetic studies indicate that the linear chain structures 3 and 4 are destroyed during the grinding process, resulting in the formation of different fragments of the polymeric chains. The mechanochemical reaction that occurred is hypothesized to be the cleavage of the ···V–O···V–O···bonds [68].
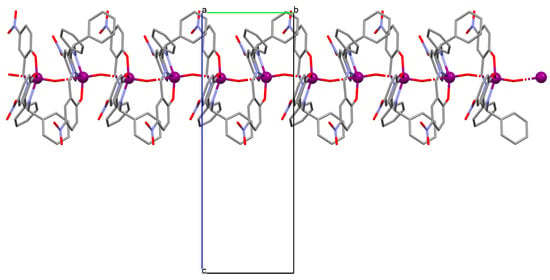
Figure 5.
Fragment of crystal structure showing formation of a 1D chain of 4 (Table 1) viewed along the b-axis [33,68,93]. Solvent molecules are omitted for clarity.
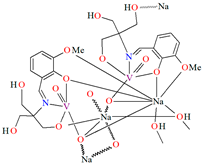
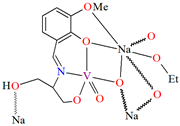
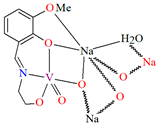
{[(VO2)2(L5)2]Na2(CH3OH)2}n (5), {[(VO2)(L6)]Na(C2H5OH)}n (6), and {[(VO2)(L7)(H2O)]Na}n (7) were obtained during reaction of the respective Schiff bases (formed in situ from o-vanillin and tromethamine (L5), 2-amino-1,3-propanediol (L6), and ethanolamine (L7)) and VO2+ and sodium hydroxide in the methanol (5) or ethanol (6, 7) solution. 5 crystallizes in the monoclinic system and its molecular structure is composed of [(VO2)2(L5)2]Na2(CH3OH)2 units, which form a 2D network (Figure 6). In 5 the coordination geometry around the two crystallographically independent vanadium centres is square pyramidal and consists of two doubly bonded oxido groups and one tridentate Schiff base ligand. The sodium ions are eight- and six-coordinated with triangular dodecahedral and distorted octahedral geometries, respectively. 6 crystallizes in the monoclinic system. As above, the coordination polyhedron around each vanadium ion can be described as a square pyramid, whereas the coordination polyhedron around each six-coordinated sodium ion adopts the geometry of a distorted trigonal prism. Schiff bases bind vanadium and sodium ions in infinite 1D chains (Figure 7a). 7 crystallizes in the orthorhombic system. In the crystal 7 the double deprotonated Schiff base acts as a tetradentate N,O,O,O-donor ligand and each vanadium ion has a square pyramidal geometry, while the coordination polyhedron around each sodium ion has the geometry of a distorted trigonal prism. A water molecule bridges adjacent sodium ions of {[(VO2)(L7)]Na(C2H5OH)} units to form a final 1D polymeric structure (Figure 7b). The obtained compounds may be potentially investigated as anti-diabetic vanadodrugs [69].
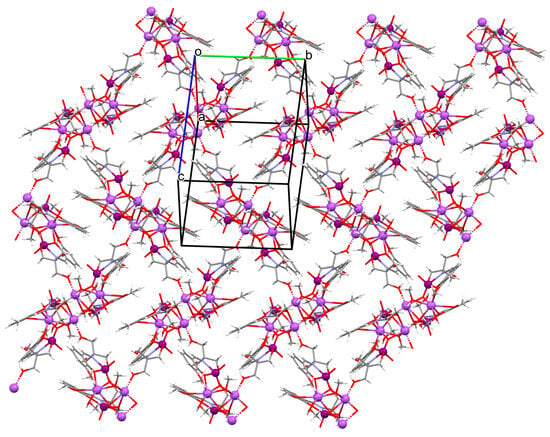
Figure 6.
Fragment of crystal structure showing formation of a 2D network of 5 (Table 1) [33,69,93].
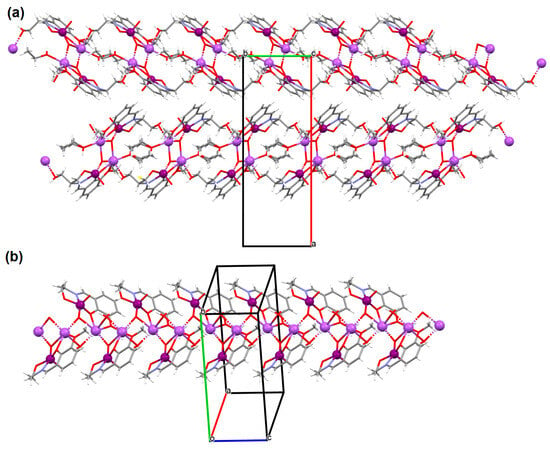
Figure 7.
Fragment of crystal structure showing formation of 1D chain of (a) 6, (b) 7 (Table 1) [33,69,93].
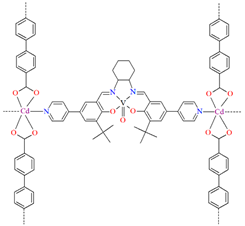
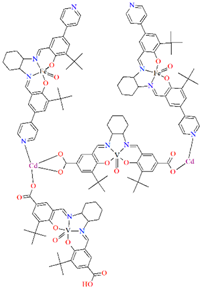
A homochiral vanadium–salen-based cadmium BPDC MOF (8) was obtained via in situ synthesis under solvothermal conditions during the reaction of the chiral salen ligand L8 = (R,R)-(−)-1,2-cyclohexanediamino-N,N′-bis(3-tert-butyl-5-(4-pyridyl)salicylidene), VO2+, CdII and biphenyl-4,4′-dicarboxylic acid (BPDC) in the presence of DMF/EtOH/H2O. 8 crystallizes in the orthorhombic system. The asymmetric unit contains two CdII ions, two V–salen units (VOL), and two biphenyldicarboxylate (BPDC) ligands.
The vanadium(II) ion is bound by the O,N,N,O donor atoms of the Schiff base and the coordination sphere is completed by an oxygen atom. The cadmium(II) ions are surrounded by five oxygen atoms of three carboxylate groups and two pyridine nitrogen atoms of two Schiff bases, which result formation. The BPDC ligand bridges two cadmium ions and results in the formation of a 3D polymeric structure (Figure 8) [70].
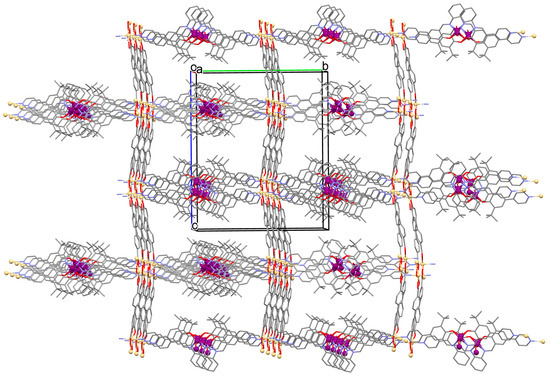
Figure 8.
Part of crystal structure of 3D coordination polymer of 8 (Table 1) viewed along the a-axis [33,70,93]. Hydrogen atoms are omitted for clarity.
The BET surface area of 8 is equal to 574 m2 g−1, and 1 has high H2 adsorption capacity (1.05 wt% at 77 K, 1 bar) and CO2 uptake (51 cm3 g−1 at 273 K, 1 bar), so it can potentially be used for the gas storage and gas separation. In addition, 8 may have a potential application in solvent-free cyanosilylation catalysis [70].
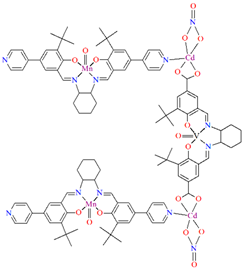
Two chiral porous metallosalen-based binary MOFs [Cd2(FeL8)2(VOL9)2](DMF)2(MeOH)3(H2O)4 (9) (Figure 9) and [Cd2(MnL8)2(VOL9)]·(DMF)(EtOH)2(H2O)3 (10) were obtained during reaction of CdII, VO(H2L9) and FeL8(OAc) in DMF/MeOH or CdII, VO(H2L9) and MnL8Cl in DMF/EtOH. 9 crystallizes in the orthorhombic system. The six-coordinated CdII centres 9 adopt a distorted octahedral coordination geometry, and their coordination sphere consists of four oxygen atoms derived from the carboxylate groups of three VOL2 units and two pyridyl nitrogen atoms from two FeL8 units. 10 crystallizes in the orthorhombic system. The coordination sphere of the seven-coordinated CdII centres 10 is formed by five oxygen atoms (three O atoms of carboxylate group of two VOL2 units, two O atoms of the chelating nitrate group) and two pyridyl nitrogen atoms of two MnL1 units. The geometry around each CdII centre can be described as a pentagonal bipyramid [71].
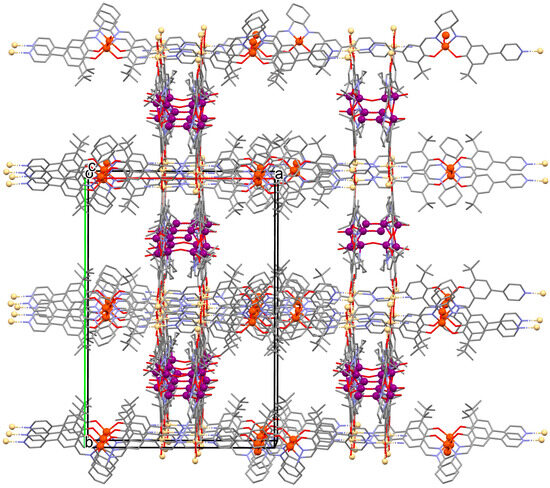
Figure 9.
Part of crystal structure of 3D coordination polymer of 9 (Table 1) [33,71,93]. Solvent molecules and hydrogen atoms are omitted for clarity.
Compounds 9 and 10 as catalysts have been demonstrated to be highly effective in a range of reactions, including asymmetric sequential alkene epoxidation and epoxide ring-opening reactions. In these reactions, the enantioselectivity achieved has been reported to reach a level of up to 99%, making them catalysts of significant interest [71].
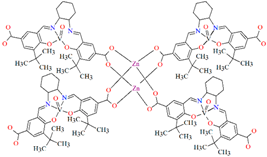
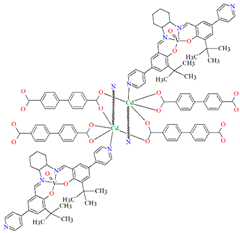
[Zn2(VOL9)2]·DMA H2O (11) (where L8 = deprotonated salen dicarboxylate ligand) and [Cd2(VOL8)2(BPDC)2]·4DMF·2H2O (12) (where L2 = deprotonated salen dipyridine ligand) were obtained during reaction of [VO(H2L9)] and ZnII or [VOL8], biphenyl-4,4′-dicarboxylic acid (H2BPDC), and CdII in the mixture of DMA/MeOH/H2O or DMF/MeOH, respectively. 11 crystallizes in the triclinic system. The five-coordinated V ion in the VOL9 core adopts a square-pyramidal geometry with the N,N,O,O atoms of the Schiff base ligand L9 in the equatorial position and one double-bonded O atom in the apical position. In crystal 11, the bidentate carboxylate groups of the {VOL9} cores connect ZnII ions forming {Zn2(O2C)4} units that are next bridged by exo-tetradentate {VOL9} units resulting in the formation of a 2D layered framework (Figure 10a). Such 2D structures are arranged in space in such a way that they generate a 3D lamellar structure with open channels along the a-axis. Complex 12 crystallizes in the orthorhombic system. The polyhedral formed by donor atoms around the V centre in VOL2 unit may be also described as a square pyramid. In crystal 12, six-coordinated CdII ions are linked by two bidentate carboxylate groups of two BPDC resulting in 2D layer structure. Adjacent 2D layers are bound into a 3D structure (Figure 10b) by cadmium ions linked by pyridyl nitrogen atoms of {VOL8} units. Following the oxidation of VIV to VV, it was determined that the compounds may have been potentially used as highly effective, recyclable, and reusable as catalysts for the asymmetric cyanosilylation of aldehydes [72].
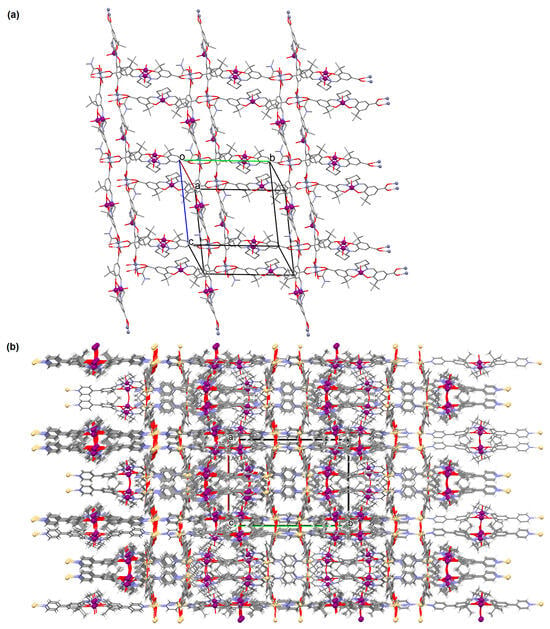
Figure 10.
The coordination polymers: (a) 2D layered framework of 11; (b) 3D framework of 12 (Table 1) [33,72,93]. Solvent molecules and hydrogen atoms are omitted for clarity.
[VO(L10)] (13), [VO(L11)] (14), and [VO(L12)] (15) were obtained during reaction of 3,3′,5,5′-tetrachloro-,3,3′,5,5′-tetrabromo- and 4,4′,6,6′-tetrachlorosalen derivatives with VO2+ in methanol. 13 and 14 are isomorphous and crystallize in the orthorhombic system. The coordination environment around the vanadium(IV) centres consists of O,N,N,O do-nor atoms of the Schiff base ligand and two oxygen ions. The oxygen ions link the VIV ions into chains running along the a-axis (Figure 11a,b). 15 crystallizes in the orthorhombic system. The coordination environment is similar to the above complexes, but the chains are formed along the [010] direction, and the vanadium ions are disordered with occupancy factors of 0.67/0.33 (Figure 11c). The presence of ferromagnetic exchange interaction between the VIV centres was observed [73].
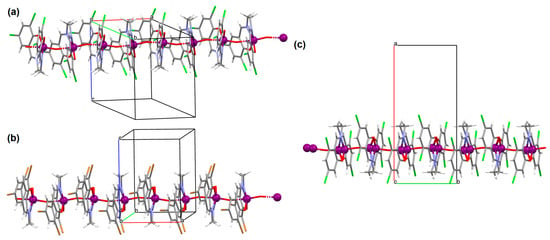
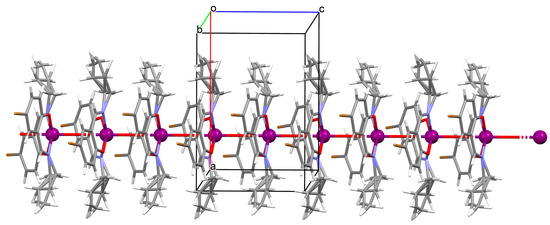
1D coordination polymer [VO(L13)2]n (16) (where L13 = 5-bromo-2-((allylimino)methyl)phenolate)) was obtained in methanol in a one pot reaction using an allylamine, 5-bromo salicylaldehyde and VO2+. 16 crystallizes in the orthorhombic system. A distorted octahedral geometry is observed around the metal centre VIV formed by O,N,N,O-donor atoms of Schiff base ligand and two oxygen ions. In crystal 16, the metal ions are linked through the oxygen atoms, resulting in the formation of 1D chains running parallel to the c-axis (Figure 12) [74].
3D chiral porous framework [Cd2(VOL14)2]·5H2O (17) was synthesized during reaction of CdII and VO(H2L14) in a solution of DMF. 17 crystallizes in the orthorhombic system. In 17, the fundamental structural element is constituted by infinite Cd-O-C chains that are connected by [VOL14] units. The coordination environment surrounding each vanadium ion is characterized by a square-pyramid geometry, with the equatorial plane occupied by the N,N,O,O atoms of a Schiff base and the apical position occupied by one double bond oxygen atom. In contrast, the coordination environment surrounding a cadmium ion adopts a distorted octahedral geometry. The oxidation of VIV to VV VO-MOF showed enhanced stereoselectivity and comparable activity in the cyanation of aldehydes. 17 can be easily recycled and reused without significant loss of catalytic activity and enantioselectivity [75].
In the case of polymeric Schiffa bases L15, L16, L19, L20, or L21 [76,79], the coordination of metal ions has not resulted in monocrystals, and there are no structures of such coordination polymers. However, these compounds (18, 19, 22–24) show interesting catalytic properties that may be of application, as is often observed for other vanadium coordination polymers (20, 21) [77,78].
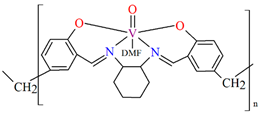
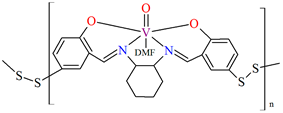
The oxidation of styrene with tert-butylhydroperoxide (TBHP) as oxidant catalyzed by coordination polymers [–CH2{VO(sal-dach)·DMF}–]n (18) and [–S2{VO(sal-dach)·DMF}–]n (19) results in formation of styrene oxide, 1-phenylethane-1,2-diol, benzaldehyde, benzoic acid along with some unidentified products. Reaction conditions: styrene (10 mmol); catalyst (equivalent to 0.032 mmol of repeating unit); acetonitrile (20 mL); temperature (75 °C) (Figure 13a). The oxidation of cyclohexene catalyzed by [–CH2{VO(sal-dach)·DMF}–]n (18) and [–S2{VO(sal-dach)·DMF}–]n (19) afforded various oxidation products, i.e., cyclohexeneoxide, 2-cyclohexene-1-ol,cyclohexane-1,2-diol, and 2-cyclohexene-1-one. Reaction conditions: cyclohexene (10 mmol); catalyst (0.032 mmol of repeating unit); acetonitrile (20 mL); temperature (75 °C) (Figure 13b). The oxidation of trans-stilbene TBHP as oxidant catalyzed by [–CH2{VO(sal-dach)·DMF}–]n (18) and [–S2{VO(sal-dach)·DMF}–]n (19) gives mainly stilbeneoxide, benzaldehyde, and 1,2-diphenylethanedione (benzil) as oxidation products (Figure 13c). Reaction conditions: trans-stilbene (5 mmol); catalyst (0.032 mmol of repeating unit); acetonitrile (20 mL); temperature (75 °C) [76].
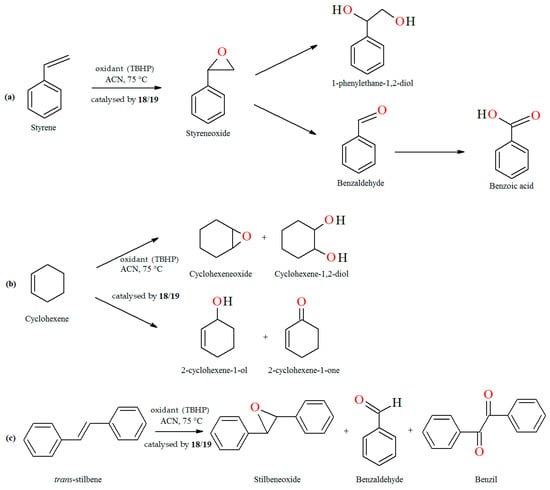
Figure 13.
The oxidation reaction of (a) styrene; (b) cyclohexene; (c) trans-stilbene catalyzed by 18 and 19. Adapted from reference [76].
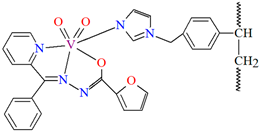
The oxidation of isoeugenol in the presence of aqueous H2O2 as oxidant (Figure 14), catalyzed by polymer-supported complex PS-im[VVO2(L17)] (20) gave dehydrodiisoeugenol, vanillic acid, and vanillin. Reaction conditions: isoeugenol (5 mmol); 30% H2O2 (10 mmol); catalyst PS-im[VVO2(L17)] (0.010–0.030); acetonitrile (7 mL); temperature (80 °C) [77].
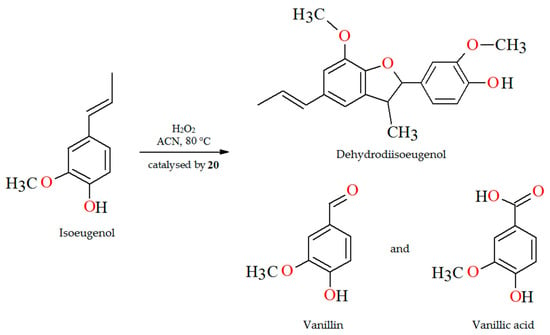
Figure 14.
Scheme of the oxidation of isoeugenol catalyzed by 20. Adapted from reference [77].
The oxidation of pyrogallol with H2O2 as oxidant catalyzed by polymer-supported complex PS-[VIVO(L18)] (21) results in the formation of purpurogallin (Figure 15). Reaction conditions: pyrogallol (25 mmol) in phosphate buffer (pH 7), 30% H2O2 (25 mmol), catalyst PS-[VIVO(L18)] (0.005–0.020 g), temperature (25 °C) [78].
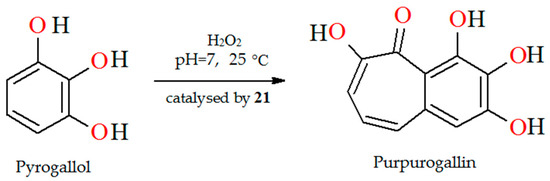
Figure 15.
Peroxidase like oxidation of pyrogallol to purpurogallin catalyzed by 21. Adapted from reference [78].
The liquid-phase catalytic hydroxylation of phenol with H2O2 as oxidant catalyzed by oxovanadium(IV) polymeric complexes [–CH2{VO(salen)}–]n (22), [–CH2{VO(sal-1,2-pn)}–]n (23) and [–CH2{VO(sal-1,3-pn)}–]n (24), respectively, leads to catechol and hydroquinone as products of oxidation (Figure 16). Reaction conditions: phenol (0.05 mol); 30% aqueous H2O2 (0.05 mol); acetonitrile (2 mL); catalyst 22, 23, and 24 (0.01 g); temperature (80 °C) [79].
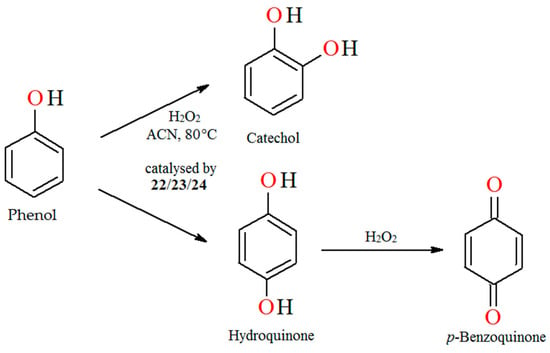
Figure 16.
Oxidation of phenol catalyzed by 22–24. Adapted from reference [79].
4. Crystal Structure and Properties of Selected Metal Ion Coordination Polymers
[Cd2(L22)2(SCN)2]n (25) and [Cd2(L22)2(N(CN)2)2]n (26) were prepared at ambient temperature by direct reaction of methanolic solution of CdII and 4-allyl-2-(((2-(benzylamino)ethyl)imino)methyl)-6-methoxyphenol L22 = C20H24N2O2 in the presence of SCN−/N(CN)2− (stoichiometric ratio 1:1:1). 25 and 26 crystallize in the monoclinic system. The distorted octahedral geometry around the metal centre CdII is formed by two bridged phenoxido oxygens, an imine and an amine nitrogen of the deprotonated Schiff base L22, and by nitrogen and sulphur atoms of two μ1,3 bridged thiocyanate ions 25/nitrogen atoms of two μ1,5 bridged dicyanamide ions 26. In the crystal structure of the coordination polymers 25 and 26 bridged thiocyanate/dicyanamide ions link neighbouring units [Cd2(L)2]2+ to form 1D chains. In addition, the units of each layer are linked together again by another μ1,3-bridged thiocyanate 25 μ1,5-bridged dicyanamide 26 ions, respectively, which creates 2D architecture (Figure 17) [80].
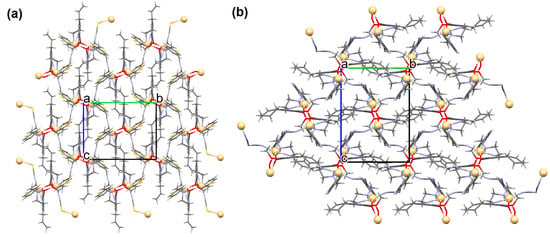
Figure 17.
Fragment of crystal structure of cadmium(II) 2D coordination polymers: (a) 25; (b) 26 (Table 2) [33,80,93].
[Cd2(L22)2(SCN)2]n 25 and [Cd2(L22)2(N(CN)2)2]n 26 may be used for monitoring toxic 2,4,6-trinitrophenol (TNP) in low detection threshold [80].
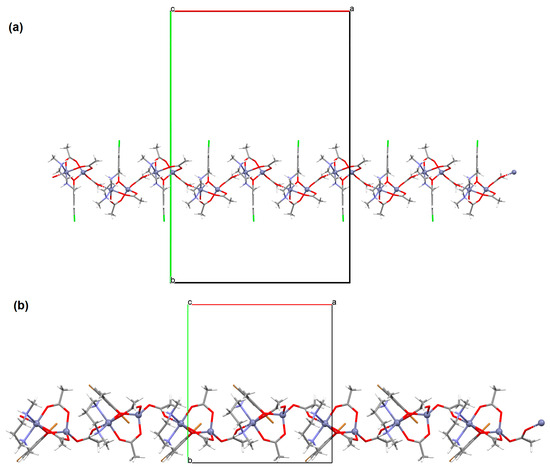
[Zn2L23(μ-OAc)3]n·H2O (27) and [Zn2L24(μ-OAc)3]n (28) forming 1D chains (Figure 18) were synthesized during reaction of ZnII and tridentate N,N,O reduced Schiff base ligands (stoichiometric ratio 2:1) L23 {4-chloro-2-(((2-(methylamino)ethyl)amino)methyl)phenol} and L24 {2,4-dibromo-6-(((3-(methylamino)propyl)amino)methyl)phenol}, respectively, in methanol. 27 and 28 crystalize in orthorhombic system. In both compounds, the coordination number of zinc ions is equal to 6 and 4, respectively, with the distorted octahedral and the distorted tetrahedral geometry around the metal centres. The six-coordinated ZnII is equatorially coordinated by two amine nitrogen, one bridging phenoxo oxygen atom of an N,N,O-donor ligand, and one oxygen atom of bridging acetate ion, and axially it is coordinated by two oxygen atoms of another bridging acetate. The four-coordinated ZnII is surrounded by one phenoxo oxygen atom of N,N,O-donor ligand and three oxygen atoms of different bridging acetate ions. The synthesized coordination polymers could potentially be investigated as compounds used in the photocatalytic degradation of various organic dyes, in the bio-mimetic catalysis, for producing a variety of optoelectronic devices and as a sensor for the detection of various nitro-aromatic explosives [81].
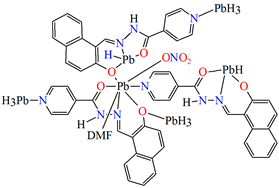
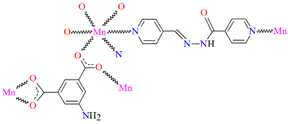
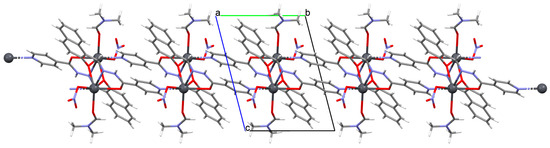
{[Pb(L25)NO3DMF]}n (29) (where L25 = anion of isonicotinic acid (2-hydroxy-naphthalen-1-ylmethylene)-hydrazide) was obtained during reaction of PbII and N,N,O,O-donor Schiff base (isonicotinic acid (L25) dissolved in the mixture MeOH/DMF. 29 crystalizes in the triclinic system. A seven-coordinated PbII is surrounded by five oxygen and two nitrogen atoms that form a distorted pentagonal bipyramidal geometry around the metal centre. Each Schiff base coordinates three metal ions leading the formation of a 1D looped chain running along [010] (Figure 19). Compound 29 indicates the n-type semiconducting behaviour, as well as the stability against photo corrosion, so it may be potentially considered as the next generation of energy materials [1].
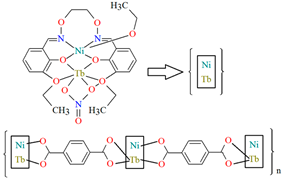
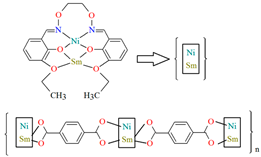
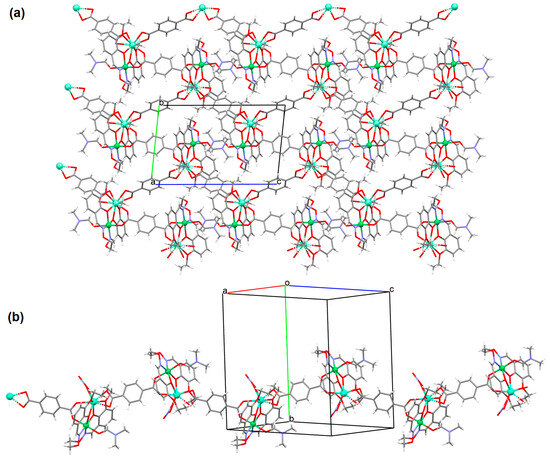
The heteronuclear coordination polymers 2∞[Ni(L26)Sm(bdc)1.5]CH3OH (30) and ∞[Ni(L26)Tb(NO3)(DMF)(bdc)] (31) (where bdc = anion of terephthalic acid, L26 = anion of 3-EtO salamo) were synthesized during reaction of methanolic 30/ethanolic 31 solution of NiII and SmIII/TbIII salts with salamo-like bisoxime (dissolved in trichloromethane) and terephthalic acid H2bdc (dissolved in DMF). 30 crystallizes in the triclinic system whereas 31 crystallizes the monoclinic system. In crystals 30 and 31 [NiII(L26)SmIII]/[Ni(L26)Tb(NO3)] units are connected by exodentate (bdc)2− ions forming 2D 30 and 1D 31 chain coordination polymers (Figure 20). The fluorescence intensities of 30 and 31 are lower than those of the free salamo-like bisoxime L26, indicating that NiII/LnIII ions influence the changes in the fluorescence properties of the ligand [82].
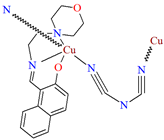
[Cu(L27)(μ1,5-dca)]n an end-to-end dicyanamide bridged (dca) coordination polymer 32 (Figure 21) was obtained during reaction of a naphthalene-based N,N,O-Schiff base blocking ligand and CuII ions. 32 crystallizes in the monoclinic system in which a five-coordinated CuII is coordinated equatorially by two nitrogen and one oxygen atoms of the tridentate Schiff base and one nitrogen atom of the dicyanamide ion and the apical position is occupied by one nitrogen atom of another bridged dicyanamide ion. The coordination polyhedron has classical distorted square pyramidal geometry [83].
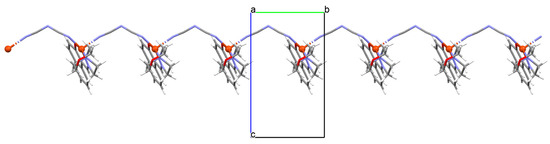
Figure 21.
Structure of 32 (Table 2) showing formation of 1D coordination polymer viewed along the a-axis [33,83,93].
3D heterometallic coordination polymer [CuNa(L28)(H2O)(OH)]n (33) was obtained by using the tridentate N,O,O-donor Schiff base (2-[(E)-(2-hydroxyphenyl)methyleneamino]terephthalic acid), CuII and NaI ions. 33 crystallizes in the monoclinic system. The penta-coordinated CuII ion adopts a distorted square pyramidal geometry with one nitrogen and two oxygen atoms of the Schiff base and one oxygen atom of another N,O,O-donor ligand in the equatorial position and one oxygen atom of the hydroxide group in the apical position. The four-coordinated NaI ion adopts a tetrahedral geometry with two carboxylate oxygen atoms, one hydroxy oxygen and one water oxygen atom. In the crystal CuII and NaI centres are connected by a bridging hydroxide group. Each Schiff base ligand adopts a tetradental coordination mode binding two copper(II) and two sodium ions. The presence of additional ligands bridging the metal ions, i.e., water molecules and hydroxyl ions, results in the formation of a 3D network (Figure 22). The antibacterial efficacy of 33 was studied in relation to both Gram-positive and Gram-negative strains in order to check if 33 can be potential used as drug and/or antimicrobial agent [84].
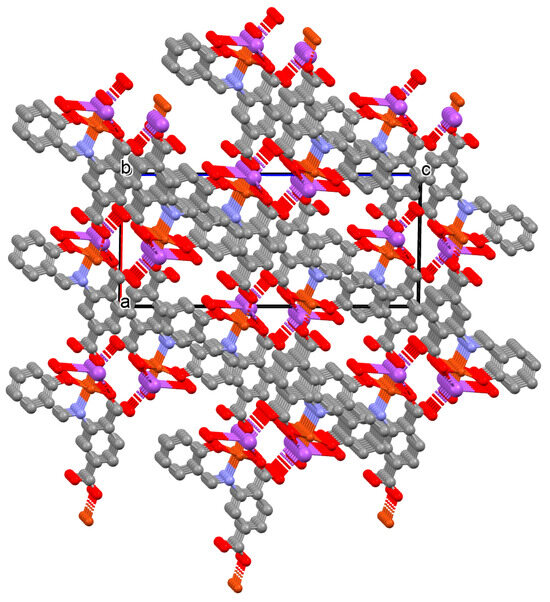
Figure 22.
Three-dimensional coordination polymer of 33 (Table 2) viewed along the b-axis [33,84,93]. Hydrogen atoms are omitted for clarity.
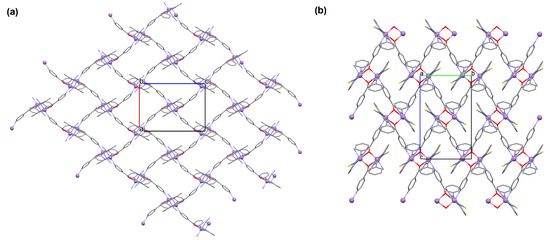
[Mn2(L29)2(μ-N3)2]n (34) 2D coordination polymer (Figure 23a) with azide bridged dinuclear unit was synthesized during reaction of the isonicotinhydrazide ligand (L29) with MnII and sodium azide in methanol. During the synthesis of [Mn2(L29)2(NCS)2]n (35), a 2D coordination polymer (Figure 23b), instead of sodium azide, potassium thiocyanate was used. 34 crystalizes in the orthorhombic system whereas 35 crystalizes in the monoclinic system. A ditopic isonicotinhydrazone-based tetradentate N,N,O-donor ligand coordinates to the MnII centre in the enolic form (=N–N=C–O). The magnetic behaviours exhibited by the dimeric samples demonstrate a pronounced intradimer exchange of antiferromagnetic nature for 34, while 35 exhibits a ferromagnetic coupling between metal centres [85].
[Mn2(L30)(μ-OCH3)2(OHCH3)2]n (36) 1D coordination polymer was obtained from the hexadentate dihydrazoneSchiff base (L30, bis[(2-hydroxynaphthalen-1-yl)methylene]-adipohydrazide) and MnII in methanol solution. 36 is a rare example of the MnIII coordination polymer (during synthesis the MnII is oxidized to MnIII by air) with methoxy-bridged groups. The coordination polyhedron built by the MnIII centres exhibits Jahn–Teller distorted octahedral arrangement. Each Schiff base binds two manganese(II) ions and acts as a hexadentate ligand coordinating a single metal centre via Ophenoxide, Nazomethine, Ocarbonyl-donor atoms. The same metal centres are also bridged by two oxygen atoms of methoxide ions. This gives rise to Mn2(μ-O)2 metallocycles. This coordination of the manganese(II) ions by the Schiff base and the methoxide ion leads to the formation of chains that run along the c-axis (Figure 24). The magnetic measurements evident the presence of a weak antiferromagnetic interaction between MnIII centres. It was also demonstrated that 36 exhibited catalase-like activity in the disproportionation of H2O2 [86].
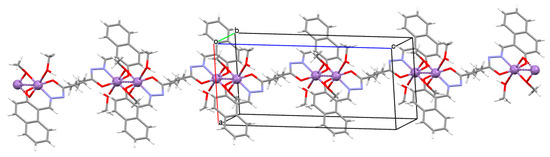
Figure 24.
Part of crystal structure showing formation of 1D coordination polymer of 36 (Table 2) [33,86,93].
Lin et al. used a racemic bispyridyl ligand (L31) to synthesize four coordination polymers of cadmium(II). To synthesize the complexes, they used four different cadmium(II) salts (chloride, iodide, bromide, and nitrate) and demonstrated the influence of the counterion on the synthesis and structure of the obtained coordination polymers {[Cd(L31)2Cl2]·2DMF}n (37), [Cd(L31)I2]n (38), {[Cd(L31)2Br2]·4H2O}n (39), and {[Cd(L31)2(H2O)2](NO3)2·2CH3OH·8H2O}n (40). All compounds crystalized in the monoclinic system in different space groups. In complex 37, the metal ions are coordinated by four nitrogens of different Schiff bases and two chloride ions, which formed an octahedral environment around CdII. Adjacent cadmium(II) ions are linked by two ligand molecules to form 24-membered metallamacrocycles arranged in a chain along [101] direction (Figure 25a). The change of the counterion from chloride to iodide (complex 38) also resulted in a 1D-corridinated polymer, but of a different type, i.e., zig-zag chains (Figure 25b). The coordination polyhedron around the CdII ion takes the form of a distorted tetrahedron and consists of by two pyridyl nitrogen atoms from different Schiff bases and two iodide ions. In the case of compound 39, metal ions are surrounded via four Schiff base ligands and two water molecules. As for 37, the coordination environment around the CdII ions is a slightly distorted octahedron, but the way the metal ions are bound and arranged results in two-dimensional coordination polymers (Figure 26a). In the last coordination polymer (40), the coordination environment around the central ion is also a distorted octahedron. The CdII ions are coordinated by the pyridyl nitrogen of the four Schiff bases and two water molecules. This coordination mode of the ligands with cadmium(II) ions results in the formation of a 3D framework (Figure 26b). The synthesized polymers were tested for sorption for iodine and dyes. The study showed that these compounds can be used to remove iodine and dyes from wastewater, e.g., complex 38 displayed selective adsorption for crystal violet and, complex 40 showed preferential adsorption for direct yellow in the mixed solution of these two dyes [87].
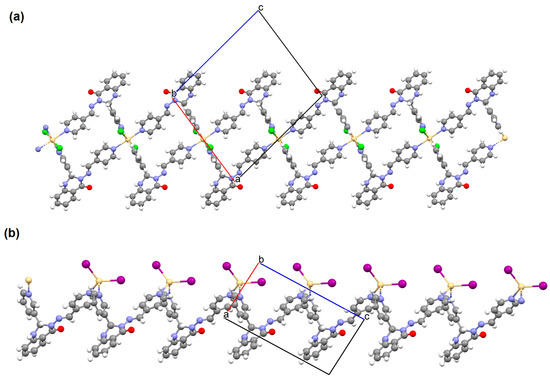
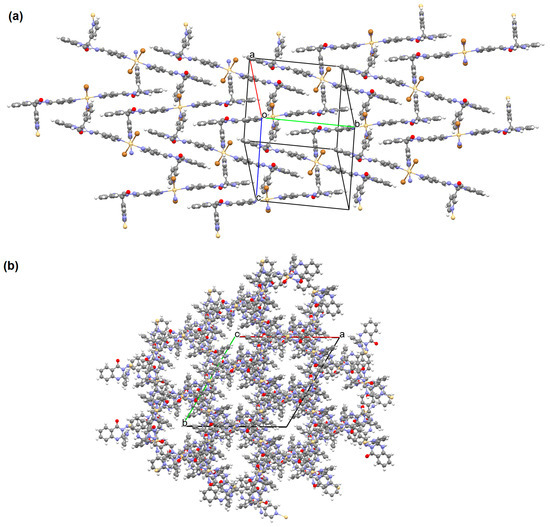
Shit and co-workers reported the synthesis of MnII 3D coordination polymer with pyridine-4-carboxaldehyde isonicotinoyl hydrazine (L32) and 5-aminoisophthalic acid (H2aisp), [Mn2(aisp)3(L32)2(solvent)]n (41). The compound crystallizes in triclinic system. The distorted octahedral environment observed around manganese(II) ions is formed by three carboxylate ions and two pyridyl nitrogens of two Schiff bases. The asip2− ligand bridges two metal ions into a 2D framework. The L32 also acts as a bridging ligand, which results in the formation of a 3D network present in Figure 27 [88].
41 shows significant cytotoxicity against some cancer cell lines (PC3, HeLa, MDA-MB-231, and A549). The complex is particularly effective in HeLa cell death through induced apoptosis. The compound was also tested for use as a functional matrix for the encapsulation of the drug, i.e., diclofenac sodium. Studies have shown that at low pH, only a small amount of drug is released (less than 10%), while at pH 7.4 (as observed in the intestinal environment), a significant increase in drug release was observed, reaching approximately 87% over 30 h. These results show the promising potential of 41 as an oral drug carrier. Moreover, this coordination polymer displays intriguing electrical and optical properties, which can be utilized in the fabrication of energy-saving electronic devices [88].
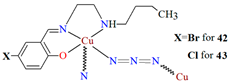
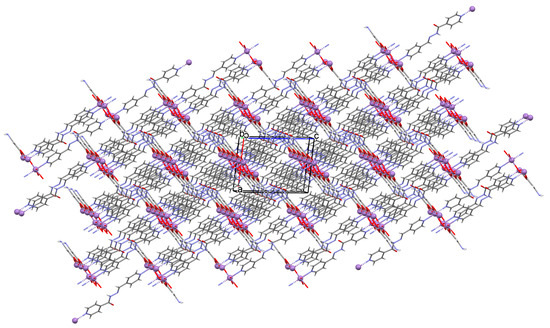
Other examples of metal-assembled Schiff base coordination polymers include a two isostructural copper complexes 42 and 43 with Schiff bases derived from N-butylethyldiamine. The reaction of copper(II) acetate with 4-bromo-2-(((2-(butylamino)ethyl)imino)methyl)phenol (L33)/4-chloro-2-(((2-(butylamino)ethyl)imino)methyl)phenol (L34) in the presence of NaN3 in methanol medium leads to the formation of zig-zag one-dimensional (1D) chains (Figure 28). In both complexes, copper(II) ions are surrounded by N,N,O atoms of a Schiff base molecule and two nitrogen atoms of azide ions, leading to the formation of a distorted square pyramidal coordination geometry around metal centres. The complexes show moderate catecholase-like activity as well as interesting optoelectronic properties [4].
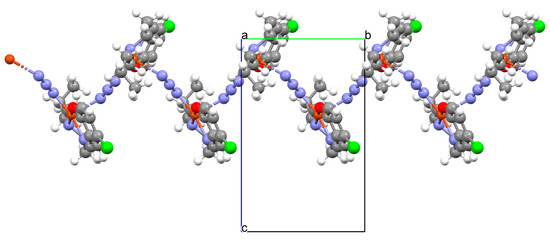
Figure 28.
Fragment of crystal structure showing formation of a zig-zag chain of 43 (Table 2) viewed along the a-axis [4,33,93].
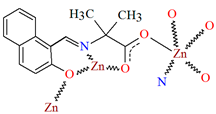
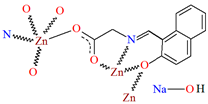
Karakousi et al. reported the formation of 2D and 3D zinc coordination polymers that emit blue light. The catena-((μ-N-[(2-oxynaphthalen-1-yl)methylidene]-2-methylalaninato)-zinc), {[ZnL35]}2n, (44), have been synthesized during reaction of zinc nitrate, 2-OH-1-napthaldehyde, and 2-aminoisobutyric acid. The complex crystalizes in monoclinic system. Zinc(II) ions are linked by three Schiff bases, which coordinate the metal centre via an azomethine nitrogen, two carboxylate and two phenolate oxygen atoms. The coordination polyhedron around ZnII adopts a slightly distorted square-pyramidal geometry. Each Schiff base ligand binds three metal ions to form a 2D coordination polymer parallel to the ab plane. (Figure 29). The other coordination polymer, catena-((μ-N-[(2-oxynaphthalen-1-yl)methylidene]glycinato)-zinc methanol solvate), {[Zn(L36)2]∙0.33MeOH}3n (45), was synthesized in a similar way to the previous one, but glycine was used as the reagent instead of 2-aminoisobutyric acid. This complex crystalizes in the trigonal system, where zinc(II) ions are coordinated in similar way to {[ZnL35]}2n, i.e., by an azomethine nitrogen, and two carboxylate and two phenolate oxygen atoms of three Schiff base ligands. A different coordination mode of the Schiff base L36 (anti, anti-η1:η1:μ) than that observed for L35 (syn, anti-η1:η1:μ) is probably responsible for the different dimensionality, i.e., 3D framework with channels running along the c-axis (Figure 30). Both coordination polymers emit light at a wavelength of around 435 nm, and such luminescent materials can be used as sensors [89].
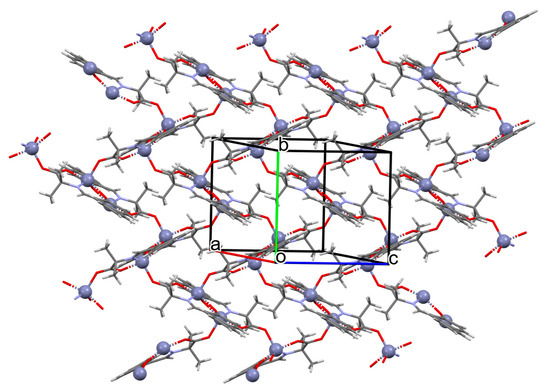
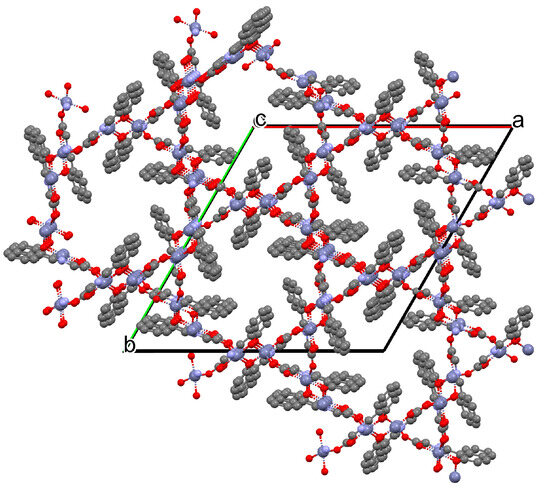
Figure 30.
Fragment of crystal structure showing a 3D framework of 45 (Table 2) viewed along the c-axis [33,89,93]. Hydrogen atoms and solvent molecules are omitted.
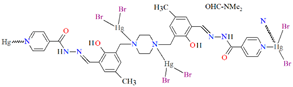
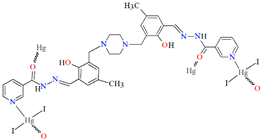
Huang and co-workers investigated complexes of two bispyridyl acylhydrazone Schiff bases (L37 and L38) with HgII ion. The first coordination polymer, {[Hg3L37Br6]⋅4DMF}n (46) crystalizes in a monoclinic system, where each mercury(II) ion is four-coordinated, and polyhedrons around the metal centres are distorted tetrahedrons. There are two types of mercury(II) ions. The first one is linked by two pyridyl nitrogen atoms from Schiff bases and two bromide ions. The second one is surrounded by two bromide ions, a piperazine nitrogen, and a phenolic oxygen atom of the Schiff base. The bridging nature of the Schiff base leads to the formation of zig-zag chains along the b-axis (Figure 31). The second coordination polymer, {[Hg2(L38)2I4]⋅2DMF}n (47), also crystalizes in a monoclinic system. In this compound, there is only one type of four-coordinated mercury(II) ion, which is linked by a pyridyl nitrogen atom and a phenoxide oxygen atom from two Schiff bases and two iodine ions. The coordination environment around metal ions is also a distorted tetrahedral geometry. This way of binding of metal ions by the N,O-donor ligand leads to the formation of a 1D looped chain structure (Figure 32). The adsorption properties of the compounds have been studied with respect to molecules such as CH3OH, N2, H2, O2, and CO2. These compounds show selective and good absorption towards methanol vapour when mixed with other gasses, which may allow them to be used in gas storage and separation [90].
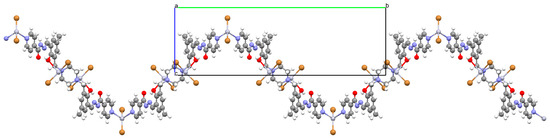
Figure 31.
Fragment of crystal structure showing formation of a zig-zag chain of 46 (Table 2) viewed along the a-axis [33,90,93].
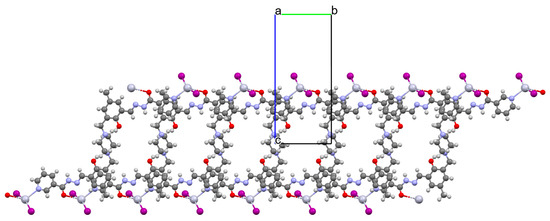
Figure 32.
Fragment of crystal structure showing formation of a 1D looped chain of 47 (Table 2) viewed along the a-axis [33,90,93]. Solvent molecules are omitted for clarity.
[Zn2L39(μ1,3-SCN)(η1SCN)]n (48) 1D chain thiocyanato bridged coordination polymer (Figure 33) was synthesized during reaction of a N,O-donor Schiff base (L39 N,N′-bis(3-methoxysalicylidenimino)-1,3-daminopropane), ZnII, and SCN− in the methanol solution. 48 crystallizes in the orthorhombic system. The coordination number of zinc ions is equal to 6 and 5, respectively, with the distorted octahedral (with two phenolate O, two methoxy O, and two thiocyanato N atoms) and the distorted square pyramidal geometry (with two phenoxido O, two imine N atoms in equatorial plane, and one thiocyanato S atom in the axial plane) around the metal centres. Compound 48 shows strong antimicrobial efficacy against some both Gram-positive and Gram-negative strains, whereas the photoluminescent investigation confirmed its credential as a potential photoactive candidate [91].
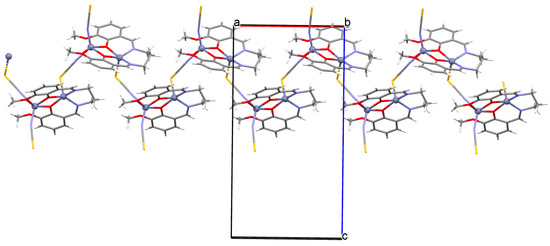
Figure 33.
Structure of 48 (Table 2) showing formation of 1D coordination polymer viewed along the b-axis [33,91,93].
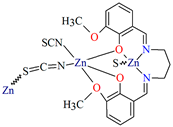
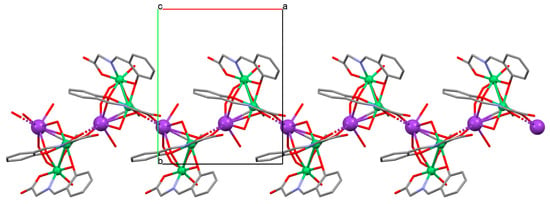
The polymeric chain ion {[Ni2K(L40)2Ac(H2O)2MeOH]·H2O}n (49) coordination polymer (Figure 34) was derived from a tridentate Schiff base and NiII in the methanol solution. 49 crystallizes in the monoclinic system. In the crystal, each NiII centre is six-coordinated and has a distorted octahedral geometry. Compound 49 may be applied as an electrocatalyst in fuel cells using H2O2 as an oxidant [92].
5. Conclusions
The synthesis, crystal structures, and selected promising properties of coordination polymers for potential applications are presented in this paper. The review may be of interest to scientists, who can read about the latest developments in the above-mentioned aspects of coordination polymers with N,O-donor Schiff base ligands. Single crystal X-ray diffraction analysis revealed that the topology of the coordination polymers is influenced by the differences in coordination modes and conformations of the N,O-donor Schiff base ligands. These compounds are mainly obtained as 1D chains due to steric effect of ligands used as well as they are built by bridging mononuclear or polynucelear units by additional linkers. The 2D layers or 3D frameworks are rarely formed.
Their unique properties, straightforward synthesis often following with green chemistry rules, and easy availability of reagents are increasing the interest of researchers. Vanadium complexes are mainly under investigation for their use as catalysts in organic synthesis or for their biological or sorption properties (Figure 35). V-CPs highly stable polymeric structures help maintain the structural feature required for catalytic activity during the reaction. The vanadium coordination polymers are mainly studied as catalysts in oxidation reactions, e.g., cyclohexene, trans-stilbene, styrene, isoeugenol, pyrogallol, but can also be used in epoxidation (e.g., alkene, cycloalkene) or cyanation of aldehyde processes. The sorption properties of vanadium polymers are also proving useful. Some complexes are being investigated for their ability to absorb small molecules such as H2 and CO2, which can be used in gas storage and separation. Coordination polymers of other metal ions also have a much wider range of applications due to their different properties, as shown in Figure 36. These compounds are mainly tested for their biological activity, e.g., antiviral, antimicrobial, anticancer. Their catalytic properties, not only in organic synthesis but also as photocatalysts or electrocatalysts, are of great interest. In recent years, there has been growing interest in the development of metals coordination polymers for use in new technologies, such as energy and electronic materials. Similarly to V-CPs, the coordination polymers of other metals are characterized by a good sorption capacity not only for small molecules such as CH3OH, N2, H2, O2, and CO2 but also for larger molecules such as dyes.
Figure 35.
Some potential applications of vanadium coordination polymers.
Figure 36.
Some potential applications of selected metal ions coordination polymers.
Author Contributions
Conceptualization, B.C., A.B. and D.O.; software, A.B., D.O. and B.C.; writing—original draft preparation, B.C., D.O. and A.B; writing—review and editing, B.C., D.O. and A.B; visualization, D.O., A.B. and B.C; supervision, B.C. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Debsharma, K.; Dey, S.; Pal, S.; Dutta, B.; Ghosh, S.; Sinha, C. Structural characterization and photoelectrochemistry of coordination polymer of Pb(II)-naphthyl-isonicotinohydrazide Schiff base. Appl. Organomet. Chem. 2023, 37, e7157. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the art and prospects in metal−organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438. [Google Scholar] [CrossRef] [PubMed]
- Basu Baul, T.S.; Brahma, S.; Addepalli, M.R.; Duthie, A.; Mahmoud, A.G.; Guedes da Silva, M.F.C.; Parkin, S. Structures and NMR solution dynamics of diorganotin complexes with bulky quadridentate salen- and salphen-type Schiff bases (N2O2) incorporating diazenylpyridyl side arms. Eur. J. Inorg. Chem. 2024, 27, e202400377. [Google Scholar] [CrossRef]
- Roy, I.; Brandao, P.; Majumder, A.; Bera, M.; Banerjee, A.; Saha, S. Structures, electrical properties and catecholase-like activity of a series of new copper(II) coordination polymers supported by azido bridges. J. Mol. Struct. 2024, 1317, 139085. [Google Scholar] [CrossRef]
- Husain, A.; Rani, P.; Nar, K.K.; Singh, A.P.; Kumar, R.; Bhasin, K.; Kumar, G. A tryptophan-based copper(II) coordination polymer: Catalytic activity towards Suzuki-Miyaura cross-coupling reactions. CrystEngComm 2021, 23, 7855–7864. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dasgupta, S. Synthesis of one-dimensional coordination polymer using dicyanamide spacer to explore catecholase like activity. Polyhedron 2020, 188, 114700. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, X.R.; Chen, H.X.; Niu, Z.; Hirao, H.; Braunstein, P.; Lang, J.P. Ultrafast luminescent light-up guest detection based on the lock of the host molecular vibration. J. Am. Chem. Soc. 2020, 142, 6690–6697. [Google Scholar] [CrossRef]
- Kamaal, S.; Usman, M.; Afzal, M.; Alarifi, A.; Ali, A.; Das, R.; Lama, P.; Ahmad, M. A new copper(II)-based layered coordination polymer: Crystal structure, topology, QTAIM analysis, experimental and theoretical magnetic properties based on DFT combined with broken-symmetry formalism (BS-DFT). Polyhedron 2021, 193, 114881. [Google Scholar] [CrossRef]
- Gao, Y.L.; Nishihara, S.; Suzuki, T.; Umeo, K.; Inoue, K.; Kurmoo, M. Ferroelastic-like transition and solvents affect the magnetism of a copper–organic radical one-dimensional coordination polymer. Dalton Trans. 2022, 51, 6682–6686. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H. Charge manipulation in metal-organic frameworks: Toward designer functional molecular materials. Bull. Chem. Soc. Jpn. 2021, 94, 2929–2955. [Google Scholar] [CrossRef]
- Takahashi, K.; Takeda, T.; Zheng, X.; Noro, S.I.; Akutagawa, T.; Nakamura, T. Selective gas sensing under a mixed gas flow with a one-dimensional copper coordination polymer. Inorg. Chem. 2023, 62, 14942–14948. [Google Scholar] [CrossRef] [PubMed]
- Lippi, M.; Cametti, M. Highly dynamic 1D coordination polymers for adsorption and separation applications. Coord. Chem. Rev. 2021, 430, 213661. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, B.; Das, P.; Halder, S.; Shit, M.; Ray, P.P.; Jana, K.; Sinha, C. Double advantages of 2D coordination polymer of coumarinyl-pyridyl Schiff base decorated Zn(II): The fabrication of schottky device and anti-carcinogenic activity. Appl. Organomet. Chem. 2023, 37, e7160. [Google Scholar] [CrossRef]
- Ashashi, N.A.; Kumar, M.; Ul Nisa, Z.; Frontera, A.; Sahoo, S.C.; Sheikh, H.N. Solvothermal self assembly of three lanthanide(III)-succinates: Crystal structure, topological analysis and DFT calculations on water channel. J. Mol. Struct. 2021, 1245, 131094. [Google Scholar] [CrossRef]
- Abu-Yamin, A.A.; Abduh, M.S.; Saghir, S.A.M.; Al-Gabri, N. Synthesis, characterization and biological activities of new Schiff base compound and its lanthanide complexes. Pharmaceuticals 2022, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ye, J.; Zhang, S.; Shi, L.; Akram, M.A.; Ning, G. Synthesis, structure and antibacterial activity of a copper(II) coordination polymer based on thiophene-2,5-dicarboxylate ligand. Polyhedron 2019, 166, 130–136. [Google Scholar] [CrossRef]
- Narayanaswamy, S.; Bhargavi, G.; Rajasekharan, M.V. Synthesis, characterization, and structural analysis of hetero Ni(II)–Na(I) and Homo Ni(II)–Ni(II) dinuclear complexes with a flexible tridendate schiff base ligand. J. Mol. Struct. 2024, 1317, 139027. [Google Scholar] [CrossRef]
- Xie, Y.; Dou, Z.; Liu, Z.; Huang, N.; Fei, C.; Wu, H. Cd-Ln (Ln = Pr, Sm, Yb and Nd) heteronuclear complexes based on the linear chain ether Schiff base: Structural regulation and fluorescence properties. J. Mol. Struct. 2024, 1318, 139445. [Google Scholar] [CrossRef]
- Dutta, B.; Debsharma, K.; Dey, S.; Sinha, C. Advancement and future challenges of metal–organic coordination polymers: A case study of optical sensor for the detection of the environmental contaminants. Appl. Organomet. Chem. 2023, 37, e6919. [Google Scholar] [CrossRef]
- Sarkar, S.; Singha, D.K.; Majee, P.; Daga, P.; Mondal, S.K.; Mahata, P. Stabilization of CO2 as zwitterionic carbamate within a coordination polymer (CP): Synthesis, structure and anion sensing behaviour of a Tb-CP composite. CrystEngComm 2022, 24, 5890–5899. [Google Scholar] [CrossRef]
- Manna, P.; Chandra, A.K.; Mahata, P. A two-dimensional mixed ligands based coordination polymer of cadmium: Synthesis, single crystal structure and luminescence quenching based sensing behaviors towards Fe3+, Cr3+, Al3+ ions and TNP in aqueous phase. Inorg. Chim. Acta 2025, 580, 122592. [Google Scholar] [CrossRef]
- Bibik, Y.S.; Fritsky, I.O.; Kucheriv, O.I.; Marynin, A.I.; Molnár, G.; Salmon, L.; Bousseksou, A.; Gural’skiy, I.A. Switchable nanoparticles based on Fe(II)-Au(I) spin-crossover coordination polymer. J. Mol. Struct. 2024, 1318, 139302. [Google Scholar] [CrossRef]
- Debsharma, K.; Dey, S.; Das, D.; Halder, S.; Ortega-Castro, J.; Sarkar, S.; Dutta, B.; Maity, S.; Jana, K.; Frontera, A.; et al. Designing of a Zn(II)-isonicotinohydrazido thiophenyl based 2D coordination polymer: Structure, augmented photoconductivity and superior biological activity. CrystEngComm 2023, 25, 162. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E.; Noor, T.; Rabi, O.; Zahra, R.; Yang, M. Recent advancements in MOF-based catalysts forapplications in electrochemical and photoelectrochemicalwater splitting: A review. Int. J. Energy Res. 2021, 45, 1190. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bera, S.; Das, R.; Chakraborty, D.; Basu, A.; Banerjee, P.; Ghosh, S.; Bhaumik, A. A Ni(II) metal-organic framework with mixed carboxylate and bipyridine ligands for ultrafast and selective sensing of explosives and photoelectrochemical hydrogen evolution. ACS Appl. Mater. Interfaces 2022, 14, 20907. [Google Scholar] [CrossRef]
- Pu, L.M.; An, X.X.; Liu, C.; Long, H.T.; Zhao, L. Insights into crystal structures, supramolecular architectures and antioxidant activities of self-assembled fluorescent hetero-multinuclear [Cu(II)–Ln(III)](Ln = La, Ce, Pr and Nd) salamo-like complexes. Appl. Organomet. Chem. 2020, 34, e5980. [Google Scholar] [CrossRef]
- Xu, J.H.; Xia, X.Z.; Xia, L.X.; Dong, J.P.; Jiang, Y.X.; Sun, F.G.; Wu, H.L. Syntheses, structures and fluorescence properties of two Ce/Zn-Ce complexes with an open- chain ether Schiff base ligand. J. Coord. Chem. 2021, 74, 2263–2273. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, C.; Wu, Y.C.; Wu, H.L.; Han, X.T.; Xu, J.H.; Xia, X.Z. Construction of d-f heteronuclear complexes with open-chain ether Schiff base ligand: Regulation effects of Zn(II) and Cd(II) on structures and luminescence properties. J. Lumin. 2020, 226, 117437. [Google Scholar] [CrossRef]
- Fei, C.L.; Gao, W.; Zhang, J.Q.; Wu, Z.W.; Lv, Y.; Wu, H.L. Structural regulation and luminescence properties of Tb/Zn-Tb complexes based on aliphatic ether Schiff base. J. Mol. Struct. 2024, 1308, 138069. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Garcia-Santos, I.; Safin, D.A.; Fernández-Vazquez, R.; Eftekharisis, B.; Gomila, R.M.; Frontera, A. A joint action of coordination, hydrogen and tetrel bonds toward a new 2D coordination polymer of lead(II) perchlorate and N’-isonicotinoylpyrazine-2-carbohydrazonamide. Inorg. Chem. Commun. 2025, 174, 113914. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, X.Z.; Xia, L.X.; Sun, F.G.; Dong, J.P.; Li, R.X.; Wu, H.L. A series of new hetero-decanucleus sandwich Cd–Ln clusters constructed from open-chain ether Schiff base and niacin ligands: Synthesis, structure, luminescence and antioxidant activity. Inorg. Chim. Acta. 2021, 522, 120371. [Google Scholar] [CrossRef]
- Dong, J.P.; Li, R.X.; Sun, F.G.; Jiang, Y.X.; Wu, H.L. Structures, fluorescence, and superoxide radical scavenging activities of two Cd–Ln (Ln = Gd, Er) coordination polymers with an open-chain ether Schiff base and isonicotinate. J. Coord. Chem. 2022, 75, 107–119. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ferraz-Caetano, J.; Teixeira, F.; Cordeiro, M.N.D.S. Systematic development of vanadium catalysts for sustainable epoxidation of small alkenes and allylic alcohols. Int. J. Mol. Sci. 2023, 24, 12299. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, G.; Budka, J.; Inkielewicz-Stepniak, I. Synthesis, spectroscopic characterization, catalytic and biological activity of oxidovanadium(V) complexes with chiral tetradentate Schiff bases. Molecules 2023, 28, 7408. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, G.; Budka, J.; Inkielewicz-Stepniak, I. Oxidovanadium(V) Schiff base complexes derived from chiral 3-amino-1,2-propanediol enantiomers: Synthesis, spectroscopic studies, catalytic and biological activity. Int. J. Mol. Sci. 2024, 25, 5010. [Google Scholar] [CrossRef]
- Romanowski, G.; Budka, J.; Inkielewicz-Stepniak, I. Oxidovanadium(V) complexes with chiral tetradentate Schiff bases: Synthesis, spectroscopic characterization, catalytic and biological activity. J. Mol. Struct. 2024, 1306, 137929. [Google Scholar] [CrossRef]
- Salubi, C.A. Heterogeneous vanadium Schiff base complexes in catalytic oxidation reactions. Curr. Chem. Lett. 2023, 12, 91–106. [Google Scholar] [CrossRef]
- Bendia, S.; Benabid, W.; Bourzami, R.; Ouari, K. Spectroscopic characterization of a mononuclear oxovanadium(IV) Schiff base complex. Oxidation catalysis applications and antibacterial activities. J. Mol. Struct. 2023, 1281, 135131. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Liu, J.; Xiao, W.; Lei, Y.; Xiao, X.; Liu, Y. Synthesis, crystal structures and catalytic oxidation property of two oxidovanadium(V) complexes with Schiff bases. Acta Chim. Slov. 2024, 71, 288–294. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Maurya, M.R. Vanadium complexes supported on organic polymers as sustainable systems for catalytic oxidations. Coord. Chem. Rev. 2017, 455, 415–428. [Google Scholar]
- Mahboubi-Anarjan, P.; Bikas, R.; Hosseini-Monfared, H.; Aleshkevych, P.; Mayer, P. Synthesis, characterization, EPR spectroscopy and catalytic activity of a new oxidovanadium(IV) complex with N2O2-donor ligand. J. Mol. Struct. 2017, 1131, 258–265. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Emami, M.; Bikas, R.; Sanchiz, J.; Otreba, M.; Aleshkevych, P.; Lis, T. Synthesis, characterization and magnetic properties of a dinuclear oxidovanadium(IV) complex: Magneto-structural DFT studies on the effects of out-of-plane-OCH3 angle. Polyhedron 2017, 122, 194–202. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301–302, 200–239. [Google Scholar] [CrossRef]
- Zhang, W.; Basak, A.; Kosugi, Y.; Hoshino, Y.; Yamamoto, H. Enantioselective epoxidation of allylic alcohols by a chiral complex of vanadium: An effective controller systemand a rational mechanistic model. Angew. Chem. Int. Ed. Engl. 2005, 44, 4389–4391. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Conte, V.; Coletti, A.; Floris, B.; Licini, G.; Zonta, C. Mechanistic aspects of vanadium catalysed oxidations with peroxides. Coord. Chem. Rev. 2011, 255, 2165–2177. [Google Scholar] [CrossRef]
- Trindade, A.F.; Gois, P.M.P.; Afonso, C.A.M. Recyclable stereoselective catalysts. Chem. Rev. 2009, 109, 418–514. [Google Scholar] [CrossRef]
- Naglah, A.M.; Al-Omar, M.A.; Almehizia, A.A.; Obaidullah, A.J.; Bhat, M.A.; Kalmouch, A.; Al-Wasidi, A.S.; Al-Humaidi, J.Y.; Refat, M.S. Synthesis, characterization, and anti-diabetic activity of some novel vanadium-folate-amino acid materials. Biomolecules 2020, 10, 781. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Misinterpretations in evaluating interactions of vanadium complexes with proteins and other biological targets. Inorganics 2021, 9, 17. [Google Scholar] [CrossRef]
- Kostenkova, K.; Levina, A.; Walters, D.; Murakami, H.A.; Lay, P.A.; Crans, D.C. Vanadium(V) pyridine-containing Schiff base catecholate complexes are lipophilic, redox-active and selectively cytotoxic in glioblastoma (T98G) cells. Chem. Eur. J. 2023, 29, e202302271. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Koehn, J.T.; Petry, S.M.; Glover, C.M.; Wijetunga, A.; Kaur, R.; Levina, A.; Lay, P.A. Hydrophobicity may enhance membrane affinity and anti-cancer effects of Schiff base vanadium(V) catecholate complexes. Dalton Trans. 2019, 48, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Krośniaka, M.; Szklarzewicz, J.; Gryboś, R.; Tatar, B.; Yildirim, M.; Sahin, B.; Yuksek, N.; Ustunda, M. The influence of chronic supply of vanadium compounds on organ weights and body mass in animal diabetes model (NZO). Sci. Tech. Innov. 2019, 4, 63–73. [Google Scholar] [CrossRef]
- Levina, A.; Vieira, A.P.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Crans, D.C.; Lay, P.A. A short-lived but highly cytotoxic vanadium(V) complex as a potential drug lead for brain cancer treatment by intratumoral injections. Angew. Chem. Int. Ed. 2020, 59, 15834–15838. [Google Scholar] [CrossRef]
- Manganaro, J.; Levina, A.; Lay, P.A.; Crans, D.C. Potential applications of vanadium-based anticancer drugs for intratumoral injections. Med. Sci. Forum. 2022, 11, 10. [Google Scholar]
- Irving, E.; Stoker, A.W. Vanadium compounds as PTP inhibitors. Molecules 2017, 22, 2269. [Google Scholar] [CrossRef]
- Suzuki, T.; Ueda, K.; Kita, R.; Kokado, S.; Harigaya, K. Synthesis, crystal structure and magnetic property of bis(tetra-n-butylammonium) bis(4,5-dicyanobenzene-1,2-dithiolato)oxovanadium complex. Polyhedron 2005, 24, 2491–2496. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord. Chem. Rev. 2001, 2019, 1033. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Archer, S.D.; Beale, R. Ocean-atmosphere trace gas exchange. Chem. Soc. Rev. 2012, 41, 6473–6506. [Google Scholar] [CrossRef]
- Tsuchida, E.; Oyaizu, K. Oxovanadium(III–V) mononuclear complexes and their linear assemblies bearing tetradentate Schiff base ligands: Structure and reactivity as multielectron redox catalysts. Coord. Chem. Rev. 2003, 237, 213. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Patra, A.K.; Chakravarty, A.R. Synthesis, structure, DNA binding and DNA cleavage activity of oxovanadium(IV) N-salicylidene-S-methyldithiocarbazate complexes of phenanthroline bases. J. Inorg. Biochem. 2008, 102, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Eady, R.R. Current status of structure function relationships of vanadium nitrogennase. Coord. Chem. Rev. 2003, 237, 23–30. [Google Scholar] [CrossRef]
- Bastos, A.M.B.; da Silva, J.G.; Maia, P.I.S.; Deflon, V.M.; Batista, A.A.; Ferreira, A.V.M.; Botion, L.M.; Niquet, E.; Beraldo, H. Oxovanadium(IV) and (V) complexes of acetylpyridinederived semicarbazones exhibit insulin-like activity. Polyhedron 2008, 27, 1787–1794. [Google Scholar] [CrossRef]
- Tracey, A.S.; Willsky, G.R.; Takeuchi, E.S. Vanadium Chemistry, Biochemistry, Pharmacology and Practical Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Shechter, Y. Insulin-mimetic effects of vanadate: Possible implications for future treatment of diabetes. Diabetes 1990, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Grivani, G.; Delkhosh, S.; Fejfarová, K.; Dušek, M.; Khalaji, A.D. Polynuclear oxovanadium(IV) Schiff base complex [VOL2]n (L = (5-bromo-2-hydroxybenzyl-2-furylmethyl)imine): Synthesis, characterization, crystal structure, catalytic properties and thermal decomposition into V2O5 nano-particles. Inorg. Chem. Commun. 2013, 27, 82–87. [Google Scholar] [CrossRef]
- Matsuoka, N.; Kawamura, H.; Yoshioka, N. Magnetic property and crystal structure of bis [N-(4-chlorophenyl)salicylideneaminato] oxovanadium(IV). Chem. Phys. Lett. 2010, 488, 32–37. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Hoshina, G.; Yoshioka, N.; Inoue, H.; Nakajima, K.; Kamishima, M.; Kojima, M.; Ohba, S. Mechanochemical reaction of polymeric oxovanadium(IV) complexes with Schiff base ligands derived from 5-nitrosalicylaldehyde and diamines. J. Solid State Chem. 2000, 153, 9–15. [Google Scholar] [CrossRef]
- Halevas, E.; Tsave, O.; Yavropoulou, M.P.; Hatzidimitriou, A.; Yovos, J.G.; Psycharis, V.; Gabriel, C.; Salifoglou, A. Design, synthesis and characterization of novel binary V(V)-Schiff base materials linked with insulin-mimetic vanadium-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Structure–function correlations at the molecular level. J. Inorg. Biochem. 2015, 147, 99–115. [Google Scholar] [CrossRef]
- Bhunia, A.; Dey, S.; Moreno, J.M.; Diaz, U.; Concepcion, P.; Van Hecke, K.; Janiak, C.; Van Der Voort, P. A homochiral vanadium-salen based cadmium bpdc MOF with permanent porosity as an asymmetric catalyst in solvent-free cyanosilylation. Chem. Commun. 2016, 52, 1401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Xia, Q.; Cui, Y. Chiral binary metal–organic frameworks for asymmetric sequential reactions. Chem. Commun. 2017, 53, 12313. [Google Scholar] [CrossRef]
- Xi, W.; Liu, Y.; Xia, Q.; Li, Z.; Cui, Y. Direct and post-synthesis incorporation of chiral metallosalen catalysts into metal–organic frameworks for asymmetric organic transformations. Chem. Eur. J. 2015, 21, 12581–12585. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Ishida, T. Ferromagnetic oxovanadium(IV) complexes chelated with tetrahalosalen ligands. Polyhedron 2011, 30, 3073–3078. [Google Scholar] [CrossRef]
- Deilami, A.B.; Salehi, M.; Amiri, A.; Arab, A. New copper(II) and vanadium(IV) complexes based on allylaminederived Schiff base ligand; synthesis, crystal structure, electrochemical properties and DFT calculations. J. Mol. Struct. 2019, 1181, 190–196. [Google Scholar] [CrossRef]
- Zhu, C.; Xia, Q.; Chen, X.; Liu, Y.; Du, X.; Cui, Y. Chiral metal-organic framework as a platform for cooperative catalysis in asymmetric cyanosilylation of aldehydes. ACS Catal. 2016, 6, 7590–7596. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A. Oxovanadium(IV) based coordination polymers and their catalytic potentials for the oxidation of styrene, cyclohexene and trans-stilbene. J. Mol. Catal. A Chem. 2006, 250, 190–198. [Google Scholar] [CrossRef]
- Maurya, M.R.; Uprety, B.; Chaudhary, N.; Avecilla, F. Synthesis and characterization of di-l-oxidovanadium(V), oxidoperoxido-vanadium(V) and polymer supported dioxidovanadium(V) complexes and catalytic oxidation of isoeugenol. Inorganica Chim. Acta. 2015, 434, 230–238. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chaudhary, N.; Avecilla, F.; Correia, I. Mimicking peroxidase activity by a polymer-supported oxidovanadium(IV) Schiff base complex derived from salicylaldehyde and 1,3-diamino-2-hydroxypropane. J. Inorg. Biochem. 2015, 147, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Maurya, M.R.; Kumar, A.; Manikandan, P.; Chand, S. Synthesis, characterisation and catalytic potential of oxovanadium(IV) based coordination polymers having a bridging methylene group. Appl. Catal. 2004, 277, 45–53. [Google Scholar] [CrossRef]
- Malik, S.; Mondal, U.; Jana, N.C.; Banerjee, P.; Saha, A. Using eugenol scaffold to explore the explosive sensing properties of Cd(II)-based coordination polymers: Experimental studies and real sample analysis. Dalton Trans. 2024, 53, 12995. [Google Scholar] [CrossRef] [PubMed]
- Middya, P.; Frontera, A.; Chattopadhyay, S. Thecrucial role of hydrogen bonding in shaping the structures of zinc-based coordination polymers using tridentate N,N,O donor reduced Schiff base ligands and bridging acetates. RSC Adv. 2024, 14, 13905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Zhang, Y.; Cui, Y.F.; Yu, M.; Dong, W.K. Heterobimetallic [NiIILnIII] (Ln = Sm and Tb) N2O4-donor coordination polymers: Syntheses, crystal structures and fluorescence properties. Inorganica Chim. Acta 2020, 506, 119534. [Google Scholar] [CrossRef]
- Khan, S.; Giri, P.; Bauzá, A.; Harms, K.; Frontera, A.; Chattopadhyay, S. Synthesis, characterization and DFT study on two copper(II) complexes with a naphthalene-based Schiff base: Examples of stronger chelate-chelate interactions than those reported for classical p–p complexes. Polyhedron 2019, 157, 487–494. [Google Scholar] [CrossRef]
- Saha, S.; Biswas, N.; Chowdhury, M.; Ghosh, K.K.; Rizzoli, C.; Sepay, N.; Chakraborty, S.; Chakraborty, M.; Choudhury, C.R. Self-assembled heterometallic Cu(II)–Na(I) coordination polymer with salen-type Schiff base ligand: Structural analysis, antimicrobial, DFT and molecular docking study. Transit. Met. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Bikas, R.; Hosseini-Monfared, H.; Siczek, M.; Gutiérrez, A.; Krawczyk, M.S.; Lis, T. Syntheses, crystal structures and magnetic studies of new 2D coordination polymers containing dinuclear manganese(II) repetitive units using a ditopic isonicotinhydrazone based N,N,O-donor ligand. Polyhedron 2014, 67, 396–404. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Bikas, R.; Mohammadi, S.; Percino, T.M.; Demeshko, S.; Meyer, F.; Leyva Ramírez, M.A. Synthesis, structure, magnetic properties, and catalase-like activity of methoxy-bridged manganese(III) coordination polymer containing hydrazone based (O,N,O)2-donor ligand. Z. Anorg. Allg. Chem. 2014, 640, 405–411. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Tian, X.M.; Xiong, Y.; Huang, C.; Chen, D.M.; Zhu, B.X. Coordination-driven heterochiral self-assembly: Construction of Cd(II) coordination polymers with sorption behaviors for iodine and dyes. Inorg. Chem. 2023, 62, 19887–19897. [Google Scholar] [CrossRef] [PubMed]
- Shit, M.; Halder, S.; Manna, K.; Karan, A.K.; Samanta, A.; Manik, N.B.; Pal, S.; Jana, K.; Sinha, C. Mn(II) 3D coordination framework with mixed 5-aminoisophthalato and pyridyl-isonicotinoyl hydrazone bridges: Structure, electrical conductivity, anticancer activity, and drug delivery. ACS Appl. Polym. Mater. 2024, 6, 2637–2648. [Google Scholar] [CrossRef]
- Karakousi, R.; Tsami, P.A.; Spanoudaki, M.A.; Dalgarno, S.J.; Papadimitriou, V.C.; Milios, C.J. Blue-emitting 2D- and 3D-zinc coordination polymers based on Schiff-base amino acid ligands. Chemistry 2023, 5, 1770–1780. [Google Scholar] [CrossRef]
- Huang, X.; Yan, S.Y.; Chen, Y.M.; Zhang, D.S.; Huang, C.; Zhu, B.X.; Lu, J.H. Synthesis, structures, and gas adsorption properties of Hg(II) and Cd(II) complexes constructed from two acylhydrazone ligands with multiple coordination sites. Inorg. Chim. Acta 2023, 555, 121588. [Google Scholar] [CrossRef]
- Majumdar, D.; Surendra Babu, M.S.; Das, S.; Biswas, J.K.; Mondal, M.; Hazra, S. Synthesis, X-ray crystal structure, photo luminescent property, antimicrobial activities and DFT computational study of Zn(II) coordination polymer derived from multisite N,O donor Schiff base ligand (H2L1). J. Mol. Struct. 2017, 1138, 161–171. [Google Scholar] [CrossRef]
- Santos, R.; Freire dos Santos, S.; da Silva Moura, F.; Sousa Maia, P.J.; Teixeira da Fonseca, B.; de Almeida Santos, R.H.; Medeiros, M.E.; Manoel dos Santos Garrido, F.; Casellato, A. A nickel(II) coordination polymer derived from a tridentate Schiff base ligand with N,O-donor groups: Synthesis, crystal structure, spectroscopy, electrochemical behavior and electrocatalytic activity for H2O2 electroreduction in alkaline medium. Transit. Met. Chem. 2017, 42, 301–310. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).