The Role of Synthesis Methods of Ceria-Based Catalysts in Soot Combustion
Abstract
1. Introduction
2. Results and Discussion
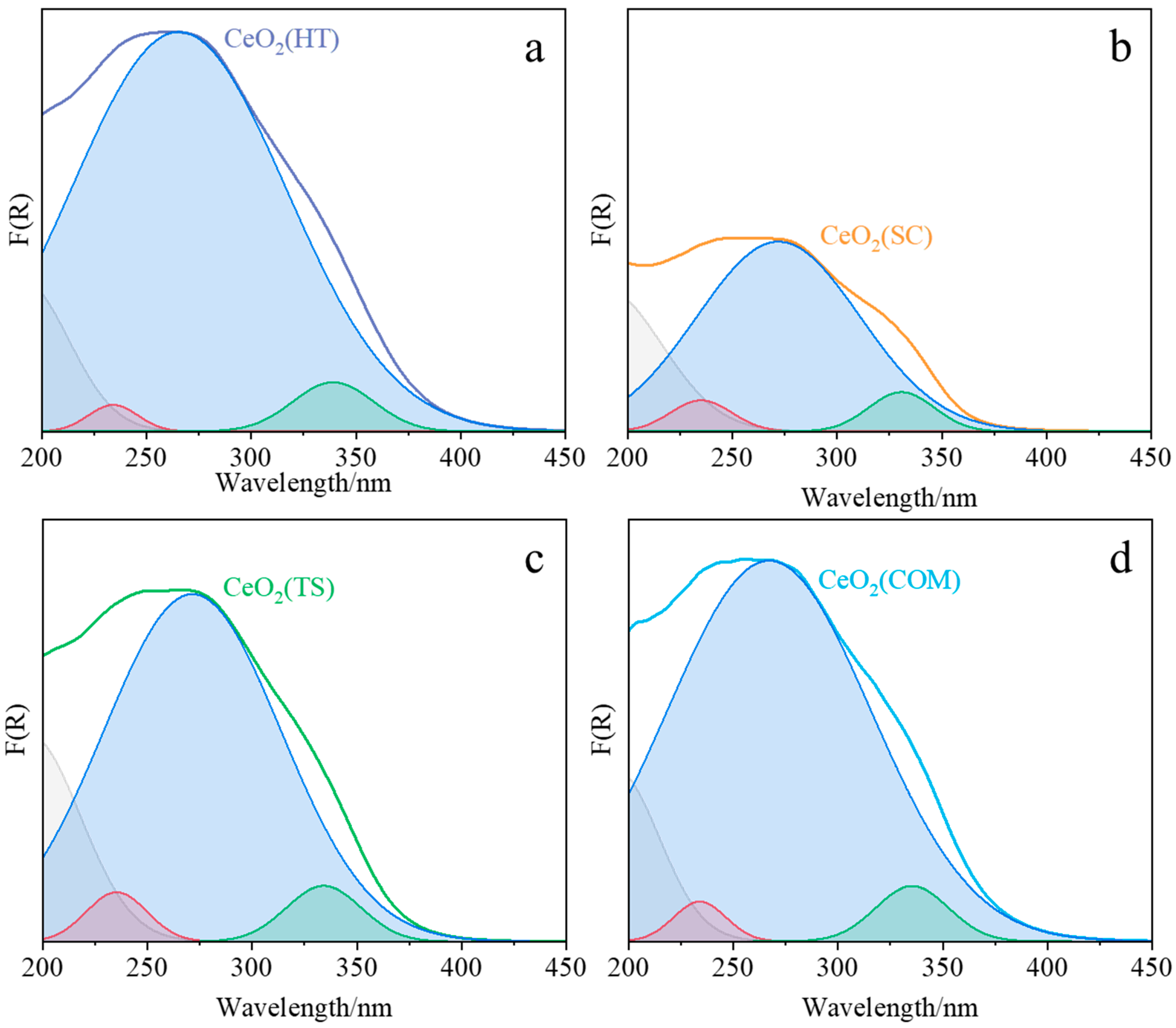
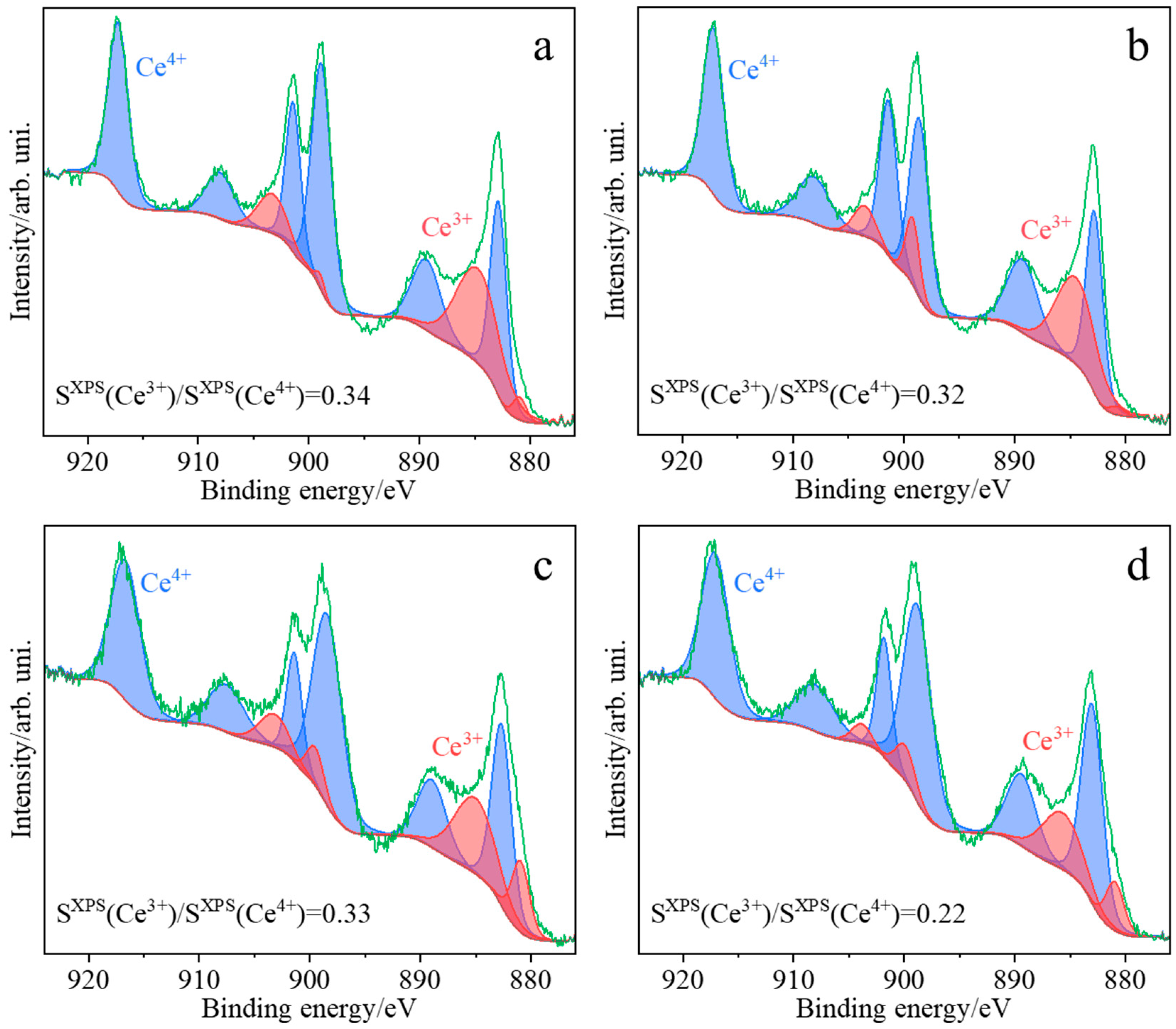
2.1. Characterization of the Synthesized Materials
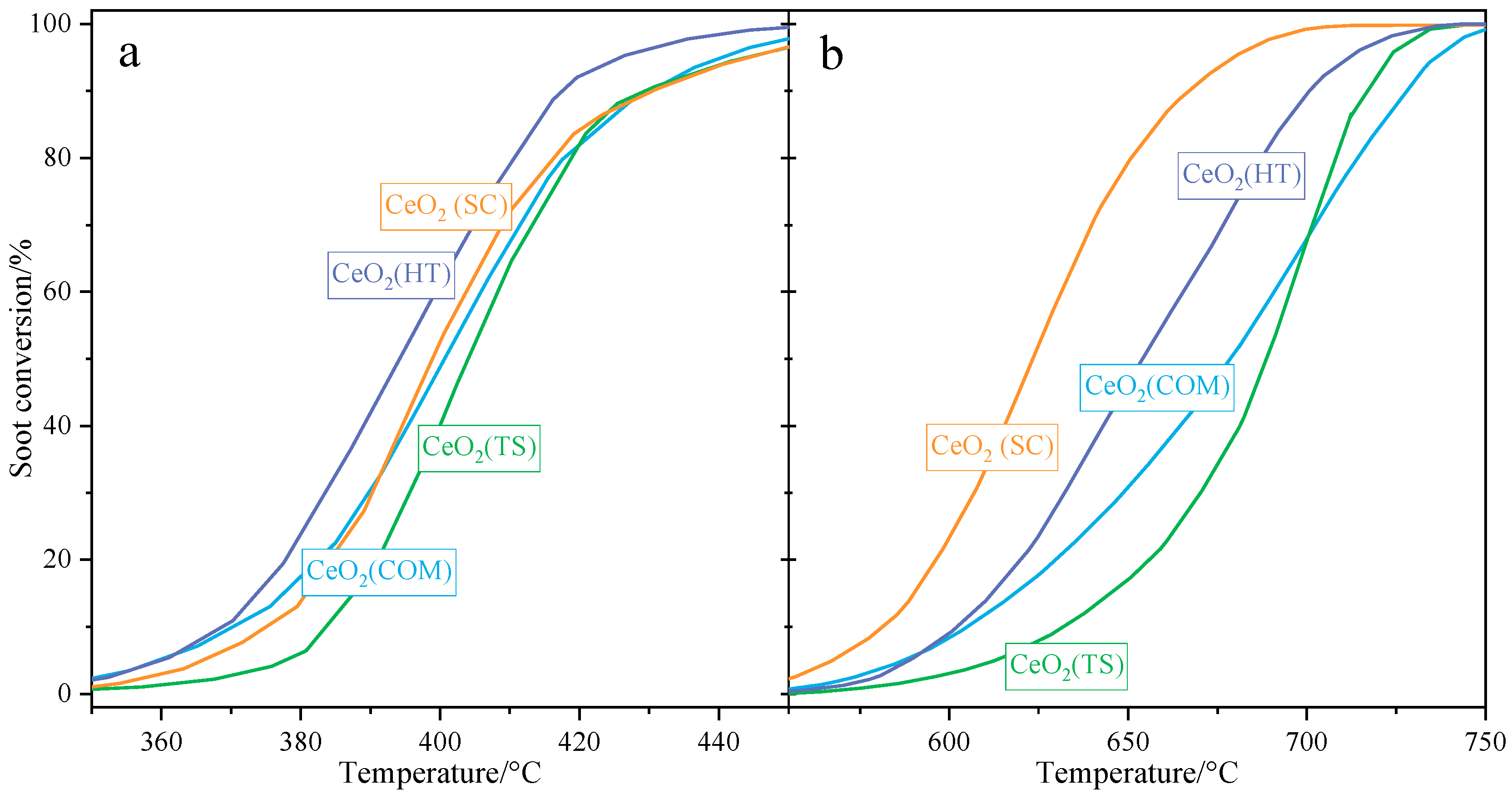
2.2. The Activity of the Ceria-Based Catalyst in the Soot Combustion Process
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Hydrothermal Synthesis
3.1.2. Sonochemical Synthesis
3.1.3. Template Synthesis
3.1.4. Commercial Cerium Oxide
3.2. Characterization of Materials
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, R.; Shang, J.; Qiu, X.; Gong, J.; Xue, T.; Zhu, T. Origin, Structural Characteristics, and Health Effects of Atmospheric Soot Particles: A Review. Curr. Pollut. Rep. 2024, 10, 532–547. [Google Scholar] [CrossRef]
- Das, A.; Pantzke, J.; Jeong, S.; Hartner, E.; Zimmermann, E.J.; Gawlitta, N.; Offer, S.; Shukla, D.; Huber, A.; Rastak, N.; et al. Generation, Characterization, and Toxicological Assessment of Reference Ultrafine Soot Particles with Different Organic Content for Inhalation Toxicological Studies. Sci. Total Environ. 2024, 951, 175727. [Google Scholar] [CrossRef]
- Li, W.; Riemer, N.; Xu, L.; Wang, Y.; Adachi, K.; Shi, Z.; Zhang, D.; Zheng, Z.; Laskin, A. Microphysical Properties of Atmospheric Soot and Organic Particles: Measurements, Modeling, and Impacts. NPJ Clim. Atmos. Sci. 2024, 7, 65. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Zuhra, Z.; Li, S.; Xie, G.; Wang, X. Soot Erased: Catalysts and Their Mechanistic Chemistry. Molecules 2023, 28, 6884. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.D.; Yan, C.H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Putla, S.B.; Kamali, M.; Swapna, B.; Reddy, B.M.; Sudarsanam, P. Review of Shape-Controlled CeO2 Nanocatalysts for Purification of Auto-Exhaust Pollutants. ACS Appl. Nano Mater. 2024, 7, 6749–6771. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-Based Catalysts for Soot Oxidation: A Review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Mishra, U.K.; Chandel, V.S.; Singh, O.P. A Review on Cerium Oxide–Based Catalysts for the Removal of Contaminants. Emergent Mater. 2022, 5, 1443–1476. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A. Catalytic Soot Combustion—General Concepts and Alkali Promotion. ACS Catal. 2023, 13, 3395–3418. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; De Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. CeO2-Based Catalysts with Engineered Morphologies for Soot Oxidation to Enhance Soot-Catalyst Contact. Nanoscale Res. Lett. 2014, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured Ceria-Based Catalysts for Soot Combustion: Investigations on the Surface Sensitivity. Appl. Catal. B 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured Ceria-Based Materials: Effect of the Hydrothermal Synthesis Conditions on the Structural Properties and Catalytic Activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef]
- Bensaid, S.; Russo, N.; Fino, D. CeO2 Catalysts with Fibrous Morphology for Soot Oxidation: The Importance of the Soot-Catalyst Contact Conditions. Catal. Today 2013, 216, 57–63. [Google Scholar] [CrossRef]
- Kumar, P.A.; Tanwar, M.D.; Bensaid, S.; Russo, N.; Fino, D. Soot Combustion Improvement in Diesel Particulate Filters Catalyzed with Ceria Nanofibers. Chem. Eng. J. 2012, 207–208, 258–266. [Google Scholar] [CrossRef]
- Piumetti, M.; van der Linden, B.; Makkee, M.; Miceli, P.; Fino, D.; Russo, N.; Bensaid, S. Contact Dynamics for a Solid-Solid Reaction Mediated by Gas-Phase Oxygen: Study on the Soot Oxidation over Ceria-Based Catalysts. Appl. Catal. B 2016, 199, 96–107. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. Effect of the Morphological and Surface Properties of CeO2-Based Catalysts on the Soot Oxidation Activity. Chem. Eng. J. 2015, 278, 190–198. [Google Scholar] [CrossRef]
- Legutko, P.; Gryboś, J.; Fedyna, M.; Janas, J.; Wach, A.; Szlachetko, J.; Adamski, A.; Yu, X.; Zhao, Z.; Kotarba, A.; et al. Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts 2020, 10, 1390. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Tuler, F.E.; Gaigneaux, E.M.; Debecker, D.P.; Miró, E.E.; Milt, V.G. Novel Ceramic Paper Structures for Diesel Exhaust Purification. Environ. Sci. Pollut. Res. 2018, 25, 35276–35286. [Google Scholar] [CrossRef]
- Cooper, A.; Davies, T.E.; Morgan, D.J.; Golunski, S.; Taylor, S.H. Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and Nox. Catalysts 2020, 10, 294. [Google Scholar] [CrossRef]
- Wang, K.; Guan, B.; Li, K.; Zhan, R.; Lin, H.; Huang, Z. Study on Oxidation Activity of CuCeZrOx Doped with K for Diesel Engine Particles in NO/O2. J. Shanghai Jiaotong Univ. Sci. 2018, 23, 18–27. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.H.; Bass, K.C.; McBride, J.R. Raman study of CeO2. Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Lefferts, L.; Mojet, B.L. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalyst. Sci. Rep. 2019, 9, 3875. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Novara, C.; Paganini, M.C.; Chiesa, M.; Castellino, M.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Fino, D.; Russo, N. Investigation of Cu-doped ceria through a combined spectroscopic approach: Involvement of different catalytic sites in CO oxidation. Catal. Today 2023, 420, 114037. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Kierys, A. Polymer Templated Production of Highly Porous Cerium Oxide in Direct Temperature Driven Transformation of Cerium(III) Salt. Microporous Mesoporous Mater. 2021, 318, 111032. [Google Scholar] [CrossRef]
- Legutko, P.; Kozieł, M.; Kowalczyk, A.; Michalik, M.; Adamski, A. Effect of Active Phase Precursor on Structural, Textural and Catalytic Properties of the Model NiOx/CeO2 System Active in Dry Reforming of Methane. Crystals 2024, 14, 634. [Google Scholar] [CrossRef]
- Shan, W.; Guo, H.; Liu, C.; Wang, X. Controllable Preparation of CeO2 Nanostructure Materials and Their Catalytic Activity. J. Rare Earths 2012, 30, 665–669. [Google Scholar] [CrossRef]
- Song, Z.; Liu, P.; Fu, Y.; Liu, H.; Huang, Z.; Kang, H.; Mao, Y.; Liu, B.; Guo, Y. Promotional Effect of Acidic Oxide on Catalytic Activity and N2 Selectivity over CeO2 for Selective Catalytic Reduction of NOx by NH3. Appl. Organomet. Chem. 2019, 33, e4919. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Zhang, X.; Liu, J. Flower-Like Mn-Doped CeO2 Microstructures: Synthesis, Characterizations, and Catalytic Properties. J. Chem. 2015, 2015, 254750. [Google Scholar] [CrossRef]
- Lupa, J.; Morlo, K.; Dobrowolski, R.; Legutko, P.; Sienkiewicz, A.; Kierys, A. Highly Porous Cerium Oxide Prepared via a One-Step Hard Template Method as an Extremely Effective Adsorbent for Arsenic Species Removal from Water. Chem. Eng. J. 2023, 474, 145750. [Google Scholar] [CrossRef]
- Bensalem, A.; Muller, J.C.; Bozon-Verduraz, F. From Bulk CeO2 to Supported Cerium-Oxygen Clusters: A Diffuse Reflectance Approach. J. Chem. Soc. Faraday Trans. 1992, 88, 153–154. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Comini, N.; Huthwelker, T.; Diulus, J.T.; Osterwalder, J.; Novotny, Z. Factors Influencing Surface Carbon Contamination in Ambient-Pressure X-Ray Photoelectron Spectroscopy Experiments. J. Vac. Sci. Technol. A Vac. Surf. Film. 2021, 39, 043203. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel Soot Combustion Ceria Catalysts. Appl. Catal. B 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. On the Soot Combustion Mechanism Using 3DOM Ceria Catalysts. Appl. Catal. B 2018, 234, 187–197. [Google Scholar] [CrossRef]
- Sorolla-Rosario, D.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; Bueno-López, A. Key-Lock Ceria Catalysts for the Control of Diesel Engine Soot Particulate Emissions. ChemCatChem 2020, 12, 1772–1781. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A Review on the Catalytic Combustion of Soot in Diesel Particulate Filters for Automotive Applications: From Powder Catalysts to Structured Reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ma, Y.; Xiong, J.; Zhang, P.; Lai, K.; Liu, X.; Zhao, Z.; Liu, J.; Chen, L.; et al. Revealing Active Edge Sites Induced by Oriented Lattice Bending of Co-CeO2 Nanosheets for Boosting Auto-Exhaust Soot Oxidation. J. Catal. 2023, 421, 351–364. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Xiong, J.; Ma, Y.; Li, X.; Zhang, P.; Zhang, S.; Wei, Y. The Catalyst of Ruthenium Nanoparticles Decorated Silicalite-1 Zeolite for Boosting Catalytic Soot Oxidation. Catalysts 2023, 13, 1167. [Google Scholar] [CrossRef]
- Cao, C.; Yang, H.; Xiao, J.; Yang, X.; Ren, B.; Xu, L.; Liu, G.; Li, X. Catalytic Diesel Soot Elimination over Potassium Promoted Transition Metal Oxide (Co/Mn/Fe) Nanosheets Monolithic Catalysts. Fuel 2021, 305, 121446. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Z.; Zou, X.; Liu, M.; Liu, W.; Wu, X.; Weng, D. Activation and Deactivation of Ag/CeO2 during Soot Oxidation: Influences of Interfacial Ceria Reduction. Catal. Sci. Technol. 2017, 7, 2129–2139. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Jiang, M.; Gao, X.; Huang, M.; Li, Y.; Ren, L.; Yang, Y.; Yang, Z. Controlled Synthesis of CeO2 Nanorods and Their Promotional Effect on Catalytic Activity and Aging Resistibility for Diesel Soot Oxidation. Appl. Surf. Sci. 2020, 510, 145401. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, J.; Wang, X. Oxidation Treatment of Diesel Soot Particulate on CexZr1−xO2. J. Hazard. Mater. 2007, 140, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Stegmayer, M.Á.; Milt, V.G.; Miró, E.E. Biomorphic Synthesis of Cobalt Oxide and Ceria Microfibers. Their Application in Diesel Soot Oxidation. Catal. Commun. 2020, 139, 105984. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Li, X.; Tao, Q.; Huang, Z.; Zuo, Q.; Chen, Y.; Li, T.; Fu, M.; Ye, D. Surface Adsorbed and Lattice Oxygen Activated by the CeO2/Co3O4 Interface for Enhancive Catalytic Soot Combustion: Experimental and Theoretical Investigations. J. Colloid Interface Sci. 2023, 638, 109–122. [Google Scholar] [CrossRef]
- Liu, P.; Liang, X.; Dang, Y.; He, J.; Shirazi-Amin, A.; Achola, L.A.; Dissanayake, S.; Chen, H.; Fu, M.; Ye, D.; et al. Effects of Zr substitution on soot combustion over cubic fluorite-structured nanoceria: Soot-Ceria contact and interfacial oxygen evolution. J. Environ. Sci. 2021, 101, 293–303. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Kopacz, A.; Legutko, P.; Indyka, P.; Wojtasik, M.; Ziemianski, L.; Żak, G.; Sojka, Z.; Kotarba, A. The role of crystallite size of iron oxide catalyst for soot combustion. Catal. Today 2015, 257, 111–116. [Google Scholar] [CrossRef]
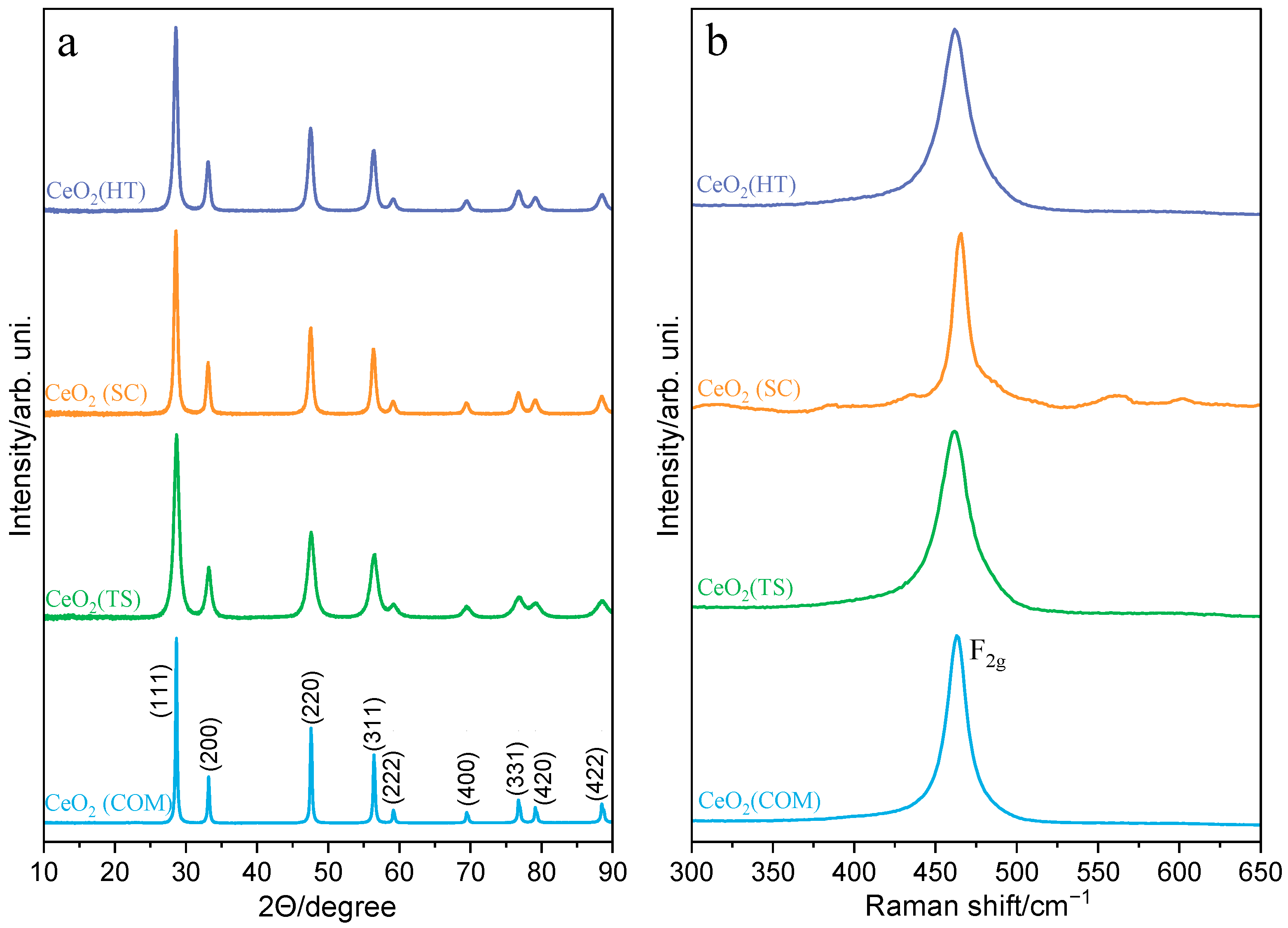

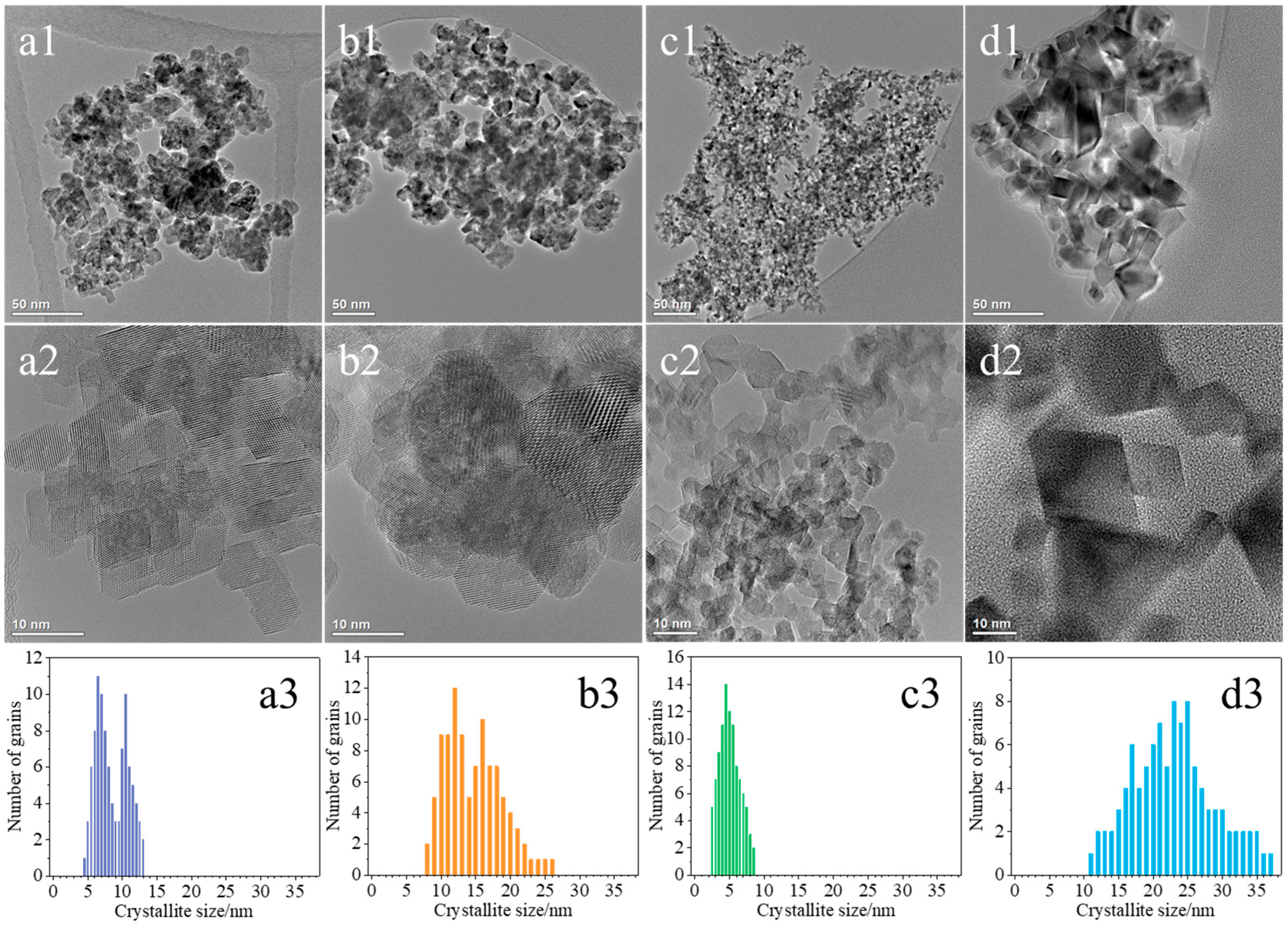


| Sample | Crystallites Size—Scherrer Method (nm) | Average Crystallites Size—TEM (nm) |
|---|---|---|
| CeO2(HT) | 16 | 8 |
| CeO2(SC) | 17 | 14 |
| CeO2(TS) | 11 | 5 |
| CeO2(COM) | 48 | 22 |
| Sample | LM CT Ce3+ ← O2− (nm) | LM CT Ce4+ ← O2− (nm) | Interband (nm) | Ce (III): Ce (IV) Area Ratio | Band Gap (eV) |
|---|---|---|---|---|---|
| CeO2(HT) | 234 | 265 | 339 | 0.0152 | 3.48 |
| CeO2(SC) | 235 | 272 | 331 | 0.0615 | 3.53 |
| CeO2(TS) | 235 | 271 | 334 | 0.0485 | 3.52 |
| CeO2(COM) | 234 | 267 | 335 | 0.0268 | 3.48 |
| Sample | Atomic Concentration (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce(III) | Ce(IV) | Ce (All) | Olatt | Oads | O (All) | Caliph | Ccarbox | C (All) | Ce/O | Ce(III)/Ce(IV) | Oads/Olatt | |
| CeO2(HT) | 7.42 | 21.82 | 29.24 | 38.91 | 9.8 | 48.71 | 11.16 | 10.9 | 22.06 | 0.60 | 0.34 | 0.25 |
| CeO2(SC) | 6.69 | 21.19 | 27.88 | 37.39 | 10.95 | 48.34 | 15.58 | 8.21 | 23.79 | 0.58 | 0.32 | 0.29 |
| CeO2(TS) | 2.47 | 7.44 | 9.91 | 41.79 | 10.25 | 52.04 | 31.31 | 6.73 | 38.04 | 0.19 | 0.33 | 0.25 |
| CeO2(COM) | 3.42 | 15.49 | 18.91 | 24.47 | 4.31 | 28.78 | 40.37 | 11.94 | 52.31 | 0.66 | 0.22 | 0.18 |
| Sample | Characteristic Temperatures of Soot Conversion/°C | |||||
|---|---|---|---|---|---|---|
| Tight Contact Mode | Loose Contact Mode | |||||
| T10 | T50 | T90 | T10 | T50 | T90 | |
| CeO2(HT) | 368 | 394 | 418 | 602 | 654 | 701 |
| CeO2(SC) | 375 | 400 | 431 | 581 | 623 | 668 |
| CeO2(TS) | 383 | 404 | 430 | 632 | 689 | 717 |
| CeO2(COM) | 370 | 402 | 430 | 605 | 679 | 728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzybek, G.; Wójtowicz, A.; Legutko, P.; Greluk, M.; Słowik, G.; Sienkiewicz, A.; Adamski, A.; Kotarba, A. The Role of Synthesis Methods of Ceria-Based Catalysts in Soot Combustion. Molecules 2025, 30, 358. https://doi.org/10.3390/molecules30020358
Grzybek G, Wójtowicz A, Legutko P, Greluk M, Słowik G, Sienkiewicz A, Adamski A, Kotarba A. The Role of Synthesis Methods of Ceria-Based Catalysts in Soot Combustion. Molecules. 2025; 30(2):358. https://doi.org/10.3390/molecules30020358
Chicago/Turabian StyleGrzybek, Gabriela, Andrzej Wójtowicz, Piotr Legutko, Magdalena Greluk, Grzegorz Słowik, Andrzej Sienkiewicz, Andrzej Adamski, and Andrzej Kotarba. 2025. "The Role of Synthesis Methods of Ceria-Based Catalysts in Soot Combustion" Molecules 30, no. 2: 358. https://doi.org/10.3390/molecules30020358
APA StyleGrzybek, G., Wójtowicz, A., Legutko, P., Greluk, M., Słowik, G., Sienkiewicz, A., Adamski, A., & Kotarba, A. (2025). The Role of Synthesis Methods of Ceria-Based Catalysts in Soot Combustion. Molecules, 30(2), 358. https://doi.org/10.3390/molecules30020358










