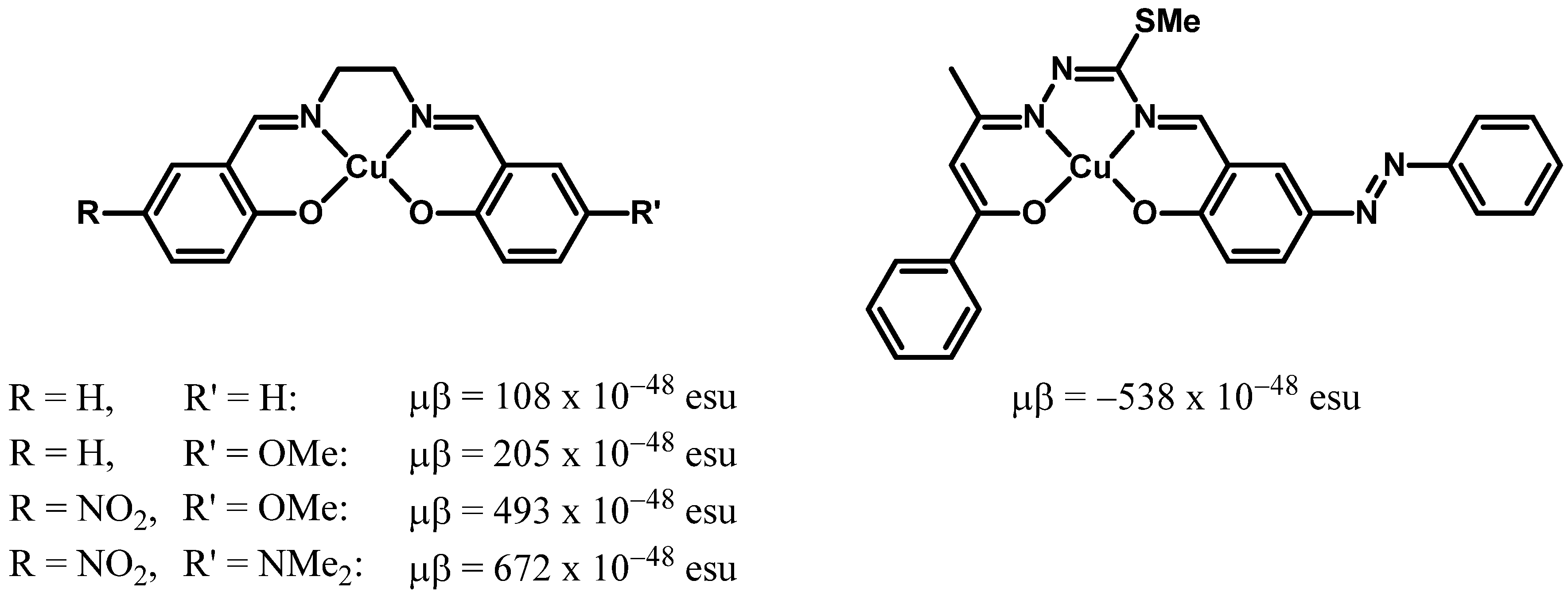
Dipolar Copper(I) Complexes: A Novel Appealing Class of Highly Active Second-Order NLO-Phores
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of the Complexes
2.2. UV-Vis Absorption Spectra and Computational Modeling
2.3. Study of the Second-Order NLO Properties
3. Materials and Methods
3.1. Procedure for the Synthesis of Complexes 1–3
3.2. Computational Detail
3.3. EFISH Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, N.P.; Williams, D.J. Introduction to Nonlinear Optical Effects in Molecules and Polymers; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Zyss, J. Molecular Nonlinear Optics: Materials, Physics and Devices; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Roundhill, D.M. Optoelectronic Properties of Inorganic Compounds; Fackler, J.P., Jr., Ed.; Plenum Press: New York, NY, USA, 1999. [Google Scholar]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Ledoux, I.; Zyss, J. Influence of the molecular environment in solution measurements of the Second-order optical susceptibility for urea and derivatives. Chem. Phys. 1982, 73, 203–213. [Google Scholar] [CrossRef]
- Maker, P.D. Spectral Broadening of Elastic Second-Harmonic Light Scattering in Liquids. Phys. Rev. A 1970, 1, 923–951. [Google Scholar] [CrossRef]
- Clays, K.; Persoons, A. Hyper-Rayleigh scattering in solution. Phys. Rev. Lett. 1991, 66, 2980–2983. [Google Scholar] [CrossRef] [PubMed]
- Zyss, J. Molecular engineering implications of rotational invariance in quadratic nonlinear optics: From dipolar to octupolar molecules and materials. J. Chem. Phys. 1993, 98, 6583–6599. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, C.; Huo, F.; He, W.; Cao, A. Progress in the enhancement of electro-optic coefficients and orientation stability for organic second-order nonlinear optical materials. Dye. Pigment. 2020, 181, 108509. [Google Scholar] [CrossRef]
- Le Bozec, H.; Renouard, T. Dipolar and Non-Dipolar Pyridine and Bipyridine Metal Complexes for Nonlinear Optics. Eur. J. Inorg. Chem. 2000, 229–239. [Google Scholar] [CrossRef]
- Di Bella, S. Second-order nonlinear optical properties of transition metal complexes. Chem. Soc. Rev. 2001, 30, 355–366. [Google Scholar] [CrossRef]
- Powell, C.E.; Humphrey, M.G. Nonlinear optical properties of transition metal acetylides and their derivatives. Coord. Chem. Rev. 2004, 248, 725–756. [Google Scholar] [CrossRef]
- Coe, B.J.; Curati, N.R.M. Metal complexes for molecular electronics and photonics. Comments Inorg. Chem. 2004, 25, 147–184. [Google Scholar] [CrossRef]
- Maury, O.; Le Bozec, H. Molecular Engineering of Octupolar NLO Molecules and Materials Based on Bipyridyl Metal Complexes. Acc. Chem. Res. 2005, 38, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J. Switchable Nonlinear Optical Metallochromophores with Pyridinium Electron Acceptor Groups. Acc. Chem. Res. 2006, 39, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Roberto, D.; Tavazzi, S.; Escadeillas, M.; Guerchais, V.; Le Bozec, H.; Boucekkine, A.; et al. Cyclometallated 4-styryl-2-phenylpyridine Pt (II) acetylacetonate complexes as second-order NLO building blocks for SHG active polymeric films. Organometallics 2013, 32, 3890–3894. [Google Scholar] [CrossRef]
- Reedijk, J.; Poepperlmeier, K. Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Attar, S.; Espa, D.; Artizzu, F.; Pilia, L.; Serpe, A.; Pizzotti, M.; Di Carlo, G.; Marchiò, L.; Deplano, P. Optically Multiresponsive Heteroleptic Platinum Dithiolene Complex with Proton-Switchable Properties. Inorg. Chem. 2017, 56, 6763–6767. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Malandrino, G.; Dragonetti, C.; Pellegrino, A.L. Luminescent 1,10-Phenanthroline -Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties. Molecules 2022, 27, 6990. [Google Scholar] [CrossRef]
- Pizzotti, M.; Ugo, R.; Roberto, D.; Bruni, S.; Fantucci, P.C.; Rovizzi, C. Organometallic Counterparts of Push–Pull Aromatic Chromophores for Nonlinear Optics: Push–Pull Heteronuclear Bimetallic Complexes with Pyrazine and trans-1,2-Bis (4-pyridyl) ethylene as Linkers. Organometallics 2002, 21, 5830–5840. [Google Scholar] [CrossRef]
- Durand, R.J.; Gauthier, S.; Achelle, S.; Groizard, T.; Kahlal, S.; Saillard, J.-Y.; Barsella, A.; Le Poul, N.; Robin Le Guena, F. Push–Pull D-π-Ru-π-A chromophores: Synthesis and electrochemical, photophysical and second order nonlinear optical properties. Dalton Trans. 2018, 47, 3965–3975. [Google Scholar] [CrossRef]
- Lacroix, P.G. Second-Order Optical Nonlinearities in Coordination Chemistry: The Case of Bis (salicylaldiminato) metal Schiff Base Complexes. Eur. J. Inorg. Chem. 2001, 339–348. [Google Scholar] [CrossRef]
- Tessore, T.; Roberto, D.; Ugo, R.; Mussini, P.; Quici, S.; Ledoux-Rak, I.; Zyss, J. Large, Concentration-Dependent Enhancement of the Quadratic Hyperpolarizability of [Zn(CH3CO2)2(L)2] in CHCl3 on Substitution of Acetate by Triflate. Angew. Chem. Int. Engl. Ed. 2003, 42, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Maury, O.; Viau, L.; Senechal, K.; Corre, B.; Guegan, J.P.; Renouard, T.; Ledoux, I.; Zyss, J.; Le Bozec, H. Synthesis, Linear, and Quadratic-Nonlinear Optical Properties of Octupolar D3 and D2d Bipyridyl Metal Complexes. Chem. Eur. J. 2004, 10, 4454–4466. [Google Scholar] [CrossRef] [PubMed]
- Viau, L.; Bidault, S.; Maury, O.; Brasselet, S.; Ledoux, I.; Zyss, J.; Ishow, E.; Nakatani, K.; Le Bozec, H. All-Optical Orientation of Photoisomerizable Octupolar Zinc (II) Complexes in Polymer Films. J. Am. Chem. Soc. 2004, 126, 8386–8387. [Google Scholar] [CrossRef] [PubMed]
- Bidault, S.; Viau, L.; Maury, O.; Brasselet, S.; Zyss, J.; Ishow, E.; Nakatani, K.; Le Bozec, H. Optically Tunable Nonlinearities in Polymers Based on Photoisomerizable Metal-Based Coordination Complexes. Adv. Funct. Mater. 2006, 16, 2252–2262. [Google Scholar] [CrossRef]
- Coe, B.J. Developing iron and ruthenium complexes for potential nonlinear optical applications. Coord. Chem. Rev. 2013, 257, 1438. [Google Scholar] [CrossRef]
- Kabali, S.; Thirumoorthy, K.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Colombo, A.; Haukka, M.; Nallasamy, P. Ferrocene-quinoxaline Y-shaped chromophores as fascinating second-order NLO building blocks for long lasting highly active SHG polymeric films. Dalton Trans. 2016, 45, 11939–11943. [Google Scholar]
- Prabu, S.; Fagnani, F.; Colombo, A.; Dragonetti, C.; Biagini, P.; Melchiorre, F.; Palanisami, N. Nonlinear optical-active ferrocene conjugated Y-shaped imidazole donor–π–acceptor [(D–π)2–IM–π–A] compounds for dye-sensitized solar cells using non-corrosive copper complexes as a redox mediator. New J. Chem. 2024, 48, 394–405. [Google Scholar] [CrossRef]
- Prabu, S.; Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Mathivathanan, L.; Palanisami, N. Effect of substitution on second-order nonlinear optical properties of ferrocene appended donor–π–acceptor Y-shaped trifluoromethyl imidazole chromophores. New J. Chem. 2024, 48, 14764–14772. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, M.; Kaur, P.; Clays, K.; Singh, K. Ferrocene chromophores continue to inspire. Fine-tuning and switching of the second-order nonlinear optical response. Coord Chem. Rev. 2017, 343, 185–219. [Google Scholar]
- Dragonetti, C.; Colombo, A.; Magni, M.; Mussini, P.; Nisic, F.; Roberto, D.; Ugo, R.; Valore, A.; Valsecchi, A.; Salvatori, P.; et al. Thiocyanate-Free Ru (II) Sensitizer with a pyrid-2-yl tetrazolate ligand for Dye-Sensitized Solar Cells. Inorg. Chem. 2013, 52, 10723–10725. [Google Scholar] [CrossRef]
- Sandroni, M.; Favereau, L.; Planchat, A.; Akdas-Kilig, H.; Szuwarski, N.; Pellegrin, Y.; Blart, E.; Le Bozec, H.; Boujtita, M.; Odobel, F.J. Heteroleptic copper (I)–polypyridine complexes as efficient sensitizers for dye sensitized solar cells. J. Mater. Chem. A 2014, 2, 9944–9947. [Google Scholar] [CrossRef]
- Malzner, F.J.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Exploring simple ancillary ligands in copper-based dye-sensitized solar cells: Effects of a heteroatom switch and of co-sensitization. J. Mater. Chem. A 2017, 5, 4671–4685. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Kavana, L.; Saygili, Y.; Freitag, M.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Electrochemical Properties of Cu (II/I)-Based Redox Mediators for Dye-Sensitized Solar Cells. Electrochim. Acta 2017, 227, 194–202. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards efficient sustainable full-copper dye-sensitized solar cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Copper Complexes as Alternative Redox Mediators in Dye-Sensitized Solar Cells. Molecules 2021, 26, 194. [Google Scholar] [CrossRef] [PubMed]
- Srivishnu, K.S.; Prasanthkumar, S.; Giribabu, L. Cu (II/I) redox couples: Potential alternatives to traditional electrolytes for dye-sensitized solar cells. Mater. Adv. 2021, 2, 1229–1247. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. Solar energy conversion using first row d-block metal coordination compound sensitizers and redox mediators. Chem. Sci. 2022, 13, 1225–1262. [Google Scholar] [CrossRef] [PubMed]
- Conradie, J. Polypyridyl copper complexes as dye sensitizer and redox mediator for dye-sensitized solar cells. Electrochem. Commun. 2022, 134, 107182. [Google Scholar] [CrossRef]
- Selvaraj, B.; Shanmugam, G.; Kamaraj, S.; Thirugnanasambandam, E.; Peters, S.; Gunasekeran, A.; Sambandam, A.; Pillai, R.S. Effect of copper and cobalt metal complex redox mediator based xanthan gum gel electrolyte materials on performance of dye sensitized solar cells. ChemistrySelect 2022, 7, e202203197. [Google Scholar] [CrossRef]
- Risi, G.; Devereux, M.; Prescimone, A.; Housecroft, C.E.; Constable, E.C. Back to the future: Asymmetrical DπA 2,2’-bipyridine ligands for homoleptic copper (i)-based dyes in dye-sensitised solar cells. RSC Adv. 2023, 13, 4122. [Google Scholar] [CrossRef] [PubMed]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D. Recent Investigations on the Use of Copper Complexes as Molecular Materials for Dye-Sensitized Solar Cells. Molecules 2024, 29, 6. [Google Scholar] [CrossRef]
- Minozzi, C.; Caron, A.; Grenier-Petel, J.-C.; Santandrea, J.; Collins, S.K. Heteroleptic Copper (I)-Based Complexes for Photocatalysis: Combinatorial Assembly, Discovery, and Optimization. Angew. Chem. Int. Ed. 2018, 57, 5477–5481. [Google Scholar] [CrossRef]
- Wang, C.; Guo, M.; Qi, R.; Shang, Q.; Liu, Q.; Wang, S.; Zhao, L.; Wang, R.; Xu, Z. Visible-Light-Driven, Copper-Catalyzed Decarboxylative C (sp3)−H Alkylation of Glycine and Peptides. Angew. Chem. Int. Ed. 2018, 57, 15841–15846. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhou, B.; Luo, S.P.; Xu, Z.; Jin, H.; Liu, Y. P/N Heteroleptic Cu (I)-Photosensitizer-Catalyzed Deoxygenative Radical Alkylation of Aromatic Alkynes with Alkyl Aldehydes Using Dipropylamine as a Traceless Linker Agent. ACS Catal. 2020, 10, 7563–7572. [Google Scholar] [CrossRef]
- Neerathilingam, N.; Prasanth, K.; Anandhan, R. Substituent-controlled selective synthesis of 1,2-diketones and internal alkynes from terminal alkynes and arylboronic acids via α-stilbene radicals obtained from heteroleptic Cu (i) complexes under visible light. Green Chem. 2022, 24, 8685–8690. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. TADF: Enabling luminescent copper (i) coordination compounds for light-emitting electrochemical cells. J. Mater. Chem. C 2022, 10, 4456–4482. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu (I) hybrid inorganic—organic materials with intriguing stimuli responsive and optoelectronic properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Cariati, E.; Roberto, D.; Ugo, R.; Ford, P.C.; Galli, S.; Sironi, A. X-ray Structures and Emissive and Second-Order Nonlinear Optical Properties of Two Inorganic−Organic Polymeric Adducts of CuI with 4-Acetylpyridine. The Role of Both “Intrastrand” Charge Transfers and Structural Motifs on the Nonlinear Optical Response of Cu (I) Polymeric Adducts with Pseudoaromatic η1-Nitrogen Donor Ligands. Chem. Mater. 2002, 14, 5116–5123. [Google Scholar]
- Anthony, S.P.; Radhakrishnan, T.P. Helical and network coordination polymers based on a novel C2-symmetric ligand: SHG enhancement through specific metal coordination. Chem. Commun. 2004, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.P.; Radhakrishnan, T.P. Coordination Polymers of Cu(I) with a Chiral Push−Pull Ligand: Hierarchical Network Structures and Second Harmonic Generation. Cryst. Growth Des. 2004, 4, 1223–1227. [Google Scholar] [CrossRef]
- Kang, Y.; Yao, Y.G.; Qin, Y.Y.; Zhang, J.; Chen, Y.B.; Li, Z.J.; Wen, Y.H.; Cheng, J.K.; Hu, R.F. A novel ligand-unsupported 3D framework polymer of trimeric copper (i) and its NLO property. Chem. Commun. 2004, 1046–1047. [Google Scholar] [CrossRef]
- Han, L.; Hong, M.; Wang, R.; Wu, B.; Xu, Y.; Lou, B.; Lin, Z. Red luminescent polymeric cuprous organosulfide generated by solvothermal redox reaction. Chem. Commun. 2004, 2578–2579. [Google Scholar] [CrossRef]
- Huang, X.F.; Li, Y.H.; Wu, Q.; Ye, Q.; Xiong, R.G. A novel one-dimensional acentric copper(I) coordination polymer with second-harmonic generation response. Inorg. Chim. Acta 2004, 358, 2097–2100. [Google Scholar] [CrossRef]
- Li, Z.; Du, S.; Wu, X. Construction of two novel 2D layer Mo(W)/Cu/S polymers using bidentate thiolato ligands as linkers. Inorg. Chem. Commun. 2006, 9, 79–81. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Yu, R.; Chen, S.M.; Wu, X.Y.; Chen, S.C.; Xie, Y.M.; Zhou, W.W.; Lu, C.Z. Topological derivation from centrosymmetry to noncentrosymmetry in a three-dimensional polar framework material. Cryst. Eng. Commun. 2010, 12, 671–673. [Google Scholar] [CrossRef]
- Macchi, R.; Cariati, E.; Marinotto, D.; Tordin, E.; Ugo, R.; Santoro, G.; Ubaldi, M.C.; Pietralunga, S.M.; Mattei, G. In situ growth in a PMMA film of oriented nanocrystals of the hybrid inorganic—organic acentric material [(E)-N,N-dimethylamino-N’-methylstilbazolium][Cu5I6]. J. Mater. Chem. 2011, 21, 9778. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Ledoux, I.; Marks, T.J. Role of Metal Electronic Properties in Tuning the Second-Order Nonlinear Optical Response of Coordination Complexes. A Combined Experimental and Theoretical Investigation of a Homologous Series of (N,N′-Disalicylidene-1,2-phenylenediaminato)M(II) (M = Co, Ni, Cu) Complexes. J. Am. Chem. Soc. 1995, 117, 9481–9485. [Google Scholar]
- Di Bella, S.; Fragalà, I.; Marks, T.J.; Ratner, M.A. Large Second-Order Optical Nonlinearities in Open-Shell Chromophores. Planar Metal Complexes and Organic Radical Ion Aggregates. J. Am. Chem. Soc. 1996, 118, 12747–12751. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Di Bella, S.; Ledoux, I. Synthesis and Second-Order Nonlinear Optical Properties of New Copper (II), Nickel (II), and Zinc (II) Schiff-Base Complexes. Toward a Role of Inorganic Chromophores for Second Harmonic Generation. Chem. Mater. 1996, 8, 541–545. [Google Scholar] [CrossRef]
- Rigamonti, L.; Demartin, F.; Forni, A.; Righetto, S.; Pasini, A. Copper (II) Complexes of salen Analogues with Two Differently Substituted (Push–Pull) Salicylaldehyde Moieties. A Study on the Modulation of Electronic Asymmetry and Nonlinear Optical Properties. Inorg. Chem. 2006, 45, 10976–10989. [Google Scholar] [CrossRef]
- Gradinaru, J.; Forni, A.; Druta, V.; Tessore, F.; Zecchin, S.; Quici, S.; Garbalau, N. Structural, Spectral, Electric-Field-Induced Second Harmonic, and Theoretical Study of Ni (II), Cu (II), Zn (II), and VO (II) Complexes with [N2O2] Unsymmetrical Schiff Bases of S-Methylisothiosemicarbazide Derivatives. Inorg. Chem. 2007, 46, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.J.; Ward, J.F. Perturbation theory of the non-linear optical polarization of an isolated system. Mol. Phys. 1971, 20, 513–520. [Google Scholar] [CrossRef]
- Renouard, T.; Le Bozec, H.; Brasselet, S.; Ledoux, I.; Zyss, J. Tetrahedral bipyridyl copper (I) complexes: A new class of non-dipolar chromophore for nonlinear optics. Chem. Commun. 1999, 871–872. [Google Scholar] [CrossRef]
- Akdas-Kilig, H.; Malval, J.P.; Morlet-Savary, F.; Singh, A.; Toupet, L.; Ledoux, I.; Zyss, J.; Le Bozec, H. The synthesis of tetrahedral bipyridyl metallo-octupoles with large second- and third-order nonlinear optical properties. Dye. Pigment. 2011, 92, 681–688. [Google Scholar] [CrossRef]
- Nitadori, H.; Ordronneau, L.; Boixel, J.; Jacquemin, D.; Boucekkine, A.; Singh, A.; Akita, M.; Ledoux, I.; Guerchais, V.; Le Bozec, H. Photoswitching of the second-order nonlinearity of a tetrahedral octupolar multi DTE-based copper (I) complex. Chem. Commun. 2012, 48, 10395–10397. [Google Scholar] [CrossRef]
- Colombo, A.; Di Carlo, G.; Dragonetti, C.; Magni, M.; Orbelli Biroli, A.; Pizzotti, M.; Roberto, D.; Tessore, F.; Benazzi, E.; Bignozzi, C.A.; et al. Coupling of Zinc Porphyrin Dyes and Copper Electrolytes: A Springboard for Novel Sustainable Dye-Sensitized Solar Cells. Inorg. Chem. 2017, 56, 14189–14197. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D.; Demartin, F.; Caramori, S.; Bignozzi, C.A. Efficient Copper Mediators Based on Bulky Asymmetric Phenanthrolines for DSSCs. ACS Appl. Mater. Interfaces 2014, 6, 13945–13955. [Google Scholar] [CrossRef] [PubMed]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis (acetonitirile) copper (I) Hexaflurorophosphate. Inorg. Synth. 1990, 28, 68–69. [Google Scholar]
- Singer, K.D.; Sohn, J.E.; King, L.A.; Gordon, H.M.; Katz, H.E.; Dirk, C.W. Second-order nonlinear-optical properties of donor- and acceptor-substituted aromatic compounds. J. Opt. Soc. Am. B 1989, 6, 1339–1350. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Guerchais, V.; Roisnel, T.; Marinotto, D.; Fantacci, S. Multifunctional Organometallic Compounds: An Interesting Luminescent NLO-Active Alkynylplatinum (II) Complex. Eur. J. Inorg. Chem. 2024, 27, e202400478. [Google Scholar]
- Kanis, D.R.; Lacroix, P.G.; Ratner, M.A.; Marks, T.J. Electronic Structure and Quadratic Hyperpolarizabilities in Organotransition-Metal Chromophores Having Weakly Coupled. pi.-Networks. Unusual Mechanisms for Second-Order Response. J. Am. Chem. Soc. 1994, 116, 10089–10102. [Google Scholar] [CrossRef]
- Valore, A.; Cariati, E.; Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; De Angelis, F.; Fantacci, S.; Sgamellotti, A.; Macchioni, A.; et al. Cyclometalated IrIII Complexes with Substituted 1,10-Phenanthrolines: A New Class of Efficient Cationic Organometallic Second-Order NLO Chromophores. Chem. Eur. J. 2010, 16, 4814–4825. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://internationalcopper.org/resource/circular-copper-building-a-culture-of-sustainability/ (accessed on 15 December 2024).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Bois-Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11 – 18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third-row atoms. J. Chem. Phys. 1970, 52, 1033. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Willetts, A.; Rice, J.E.; Burland, D.M.; Shelton, D.P.J. Problems in the comparison of theoretical and experimental hyperpolarizabilities. Chem. Phys. 1992, 97, 7590–7599. [Google Scholar] [CrossRef]
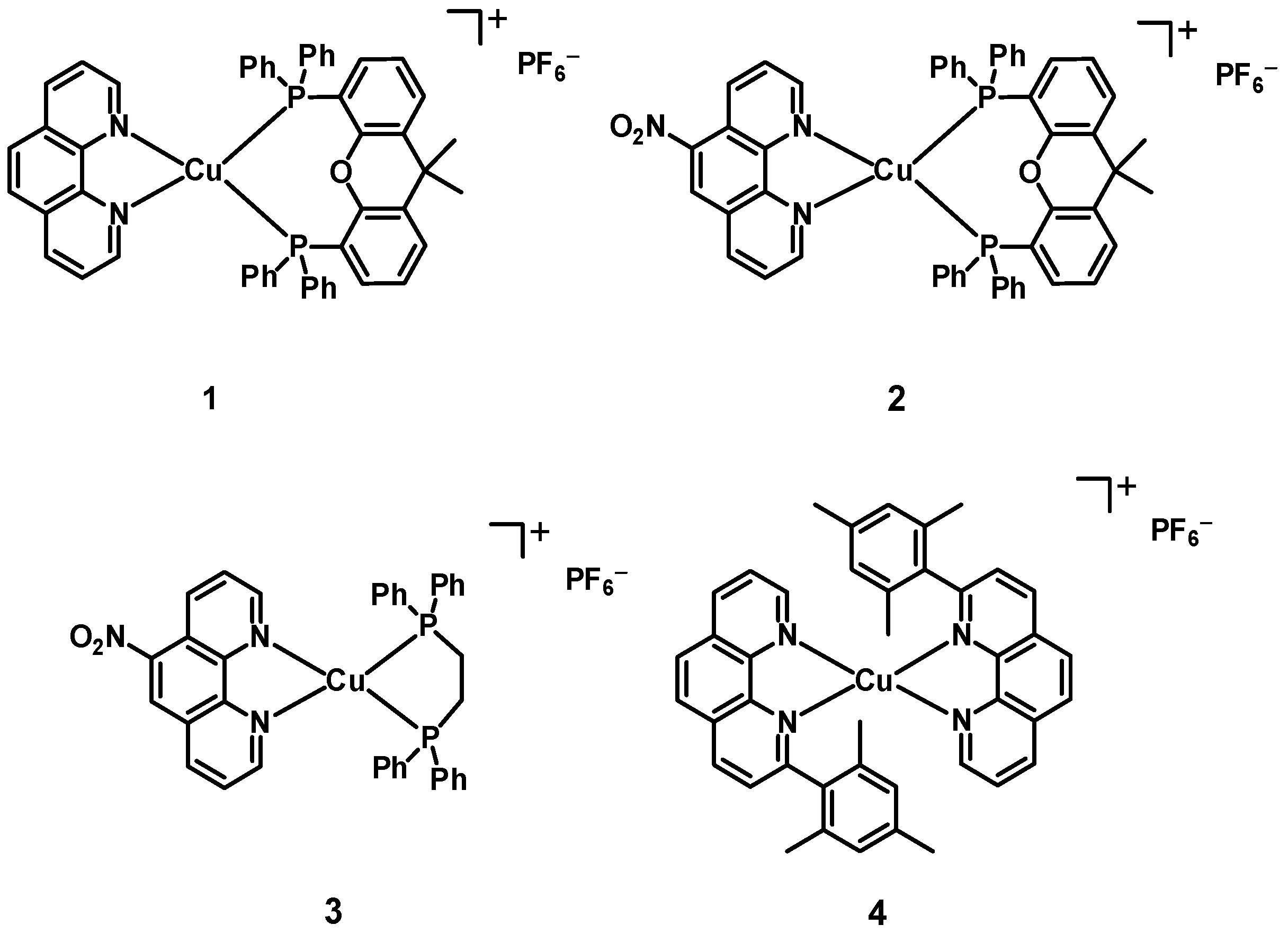

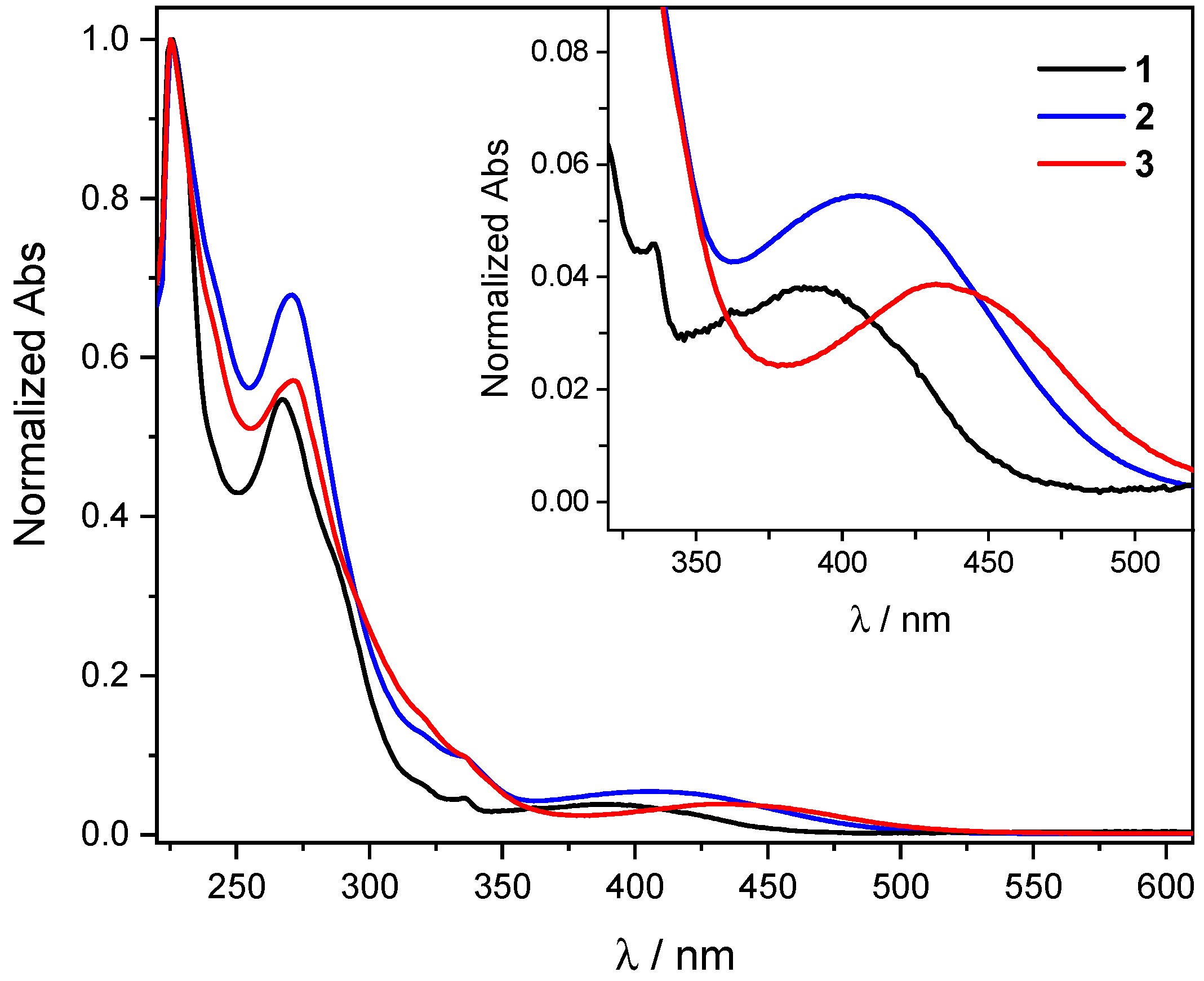

| Complex | λmax,abs/nm [ε/103 M−1 cm−1] | μβ/10−48 esu a |
|---|---|---|
| 1 | 388 [3.4] b | 995 |
| 2 | 405 [7.0] b | 1100 |
| 3 | 434 [4.6] b | 957 |
| 4 | 451 [3.6] c | 960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Fantacci, S. Dipolar Copper(I) Complexes: A Novel Appealing Class of Highly Active Second-Order NLO-Phores. Molecules 2025, 30, 1009. https://doi.org/10.3390/molecules30051009
Colombo A, Dragonetti C, Fagnani F, Roberto D, Fantacci S. Dipolar Copper(I) Complexes: A Novel Appealing Class of Highly Active Second-Order NLO-Phores. Molecules. 2025; 30(5):1009. https://doi.org/10.3390/molecules30051009
Chicago/Turabian StyleColombo, Alessia, Claudia Dragonetti, Francesco Fagnani, Dominique Roberto, and Simona Fantacci. 2025. "Dipolar Copper(I) Complexes: A Novel Appealing Class of Highly Active Second-Order NLO-Phores" Molecules 30, no. 5: 1009. https://doi.org/10.3390/molecules30051009
APA StyleColombo, A., Dragonetti, C., Fagnani, F., Roberto, D., & Fantacci, S. (2025). Dipolar Copper(I) Complexes: A Novel Appealing Class of Highly Active Second-Order NLO-Phores. Molecules, 30(5), 1009. https://doi.org/10.3390/molecules30051009