Construction of ZnO/r-GO Composite Photocatalyst for Improved Photodegradation of Organic Pollutants
Abstract
1. Introduction
2. Results and Discussion
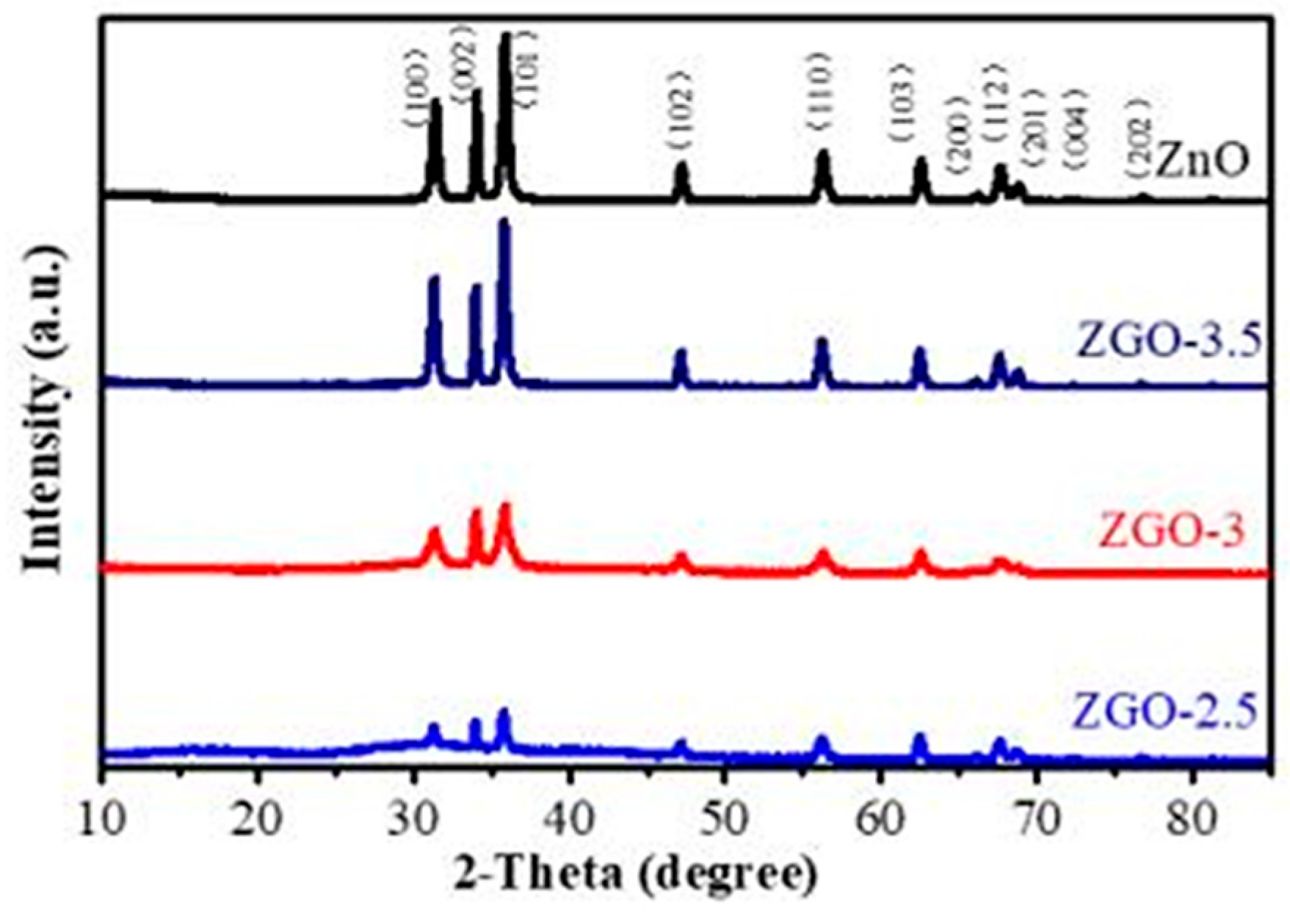
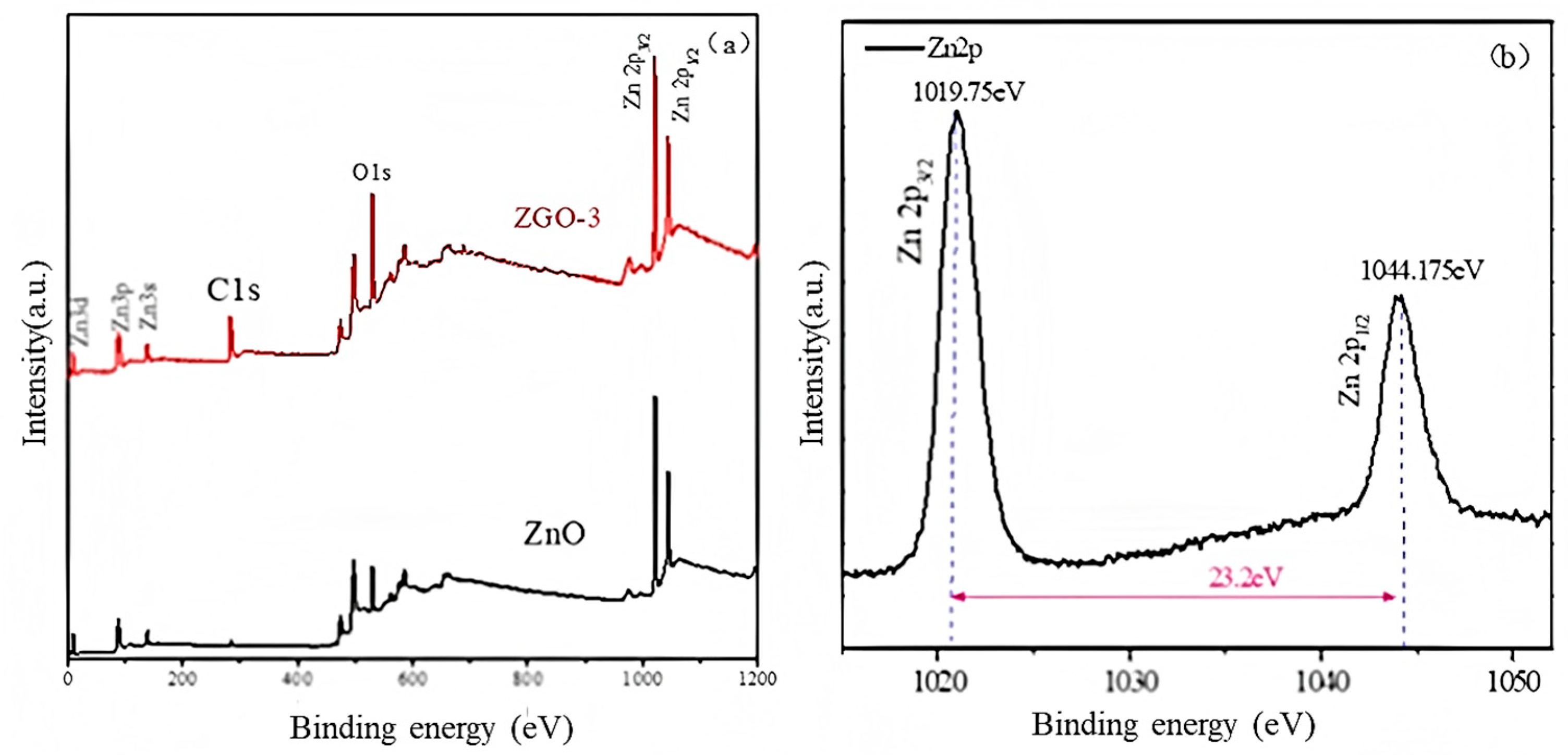
2.1. Structure and Physical Properties
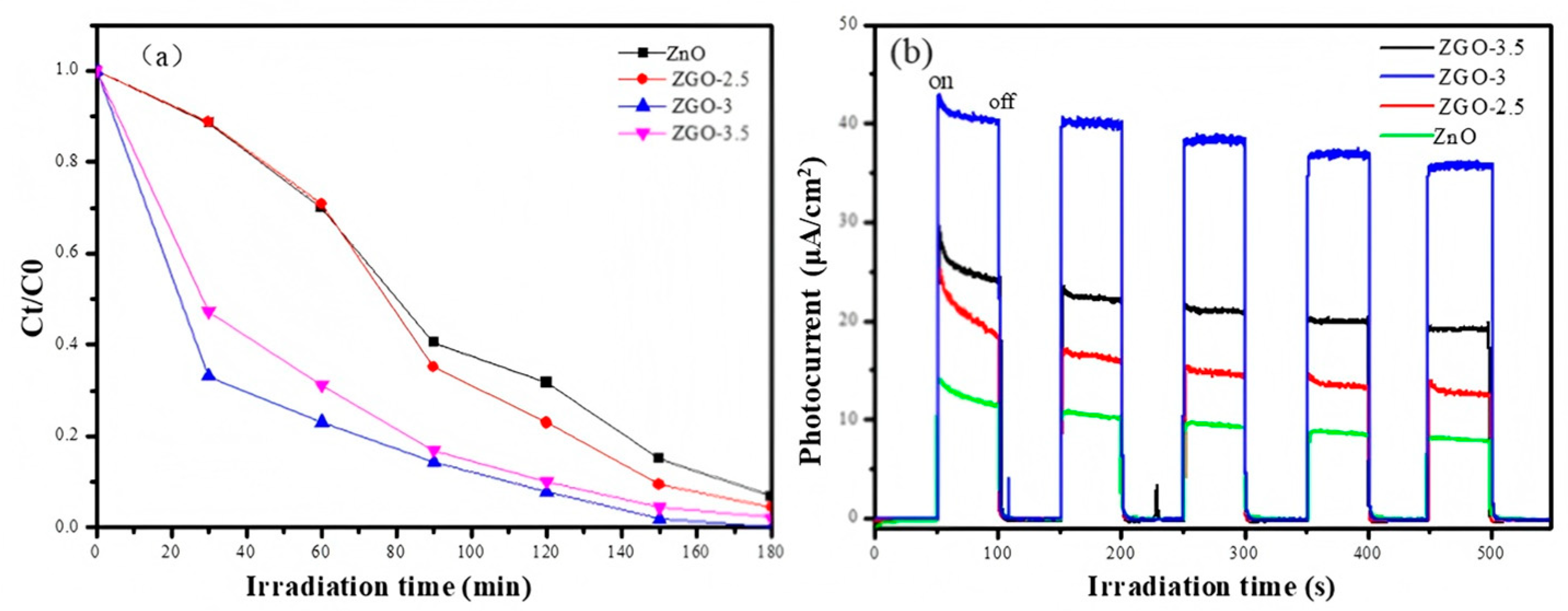
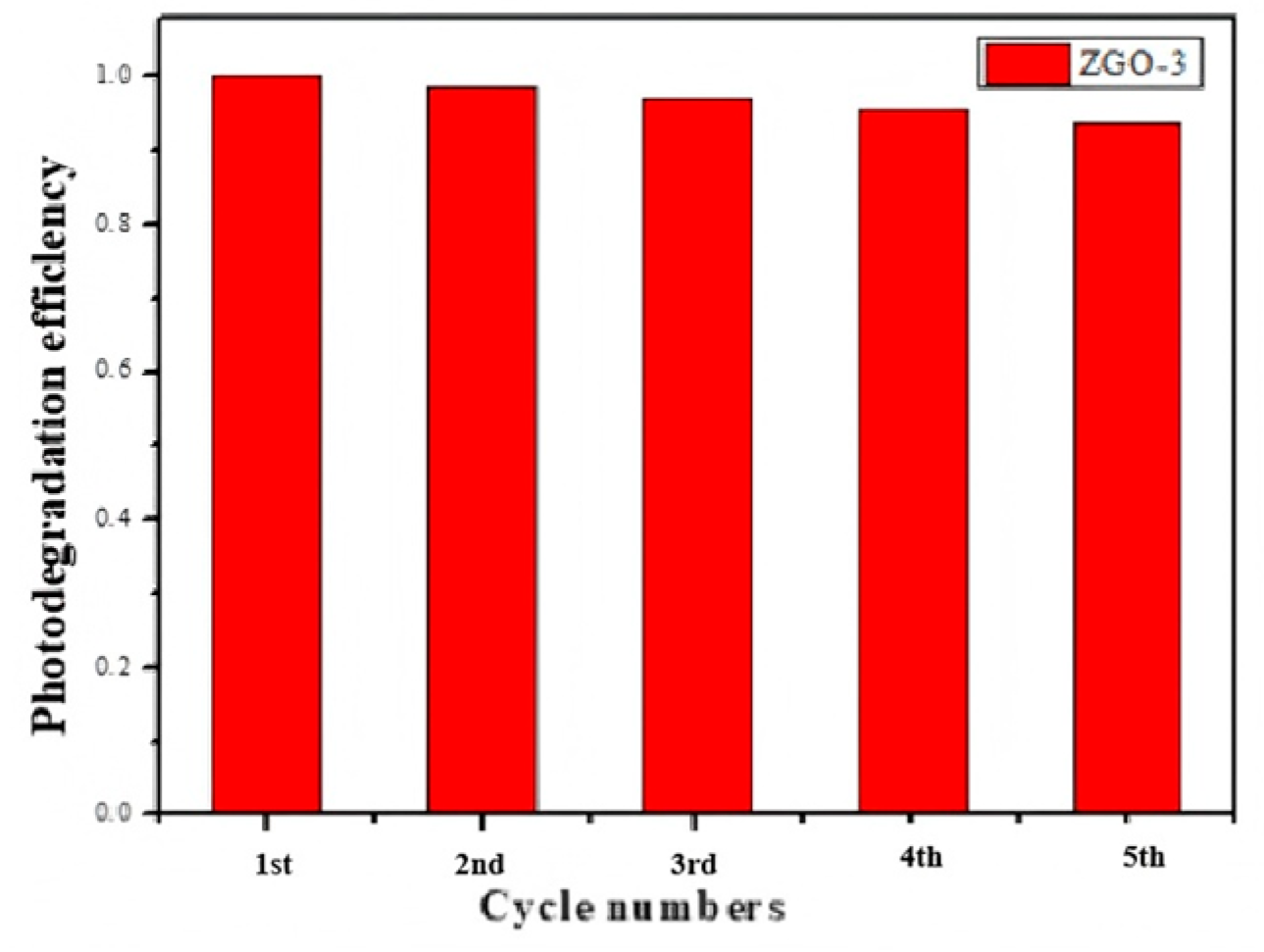
2.2. Catalytic Analysis
3. Experiment
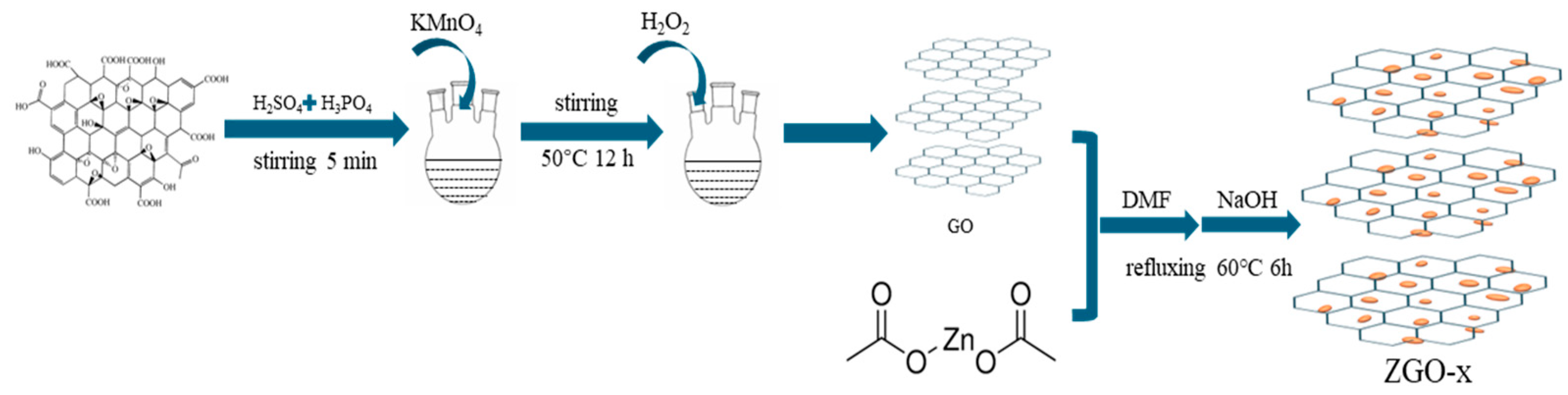
3.1. Synthesis of r-GO
3.2. Synthesis of ZnO Nanoparticles/r-GO
3.3. Catalyst Characterization
3.4. Catalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.; Khiadani, M.; Zhong, Y.; Wang, W.; Su, C.; Shao, Z. Prussian blue-conjugated ZnO nanoparticles for near-infrared light-responsive photocatalysis-ScienceDirect. Mater. Today Energy 2021, 23, 100895. [Google Scholar] [CrossRef]
- Smazna, D.; Shree, S.; Polonskyi, O.; Lamaka, S.; Baum, M.; Zheludkevich, M.; Faupel, F.; Adelung, R.; Mishra, Y.K. Mutual Interplay of ZnO Micro- and Nanowires and Methylene Blue during Cyclic Photocatalysis Process. J. Environ. Chem. Eng. 2019, 7, 103016. [Google Scholar] [CrossRef]
- Nawaz, R.; Ullah, H.; Ghanim, A.A.J.; Irfan, M.; Anjum, M.; Rahman, S.; Ullah, S.; Baki, Z.A.; Oad, V.K. Green Synthesis of ZnO and Black TiO2 Materials and Their Application in Photodegradation of Organic Pollutants. ACS Omega 2023, 8, 36076–36087. [Google Scholar] [CrossRef] [PubMed]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2016, 418, 103–112. [Google Scholar] [CrossRef]
- Ji, X.; Chen, L.; Xu, M.X.; Done, M. Crystal Imperfection Modulation Engineering for Functionalization of Wide Band Gap Semiconductor Radiation Detector. Adv. Electron. Mater. 2018, 4, 1700307. [Google Scholar] [CrossRef]
- Padmanaban, V.C.; Nandagopal, M.S.G.; Priyadharshini, G.M.; Maheswari, N.; Sree, G.J.; Selvaraju, N. Advanced approach for degradation of recalcitrant by nanophotocatalysis using nanocomposites and their future perspectives. Int. J. Environ. Sci. Technol. 2016, 13, 1591–1606. [Google Scholar] [CrossRef]
- Guo, J.; Si, M.; Zhao, X.; Wang, L.; Wang, K.; Hao, J.; Wang, H.; Randall, C.A. Altering interfacial properties through the integration of C-60 into ZnO ceramic via cold sintering process. Carbon 2022, 190, 255–261. [Google Scholar] [CrossRef]
- Aydogan, S.; Canpolat, N.; Kocyigit, A.; Yilmaz, M. Synergistic enhancement of simazine degradation using ZnO nanosheets and ZnO/GO nanocomposites: A sol-gel synthesis approach. Ceram. Int. 2024, 50, 25080–25094. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Highly efficient visible-light-driven plasmonic photocatalysts based on graphene oxide-hybridized one-dimensional Ag/AgCl heteroarchitectures. J. Mater. Chem. 2012, 22, 21487. [Google Scholar] [CrossRef]
- He, H.; Cheng, Y.; Qiu, S.; Sun, L.; Jin, B.; Yuan, X. Construction and mechanistic insights of a novel ZnO functionalized rGO composite for efficient adsorption and reduction of Cr(VI). Environ. Sci. Pollut. Res. 2024, 31, 34607–34621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Shang, J.; Peng, J.; Zhu, T. Construction of a novel metal-free heterostructure photocatalyst PRGO/TP-COF for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. Energy 2024, 350, 123937. [Google Scholar] [CrossRef]
- Rodwihok, C.; Wongratanaphisan, D.; Ngo, Y.L.T.; Khandelwal, M.; Hur, S.H.; Chung, J.S. Effect of GO Additive in ZnO/rGO Nanocomposites with Enhanced Photosensitivity and Photocatalytic Activity. Nanomaterials 2019, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, C.; Hristea, G.; Iordoc, M.; Sbarcea, B.-G.; Marinescu, V. Hydrothermal growth of ZnO/GO hybrid as an efficient electrode material for supercapacitor applications. Scr. Mater. 2021, 195, 113708. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Kiran, G.R.; Acharyya, S.G. Remarkable performance of GO/ZnO nanocomposites under optimized parameters for remediation of Cd (II) from water. Appl. Surf. Sci. 2023, 626, 157238. [Google Scholar] [CrossRef]
- Prabhuraj, T.; Prabhu, S.; Dhandapani, E.; Duraisamy, N. Bifunctional ZnO sphere/r-GO composites for supercapacitor and photocatalytic activity of organic dye degradation. Diam. Relat. Mater. 2021, 120, 108592. [Google Scholar] [CrossRef]
- Kumar, P.; Som, S.; Pandey, M.K.; Das, S.; Chanda, A.; Singh, J. Investigations on optical properties of ZnO decorated graphene oxide (ZnO@GO) and reduced graphene oxide (ZnO@r-GO). J. Alloys Compd. 2018, 744, 64–74. [Google Scholar] [CrossRef]
- Jammula, R.K.; Srikanth, V.V.; Hazra, B.K.; Srinath, S. ZnO nanoparticles’ decorated reduced-graphene oxide: Easy synthesis, unique polarization behavior, and ionic conductivity. Mater. Des. 2016, 110, 311–316. [Google Scholar] [CrossRef]
- Khan, H. Graphene based semiconductor oxide photocatalysts for photocatalytic hydrogen (H2) production, a review. Int. J. Hydrog. Energy 2014, 84, 356–371. [Google Scholar] [CrossRef]
- Kim, I.D.; Choi, S.J.; Cho, H.J. Graphene-Based Composite Materials for Chemical Sensor Application. In Electrospinning for High Performance Sensors; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Shanmugam, M.; Lee, Y.R. Direct solvothermal synthesis of zinc oxide nanoparticle decorated graphene oxide nanocomposite for efficient photodegradation of azodyes. J. Photochem. Photobiol. A Chem. 2017, 337, 100–111. [Google Scholar] [CrossRef]
- Ma, W.X.; Lv, M.L.; Cao, F.P.; Fang, Z. Synthesis and characterization of ZnO-GO composites with their piezoelectric catalytic and antibacterial properties. J. Environ. Chem. Eng. 2022, 10, 107840. [Google Scholar] [CrossRef]
- Elumalai, N.; Prabhu, S.; Selvaraj, M.; Silambarasan, A.; Navaneethan, M.; Harish, S.; Ramu, P.; Ramesh, R. Enhanced photocatalytic activity of ZnO hexagonal tube/rGO composite on degradation of organic aqueous pollutant and study of charge transport properties. Chemosphere 2021, 291, 132782. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chang, G.; Chen, S.; Liu, T.; Xia, Y.; Chen, C.; Yang, D. Alginate/r-GO assisted synthesis of ultrathin LiFePO4 nanosheets with oriented (010) facet and ultralow antisite defect. Chem. Eng. J. 2018, 351, 340–347. [Google Scholar] [CrossRef]
- Dadashov, S.; Demirel, E.; Suvaci, E. Tailoring microstructure of polysulfone membranes via novel hexagonal ZnO particles to achieve improved filtration performance. J. Membr. Sci. 2022, 651, 120462. [Google Scholar] [CrossRef]
- Puneetha, J.; Nagaraju, K.; Rathna, A. Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg. Chem. Commun. 2021, 125, 108460. [Google Scholar]
- Wang, X.; Tian, H.; Cui, X.; Zheng, W.; Liu, Y. One-pot hydrothermal synthesis of mesoporous Zn(x)Cd(1-x)S/reduced graphene oxide hybrid material and its enhanced photocatalytic activity. Dalton Trans 2014, 43, 12894–12903. [Google Scholar] [CrossRef]
- Ivan, R.; Popescu, C.; Pino, A.P.; Logofatu, C. Carbon–based nanomaterials and ZnO ternary compound layers grown by laser technique for environmental and energy storage applications. Appl. Surf. Sci. 2020, 509, 145359. [Google Scholar] [CrossRef]
- He, H.Y.; Fei, J.; Lu, J. High photocatalytic and photo-Fenton-like activities of ZnO–reduced graphene oxide nanocomposites in the degradation of malachite green in water. Iet Micro Nano Lett. 2015, 10, 389–394. [Google Scholar] [CrossRef]
- Mukwevho, N.; Gusain, R.; Kankeu, E.F.; Kumar, N. Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite. J. Ind. Eng. Chem. 2020, 81, 393–404. [Google Scholar] [CrossRef]
- Rani, G.J.; Rajan, M.A.J.; Kumar, G.G. Reduced graphene oxide/ZnFe2O4 nanocomposite as an efficient catalyst for the photocatalytic degradation of methylene blue dye. Res. Chem. Intermed. 2016, 43, 1–22. [Google Scholar]
- Reza, G.M.; Dinh, C.T.; Béland, F.; Do, T.-O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, S.; Ding, Y.; Tang, S.; Chen, H.; Chen, J.; Liu, J.; Yang, Z.; Hu, X.; Yuan, A. Excellent porous environmental nanocatalyst: Tactically integrating size-confined highly active MnOx in nanospaces of mesopores enables the promotive catalytic degradation efficiency of organic contaminants. New J. Chem. 2019, 43, 19020–19034. [Google Scholar] [CrossRef]

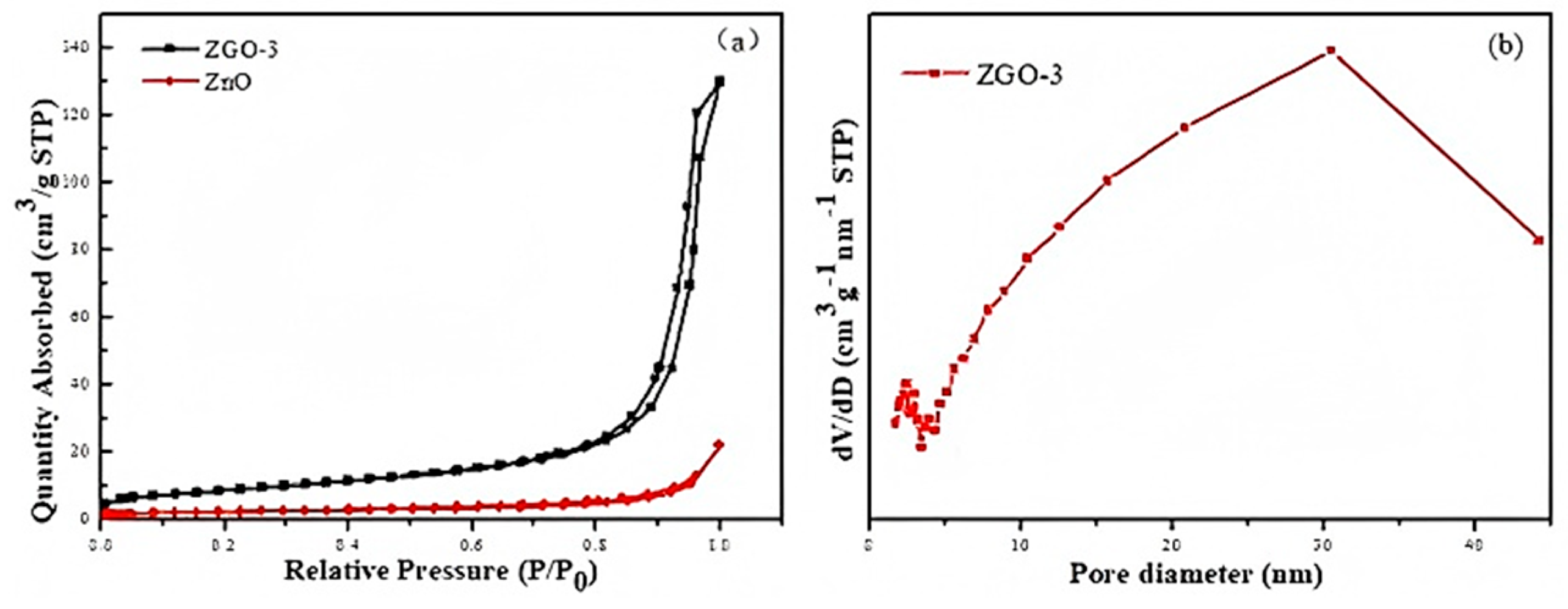
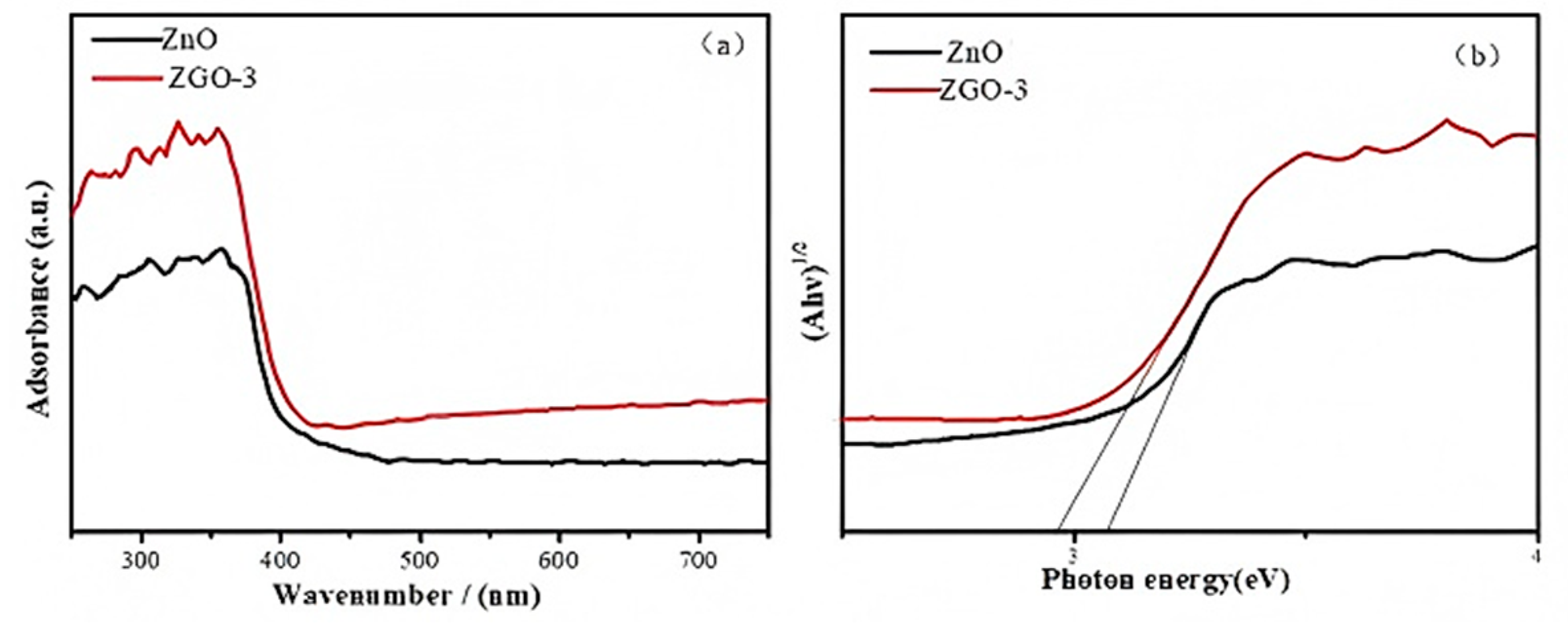


| Samples | SBET/(m2/g) | Va/(cm3/g) | Band Gap (eV) |
|---|---|---|---|
| ZnO | 8.1486 | 0.034482 | 3.172 |
| ZGO-3 | 31.5425 | 0.165617 | 2.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Qin, W.; Zhu, H.; Dai, Y.; Hong, X.; Han, S.; Xie, Y. Construction of ZnO/r-GO Composite Photocatalyst for Improved Photodegradation of Organic Pollutants. Molecules 2025, 30, 1008. https://doi.org/10.3390/molecules30051008
Ding Y, Qin W, Zhu H, Dai Y, Hong X, Han S, Xie Y. Construction of ZnO/r-GO Composite Photocatalyst for Improved Photodegradation of Organic Pollutants. Molecules. 2025; 30(5):1008. https://doi.org/10.3390/molecules30051008
Chicago/Turabian StyleDing, Yun, Wenzhen Qin, Huihua Zhu, Yuhua Dai, Xiaowei Hong, Suqin Han, and Yu Xie. 2025. "Construction of ZnO/r-GO Composite Photocatalyst for Improved Photodegradation of Organic Pollutants" Molecules 30, no. 5: 1008. https://doi.org/10.3390/molecules30051008
APA StyleDing, Y., Qin, W., Zhu, H., Dai, Y., Hong, X., Han, S., & Xie, Y. (2025). Construction of ZnO/r-GO Composite Photocatalyst for Improved Photodegradation of Organic Pollutants. Molecules, 30(5), 1008. https://doi.org/10.3390/molecules30051008







