Kinetics of Photodegradation and Durability of Inkjet Prints: A Comparative Study of Aqueous Solutions and Printed Substrates
Abstract
1. Introduction
2. Results and Discussion
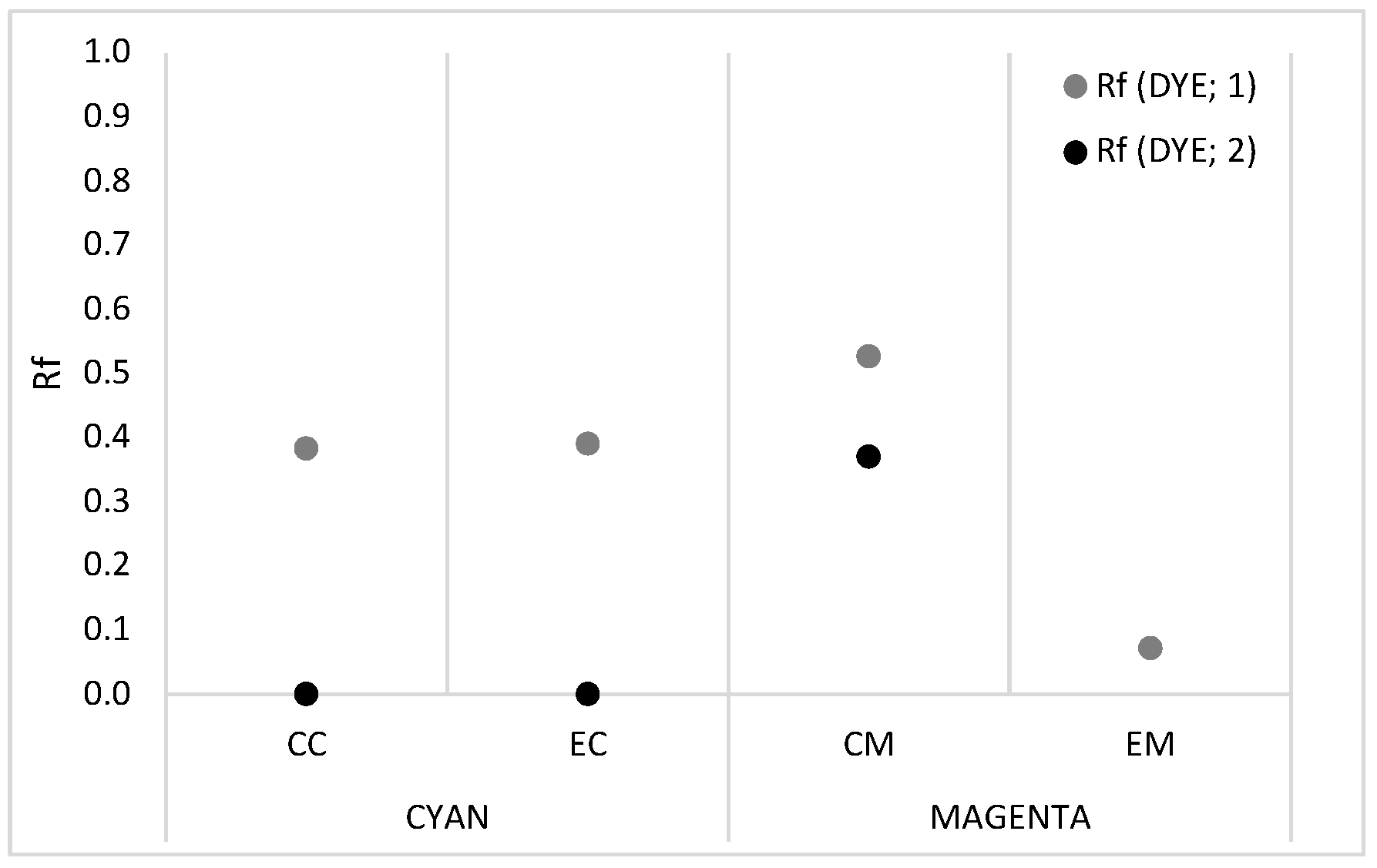
2.1. Chromatography
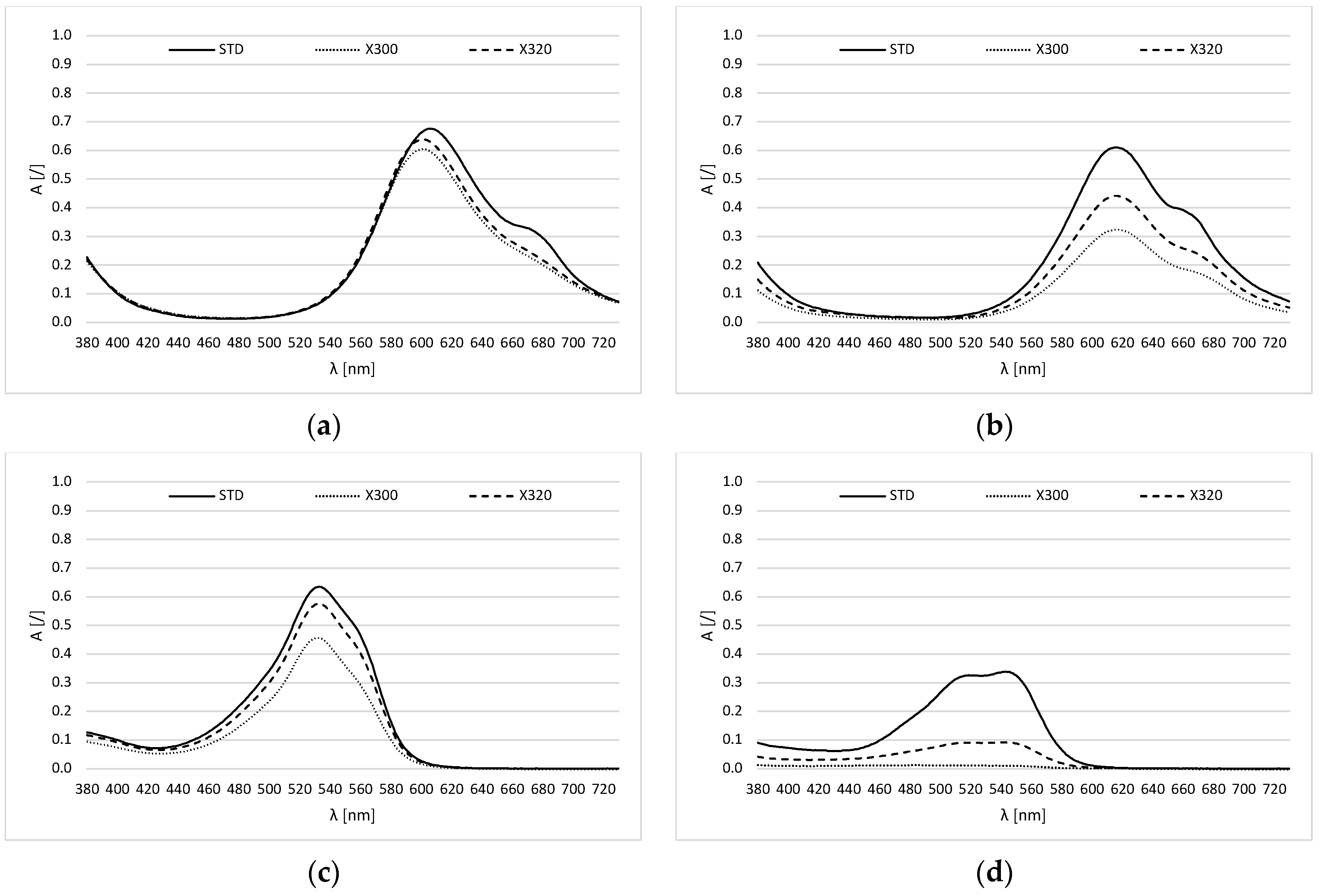
2.2. Photodegradation of Ink in Aqueous Solution
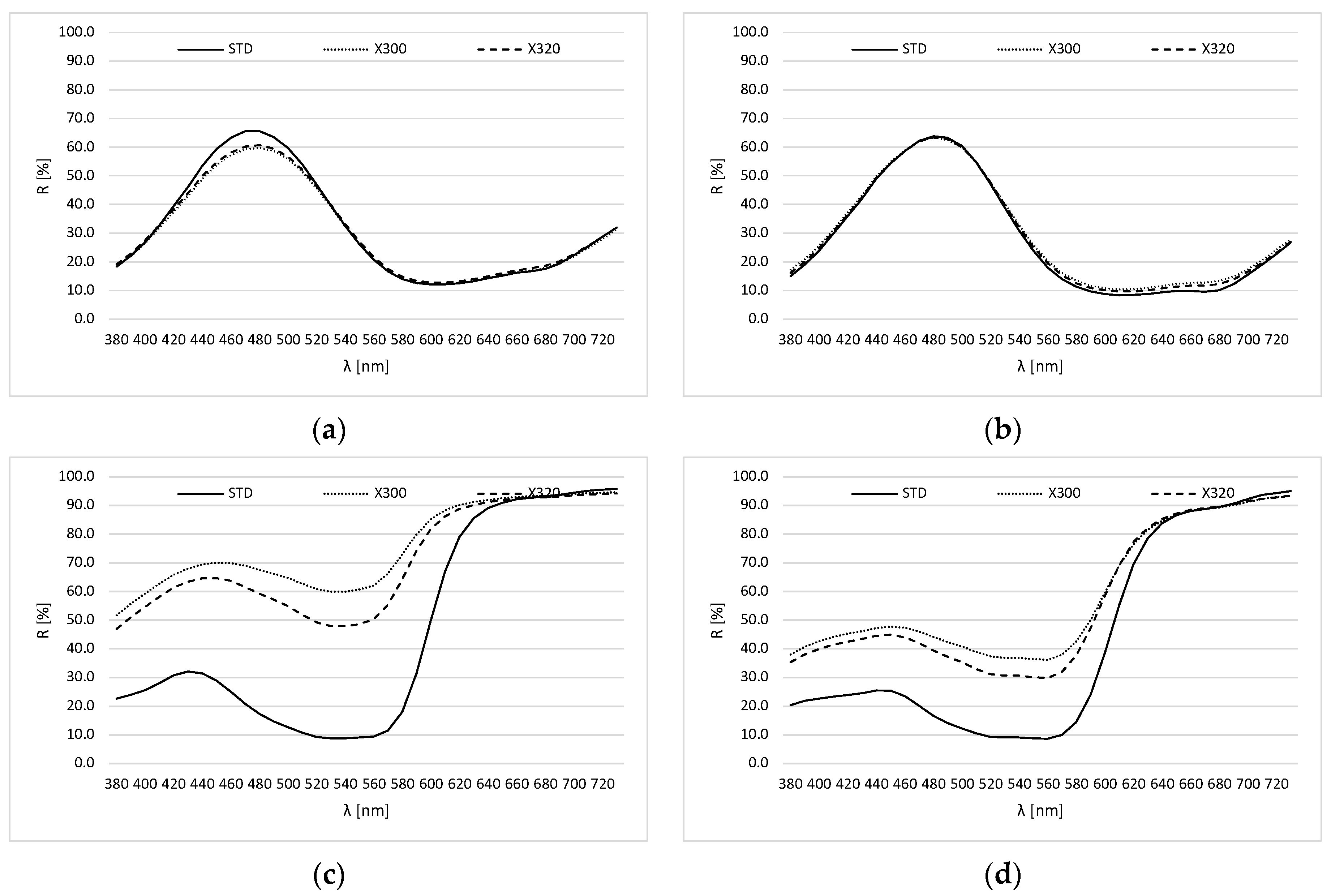
2.3. Photodegradation of Ink on the Substrate
3. Materials and Methods
3.1. Inkjet Inks and Preparation of Samples
3.2. Paper
3.3. Chromatography (TLC and GC/MS)
3.4. Lightfastness of Colour Samples
3.5. Spectrophotometrical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vikman, K. Studies on Fastness Properties of Ink Jet Prints on Coated Papers. Ph.D. Thesis, Helsinki University of Technology Helsinki, Finland, 2004; p. 49. [Google Scholar]
- Mirković Bolanča, I.; Medek, G.; Bolanča, Z.; Reháková, M. Environmentally Sustainable Offset Prints Exposed to Thermal Aging and NO2. Sustainability 2024, 16, 1681. [Google Scholar] [CrossRef]
- Vogt, B. Stability Issues and Test Methods for Ink JET Materials. Ph.D. Thesis, University of Applied Science, Department of Image Engineering, Cologne, Germany, 2001. [Google Scholar]
- Kandi, S.G. The effect of paper appearance on printed color of inkjet printer. Color Res. Appl. 2013, 38, 284–291. [Google Scholar] [CrossRef]
- Bugner, D.E. Papers and films for ink jet printing. In Handbook of Imaging Materials, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 603–627. [Google Scholar]
- Sun, J.Z.; Sun, R.; Jia, P.; Ma, M.D.; Song, Y.L. Fabricating flexible conductive structures by printing techniques and printable conductive materials. J. Mater. Chem. C 2022, 10, 9441–9464. [Google Scholar] [CrossRef]
- Escobar, L.A.; Meeker, W.Q. A Review of accelerated test models. Stat. Sci. 2006, 21, 552–577. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Material Weathering, 4th ed.; ChemTec Publishing: Toronto, ON, Canada, 2008; p. 810. [Google Scholar]
- Menart, E.; De Bruin, G.; Strlič, M. Dose–response functions for historic paper. Polym. Degrad. Stab. 2011, 96, 2029–2039. [Google Scholar] [CrossRef]
- Pugh, S.L.; Guthrie, J.T. Some characteristics of pigments that affect the kinetics of fading of prints made from water-based liquid ink formulations. Dye. Pigment. 2002, 55, 109–121. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerated Aging: Photochemical and Thermal Aspects; Getty Conservation Institute: Los Angeles, CA, USA, 1994; p. 280. [Google Scholar]
- Provost, J.; Lavery, A. Interactions of Digital Inks with Textile and Paper Substrates in Ink Jet Printing; Provost Ink Jet Consulting Ltd.: Chorley, Lancashire, 2009; p. 16. [Google Scholar]
- Herbst, W.; Hunger, K. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 87–98. [Google Scholar]
- Cristea, D.; Vilarem, G. Improving light fastness of natural dyes on cotton yarn. Dye. Pigment. 2006, 70, 238–245. [Google Scholar] [CrossRef]
- Donigian, W.D.; Wernett, C.P.; McFadden, G.M.; McKay, J.J. Ink jet dye fixation and coating pigments. In Coating Conference Proceedings; TAPPI Press: New Orleans, LA, USA, 1998; p. 514. [Google Scholar]
- Wnek, W.J.; Andreottola, M.A.; Doll, P.F.; Kelly, S.M. Ink jet ink technology. In Handbook of Imaging Materials, 2nd ed.; Diamond, A.S., Weiss, D.S., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 531–602. [Google Scholar]
- Jürgens, M.C. Preservation of Ink Jet Hardcopies: An Investigation by Martin C. Jürgens for the Capstone Project, Cross-Disciplinary Studies; At Rochester Institute of Technology; Rochester Institute of Technology: Rochester, NY, USA, 1999; p. 64. [Google Scholar]
- Doll, P.; Shi, F.; Kelly, S.; Wnek, W. The problem of catalytic fading with ink-jet inks. In Recent Progress in Ink Jet Technologies II; Hanson, E., Ed.; Society for Imaging Science and Technology: Springfield, VI, USA, 1999; pp. 325–328. [Google Scholar]
- Kim, B.H.; Danilov, E.; Yoon, S.J.; El-Shafei, A.; Freeman, H. Characterization of the photophysics of a mixed system of red disperse dyes using experimental and theoretical methods. Dye. Pigment. 2021, 184, 108745. [Google Scholar] [CrossRef]
- Fellers, C.; Iversen, T.; Lindstrom, T.; Nilsson, T.; Rigdahl, M. Ageing/Degradation of Paper: A Literature Survey; Swedish Pulp and Paper Research Institute: Stockholm, Sweden, 1989; p. 139. [Google Scholar]
- Strlič, M.; Kolar, J. Evaluating and enhancing paper stability–needs and recent trends. In Proceedings of the 5th EC Conference Cultural Heritage Research, Krakow, Poland, 16–18 May 2002; pp. 79–86. [Google Scholar]
- Cocca, M.; D’arienzo, L.; D’orazio, L. Effects of different artificial agings on structure and properties of Whatman paper samples. ISRN Mater. Sci. 2011, 2011, 863083. [Google Scholar] [CrossRef]
- Bolanča, Z.; Bolanča Mirković, I.; Majnarić, I. Ageing of substrate and the quality of digital prints. In Proccedings of International Congres of Imaging Science; Easterman, A.A.M., Ed.; Society of Imaging Science and Technology: Rochester, NY, USA, 2006; pp. 591–594. [Google Scholar]
- Bamfield, P. Chromic Phenomena: Technological Applications of Colour Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2001; p. 374. [Google Scholar]
- Brown, R.P. VAMAS Technical Report: A Review of Accelerated Durability Tests; VAMAS: Teddington, UK, 1995; p. 38. [Google Scholar]
- Kato, K.L.; Cameron, R.E. A review of the relationship between thermally-accelerated ageing of paper and horrification. Cellulose 1999, 6, 23–40. [Google Scholar] [CrossRef]
- Blaznik, B.; Kovač, F.; Bizjak, G.; Bračko, S. Fastness of dye-based ink-jet printing inks in aqueous solution in the presence and absence of oxygen. Color Res. Appl. 2022, 47, 1193–1199. [Google Scholar] [CrossRef]
- Bevk, E.; Blaznik, B.; Bračko, S. Impact of protective glass on photodegradation of ink-jet printed documents. J. Cult. Herit. 2023, 62, 356–362. [Google Scholar] [CrossRef]
- Pekarovicova, A.; Husovska, V. Printing ink formulations. In Printing on Polymers: Fundamentals and Applications; Izdebska, J., Thomas, S., Eds.; Elsevier: Oxford, UK, 2016; pp. 41–56. [Google Scholar]
- Svanholm, E. Printability and Ink-Coating Interactions in Inkjet Printing. Ph.D. Thesis, Karlstad University Studies, Karlstad, Sweden, 2007; p. 58. [Google Scholar]
- Foucher, D.A.; Sacripante, G.G.; Wong, R.W.; Breton, M.P. Ink Compositions. Canada Patent US5969003A, 2 February 1998. [Google Scholar]
- Shi, F.; Doll, P.; Wnek, W.; Andreottola, M. Lightfast Ink Jet Inks. Billerica, USA Patent US6641257B1, 28 January 2000. [Google Scholar]
- Chou, K.P.; Liu, Y.I.; LIN, Y.T. Red Colorant Composition and Magenta Inkjet Ink Composition with Stable Ph. Taiwan Patent US7125445B2, 29 September 2005. [Google Scholar]
- Cho, I.H.; Zoh, K.D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using a response surface methodology (RSM) based on the central composite design. Dye. Pigment. 2007, 75, 533–543. [Google Scholar] [CrossRef]
- Bauer, W.; Baumgart, D.; Zöller, W. Magneta dyes for ink jet applications. In Recent Progress in Ink Jet Technologies II; Hanson, E., Ed.; Society for Imaging Science and Technology: Springfield, VI, USA, 1999; pp. 474–480. [Google Scholar]
- Valentini, J.E.; Jackson, C.; Kung, K.H. Magenta Inkjet Ink and an Inkjet Ink Set Containing Same. Patent US8313186B2, 10 December 2008. [Google Scholar]
- Palenik, C.; Groves, E.; Palenik, S. Development of an Analytical System for the Forensic Comparison and Identification of Fiber Dyes on Casework-Sized Fibers; Office of Justice Programs’ National Criminal Justice Reference Service: Elgin, IL, USA, 2016; p. 16. [Google Scholar]
- EN ISO 9706; Information and Documentation—Paper for Documents—Requirements for Permanence. ISO: Geneva, Switzerland, 1994.
- ISO 11108; Information and Documentation—Archival Paper—Requirements for Permanence and Durability. ISO: Geneva, Switzerland, 1996.
- ISO 13655; Graphic Technology—Spectral Measurement and Colorimetric Computation for Graphic Arts Images. ISO: Geneva, Switzerland, 2017.
- CIE 15:2024 Technical Report–Colorimetry, 3rd ed.; CIE: Bilbao, Spain, 2024.

| Sample Name | λmax [nm] | X300 | X320 | ||
|---|---|---|---|---|---|
| IA [%] | t1/2 [h] | IA [%] | t1/2 [h] | ||
| CC | 610 | 87 | 693 | 91 | 1155 |
| EC | 641 | 53 | 144 | 72 | 301 |
| CM | 533 | 72 | 301 | 91 | 866 |
| EM | 544 | 3 | 28 | 28 | 75 |
| Sample Name | λmax [nm] | X300 | X320 | ||
|---|---|---|---|---|---|
| IA [%] | t1/2 [h] | IA [%] | t1/2 [h] | ||
| CC | 610 | 100 | 1155 | 97 | 1386 |
| EC | 640 | 91 | 1155 | 94 | 1733 |
| CM | 530 | 21 | 49 | 30 | 60 |
| EM | 540 | 42 | 80 | 49 | 99 |
| X300 | X320 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paper | CC | EC | CM | EM | paper | CC | EC | CM | EM | |
| ΔL* | 0.09 | −0.89 | 1.28 | 31.31 | 20.67 | 0.05 | −0.21 | 0.80 | 26.83 | 17.53 |
| Δa* | 0.16 | −0.15 | 2.65 | −44.02 | −30.09 | 0.19 | 0.37 | 1.57 | −39.90 | −24.01 |
| Δb* | −0.53 | 3.66 | 1.67 | 1.19 | −0.14 | −0.68 | 3.80 | 1.22 | −2.77 | −1.32 |
| ΔE*00 | 0.37 | 1.47 | 1.48 | 30.46 | 21.34 | 0.46 | 1.25 | 0.92 | 25.95 | 17.85 |
| Paper Characteristics | Paper |
|---|---|
| Grammage [g/m2] | 80 |
| Thickness [μm] | 102 |
| Ash content [%] | 11.7 |
| CIE whiteness | 81.85 |
| Yellowness | 5.83 |
| CIE L* | 97.79 |
| CIE a* | −0.19 |
| CIE b* | 2.83 |
| Roughness | 3.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaznik, B.; Kovač, F.; Bračko, S. Kinetics of Photodegradation and Durability of Inkjet Prints: A Comparative Study of Aqueous Solutions and Printed Substrates. Molecules 2025, 30, 968. https://doi.org/10.3390/molecules30040968
Blaznik B, Kovač F, Bračko S. Kinetics of Photodegradation and Durability of Inkjet Prints: A Comparative Study of Aqueous Solutions and Printed Substrates. Molecules. 2025; 30(4):968. https://doi.org/10.3390/molecules30040968
Chicago/Turabian StyleBlaznik, Barbara, Franci Kovač, and Sabina Bračko. 2025. "Kinetics of Photodegradation and Durability of Inkjet Prints: A Comparative Study of Aqueous Solutions and Printed Substrates" Molecules 30, no. 4: 968. https://doi.org/10.3390/molecules30040968
APA StyleBlaznik, B., Kovač, F., & Bračko, S. (2025). Kinetics of Photodegradation and Durability of Inkjet Prints: A Comparative Study of Aqueous Solutions and Printed Substrates. Molecules, 30(4), 968. https://doi.org/10.3390/molecules30040968






