Supercritical Fluid Extraction of Lipids from Rowanberry Pomace with Pure CO2 and Its Mixtures with Ethanol Followed by the On-Line Separation of Fractions
Abstract
1. Introduction
2. Results and Discussion
2.1. The Yields of Extracts, Fractions and β-Carotene
2.2. Composition of Fatty Acids and Triacylglycerols (TAGs)
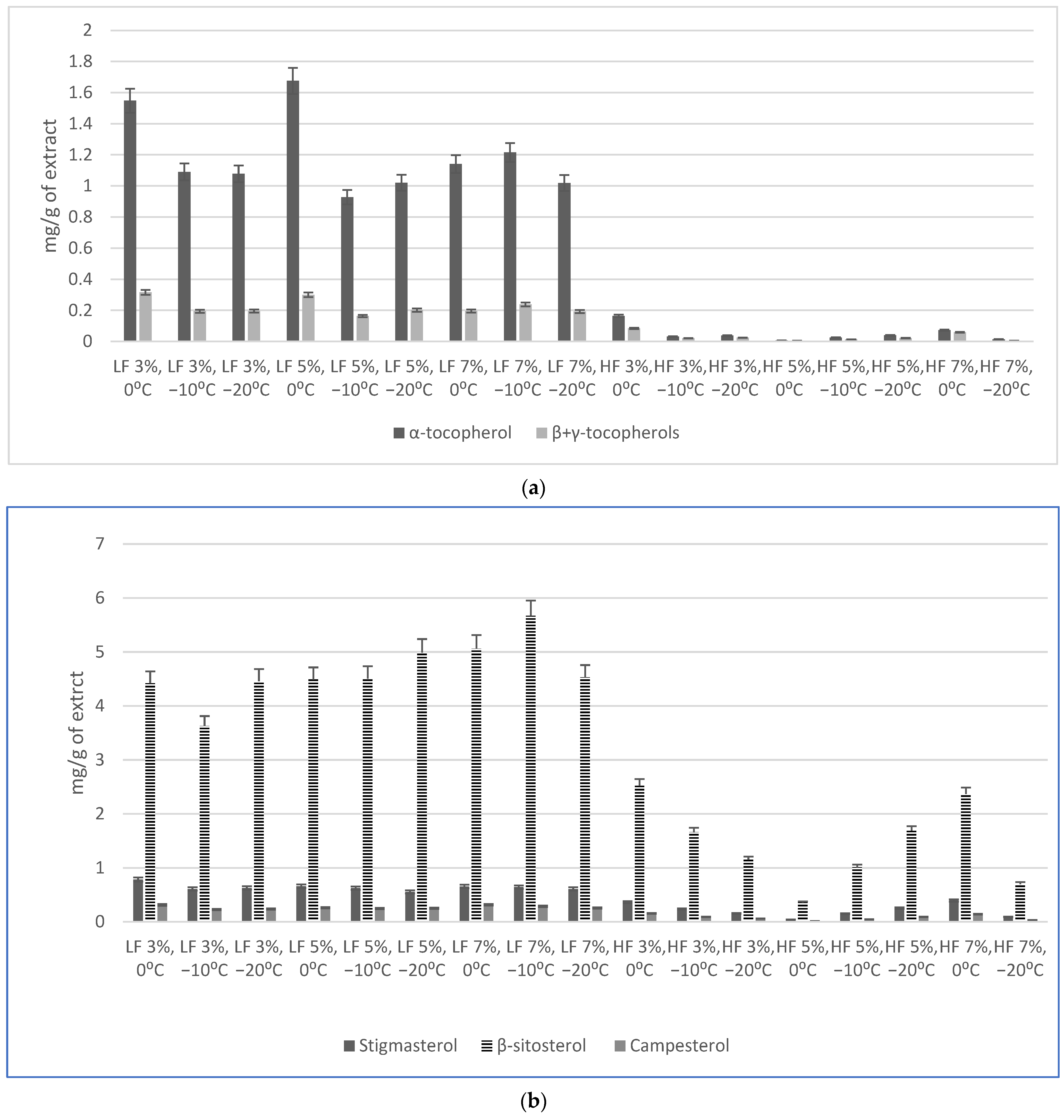
2.3. Tocopherols and Phytosterols
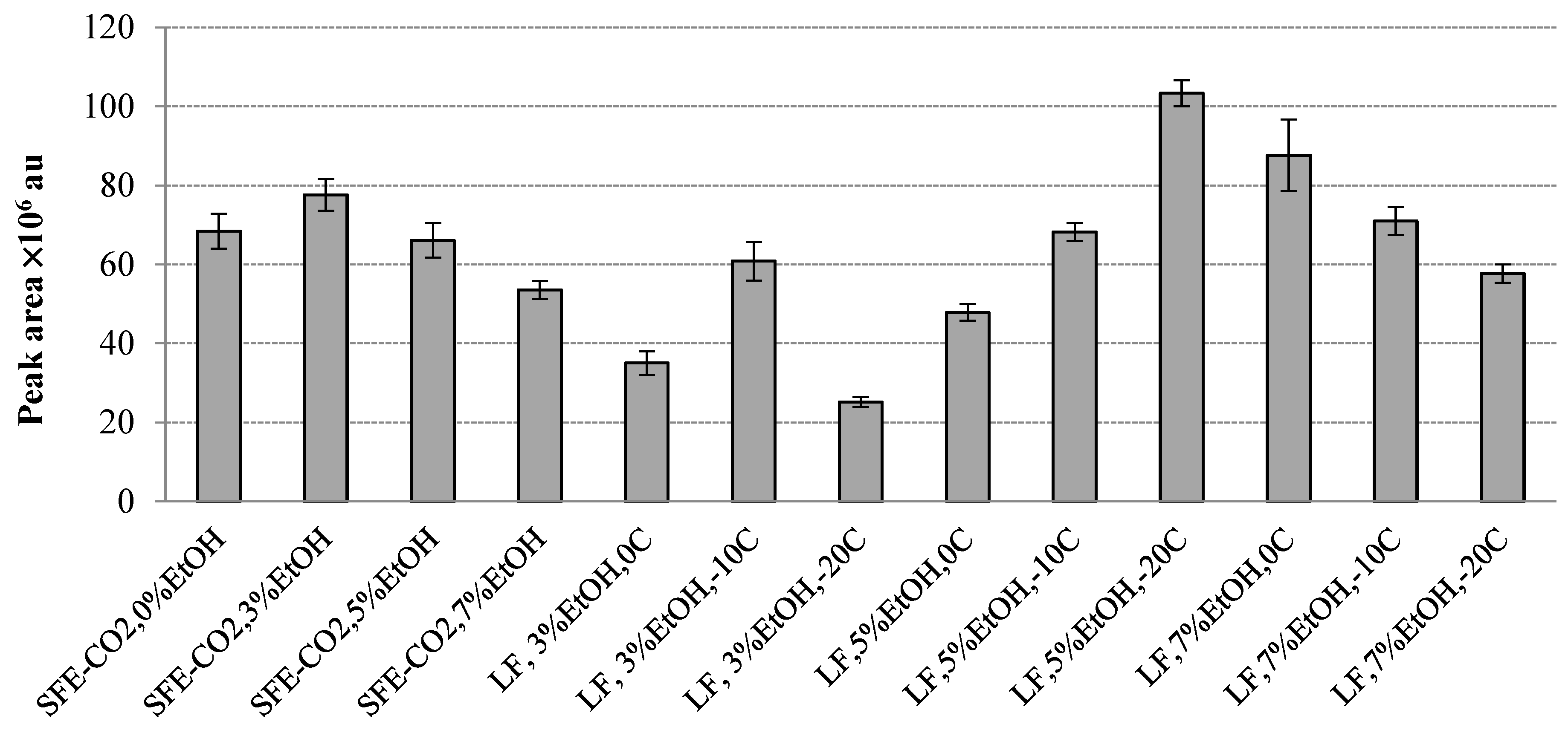
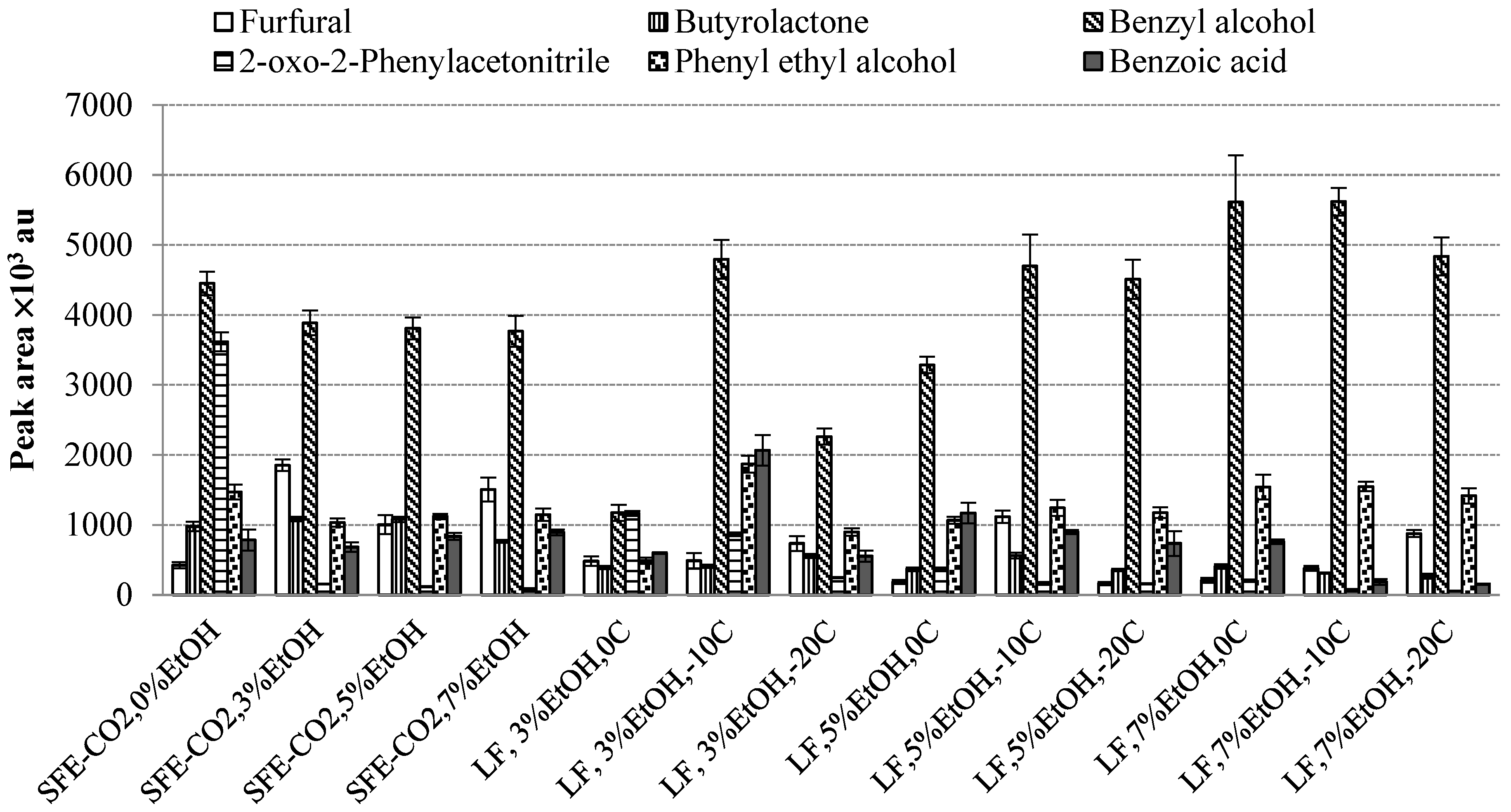
2.4. Volatile Constituents
3. Materials and Methods
3.1. Chemicals, Solvents and Gasses
3.2. Extraction and Fractionation of Rowanberry Pomace by SFE−CO2
3.3. Analysis of β-Carotene
3.4. Determination of Fatty Acids and Triacylglycerols (TAGs)
3.5. Determination of Tocopherols and Sterols
3.6. Evaluation of Volatile Aromatic Compounds
3.7. Statistical Data Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Farm to Fork Strategy; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Venskutonis, P.R. Berries. In Valorization of Fruit Processing By-products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–125. [Google Scholar]
- Górnaś, P.; Rudzińska, M. Seeds Recovered from Industry By-products of Nine Fruit Species with a High Potential Utility as a Source of Unconventional Oil for Biodiesel and Cosmetic and Pharmaceutical Sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Galanakis, C.M. Valorization of Fruit Processing By-products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128171066. [Google Scholar]
- Kryževičiūtė, N.; Kraujalis, P.; Venskutonis, P.R. Optimization of High Pressure Extraction Processes for the Separation of Raspberry Pomace into Lipophilic and Hydrophilic Fractions. J. Supercrit. Fluids 2016, 108, 61–68. [Google Scholar] [CrossRef]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of Black Chokeberry Pomace into Functional Ingredients Using High Pressure Extraction Methods and Evaluation of their Antioxidant Capacity and Chemical Composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of Bioactive Substances from Rowanberry Pomace by Consecutive Extraction with Supercritical Carbon Dioxide and Pressurized Solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Commission Regulation (EU). Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Official Journal of the European Union, L 136, 25 May 2012; Commission Regulation (EU): Brussels, Belgium, 2012; Volume 136, Available online: https://eur-lex.europa.eu/eli/reg/2012/432/oj/eng (accessed on 10 February 2025).
- Ryszczyńska, S.; Gumulak-Wołoszyn, N.; Urbaniak, M.; Stępień, Ł.; Bryła, M.; Twarużek, M.; Waśkiewicz, A. Inhibitory Effect of Sorbus aucuparia Extracts on the Fusarium proliferatum and F. culmorum Growth and Mycotoxin Biosynthesis. Molecules 2024, 29, 4257. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Porcedda, S.; Smeriglio, A.; Trombetta, D.; Nieddu, M.; Piras, F.; Sogos, V.; Rosa, A. Chemical Composition, Nutritional, and Biological Properties of Extracts Obtained with Different Techniques from Aronia melanocarpa Berries. Molecules 2024, 29, 2577. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Mazurek, B.; Tyśkiewicz, K.; Kondracka, M.; Wójcicka, G.; Blicharski, T.; Sowa, I. Blackcurrant (Ribes nigrum L.) Seeds—A Valuable Byproduct for Further Processing. Molecules 2022, 27, 8679. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Mazurek, B.; Kostrzewa, M.; Jóźwik, E. Carotenoids and Fatty Acids Obtained from Paprika Capsicum annuum by Supercritical Carbon Dioxide and Ethanol as Co-Extractant. Molecules 2023, 28, 5438. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Sarv, V.; Venskutonis, P.R.; Rätsep, R.; Aluvee, A.; Kazernavičiūtė, R.; Bhat, R. Antioxidants Characterization of the Fruit, Juice, and Pomace of Sweet Rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants 2021, 10, 1779. [Google Scholar] [CrossRef]
- Ivakhnov, A.D.; Sadkova, K.S.; Sobashnikova, A.S.; Skrebets, T.E. Optimization of Oil Extraction from Rowanberry Waste in Alcoholic Beverage Production. Russ. J. Phys. Chem. B 2019, 13, 1135–1138. [Google Scholar] [CrossRef]
- Tamkutė, L.; Pukalskas, A.; Syrpas, M.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Fractionation of Cranberry Pomace Lipids by Supercritical Carbon Dioxide Extraction and On-Line Separation of Extracts At Low Temperatures. J. Supercrit. Fluids 2020, 163, 104884. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical Fluid Technologies Applied to the Extraction of Compounds of Industrial Interest from Cannabis sativa L. and their Pharmaceutical Formulations: A Review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Mu, H.; Høy, C.E. The Digestion of Dietary Triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Analysis of Triacylglycerols in Refined Edible Oils by Isocratic HPLC-ESI-MS. Eur. J. Lipid Sci. Technol. 2010, 112, 844–851. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant. 2022, 44, 20. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Supercritical carbon dioxide extraction of squalene and tocopherols from amaranth and assessment of extracts antioxidant activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phyther. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Klavins, L.; Klavins, M. Cuticular Wax Composition of Wild and Cultivated Northern Berries. Foods 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Doležal, M.; Velíšek, J.; Famfulikova, P. Aroma of Less-Known Wild Fruits. In Proceedings of the Flavour Research at the Dawn of the Twenty-first Century-Proceedings of the 10th Weurman Flavour Research Symposium, Beaune, France, 25–28 June 2002; Editions Tec & Doc. pp. 576–579. [Google Scholar]
- Doležal, M.; Velíšek, J.; Famfulíková, P. Chemical Composition of Less-Known Wild Fruits. In Proceedings of the Biologically-active phytochemicals in food: Analysis, metabolism, bioavailability and function. Proceedings of the EUROFOODCHEM XI Meeting, Norwich, UK, 26–28 September 2001; Royal Society of Chemistry; pp. 241–244. [Google Scholar]
- Houlberg, S.; Westh, B.C.; Poll, L. The Aroma Composition of Ethanol Extracts of Rowanberries (Sorbus aucuparia). In Frontiers in Flavour Science, the Proceedings of the Ninth Weurman Flavour Research Symposium, Freising, Germany, 22–25 June 1999; Schieberle, P., Engel, K.-H., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2000; pp. 56–57. Available online: https://search.worldcat.org/title/46705039 (accessed on 10 February 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Adams, R.P., Ed.; Allured Publishing: Carol Stream, IL, USA; Allured Business Media: Carol Stream, IL, USA, 2017. [Google Scholar]
- Acree, T.; Arnold, H. Flavornet. Available online: http://www.flavornet.org (accessed on 28 July 2022).
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 26 January 2023).
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Adams, T.B.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS Assessment of Benzyl Derivatives Used as Flavor Ingredients. Food Chem. Toxicol. 2005, 43, 1207–1240. [Google Scholar] [CrossRef] [PubMed]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid Method for Determination of Total Fatty Acid Content and Composition of Feedstuffs and Feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Slavin, M.; Yu, L. A Single Extraction and HPLC Procedure for Simultaneous Analysis of Phytosterols, Tocopherols and Lutein in Soybeans. Food Chem. 2012, 135, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
| EtOH, % | Separator S1 Temp, °C | Yield in LF, % | β-Carotene in LF, mg/100 g | β-Carotene Recovery in S2 with LF, mg/100 g Pomace | β-Carotene in HF, mg/100 g |
|---|---|---|---|---|---|
| 3 | 0 | 1.87 ± 0.02 ab | 133.1 ± 2.2 a | 2.5 | 187.4 ± 0.1 l |
| −10 | 3.15 ± 0.10 cd | 174.1 ± 0.6 c | 5.5 | 48.94 ± 0.15 d | |
| −20 | 3.61 ± 0.38 de | 213.3 ± 1.6 e | 7.7 | 52.38 ± 0.09 e | |
| 5 | 0 | 2.92 ± 0.10 bcd | 145.1 ± 1.4 b | 4.2 | 131.5 ± 0.2 f |
| −10 | 5.53 ± 0.17 fgh | 219.0 ± 0.8 e | 12.1 | 17.82 ± 0.10 a | |
| −20 | 4.51 ± 0.27 ef | 236.3 ± 1.2 f | 10.7 | 31.39 ± 0.18 b | |
| 7 | 0 | 3.84 ± 0.37 de | 186.2 ± 1.5 d | 7.2 | 180.3 ± 0.1 k |
| −10 | 6.00 ± 0.42 gh | 178.2 ± 1.0 c | 10.7 | nd | |
| −20 | 7.75 ± 0.25 ij | 238.1 ± 0.6 f | 18.5 | 37.41 ± 0.28 c |
| No | TAG | 0% EtOH | 3% EtOH | 5% EtOH | 7% EtOH | Light Fraction (LF) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 °C | −10 °C | −20 °C | ||||||||||||
| 3% EtOH | 5% EtOH | 7% EtOH | 3% EtOH | 5% EtOH | 7% EtOH | 3% EtOH | 5% EtOH | 7% EtOH | ||||||
| 1 | LLLn | 1.76 ± 0.01 a | 1.59 ± 0.05 a | 1.64 ± 0.03 a | 1.65 ± 0.03 a | 1.74 ± 0.02 a | 1.62 ± 0.08 a | 1.74 ± 0.06 a | 1.71 ± 0.08 a | 1.62 ± 0.11 a | 1.69 ± 0.03 a | 1.63 ± 0.01 a | 1.62 ± 0.08 a | 1.57 ± 0.01 a |
| 2 | SLO | 3.09 ± 0.06 a | 3.18 ± 0.01 ab | 3.35 ± 0.14 ab | 3.66 ± 0.21 b | 3.24 ± 0.12 ab | 3.30 ± 0.05 ab | 3.49 ± 0.26 ab | 3.45 ± 0.01 ab | 3.38 ± 0.13 ab | 3.37 ± 0.11 ab | 3.36 ± 0.15 ab | 3.45 ± 0.06 ab | 3.43 ± 0.06 ab |
| 3 | PLLn | 1.04 ± 0.02 d | 0.92 ± 0.00 cd | 0.88 ± 0.05 bcd | 0.92 ± 0.09 cd | 0.92 ± 0.06 cd | 0.86 ± 0.04 abc | 0.78 ± 0.02 abc | 0.83 ± 0.04 abc | 0.83 ± 0.01 abc | 0.74 ± 0.02 ab | 0.79 ± 0.04 abc | 0.83 ± 0.04 abc | 0.70 ± 0.03 a |
| 4 | PLS | 1.74 ± 0.02 ab | 1.80 ± 0.02 ab | 1.67 ± 0.16 a | 2.15 ± 0.29 b | 1.70 ± 0.19 ab | 1.59 ± 0.05 a | 1.67 ± 0.06 a | 1.83 ± 0.06 ab | 1.55 ± 0.04 a | 1.75 ± 0.05 ab | 1.71 ± 0.14 ab | 1.70 ± 0.05 ab | 1.82 ± 0.01 ab |
| 5 | OLL | 26.77 ± 0.50 ab | 27.00 ± 0.08 abc | 27.44 ± 0.11 abc | 27.09 ± 0.30 abc | 26.52 ± 0.50 a | 27.05 ± 0.00 abc | 27.35 ± 0.43 abc | 27.17 ± 0.02 abc | 27.87 ± 0.00 c | 27.18 ± 0.15 abc | 27.76 ± 0.09 bc | 27.59 ± 0.27 bc | 27.08 ± 0.12 abc |
| 6 | PLL | 11.65 ± 0.09 d | 10.70 ± 0.04 ab | 10.71 ± 0.02 ab | 10.64 ± 0.25 ab | 11.42 ± 0.08 cd | 11.24 ± 0.40 bcd | 10.67 ± 0.02 ab | 11.02 ± 0.10 abcd | 10.79 ± 0.09 abc | 10.60 ± 0.06 ab | 10.67 ± 0.33 ab | 10.63 ± 0.02 ab | 10.43 ± 0.16 a |
| 7 | LLL | 35.26 ± 0.27 a | 36.13 ± 0.25 a | 35.52 ± 0.60 a | 34.93 ± 0.16 a | 35.57 ± 0.22 a | 35.57 ± 0.64 a | 35.68 ± 0.13 a | 35.01 ± 0.43 a | 34.88 ± 0.16 a | 36.01 ± 0.00 a | 35.62 ± 0.38 a | 35.28 ± 0.32 a | 36.12 ± 0.42 a |
| 8 | SLL | 11.92 ± 0.09 a | 12.54 ± 0.15 b | 12.55 ± 0.04 b | 12.62 ± 0.22 b | 12.54 ± 0.13 b | 12.35 ± 0.32 ab | 12.48 ± 0.06 b | 12.62 ± 0.15 b | 12.82 ± 0.00 b | 12.57 ± 0.07 b | 12.38 ± 0.13 ab | 12.79 ± 0.06 b | 12.72 ± 0.04 b |
| 9 | PLO | 6.49 ± 0.08 b | 6.13 ± 0.11 ab | 6.22 ± 0.05 ab | 6.35 ± 0.02 ab | 6.35 ± 0.08 ab | 6.43 ± 0.03 ab | 6.13 ± 0.03 ab | 6.36 ± 0.14 ab | 6.25 ± 0.14 ab | 6.07 ± 0.05 a | 6.06 ± 0.15 a | 6.11 ± 0.07 a | 6.12 ± 0.12 a |
| No # | Compound A | RI-E | RI-L | Total | Light Fractions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFE-CO2 + EtOH, % | SFE-CO2 + 3% EtOH | SFE-CO2 + 5% EtOH | SFE-CO2 + 7% EtOH | |||||||||||||
| 0 | 3 | 5 | 7 | 0 | −10 | −20 | 0 | −10 | −20 | 0 | −10 | −20 | ||||
| 1 | Furfural | 832 | 828 | 0.38 ± 0.04 | 1.98 ± 0.07 | 1.17 ± 0.11 | 1.94 ± 0.18 | 1.07 ± 0.05 | 0.60 ± 0.09 | 2.06 ± 0.20 | 0.30 ± 0.05 | 1.28 ± 0.06 | 0.14 ± 0.01 | 0.19 ± 0.02 | 0.39 ± 0.04 | 1.06 ± 0.09 |
| 4 | Butyrolactone | 938 | 941 | 0.88 ± 0.02 | 1.16 ± 0.06 | 1.27 ± 0.04 | 1.00 ± 0.05 | 0.88 ± 0.04 | 0.51 ± 0.02 | 1.57 ± 0.05 | 0.56 ± 0.04 | 0.65 ± 0.02 | 0.30 ± 0.00 | 0.37 ± 0.02 | 0.32 ± 0.02 | 0.32 ± 0.04 |
| 18 | Benzyl alcohol | 1040 | 1031 | 4.02 ± 0.10 | 4.15 ± 0.08 | 4.47 ± 0.16 | 4.90 ± 0.25 | 2.61 ± 0.03 | 5.99 ± 0.19 | 6.35 ± 0.03 | 5.01 ± 0.12 | 5.39 ± 0.28 | 3.83 ± 0.08 | 5.12 ± 0.22 | 5.72 ± 0.09 | 5.83 ± 0.23 |
| 25 | 2-oxo-2-Phenylacetonitrile | 1104 | 1095 | 3.27 ± 0.08 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.11 ± 0.01 | 2.60 ± 0.16 | 1.09 ± 0.06 | 0.69 ± 0.05 | 0.58 ± 0.03 | 0.19 ± 0.01 | 0.13 ± 0.00 | 0.19 ± 0.01 | 0.08 ± 0.00 | 0.06 ± 0.00 |
| 27 | Phenyl ethyl alcohol | 1119 | 1107 | 1.33 ± 0.02 | 1.11 ± 0.03 | 1.31 ± 0.06 | 1.49 ± 0.10 | 1.09 ± 0.01 | 2.33 ± 0.06 | 2.52 ± 0.02 | 1.63 ± 0.04 | 1.43 ± 0.07 | 1.00 ± 0.02 | 1.41 ± 0.07 | 1.58 ± 0.04 | 1.71 ± 0.10 |
| 33 | Benzoic acid | 1202 | 1197 | 0.71 ± 0.13 | 0.73 ± 0.08 | 0.98 ± 0.08 | 1.17 ± 0.04 | 1.32 ± 0.17 | 2.57 ± 0.05 | 1.55 ± 0.22 | 1.78 ± 0.21 | 1.03 ± 0.03 | 0.62 ± 0.14 | 0.70 ± 0.05 | 0.19 ± 0.04 | 0.19 ± 0.01 |
| Total identified, % | 92.06 | 94.67 | 91.62 | 88.38 | 94.59 | 94.36 | 90.54 | 90.70 | 94.90 | 96.43 | 94.78 | 96.92 | 96.47 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarv, V.; Bhat, R.; Jūrienė, L.; Baranauskienė, R.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Supercritical Fluid Extraction of Lipids from Rowanberry Pomace with Pure CO2 and Its Mixtures with Ethanol Followed by the On-Line Separation of Fractions. Molecules 2025, 30, 964. https://doi.org/10.3390/molecules30040964
Sarv V, Bhat R, Jūrienė L, Baranauskienė R, Urbonavičienė D, Viškelis P, Venskutonis PR. Supercritical Fluid Extraction of Lipids from Rowanberry Pomace with Pure CO2 and Its Mixtures with Ethanol Followed by the On-Line Separation of Fractions. Molecules. 2025; 30(4):964. https://doi.org/10.3390/molecules30040964
Chicago/Turabian StyleSarv, Viive, Rajeev Bhat, Laura Jūrienė, Renata Baranauskienė, Dalia Urbonavičienė, Pranas Viškelis, and Petras Rimantas Venskutonis. 2025. "Supercritical Fluid Extraction of Lipids from Rowanberry Pomace with Pure CO2 and Its Mixtures with Ethanol Followed by the On-Line Separation of Fractions" Molecules 30, no. 4: 964. https://doi.org/10.3390/molecules30040964
APA StyleSarv, V., Bhat, R., Jūrienė, L., Baranauskienė, R., Urbonavičienė, D., Viškelis, P., & Venskutonis, P. R. (2025). Supercritical Fluid Extraction of Lipids from Rowanberry Pomace with Pure CO2 and Its Mixtures with Ethanol Followed by the On-Line Separation of Fractions. Molecules, 30(4), 964. https://doi.org/10.3390/molecules30040964









